ABSTRACT
Heterotopic ossification (HO) is the de novo formation of bone that occurs in soft tissue, through recruitment, expansion, and differentiation of multiple cells types including transient brown adipocytes, osteoblasts, chondrocytes, mast cells, and platelets to name a few. Much evidence is accumulating that suggests changes in metabolism may be required to accomplish this bone formation. Recent work using a mouse model of heterotopic bone formation reliant on delivery of adenovirus‐transduced cells expressing low levels of BMP2 showed the immediate expansion of a unique brown adipocyte‐like cell. These cells are undergoing robust uncoupled oxidative phosphorylation to a level such that oxygen in the microenvironment is dramatically lowered creating areas of hypoxia. It is unclear how these oxygen changes ultimately affect metabolism and bone formation. To identify the processes and changes occurring over the course of bone formation, HO was established in the mice, and tissues isolated at early and late times were subjected to a global metabolomic screen. Results show that there are significant changes in both glucose levels, as well as TCA cycle intermediates. Additionally, metabolites necessary for oxidation of stored lipids were also found to be significantly elevated. The complete results of this screen are presented here, and provide a unique picture of the metabolic changes occurring during heterotopic bone formation. J. Cell. Biochem. 117: 1044–1053, 2016. © 2015 The Authors. Journal of Cellular Biochemistry Published by Wiley Periodicals, Inc.
Keywords: BMP2, TRANSIENT BROWN FAT, TRICARBOXYLIC ACID CYCLE, METABOLOMICS
Heterotopic ossification (HO) is the formation of bone at extraskeletal sites. Much evidence suggests that this disease process occurs with specific injuries, known to change BMP signaling and selectively active selective immune responses. Clinically, HO is a significant concern due to the fact that the bone formation is not detected until there is mineralized matrix. At this point, there can be significant damage to the soft tissues that is not recoverable. Further, one of the only treatments is surgical removal, which is often difficult in that the new bone often is adjacent to, or encompassing peripheral nerves [Nauth et al., 2012] and in many cases the bone immediately grows back [Alfieri et al., 2012].
Much emphasis has been placed on developing models that are representative of the human clinical scenarios. Many models have evolved that consist of delivering high levels of recombinant protein in Matrigel®, which will provide the inflammatory component [Lounev et al., 2009]. However, much criticism has led investigators to develop other methods for inducing HO that do not rely on delivery of BMPs. One of these models in rats is an amputation model, resulting from a high‐pressure water blast, to mimic blast injuries in the military population, which often result in HO [Qureshi et al., 2015]. Another model that involves the deficiency of plasminogen (Plg‐/‐), the precursor of plasmin, develops robust HO in skeletal muscle after injury [Yuasa et al., 2015]. The only other models of HO are the result of genetic modification in mice [Chakkalakal et al., 2012]. A consistent theme in all the models is constitutive activation of BMP signaling as well as changes in inflammatory processes essential to the bone formation. However, collective results from our laboratory suggest that the changes in BMP signaling are not the cause of changes in inflammation.
The model utilized for these experiments involves the delivery of low levels of sustained‐release BMP2, through delivery of an adenovirus‐transduced cells. This model involves both changes in BMP signaling, because of BMP2 production, as well as inflammation because of delivery of the adenovirus‐transduced cells. Characterization of this model, shows one of the first changes in the tissues is in peripheral nerves [Salisbury et al., 2011, 2012; Lazard et al., 2015], followed by the release of cells from the perineurial layer of the adjacent peripheral nerves of cells that express uncoupling protein 1 (UCP1) [Olmsted‐Davis et al., 2007]. UCP1 is thought to be the hallmark of brown adipocytes, and further characterization of these cells suggest they match almost identically brown adipocytes present in depots, with the exception that they lack expression of PMDR16 [Salisbury et al., 2012]. Simultaneous with these changes in the perineurium, cells within the endoneurium undergo early osteogenesis, and express dlx 5 and osterix [Lazard et al., 2015]. These cells exit the nerve and circulate, where they extravasate through new vasculature being established at the site of bone formation [Lazard et al., 2015]. Recent analysis of human tissues of early heterotopic ossification, taken from surgical resection of damaged tissues, suggests a similar process is occurring [Salisbury et al., unpublished]. Characterization of the newly forming brown adipocyte‐like cells suggests they utilize metabolism to regulate oxygen tension within the micro‐environment and also express vascular growth factors. Regulation of oxygen tension in the microenvironment is critical during HO not only for chondrogenesis [Zuscik et al., 2008] and neurogenesis [Pistollato et al., 2007], both of which require an hypoxic microenvironment, but also for extensive vascularization, which requires normoxia [Joyal et al., 2011]. The presence of vasorepulsive force originating from significantly hypoxic areas has also been proposed [Joyal et al., 2011] and these forces may also be operative during HO. These results parallel recent observations in tissues encompassing the site of heterotopic ossification in patients after traumatic injury where significant areas of brown adipocytes appear to be present and undergoing robust metabolism [Salisbury et al., unpublished]. Recently, active MMP9 has been implicated as a key factor in this process [Rodenberg et al., 2011]. If activation of MMP9 is suppressed, heterotopic bone formation is dramatically reduced or completely absent [Davis et al., unpublished]. Activation of MMP9 is a complex mechanism that requires the presence of plasmin, the active form of plasminogen, thus, linking heterotopic ossification with platelet activation and recruitment to the site of new bone formation [Davis et al., unpublished]. In our model of HO platelet activation using FACS for integrin α2bβ3, is always observed [Davis et al., unpublished], which is consistent with the elevation of adenosine diphosphate (ADP) observed in the studies described here, since the P2Y12, an (ADP) chemoreceptor on platelet cell membranes is another key receptor in platelet activation. Such changes in heterotopic ossification in burns have already been reported by others, but without reference to platelet activation [Peterson et al., 2014]. Interestingly, it is known that regulation of platelet activation is controlled by cd39/ATP diphosphohydrolase, which controls the level of ADP [Enjyoji et al., 1999].
