Abstract
To understand the effect of low dietary phosphorus (P) intake on the vertebral column of Atlantic salmon Salmo salar, a primary P deficiency was induced in post‐smolts. The dietary P provision was reduced by 50% for a period of 10 weeks under controlled conditions. The animal's skeleton was subsequently analysed by radiology, histological examination, histochemical detection of minerals in bones and scales and chemical mineral analysis. This is the first account of how a primary P deficiency affects the skeleton in S. salar at the cellular and at the micro‐anatomical level. Animals that received the P‐deficient diet displayed known signs of P deficiency including reduced growth and soft, pliable opercula. Bone and scale mineral content decreased by c. 50%. On radiographs, vertebral bodies appear small, undersized and with enlarged intervertebral spaces. Contrary to the X‐ray‐based diagnosis, the histological examination revealed that vertebral bodies had a regular size and regular internal bone structures; intervertebral spaces were not enlarged. Bone matrix formation was continuous and uninterrupted, albeit without traces of mineralization. Likewise, scale growth continues with regular annuli formation, but new scale matrix remains without minerals. The 10 week long experiment generated a homogeneous osteomalacia of vertebral bodies without apparent induction of skeletal malformations. The experiment shows that bone formation and bone mineralization are, to a large degree, independent processes in the fish examined. Therefore, a deficit in mineralization must not be the only cause of the alterations of the vertebral bone structure observed in farmed S. salar. It is discussed how the observed uncoupling of bone formation and mineralization helps to better diagnose, understand and prevent P deficiency‐related malformations in farmed S. salar.
Keywords: malformations, mineral deficiency, scales, skeletal development, vertebral column
Introduction
Vertebrates depend on dietary phosphorus (P) intake for proper mineralization of their calcium phosphate‐based skeleton (Penido & Alo, 2012; Santos et al., 2013). Different from tetrapods, which also depend on dietary calcium intake, bony fishes can obtain calcium from the water via the gills. Only a minimal amount of phosphorus can be obtained through the gills and thus it remains a crucial component of the diet (Ketola, 1975; 1998, 1998; Sullivan et al., 2007a ; Witten & Huysseune, 2009). Accordingly, skeletal development in the fast‐growing Atlantic salmon Salmo salar L. 1758 under farming conditions requires sufficient dietary P supply (Baeverfjord et al., 1998; Sugiura et al., 2004; Fjelldal et al., 2012). The increased P demand of S. salar after smoltification is well documented (Albrektsen et al., 2009; Fjelldal et al., 2009), and several types of skeletal deformities, diagnosed on radiographs, have been related to dietary P deficiency: vertebral body compression, abnormal softness and structural distortion (Baeverfjord et al., 1998; 2007a, 2007b; Fjelldal et al., 2009). Deschamps et al. (2014) observed on X‐rays from P‐deficient rainbow trout Oncorhynchus mykiss (Walbaum 1792) decreased intervertebral spaces, homogenous compression of vertebral bodies, a combination of abnormalities, irregular compression and undersized vertebral bodies. Also, hyper radio‐dense vertebral bodies have been linked to mineral deficiency due to the development of ectopic cartilage in bone marrow spaces (Helland et al., 2006). Thus, radiographs of P‐deficient fishes show a wide array of vertebral column pathologies and there is no unified P‐deficiency pathology. The life stage in which the animal suffers insufficient P supply as well as the degree and period of P deficiency are likely to cause the different deformity phenotypes observed. Whether all of the aforementioned pathologies are primarily the results of P deficiency, if some are secondary phenomena or if some even relate to other factors is still a matter of discussion (Witten et al., 2009; Baeverfjord et al., 2012; Fjelldal et al., 2012). In a group of harvest size, S. salar in Norway, Fjelldal et al. (2012) diagnosed 70% of the fish as deformed based on radiographs, 26% as deformed based on palpation and 4% of the fish were actually downgraded at slaughter due to severe spinal deformities.
Four hundred and fifty million years ago, the availability of P could have been a limiting factor for the population size of early vertebrates, and it has been hypothesized that skeleton evolved as a phosphorus storage organ system (Tarlo, 1964; Carroll, 1988; Hall & Witten, 2007). Today, phosphorus is also a limiting resource for agriculture and aquaculture. To protect marine fish stocks, fishmeal, a common source of P in fish feeds must be replaced. At the same time, there is a limited availability of inorganic P. In addition, P discharge from freshwater farms has raised environmental concerns (Le Luyer et al., 2014). Clearly, there is a need to increase the efficiency of phosphorus use in terrestrial and aquatic farming practices (Sugiura et al., 2004; Naylor et al., 2009; Cordell et al., 2011). The increased efficiency in P use must, of course, not come at the expense of animal health and welfare. Thus, there is a growing demand to increase the digestibility of P in fish diets (Sullivan et al., 2007a ) and to determine the quantity of dietary P content that is required for healthy growth and healthy skeletal development in farmed fishes (Åsgård & Shearer, 1997; Roy et al., 2002; Deschamps et al., 2014). Greater knowledge of the mechanisms by which P deficiency causes skeletal malformations can contribute to improve the management of P resources in aquaculture.
It is here shown for the first time how a primary P deficiency affects the skeleton of S. salar at the cellular and at the micro‐anatomical level. It is also the first experiment that generated a homogenous primary P‐deficient skeletal phenotype in the absence of other pathologies. Bone growth and bone structures were not affected. The uncoupling of bone formation and bone mineralization has consequences for the interpretation of radiographs. Uncoupling of matrix formation and mineralization in scales offers the possibility for fast and minimal‐invasive future diagnosis of the animals' skeletal P status by scale analysis. A better understanding of this primary P deficiency should help to understand which of the P deficiency‐related pathologies are primary, which are secondary and how primary and secondary pathologies develop.