Studies presented here, utilize the model of HO caused by injection of cells transduced by AdBMP2, to define changes in metabolites immediately following induction, and then just prior to mineral deposition during the progression of HO. The study design, therefore, permitted the identification of significant changes as compared to a control, which received similar transduced cells lacking the BMP2 transgene and did not result in bone formation, as well as changes associated with the progression of bone formation. This study is the first to describe changes in metabolites during heterotopic bone formation, and may provide novel targets that could be used for early detection of this disease process.
MATERIALS AND METHODS
CELLS AND VIRUSES
Ad5BMP2 (particle to PFU ratio less than 100), an E1‐ and E3‐region‐deleted replication defective recombinant adenovirus type 5 vectors possessing either a cDNA for BMP2 or no transgene (empty) were used to transduce C57BL/6 mouse skin fibroblasts. Cells were transduced using Genejammer® as previously described [Fouletier‐Dilling et al., 2005] at a multiplicity of 5,000 viral particles per cell. The virus particle to infectious particles or plaque forming units (PFU) was approximately 1:120 and 1:106 Ad5BMP2 and Ad5empty, respectively.
HETEROTOPIC OSSIFICATION MODEL
C57BL/6 mice were intramuscularly injected into the mice hind limb muscle with approximately 5 × 106 mouse skin fibroblasts transduced with Ad5BMP2 or Adempty cassette virus as described above. Mice were euthanized at the time points indicated and the tissue surrounding the site of injection was weighed and then extracted for metabolite isolation and fractionation. All experiments were conducted under an IACUC approved protocol in accordance with OLAW. All animals are housed in an ALAAC accredited vivarium under standard conditions in accordance with OLAW. Animals were randomly selected based on age and health, and placed in an experimental group. Each animal was given an experimental number that is linked to its group only in the medical record. Therefore, experimenters involved in data collection and analysis were blinded, and the animal numbers only linked to groups for the final data analysis. Group sizes were based on historical power analysis data using this model, however, all power analysis was repeated after data collection to confirm group sizes were adequate.
EXTRACTION OF METABOLITES FROM TISSUE
Chilled methanol:water (4:1 v/v, 750 µl) was added to each tissue sample. Then, approximately 200 µl of internal standards in methanol:water (4:1 v/v) was added to each sample. The final volume was made to 10 ml and the solution vortexed, homogenized, and divided into four equal aliquots. HPLC grade chloroform was then added (450 µl to each aliquot). The solution was vortexed, centrifuged, and the bottom organic layer was used for gas chromatography and the top aqueous layer for liquid chromatography/mass spectroscopy.
METABOLITE ANALYSIS
Samples were analyzed on a Agilent 6490 triple quadrupole (QQQ) mass spectrometer connected to an Agilent UHPLC. Metabolites were identified with isotopically labeled standards added to the tissue homogenates as well as using Agilent MassHunter Metabolite ID software.
RESULTS
GLYCOLYSIS
Heterotopic ossification was established in the mice through delivery of AdBMP2‐transduced cells and mice were euthanized at two time points. Tissues were then isolated and evaluated for metabolites involved in glycolysis. The first time point depicted on the graph (Fig. 1) is an early stage of heterotopic bone formation (Day 2), whereas the second is just before the appearance of bone and cartilage (Day 4). There were several metabolites involved in glycolysis that were altered relative to their respective controls (Fig. 1). In all cases, the control represents the same tissues isolated at the same time from animals that received the same cells transduced with the same adenovirus, but which lacked the BMP2 transgene cassette. Metabolites that changed significantly are shown on the graph in red text. The graph depicts the average of three replicate samples, and the error bars represent standard error of mean to a 95% confidence level (P < 0.05). Glucose and phosphoenol pyruvate were found to be elevated in the tissues at both time points. Further, comparisons between the two time points, using one way analysis of variance (ANOVA) with Tukey post hoc test showed no significant differences in this elevation between days 2 and 4. Alternatively, pyruvate and fructose 1,6‐bisphosphate, intermediate steps in glycolysis just prior to the branch between synthesis of pyruvate and glycerol, were both found to be elevated over control at the early stages of HO, but then dropped to control levels towards bone formation.
Figure 1.