Materials and methods
Feeding experiment
The experiment was performed at Lerang Research Station (Skretting ARC, Lerang, Forsand, Norway). Salmo salar post‐smolts, with a mean ± s.d. mass of 199 ± 1 g, were obtained as eggs from Erfjord Stamfisk (SalmoBreed strain) and hatched and reared in‐house. Fish were hatched in March 2012, transferred to seawater on 29 October 2012, and the trial started in January 2013. Prior to the experiment, fish were held in 1 m diameter tanks and fed a commercial standard diet, Skretting Spirit 75, pellet size 3 mm (www.skretting.com).
At the start of the experiment, 120 post‐smolt fish were anaesthetized with MS‐222 (www.sigmaaldrich.com) and evenly distributed into four, 1 m diameter tanks supplied with a constant flow of clean seawater at 12° C and salinity of 33. Fish were fed to satiation twice a day, with waste feed collected, and feed intake was monitored throughout the experiment. For the duration of the experiment, the fish were kept in constant artificial light from fluorescent strip lighting above the experimental tanks. Duplicate tanks of fish were fed one of the two iso‐nitrogenous, iso‐calorific diets: a phosphorus‐sufficient diet (see Table I) formulated to have a total P content of 0·99% and a P‐deficient diet with a total P of 0·47. All diets were extruded via a Wenger twin screw extruder (www.wenger.com) at the Feed Trial Plant, Skretting ARC, Stavanger, Norway (Table I). After dietary conditioning for 10 weeks (Fjelldal et al., 2012), all the experimental animals were euthanized with an overdoses of MS‐222, weighed and fork length (L F) recorded prior to being radiographed.
Table I.
Composition of the phosphorus (P)‐sufficient and the P‐deficient diets for Salmo salar. Both diets were iso‐nitrogenous, iso‐lipidic and were formulated to have same digestible amino‐acid content, hence the use of crystalline amino acids. Ingredients were provided by Skretting Norway (www.skretting.com). Vitamin mix and mineral mix were provided by Trouw Nutrition International (www.trouwnutrition.com). Premixes formulated to meet all National Research Council (U.S.A.) (2011) nutritional requirements for salmonids excluding phosphorus (NRC, 2011)
| Ingredients (%) | P‐sufficient diet | P‐deficient diet |
|---|---|---|
| Wheat | 19·08 | 21·46 |
| Wheat gluten | 24·00 | 24·00 |
| Soya protein concentrate | 20·00 | 20·00 |
| Fish meal | 10·00 | 10·00 |
| Fish oil | 22·40 | 22·33 |
| Vitamin mix | 0·11 | 0·11 |
| Mineral mix | 0·10 | 0·10 |
| Monoammonium phosphate | 2·03 | 0·00 |
| Total phosphate (%) | 0·99 | 0·47 |
Radiology
All fish were radiographed using a Gierth XMF80 emitter (www.gierth‐x‐ray.de) and AGFA Structurix D4DW film (www.gemeasurement.com). No screen for intensifying the X‐ray beam was used. The settings of the X‐ray unit were 80 kV, 15 mA, 4 s exposure time at a distance of 80 cm between the X‐ray tube and the film. Radiographs were developed according to the protocol of the manufacturer at the Teknologisk Institutt, Stavanger, Norway (www.ti‐norway.com). For further analysis and measurements, radiographs were digitalized with a Heidelberg transparency scanner Linoscan‐1400 (www.heidelberg.com) at 1200 dpi.
Histology and mineral detection
Ten fish per tank, chosen at random (20 per treatment) were filleted, and the whole spine, excluding the head, fixed in 10% neutral‐buffered formalin for subsequent histological analysis. Twenty individual scales from the left‐hand side of the fish were removed with forceps for histological staining and stored at −20° C.
Spine sections for histological analysis (vertebrae numbers 32–42) were fixed in 10% neutral‐buffered formalin immediately after X‐raying. Prior to decalcification, spines were rinsed in tap water for 24 h and decalcified for 72 h in a 10% EDTA solution buffered with 0·1 M Tris base at pH 7·0. After decalcification, samples were stepwise dehydrated and embedded in paraplast. Serial sections of 7 µm thickness were prepared in the sagittal plane of the vertebral column, starting at the lateral periphery of vertebral bodies and ending in the medio‐sagittal plane. Sections were stained with Masson's trichrome as basic analytical procedure and mounted with DPX (www.sigmaaldrich.com), as described in detail by Witten & Hall (2003).
The bone minerals phosphorus and calcium on histological sections were detected with Von Kossa's protocol and Alizarin red staining on non‐decalcified paraffin and on non‐decalcified cryosections. For cryosectioning, vertebral bodies were rinsed in tap water for 24 h, washed in phosphate buffered saline (PBS), equilibrated in a 5% sucrose–PBS solution and embedded in 1·5% agar in 5% sucrose–PBS. The solidified tissue blocks were then transferred to 30% sucrose in PBS and left in the solution overnight (until equilibration). Cryosections of 12 µm were sectioned in the parasagittal plane and allowed to thaw on Superfrost Plus slides, coated with a 1 mg ml−1 poly‐L‐lysine hydrobromide solution, dried for 30 min at room temperature and stored dry at −20° C prior to staining for calcium and phosphorus. For staining minerals in paraffin sections, vertebrae were embedded and sectioned as described above, but not decalcified.
Phosphorus was visualized with the Von Kossa staining protocol according to Presnell & Schreibman (1998). In short, cryosections and dewaxed paraffin sections were rinsed in tap water and subsequently placed in a 1% silver nitrate solution under UV light for 45 min. The sections were then washed in distilled water and subsequently treated with 3% sodium thiosulphate for 5 min, rinsed in distilled water and then stained with van Gieson solution for 5 min. Bone calcium was visualized on cryosections and on dewaxed paraffin sections with a 0·5% Alizarin red‐S solution (pH 9). Staining lasted for 1 min followed by rinsing for another minute in demineralized water. No counter staining was applied. All Von Kossa and Alizarin red‐stained histological sections were mounted on slides with DPX. Scales were stained with the same 0·5% Alizarin red‐S solution for 15 min, rinsed in demineralized water and transferred into 100% glycerol via graded 1% KOH–glycerol solutions. Scales were mounted on slides using 100% glycerol. A Zeiss Axio Imager–Z compound microscope equipped with a Zeiss Axiocam (www.zeiss.com) was used for the analysis of all histological preparations.
Measurements
Measurements of the length (A) on Fig. 1(e), the height (B) on Fig. 1(e) of vertebral bodies (radiopaque area on X‐rays) and the width (C) on Fig. 1(e) of the intervertebral space (radiotranslucent area on X‐rays) were taken from vertebral bodies 32–42 from 30 individuals from each experimental group. Measurements were taken from digitized radiographs using ImageJ software (http://rsbweb.nih.gov/ij/download.html/).
Figure 1.
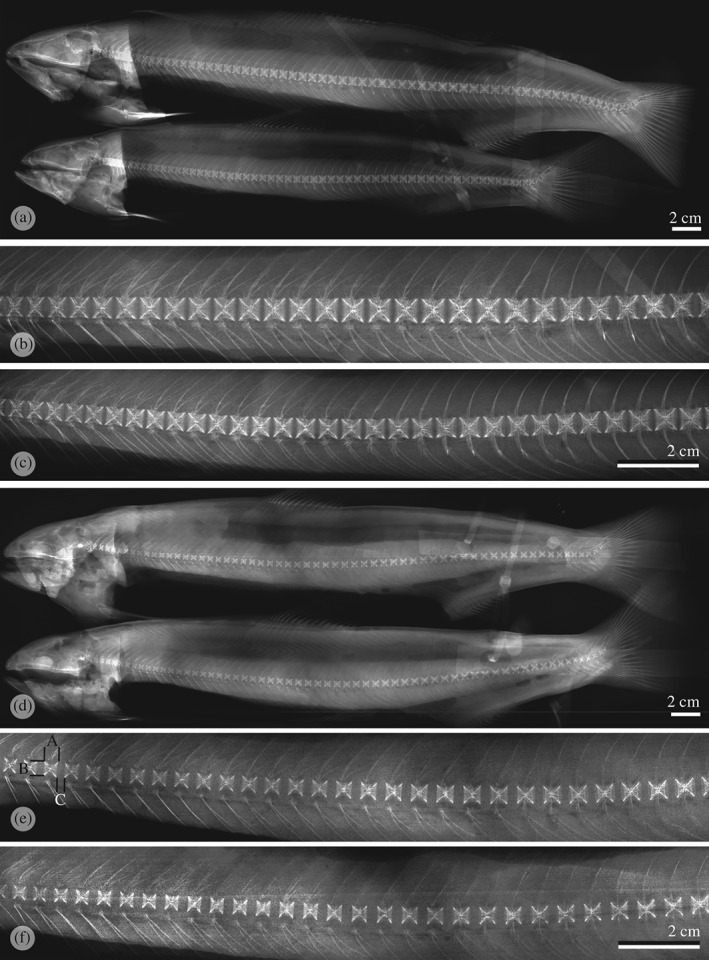
X‐rays from Salmo salar individuals that received a (a) P‐sufficient diet with (b, c) higher magnifications of the central part of their spines. Vertebral bodies display a regular shape in all parts of the spine. Individuals that received P‐deficient diet (d) with (e, f) higher magnifications of the central part of their spines. All vertebral bodies are undersized and all intervertebral spaces are enlarged. No other deformities are visible. The animals shown in this figure are representative for their experimental groups. (e) Measurements presented on Fig. 3: A, vertebral body length; B, vertebral body height; C, width of the intervertebral space.
Mineral analysis of bones and scales
From 20 animals per tank (40 animals per treatment), the entire right‐hand side of the animal was descaled, and the scales were stored in plastic bags at −20° C for scale mineral content analysis. Three adjoining vertebrae (numbers 32–35) plus the opercula from both sides of the animal were also removed and frozen at −20° C for tissue mineral content quantification. All samples were analysed for dry matter, ash, calcium and phosphorus. For mineral analysis, the soft tissue was removed and the bones were cleaned with demineralized water. Subsequently, lipids were removed from the samples by rinsing twice in a mixture of acetone and methanol (1:1, v/v). The samples were then dried for 24 h at 105° C, ashed at 550° C for 18 h and digested according to the (U.S.A.) Association of Official Analytical Chemists method (AOAC, 1995). The calcium and phosphorus content was determined colorimetrically (Taussky & Shorr, 1953). The analysis was carried out at Masterlab Analytical Services, Nutreco, The Netherlands.
Statistical analysis
Unless otherwise stated, all data are presented as mean ± s.d. All data were analysed using GraphPad Prism 5.0 for windows (www.graphpad.com/scientific‐software/prism) with percentage data arc‐sine transformed before subsequent analysis. The statistical tests used are indicated next to the relevant data table or figure legend.