Metabolite profiles of glycolytic intermediates on day 2 and day 4 after BMP2 induction during heterotopic ossification. Relative response refers to the quantitative determination of metabolite concentration relative to the amount of the internal spike of that same metabolite. Tissue was collected from BMP2‐induced and processed, and metabolites identified as described in the Materials and Methods. The average Day 2 value for each metabolite was normalized to the average day 2 control value, where AdEmpty‐transduced cells were injected; likewise, the average day 4 value for each metabolite obtained after 4 days BMP2 induction was normalized to the average day 4 value of the control.
TRICARBOXYLIC ACID CYCLE
Metabolites involved in the tricarboxylic acid cycle were also analyzed in tissues isolated that surrounded the site of heterotopic bone formation and compared to control tissues. Both citrate and isocitrate were significantly elevated both at the early time as well as just prior to the deposition of cartilage and bone (Fig. 2). None of the enzymes later in the cycle were elevated, suggesting a conversion of the intermediate α‐ketoglutarate, to glutamate. Glutamate was found to be significantly elevated on day 4, but not day 2. Also in support the biosynthesis of glutamate was the significant decrease in glutamine on day 2 as compared to its control. Since both α‐ketoglutarate and glutamine can be utilized for glutamate synthesis suggests a concerted effort to accumulate glutamate.
Figure 2.

Changes in components in the TCA cycle during heterotopic ossification.
AMINO ACID AND FATTY ACID BREAKDOWN
These are catabolic processes by which fatty acid and amino acid molecules are broken down in the mitochondria to generate acetyl‐CoA, which enters the citric acid cycle, and NADH and FADH2, which are co‐enzymes used in the electron transport chain. Several of these metabolites were analyzed as depicted in Figure 3. Three key enzymes in the breakdown and transfer of intermediates into the mitochondria for use in oxidative phosphorylation and tricarboxylic acid cycle were found to be significantly elevated in the tissues undergoing heterotopic bone formation as compared with the control tissues. Propionyl and heptanoyl carnitines were both elevated, as well as carnitine itself on day 4 of HO. All other carnitine metabolites were unchanged.
Figure 3.
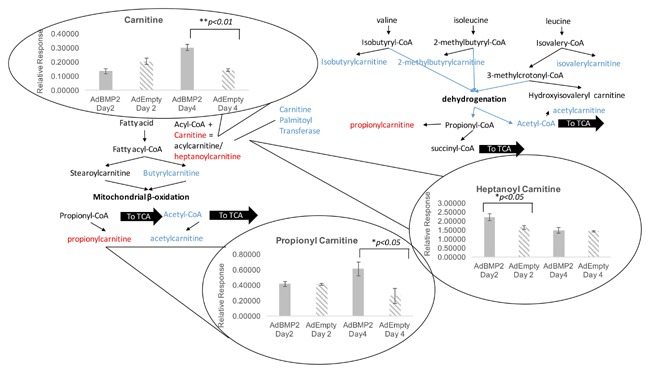
Changes in carnitine levels during heterotopic ossification.
LIPOLYSIS
Several lipid metabolites were analyzed in the tissues that showed the distinctive breakdown in both omega 3 and omega 6 fatty acids (Fig. 4). Other types of lipids remained unchanged. The saturated fat tetradecanoic, hexadecanoic, heptadecanoic, octadecanoic acids were all analyzed and there was a slight but statistically significant decrease in heptadecanoic acid in tissues undergoing HO on day 2, as compared with the control. All the others remained unchanged (Table I). Alternatively, the omega 3 and omega 6 pathways had several metabolites that were elevated on day 4, suggesting that these pathways were actively breaking down lipid to synthesize intermediates such as arachidonic acid and other regulators of arachidonic acid cascade (Fig. 4). Only one intermediate of the omega 7 pathway was analyzed and it was unchanged.
Figure 4.
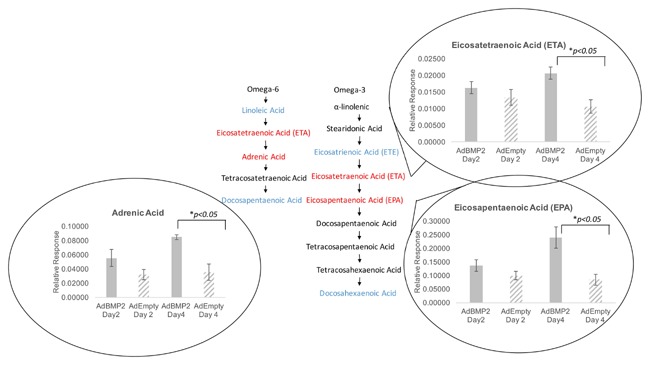
Changes in omega 3‐ and omega 6‐fatty acids during heterotopic ossification.
Table I.