Results
Growth and feed intake
After 10 weeks of dietary conditioning, mass in the P‐sufficient animals had increased from 199·0 to 684·9 ± 11·0 g, representing in excess of a tripling in body mass. The P‐deficient group had increased from 199·0 to 563·8 ± 10·0 g indicating that both diets were well received by the experimental animals. No significant differences were observed in the condition factor or feed conversion ratio of either experimental group (both P > 0·05); however, the final body mass and specific growth rate (% body mass day−1) was reduced in the P‐deficient animals (t‐test, P < 0·05) (Table II).
Table II.
Growth data for Salmo salar and t‐test comparison for significant difference (P value) between diets
| Animals that received the P‐sufficient diet | Animals that received the P‐deficient diet | P value | |
|---|---|---|---|
| Initial body mass (g) | 199·5 ± 0·8 | 198·5 ± 0·9 | >0·05 |
| Final body mass (g) | 684·9 ± 11·7 | 563·8 ± 10·0 | <0·05 |
| Condition factor | 1·39 ± 0·00 | 1·37 ± 0·01 | >0·05 |
| Specific growth rate (% body mass day−1) | 1·23 ± 0·02 | 1·04 ± 0·02 | <0·05 |
| Feed conversion ratio (dry matter) | 0·79 ± 0·00 | 0·80 ± 0·00 | >0·05 |
Mineral analysis
Mineral analysis (Fig. 2) showed a steep decrease of bone minerals in fish that received the P‐deficient diet. There was an equal reduction of calcium and phosphorus in all three analysed skeletal parts (opercula, scales and vertebrae) by c. 50%. A similar reduction in the total mineral (ash) contents was measured. There was no significant difference concerning the total calcium to phosphorus ratio between animals from the two experimental groups.
Figure 2.
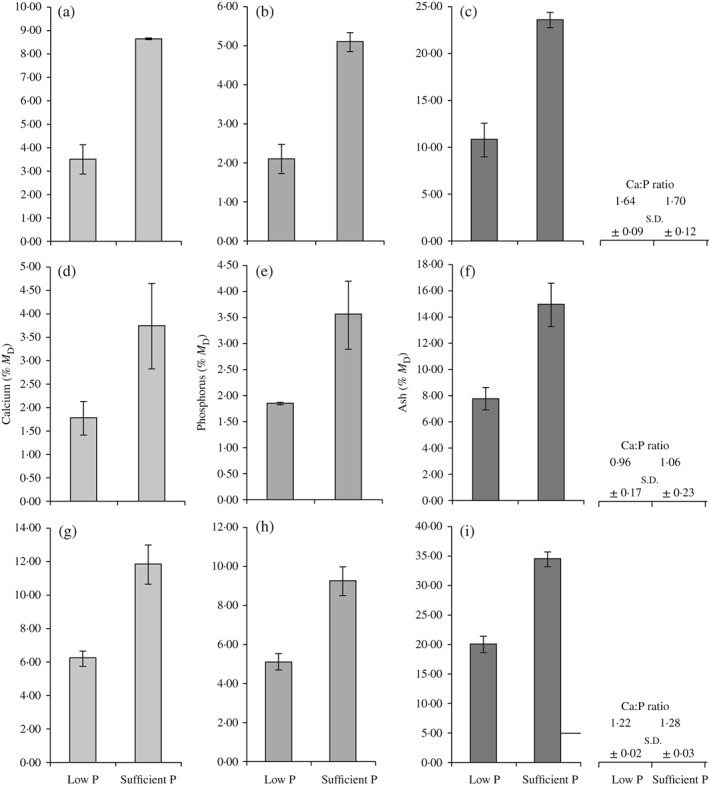
Mean ± s.d. percentage dry mass (M D) of measured (a, d, g) calcium, (b, e, h) phosphorus and (c, f, i) ash content in (a–c) opercula, (d–f) vertebrae and (g–i) scales from 40 Salmo salar individuals per experimental group. In all animals that receive a phosphorus (P)‐deficient diet (low P), values drop by c. 50% compared with animals that received a P‐sufficient diet (sufficient P). There was no significant change in the calcium to phosphorus ratio (Ca:P).
Radiology
On X‐rays, animals from both groups displayed a homogenous vertebral body phenotype. In the P‐sufficient group, vertebral bodies were regularly spaced and shaped [Fig. 1(a)–(c)]. In the P‐deficient diet group, vertebral bodies of all animals were undersized according to the radiograph readings. The width of all intervertebral spaces was significantly enlarged [Fig. 1(d)–(f)]. The phenotype corresponds to the type 10 malformation described by Witten et al. (2009). The type 10 phenotype was homogeneous in all parts of the spine from all animals assessed. There was no apparent occurrence of any other type of vertebra body malformations apart from a singular animal in each group that displayed compressed vertebral bodies in the caudal region of the spine. This incident is not discussed further. Measurements confirmed the visual observations of the vertebral bodies (Fig. 3). The length and height of vertebral bodies in the P‐deficient group were significantly reduced compared with animals of the sufficient P group. In both groups, vertebral bodies maintained a square shape in the radiograph images giving no indication of the compression of vertebral bodies in the P‐deficient group. Measuring the length of 10 vertebral bodies and the width of 10 intervertebral spaces resulted in similar total length values (10 vertebral bodies + 10 intervertebral spaces) for animals in both groups (Fig. 3).
Figure 3.