Changes in Key Metabolites Relative to Control on the Second and Fourth Day After BMP2 Induction
| Metabolite | D2 control | D2 BMP2 | Fold chg | D4 control | D4 BMP2 | Fold chg |
|---|---|---|---|---|---|---|
| TCA cycle | ||||||
| Citrate | 5.1 | 10.4 | 2.0 | |||
| Citrate | 3.9 | 16.5 | 4.2 | |||
| Isocitrate | 5.3 | 10.7 | 2.0 | |||
| Isocitrate | 3.8 | 16.2 | 4.2 | |||
| Glutamic acid | 6.3 | 7.4 | 1.2 | |||
| Glutamic acid | 4.9 | 12.0 | 2.4 | |||
| Glutamine | 0.5 | 0.32 | −1.6 | |||
| Glutamine | 0.46 | 0.42 | −1.1 | |||
| Succinate | 3.0 | 4.0 | 1.3 | |||
| Succinate | 3.2 | 1.9 | −1.7 | |||
| Carnitines | ||||||
| Carnitine | 0.20 | 0.13 | −1.5 | |||
| Carnitine | 0.14 | 0.30 | 2.2 | |||
| Butryl carnitine | 5.7 | 3.7 | ||||
| Propeonyl carnitine | 0.26 | 0.61 | 2.3 | |||
| Isobutyl carnitine | 5.0 | 3.2 | ||||
| Heptanoyl carnitine | 2.2 | 1.5 | ||||
| Heptanoyl carnitine | 1.6 | 2.2 | 1.4 | |||
| Nucleotides | ||||||
| ADP | 0.44 | 0.6 | 1.4 | |||
| Polyamines | ||||||
| Spermidine | 0.001 | 0.019 | 19 | |||
| Spermine | 0.00012 | 0.00040 | 3.3 | |||
| Spermine | 0.000073 | 0.0004 | 5.5 | |||
| 1,5 diaminopentane | 0.00031 | 0.00021 | ||||
| 1,5 diaminopentane | 0.000075 | 0.00021 | 2.8 | |||
| Lysine | 0.03 | 0.014 | −2.1 | |||
| Lysine | 0.014 | 0.007 | ||||
| Lysine | 0.028 | 0.007 | 4.0 | |||
| Vitamins | ||||||
| Ascorbic acid | 0.0014 | 0.0017 | 1.2 | |||
| Vitamin D3 | 0.0016 | 0.0021 | 1.3 | |||
| Lipids | ||||||
| Heptadecanoic acid | 0.041 | 0.030 | ||||
| Eicosapentaennoic acid | 0.08 | 0.24 | ||||
| Eicosapentaenoic acid | 0.14 | 0.24 |
The average of values is shown for control and BMP2‐treated mice on days 2 and 4. Only values, the changes in which are significant (P < 0.05) are shown except for the day 4 values for succinate.
POLYAMINES
Polyamines are organic polycations, which have variable length hydrocarbon chains and two or more primary amino groups. They are synthesized in cells via highly regulated pathways, and are involved in cell growth regulation. Cadaverine (1,5 diaminopentane) is significantly increased on day 4 during HO (Table I). Cadaverine is synthesized in one step from lysine, and in support of this, lysine was found to be significantly decreased relative to the control. Additionally, the polyamines, spermine and spermidine, both were significantly elevated at the later time point (day 4) tested during HO (Table I).
VITAMINS
Several vitamins including vitamin A (retinol), vitamin E (α‐tocopherol), vitamin B3 (nicotinamide), vitamin C (L‐ascorbic acid), and vitamin D (cholecalciferol) were also screened for significant changes during heterotopic bone formation. Two were significantly elevated as relative to control tissues, vitamin C and vitamin D. Interestingly, vitamin C was elevated at the early time (Day 2) during heterotopic bone formation, whereas vitamin D was significantly elevated at the later (Day 4). A summary containing the actual values of each of the metabolites is given in Table I.
STEROIDS
Several steroids were also measured during HO and compared to the matched controls. Several steriods did not change, which included androsterone and its down stream products androstenedione, testosterone, and dihydroxytestosterone. Several steriods were altered, including 17‐OH‐progesterone and the intermediates in the cortisone pathway (17‐deoxycorticosterone and 11 deoxycortisol) were significantly lower in the BMP2 group as compared with the control suggesting the synthesis of these steroids may be somewhat suppressed early on in the bone formation process.
NUCLEOTIDES
Several nucleotides involved in purine biosynthesis including (adenosine, xanthine, xanthosine, and hypoxanthine) were measured and found to be unchanged during HO. AMP was also found to be unchanged. Surprisingly, ADP was significantly elevated on the second day of induction relative to the AdEmpty vector control. This was the only nucleotide measured that was elevated, since the pyrimidine cytosine was measured but remained unchanged.
DISCUSSION
GLYCOLYSIS
It is obvious that there is increased flux through the glycolytic pathway during HO as several intermediates downstream from glucose, including fructose 1,6 bisphophate, phosphoenolpyruvate, and pyruvate are also increased. However, biosynthesis of glucose and phosphoenol pyruvate from oxaloacetate is also possible as described below.
Several catabolic pathways converge on the TCA cycle. Most of these reactions add intermediates to the TCA cycle and are, therefore, known as anaplerotic reactions with processes that remove intermediates from the cycle termed cataplerotic reactions. When the enzymes and throughput of the electron transport chain rapidly increase, as we have shown during HO [Olmsted‐Davis et al., 2007], an increased supply of key intermediates, such as NADH and acetyl CoA, must be provided by the TCA cycle. However, this increase necessitates increased cataplerosis since the TCA cycle cannot be a carbon sink [Owen et al., 2002]. One other way to draw off this increased supply of TCA cycle intermediates is by the synthesis of glucose. PEPCK is frequently used for this process and is a major pathway for the synthesis of glucose. Transcription of the PEPCK gene is regulated by insulin glucocorticoids, cAMP, and diet, in order to adjust glucose production to physiologic requirements [Yu et al., 1993]. We suggest that this cataplerotic reaction is another reason for the consistent increase observed in glucose and phosphoenol pyruvate during HO.
CARNITINES
Several of the carnitines increase on the fourth day after BMP2 induction. Fat is either metabolized to produce energy after activation and transportation to the mitochondria from the cytosol via the carnitine shuttle, or it is stored in the adipose tissue as lipid droplets. We have shown that transient brown fat (tBAT) is an energy powerhouse and that this cell is composed of abundant mitochondria as well as numerous lipid droplets [Olmsted‐Davis et al., 2007; Salisbury et al., 2012]. For utilization of these stored fats, the fatty acids from the lipid droplets undergo mobilization and cellular uptake by the target cell. During HO the first target cell could be tBAT itself. Once in the target cell, for the fatty acid to undergo metabolism it has to reach the mitochondria as all the enzymes required for fatty acid oxidation are present there. The fatty acids undergo activation in the cytosol through conversion to fatty acyl CoA in order to pass though the mitochondrial membrane with the help of carnitine (present as L‐carnitine, the active stereoisomer) after which they undergo β‐oxidation in the mitochondria [Flanagan et al., 2010]. Fatty acyl‐CoA formed in the cytosol can also be used for synthesis of phospholipids and triacylglycerols.
TCA CYCLE
The enzyme citrate synthase is inhibited by high ratios of ATP:ADP, acetyl‐CoA:CoA, and NADH:NAD, as high concentrations of ATP, acetyl‐CoA, and NADH show that the energy supply is high for the cell. However, during the initial phase of HO we know that the high uncoupled aerobic respiration of tBAT [Olmsted‐Davis et al., 2007; Salisbury et al., 2012], requires high energy expenditure by the cell, therefore, it is not surprising that we see an increase in citrate during the initial phases of HO. Recently, two other important facts about citrate have been determined. First, it has been found to be an important component of the bone matrix. It is strongly bound to the apatite nanocrystals in bone and accounts for 5.5 wt% of the organic fraction of the nanocomposite in bone [Davies et al., 2014], and thus, provides more COO– groups for binding to calcium of apatite than all noncollagenous proteins in bone combined [Hu et al., 2010]. We speculate that it is tBAT that carries out the addition of citrate to the bone matrix, since this is the cell that would have an elevation in citrate because of the anaplerotic effect of increased uncoupled respiration. Second, the new heterotopic bone must also be innervated and vascularized. Although citrate is metabolized through the TCA cycle, it is also exported to the cytoplasm, where it is cleaved by ATP citrate lyase to form acetyl CoA, which is a precursor to fatty acids. Again tBAT would be a candidate cell for such fatty acid production, since it indeed stores lipids in the form of lipid droplets. This would also supply the demand for lipids for new myelin synthesis since the myelin sheath of nerves is over 90% fat, although tBAT may transfer this fat to Schwann cells before myelin deposition. It has recently been determined that, the tumor suppressor LKB1 is requisite for utilization of this citrate shunt for increased production of cellular lipids for increased myelination [Patel et al., 2015].
We did not, however, see an increase in oxaloacetate, which could indicate that this component is drawn off rapidly by the highly exergonic citrate synthase reaction, which keeps intramitochondrial oxaloacetate at exceedingly low levels. Additionally, oxaloacetate is probably drawn off to make glucose as a cataplerotic reaction to maintain a homeostatic level of TCA cycle intermediates.
The levels of isocitrate mimic the levels of citrate, since the conversion of citrate to isocitrate is an isomerization carried out by aconitase with no other substrate involved other than citrate itself.
Additionally, succinate shows a statistically significant increase over control on the second day after BMP2 induction, but then although not quite achieving significance between the second and the fourth day after induction (Table I), it shows a trend toward a decline. It has now been established that an increase in succinate defines an ischemic or hypoxic state [Chouchani et al., 2014]. In the case of hypoxia, succinate dehydrogenase apparently favors the reverse reaction synthesizing succinate by reducing α‐ketogluterate [Chouchani et al., 2014]. On the second day after BMP2 induction we see rising succinate. We have previously shown that during this time period tBAT burns the oxygen in the microenvironment creating a hypoxic state in the tissue around it [Olmsted‐Davis et al., 2007], because in tBAT the dominant enzyme is UCP1 rather than ATPase [Olmsted‐Davis et al., 2007], and the end result is creation of hypoxia in the microenvironment by actual “burning” of oxygen because of the extremely high catalytic rate of UCP1. From days 2 to 4 after BMP2 induction we see a trend toward declining succinate. We would suggest that a decline in this TCA cycle intermediate indicates intense aerobic metabolic activity. This decline probably follows as hypoxia activates HIF1 and starts the cascade of vascularization that is initiated during HO [Dilling et al., 2010; Lazard et al., 2015], but in a manner such that the tissue hypoxia and the vessel formation co‐exist, adjacent to each other, but each in a distinct area. Therefore, we would argue that a shift in the metabolite profile in the TCA cycle is consistent with the actual patterning we observe during bone formation. A similar cycling is observed in glycerol 3 phosphate.