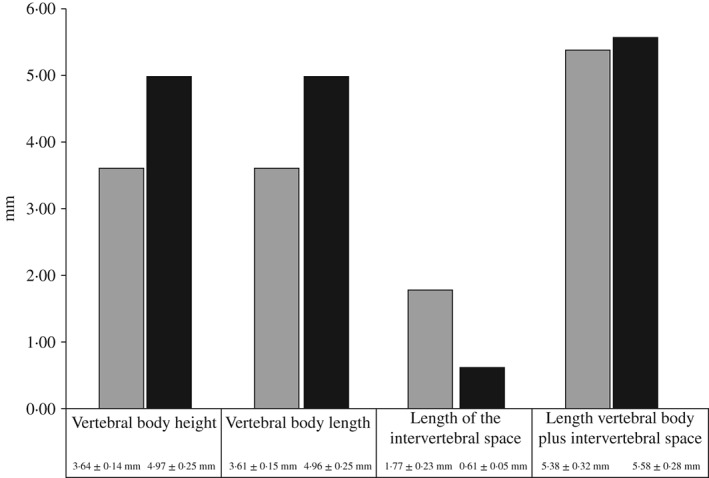
Size (mean ± s.d.) of Salmo salar vertebral bodies numbers 32–42 measured based on X‐rays. Measurements are taken from 30 individual fish per experimental group. The size of vertebral bodies from animals that received the phosphorus (P)‐deficient diet is significantly decreased. The shape of vertebra remains square with no compression. The last two columns represent the width of the intervertebral space added to the length of the vertebral bodies.  , P‐deficient diet;
, P‐deficient diet;  , P‐sufficient diet.
, P‐sufficient diet.
Histopathology and bone matrix mineralization
The radiological diagnosis suggested that the size of vertebral bodies from fish in the P‐deficient group was reduced and that the width of intervertebral spaces was enlarged. This diagnosis was not confirmed by histological examinations. The apparent smaller size of vertebral bodies on X‐rays only related to the extent of radiopaque mineralized bone matrix but not to real size of the vertebral bodies. Histological sections revealed that vertebral bodies from fish of both groups had regular sized, well‐developed vertebral bodies and non‐enlarged intervertebral spaces. Subjectively, the amount and cross‐linkage of bone trabeculae were similar in animals from both groups [Figs 4(a), (b) and 5(a)]. Animals from the P‐deficient group showed a lack of bone minerals in large parts of the bone matrix. All peripheral parts of the bone matrix were without minerals, this includes the bone of neural arches [Fig. 4(d)]. There was an abrupt cessation of matrix mineralization but the formation of bone matrix continued uninterrupted [Fig. 5(a)–(c)]. A slight bend between the mineralized and the non‐mineralized part of the vertebral endplate in P‐deficient fish [Fig. 4(d)] could indicate an upcoming vertebral body compression linked to either a decrease of the invertebrate space or a decrease of the distance between vertebral body centra (Witten et al., 2005). The measurements obtained from the X‐rays, however, show that this is not the case (Fig. 3). This bend cannot therefore be interpreted as an embedding and sectioning artefact related to the processing of non‐demineralized tissues. Staining for phosphorus and calcium showed similar patterns [Fig. 5(a)]. In animals from the P‐sufficient group, non‐mineralized bone only occurred as a narrow osteoid layer (bone prior to mineralization) on top of the regular mineralized bone [Fig. 5(d)]. The lack of minerals in the bone of P‐deficient animals did not affect the morphology of the osteoblasts. Regular spindle‐shaped osteoblasts in bone growth zones were observed; a typical morphology for productive bone‐forming cells in S. salar (Witten & Hall, 2003) [Fig. 4(e)]. Resting, non‐productive, osteoblasts have a flattened shape (Franz‐Odendaal et al., 2006) and were not observed in the P‐deficient animals. Likewise, no increase in bone resorption was observed. Bone surfaces remained smooth and without Howship's lacunae [Figs 4(f) and 5(b)]. Animals from the P‐sufficient group showed the same phenotype, but the bone matrix was fully mineralized [Figs 4(c) and 5(d)]. Tissue in bone marrow spaces showed stages of the transition from connective tissue to adipose tissue [Fig. 4(f)]. Again, no differences were observed between the P‐sufficient and the P‐deficient groups.
Figure 4.
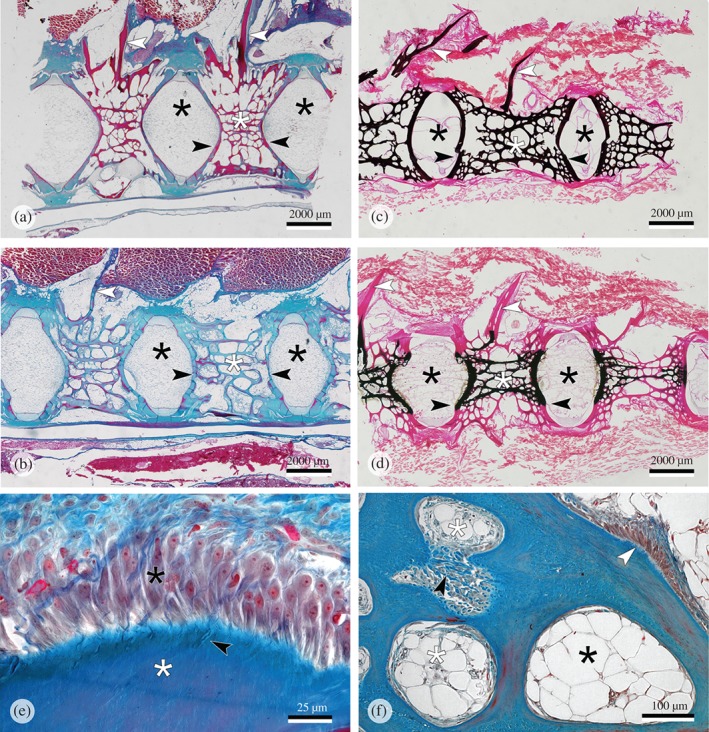
Parasagittal sections of the vertebral column of Salmo salar. (a, c) Animals that received a phosphorus (P)‐sufficient diet. (b, d–f) Animals that received a P‐deficient diet. (a, b) Regular developed internal bone structures are present in animals from both experimental groups.  , intervertebral space; white asterisks, internal vertebral bone spongiosa; white arrowheads, neural arches;
, intervertebral space; white asterisks, internal vertebral bone spongiosa; white arrowheads, neural arches;  , vertebral body endplates. Masons trichrome staining. (c, d) The relationship between bone structure and mineralization.