THE GLUTAMATE–GLUTAMINE CYCLE
One likely reason for the observed increases in glutamic acid after induction is the transamination of α ketogluterate by glutamate dehydrogenase. However, we also note that glutamine levels are significantly downregulated on the second day after BMP2 induction (not shown, P = 0.006). We have recently noted the presence of an astrocyte‐like cell that appears during HO and expresses the L‐glutamate/L‐aspartate transporter (GLAST), which is a protein specific to astrocytes [Davis et al., unpublished]. Surprisingly, this astrocyte‐like cell is the initial target of BMP2 and reacts with phosphoSMAD antibody one day after BMP2 induction. One of the main functions of GLAST is to detoxify the synaptic area of neurons conducting glutaminergic transmission (for review see ref. [Stobart and Anderson, 2013]). This is done by the astrocyte taking up excess glutamic acid via GLAST. When inside the astrocyte this glutamate is either converted to α ketogluterate by the reverse of the transamination noted above, or it is converted to glutamine via glutamine synthase. Glutamine is then shunted to the neuron where it is converted back to glutamate by a phosphate‐activated glutaminase. One other potential reason for the synthesis and secretion of glutamate by these astrocyte‐like cells is their possible participation in electrical transmission. We see these cells spaced along the sheath of peripheral nerves, a position where they could participate in neurotransmission if they were associated with the Nodes of Ranvier.
LIPIDS
It has been recently shown that both DHA and ETA are anti‐inflammatory, and act through the free fatty acid receptor 4 (FFAR4) [Yore et al., 2014]. Therefore, it is likely that on the second day after BMP2 induction, since there is a decline in DHA, this period is inflammatory, but only on day 4, with an increase in EPA, does one, therefore, see a decline in initial inflammatory response. It has recently been shown that binding of ligands to FFAR induces the incretin glucagon‐like peptide 1 (GLP1) [Hirasawa et al., 2005]. It is likely that this would indeed have an effect on bone formation since the amount of Wnt proteins binding to the LRP5 and six receptors is important in bone formation [Kato et al., 2002]. It has recently been shown that inhibitors of DKK proteins, which are themselves inhibitors of Wnt binding to LRP5/6, indeed induce GLP1 [Li et al., 2012].
The metabolite ETA, which is created by phospholipase A2 from diacyl glycerol is also called arachidonic acid and is oxygenated by cyclo‐oxygenases and lipoxygenase converting it to a number of bio‐active eicosanoids. These eiconsanoids have roles in vessel relaxation and constriction, and also have pro‐ and anti‐inflammatory effects.
NUCLEOTIDES
ADP is a very important molecule in platelet activation through the purogenic receptor P2Y12 [Dorsam and Kunapuli, 2004]. It is known that activated platelets play a critical role in vasculogensis [Daub et al., 2006; Leshem‐Lev et al., 2010] and that the second day after BMP2 induction is a critical time for the formation of new vessels to carry osteoprogenitors from the nerve to the site of bone formation [Dilling et al., 2010; Lazard et al., 2015]. Therefore, it is conceivable that one of the functions of the elevation of ADP during this time period is for stimulating P2Y12 for platelet activation to fulfill their role in new vessel formation, although there are many other potential functions for this metabolite.
Indeed, it has recently been shown that treatment with apyrase lowers the amount of heterotopic ossification that occurs during burns by lowering the amount of ATP [Peterson et al., 2014].
POLYAMINES
Polyamines have been implicated in a large number of cellular processes, including functioning of ion channels, nucleic acid packaging, DNA replication, apoptosis, transcription, and translation [Childs et al., 2003]. However, recent studies have shown that the polyamines spermine and spermidine are an essential part of the granule component of mast cells, and play an essential role in the biogenesis and homeostasis of these organelles [Garcia‐Faroldi et al., 2010]. We have previously shown that mast cells are a critical component of HO and when these cells are eliminated HO is severely inhibited [Salisbury et al., 2011]. Therefore, spermine and spermidine may participate in the function of mast cells during HO.
VITAMINS
In the synthesis of collagen, ascorbic acid is required as a cofactor for prolyl hydroxylase and lysyl hydroxylase. These two enzymes are responsible for the hydroxylation of the proline and lysine amino acids in collagen. Hydroxyproline and hydroxylysine are important for stabilizing collagen by cross‐linking the propeptides in collagen. Defective collagen fibrillogenesis would, therefore, be expected to impair bone formation. Vitamin D3 is also essential for bone formation and lack of it causes ricketts and osteomalcia.
CONCLUSIONS
Heterotopic ossification undergoes an ordered series of steps from its onset to the resultant formation of bone that has marrow and remodels, but is present in an ectopic location. In this paper, we note that small molecule metabolites increase and decrease in specific patterns during HO, seemingly in lock‐step with what is occurring in the bone formation process. This analysis may not only help us to understand this complex and multi‐step process, but may also identify metabolic processes which can be targeted to diagnosis and prevent HO. Figure 5 shows a graphical summary of we envision metabolism fits with our current hypothetical mechanism of HO.
Figure 5.
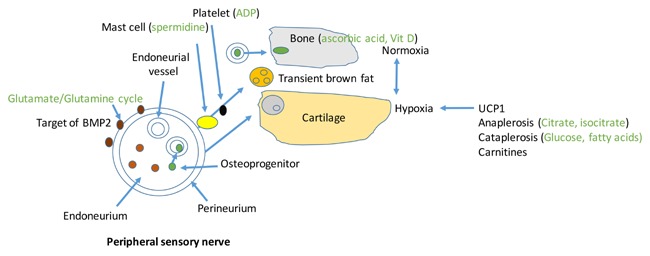
Overlay of metabolomics findings from this manuscript on our model of heterotopic ossification. HO is initiated by BMP2 binding to an astrocyte‐like glial cell in the perineurium of peripheral nerves. Glutaminergic signaling initiates a cascade causing transient brown fat and chondroprogenitors to exit the perineurium, whereas osteoprogenitors traverse the blood‐nerve barrier and enter endoneurial vessels. Transient brown fat sets up an hypoxic area immediately adjacent to an area of normoxia and vessel formation. Osteoprogenitors extravasate through the vessel wall to enter the site of bone formation. Chondroprogenitors enter the hypoxic area and differentiate to chondrocytes. Mast cells and platelets are required for release of cells from the perineurium of the nerve. Ascorbic acid and vitamin D are transported to the area of bone formation, although the cell that makes these vitamins is unknown.
ELD, EAS, EO‐D, and ARD participated in performing the experiments, collecting and interpreting data, and drafting and finalizing the manuscript.
REFERENCES
- Alfieri KA, Forsberg JA, Potter BK. 2012. Blast injuries and heterotopic ossification. Bone Joint Res 1:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, Maidment AD, Kaplan FS, Shore EM. 2012. An Acvr1 R206H knock‐in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res 27:1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs AC, Mehta DJ, Gerner EW. 2003. Polyamine‐dependent gene expression. Cell Mol Life Sci 60:1394–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord EN, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa AS, Brookes PS, Davidson SM, Duchen MR, Saeb‐Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, Murphy MP. 2014. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daub K, Langer H, Seizer P, Stellos K, May AE, Goyal P, Bigalke B, Schonberger T, Geisler T, Siegel‐Axel D, Oostendorp RA, Lindemann S, Gawaz M. 2006. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB J 20:2559–2561. [DOI] [PubMed] [Google Scholar]
- Davies E, Muller KH, Wong WC, Pickard CJ, Reid DG, Skepper JN, Duer MJ. 2014. Citrate bridges between mineral platelets in bone. Proc Natl Acad Sci USA 111:E1354–E1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilling CF, Wada AM, Lazard ZW, Salisbury EA, Gannon FH, Vadakkan TJ, Gao L, Hirschi K, Dickinson ME, Davis AR, Olmsted‐Davis EA. 2010. Vessel formation is induced prior to the appearance of cartilage in BMP‐2‐mediated heterotopic ossification. J Bone Miner Res 25:1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsam RT, Kunapuli SP. 2004. Central role of the P2Y12 receptor in platelet activation. J Clin Invest 113:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd , Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. 1999. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 5:1010–1017. [DOI] [PubMed] [Google Scholar]
- Flanagan JL, Simmons PA, Vehige J, Willcox MD, Garrett Q. 2010. Role of carnitine in disease. Nutr Metab (Lond) 7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouletier‐Dilling CM, Bosch P, Davis AR, Shafer JA, Stice SL, Gugala Z, Gannon FH, Olmsted‐Davis EA. 2005. Novel compound enables high‐level adenovirus transduction in the absence of an adenovirus‐specific receptor. Hum Gene Ther 16:1287–1297. [DOI] [PubMed] [Google Scholar]
- Garcia‐Faroldi G, Rodriguez CE, Urdiales JL, Perez‐Pomares JM, Davila JC, Pejler G, Sanchez‐Jimenez F, Fajardo I. 2010. Polyamines are present in mast cell secretory granules and are important for granule homeostasis. PLoS ONE 5:e15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. 2005. Free fatty acids regulate gut incretin glucagon‐like peptide‐1 secretion through GPR120. Nat Med 11:90–94. [DOI] [PubMed] [Google Scholar]