, vertebral body endplates. Masons trichrome staining. (c, d) The relationship between bone structure and mineralization.  , mineralized bone matrix;
, mineralized bone matrix;  , non‐mineralized bone matrix (collagen). (c) All internal bone matrix structures (white asterisk) including the neural arches (white arrowheads) are complete mineralized (P‐sufficient diet). (d) Peripheral bone structures and the neural arches (white arrowhead) are present but non‐mineralized. (e) P‐deficient diet. Highly active osteoblasts (
, non‐mineralized bone matrix (collagen). (c) All internal bone matrix structures (white asterisk) including the neural arches (white arrowheads) are complete mineralized (P‐sufficient diet). (d) Peripheral bone structures and the neural arches (white arrowhead) are present but non‐mineralized. (e) P‐deficient diet. Highly active osteoblasts ( ) in the vertebral body growth zone of an animal that received a P‐deficient diet. white asterisk, non‐mineralized bone matrix;
) in the vertebral body growth zone of an animal that received a P‐deficient diet. white asterisk, non‐mineralized bone matrix;  , regular Sharpey fibres; Masons trichrome staining. (f) Regular bone spongiosa and marrow spaces in animal that received a P‐deficient diet. Early bone marrow tissue is a mixture from connective tissue and adipose tissue (white asterisks); mature bone marrow spaces are filled with adipose tissue (
, regular Sharpey fibres; Masons trichrome staining. (f) Regular bone spongiosa and marrow spaces in animal that received a P‐deficient diet. Early bone marrow tissue is a mixture from connective tissue and adipose tissue (white asterisks); mature bone marrow spaces are filled with adipose tissue ( ); note the active bone‐forming osteoblasts on the bone surface (white arrowhead);
); note the active bone‐forming osteoblasts on the bone surface (white arrowhead); 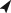 , Sharpey fibres; Masons trichrome staining.
, Sharpey fibres; Masons trichrome staining.
Figure 5.
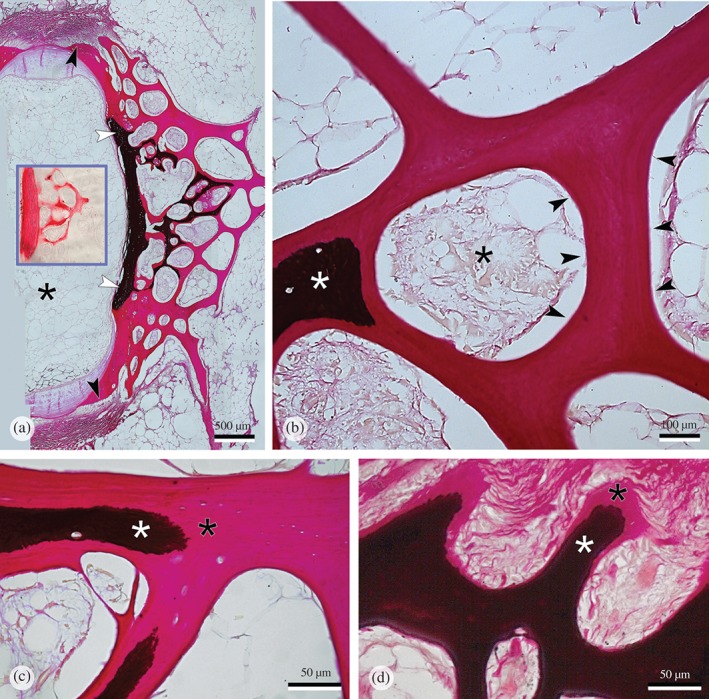
Vertebral body endplate and bone spongiosa from Salmo salar that received a phosphorus (P)‐deficient diet stained for minerals with the von Kossa's protocol. (a) The central part of the vertebral body endplate (white arrowheads) and proximal trabeculae ( ) are mineralized; peripheral bone matrix is completely void of minerals (
) are mineralized; peripheral bone matrix is completely void of minerals ( ). Formation of non‐mineralized bone continues in the vertebral body growth zones (
). Formation of non‐mineralized bone continues in the vertebral body growth zones ( ), indicated by the presence of osteoblasts shown at higher magnification in Fig. 4(e). The insert shows the same distribution of minerals visualized with Alizarin red staining; mineralized bone matrix is red, non‐mineralized bone matrix remains unstained.