- Hu YY, Rawal A, Schmidt‐Rohr K. 2010. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc Natl Acad Sci USA 107:22425–22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D, Djavari M, Kunik D, Honore JC, Picard E, Zabeida A, Varma DR, Hickson G, Mancini J, Klagsbrun M, Costantino S, Beausejour C, Lachapelle P, Smith LE, Chemtob S, Sapieha P. 2011. Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood 117:6024–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd , Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. 2002. Cbfa1‐independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol 157:303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazard ZW, Olmsted‐Davis EA, Salisbury EA, Gugala Z, Sonnet C, Davis EL, Beal E, 2nd , Ubogu EE, Davis AR. 2015. Osteoblasts have a neural origin in heterotopic ossification. Clin Orthop Relat Res 473(9):2790–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem‐Lev D, Omelchenko A, Perl L, Kornowski R, Battler A, Lev EI. 2010. Exposure to platelets promotes functional properties of endothelial progenitor cells. J Thromb Thrombolysis 30:398–403. [DOI] [PubMed] [Google Scholar]
- Li X, Shan J, Chang W, Kim I, Bao J, Lee HJ, Zhang X, Samuel VT, Shulman GI, Liu D, Zheng JJ, Wu D. 2012. Chemical and genetic evidence for the involvement of Wnt antagonist Dickkopf2 in regulation of glucose metabolism. Proc Natl Acad Sci USA 109:11402–11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. 2009. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am 91:652–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauth A, Giles E, Potter BK, Nesti LJ, O'Brien FP, Bosse MJ, Anglen JO, Mehta S, Ahn J, Miclau T, Schemitsch EH. 2012. Heterotopic ossification in orthopaedic trauma. J Orthop Trauma 26:684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted‐Davis E, Gannon FH, Ozen M, Ittmann MM, Gugala Z, Hipp JA, Moran KM, Fouletier‐Dilling CM, Schumara‐Martin S, Lindsey RW, Heggeness MH, Brenner MK, Davis AR. 2007. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol 170:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Kalhan SC, Hanson RW. 2002. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem 277:30409–30412. [DOI] [PubMed] [Google Scholar]
- Patel AV, Johansson G, Colbert MC, Dasgupta B, Ratner N. 2015. Fatty acid synthase is a metabolic oncogene targetable in malignant peripheral nerve sheath tumors. Neuro Oncol 17(12):1599–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JR, De La Rosa S, Eboda O, Cilwa KE, Agarwal S, Buchman SR, Cederna PS, Xi C, Morris MD, Herndon DN, Xiao W, Tompkins RG, Krebsbach PH, Wang SC, Levi B. 2014. Treatment of heterotopic ossification through remote ATP hydrolysis. Sci Transl Med 6:255ra132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistollato F, Chen HL, Schwartz PH, Basso G, Panchision DM. 2007. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol Cell Neurosci 35:424–435. [DOI] [PubMed] [Google Scholar]
- Qureshi AT, Crump EK, Pavey GJ, Hope DN, Forsberg JA, Davis TA. 2015. Early characterization of blast‐related heterotopic ossification in a rat model. Clin Orthop Relat Res 473:2831–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenberg E, Azhdarinia A, Lazard ZW, Hall M, Kwon SK, Wilganowski N, Salisbury EA, Merched‐Sauvage M, Olmsted‐Davis EA, Sevick‐Muraca EM, Davis AR. 2011. Matrix metalloproteinase‐9 is a diagnostic marker of heterotopic ossification in a murine model. Tissue Eng Part A 17:2487–2496. [DOI] [PubMed] [Google Scholar]
- Salisbury E, Rodenberg E, Sonnet C, Hipp J, Gannon FH, Vadakkan TJ, Dickinson ME, Olmsted‐Davis EA, Davis AR. 2011. Sensory nerve induced inflammation contributes to heterotopic ossification. J Cell Biochem 112:2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury EA, Lazard ZW, Ubogu EE, Davis AR, Olmsted‐Davis EA. 2012. Transient brown adipocyte‐like cells derive from peripheral nerve progenitors in response to bone morphogenetic protein 2. Stem Cells Transl Med 1:874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart JL, Anderson CM. 2013. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front Cell Neurosci 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yore MM, Syed I, Moraes‐Vieira PM, Zhang T, Herman MA, Homan EA, Patel RT, Lee J, Chen S, Peroni OD, Dhaneshwar AS, Hammarstedt A, Smith U, McGraw TE, Saghatelian A, Kahn BB. 2014. Discovery of a class of endogenous mammalian lipids with anti‐diabetic and anti‐inflammatory effects. Cell 159:318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Thun R, Chandrasekharappa S, Trent JM, Zhang J, Meisler MH. 1993. Human PCK1 encoding phosphoenolpyruvate carboxykinase is located on chromosome 20q13.2. Genomics 15:219–221. [DOI] [PubMed] [Google Scholar]
- Yuasa M, Mignemi NA, Nyman JS, Duvall CL, Schwartz HS, Okawa A, Yoshii T, Bhattacharjee G, Zhao C, Bible JE, Obremskey WT, Flick MJ, Degen JL, Barnett JV, Cates JM, Schoenecker JG. 2015. Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification. J Clin Invest 125:3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuscik MJ, Hilton MJ, Zhang X, Chen D, O'Keefe RJ. 2008. Regulation of chondrogenesis and chondrocyte differentiation by stress. J Clin Invest 118:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]