), indicated by the presence of osteoblasts shown at higher magnification in Fig. 4(e). The insert shows the same distribution of minerals visualized with Alizarin red staining; mineralized bone matrix is red, non‐mineralized bone matrix remains unstained.  , the location of the intervertebral space. (b) Higher magnification of non‐mineralized bone matrix (
, the location of the intervertebral space. (b) Higher magnification of non‐mineralized bone matrix ( ); note that all bone surfaces are smooth and without any trace of bone resorption (
); note that all bone surfaces are smooth and without any trace of bone resorption ( ); mineralized bone matrix (white asterisk); bone marrow (
); mineralized bone matrix (white asterisk); bone marrow ( ); von Kossa staining. (c, d) Comparison of the pattern of mineralization in P‐deficient and P‐sufficient fish at higher magnification. Like in the P‐deficient fish (c), the bone matrix of P‐sufficient fish (d) also has a non‐mineralized matrix component with a sharp border between the mineralized (white asterisk) and the non‐mineralized (
); von Kossa staining. (c, d) Comparison of the pattern of mineralization in P‐deficient and P‐sufficient fish at higher magnification. Like in the P‐deficient fish (c), the bone matrix of P‐sufficient fish (d) also has a non‐mineralized matrix component with a sharp border between the mineralized (white asterisk) and the non‐mineralized ( ) bone matrix, albeit restricted to the osteoid seam (
) bone matrix, albeit restricted to the osteoid seam ( ).
).
Scale mineralization
The pattern of scale growth and scale mineralization resembled the pattern observed in vertebral bodies: scale growth continued in animals from the P‐deficient group but without mineralization in large parts of the newly formed scale matrix (Fig. 6). No signs of increased scale resorption were observed as described by Shearer (1992). Scale phenotypes were homogenous in each of the two groups. Alizarin red staining displayed three zones of mineral content in scales from fish that received the P‐deficient diet: a modest red‐stained central zone (strongly mineralized) followed by a bright red‐stained zone (less mineralized) and broad zone of unstained (non‐mineralized) scale matrix [Fig. 6(d)]. Strongly mineralized skeletal matrix stains less with Alizarin red compared less mineralized matrix (Vandervennet et al., 2006; De Clercq et al., 2014). Higher magnification shows the same pattern in regular, fully mineralized, scales from animals of the P‐sufficient group (modest red, bright red and unstained) albeit condensed at the edge of the scale [Fig. 6(b), insert].
Figure 6.
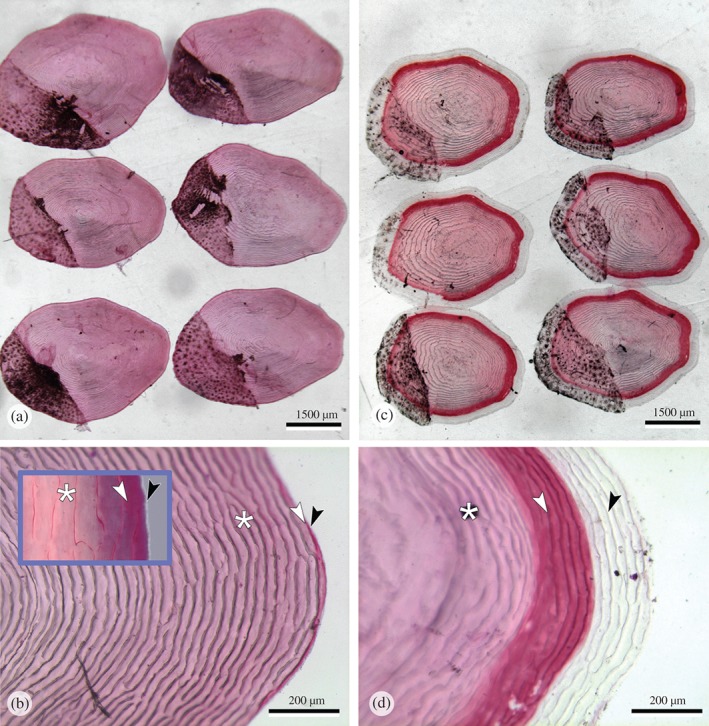
Morphology of Salmo salar scales stained for mineralization with Alizarin red. (a, b) Scales from animals that received the phosphorus (P)‐sufficient diet show homogenous red staining. (c, d) Scales from animals that received the P‐deficient diet show homogenous red staining in the centre, followed by a zone of strong red staining indicating less mineralization and a peripheral zone of non‐mineralized matrix. Note the homogeneity of the mineral staining phenotype within both groups (a, c). Higher magnifications of the distal scale edges (b, d) show regular annuli formation also in the completely non‐mineralized part of scales (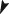 ) from fish that received the P‐deficient diet (d). The three zones of Alizarin red staining, fully mineralized (white asterisk), less mineralized (white arrowhead) and non‐mineralized (
) from fish that received the P‐deficient diet (d). The three zones of Alizarin red staining, fully mineralized (white asterisk), less mineralized (white arrowhead) and non‐mineralized (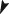 ) that are prominent in (d), are also present in fish that received the P‐sufficient diet but only visible at higher magnification, insert in (c).
) that are prominent in (d), are also present in fish that received the P‐sufficient diet but only visible at higher magnification, insert in (c).
Discussion
Beresford (1981) provides a translation of Kyle's (1927) view on fish bone: To describe the nature of bone seems to be an easy exercise; nevertheless there is hardly any more difficult question in zoology. Textbooks usually begin the description of bone with an enumeration of the typical characteristics, the matrix with Sharpey's fibers and the inorganic matter, lacunae with osteoclasts, Haversian canals, etc. Then the exceptions are mentioned, whereupon one finds out that any of the components can be absent, until finally only the matrix remains, and that perhaps could be missing. In this trial, the bone matrix did not disappear but a primary P‐deficient bone phenotype without minerals was generated. Other bone anomalies were not detected. By uncoupling bone matrix formation and mineralization, this phenotype provides new insights into the aetiology of dietary P deficiency‐related malformations and provides opportunities for further research. Obviously, in the timeframe of this 10 week experiment, low mineralization alone is not a primary cause for malformations. The bone structure of P‐deficient fish was normal despite the complete lack of mineralization in the new‐formed bone matrix. The question of whether the observed differences in vertebral bone tissues will lead to skeletal malformations over a longer period of P withdrawal, or if other factors are required to induce vertebral malformations, has not been addressed as part of this study. The findings of this experiment, however, can serve as a model for investigating these questions in a systematic manner.
True bone without minerals
It can be assumed that future challenge tests or the extension of the trial period beyond 10 weeks will reveal that regular mineralized bone has superior mechanical properties. Still, the lack of any detectable type of bone deformities in the P‐deficient group does not conform to the accepted model of low P‐causing deformities. Low mineralized fish bone has been tested for its mechanical properties, comparable with human osteoporotic bone with low bone mineral density (BMD) (Fjelldal et al., 2004; Shanthanagouda et al., 2014). Such bone could be prone to failure by fracture and, like in humans, it could be thought that vertebral body compression and fusion are a consequence of this (Sugiura et al. (2004). In S. salar, however, vertebral body fractures are rare. Typical pathologies, such as vertebral body compression and fusion, relate to alterations of tissue in the intervertebral space (Witten et al., 2005, 2006, 2009; Ytteborg et al., 2010). In human infants, low mineralized bone will bend but will not fracture. Likewise, the toughest known vertebrate bones, deer antlers, are characterized by their low mineral content (Currey, 1999, 2003). Bone is a composite material, made from a collagenous matrix and from minerals. The mechanical properties of the two components have been analysed separately. The collagenous matrix provides toughness (fracture resistant) and the minerals increase the bone's stiffness (bending resistant) (Viguet‐Carrin et al., 2006). The mineral phase alone is brittle and fractures easily. Bone strength largely depends on the non‐mineralized matrix, specifically on the orientation of collagen fibres that are arranged according to the direction of mechanical load. Consequently, collagenous bone matrix without minerals can be tougher than mineralized bone (Currey, 2003; Viguet‐Carrin et al., 2006). The fact that this experiment generated non‐mineralized bone structures that are regularly spaced and shaped shows that they grow according to mechanical requirements. Formation of internal bone structures is not genetically determined (Witmer, 1995); it follows biomechanical requirements (Burger et al., 2003; Bonucci, 2009). The validity of this mechanism, known as Wolffs' law (Wolff, 1892), has been shown for several fish species (Huysseune et al., 1994; Fiaz et al., 2012, 2014; Owen et al., 2012; Gunter & Meyer, 2014). Obviously, under the given experimental conditions and without further challenges, such as grading, transport or vaccination, the mechanical stability of the non‐mineralized bone matrix in the P‐deficient fish was sufficient; however, these and other relevant factors can be investigated using the model described here.
A notochord‐based vertebral column
Actinopterygians and tetrapods have mineralized, bony, vertebral bodies but the fossil record provides ample examples that the axial skeleton can function without mineralized vertebrae. The notochord has been the main axial skeletal support for vertebrates for over 400 million years. The vertebral column of sharks is cartilaginous and this cartilage may only mineralize later in life. Basal extant actinopterygians and sarcopterygians have an uninterrupted notochord as functional axial skeletal support and no mineralized vertebral bodies. Among these are large animals, such as sturgeons, coelacanths or lungfishes (Arratia et al., 2001). The function of the full‐sized notochord as part of the skeletal axis is preserved in S. salar, in every intervertebral space (Arratia & Schultze, 1992; Nordvik et al., 2005). Consequently, several studies show that salmon spinal deformities relate to alterations in the intervertebral space and that a healthy notochord is critically important for maintaining a healthy S. salar spine (Witten et al., 2005, 2006; Ytteborg et al., 2010). The same is even true for the vestige notochord in adult humans (Risbud & Shapiro, 2011). The size and structure of the notochord located in the intervertebral spaces correspond in animals from both experimental groups. The presence of an unchanged, healthy, notochord in the fish from the P‐deficient group can be a part of the explanation why these animals do not show signs of spinal deformities.
Validation of radiological observations
Radiographs are used to diagnose low mineralized spines in S. salar (Poirier Stewart et al., 2014). In the literature, low mineralized vertebral bodies are described as undersized and intervertebral spaces as being enlarged. Based on X‐rays, Witten et al. (2009) show three types of malformations with undersized vertebral bodies and enlarged intervertebral space. The study also shows several types of malformations with a variable size of the intervertebral space. The notion of undersized vertebral bodies and enlarged intervertebral spaces does not apply to animals from this experiment. This raises the question if all vertebral bodies that have been described as undersized based on X‐rays are in fact too small. They could be of normal size with non‐mineralized peripheral matrix. Whole mount‐stained vertebral bodies shown by Helland et al. (2006) suggest that such phenotype was generated in the frame of a dietary P‐deficiency experiment. For the compressed vertebral bodies, Fjelldal et al. (2007) described, based on high quality radiographs, the presence of low mineralized vertebral body bone matrix in radiotranslucent areas. Radiographs taken in the context of production monitoring can, for obvious reasons, not be of the same resolution as X‐rays for research purposes (Witten et al., 2009). The distinction between bone growth retardation (small vertebra bodies) and insufficient mineralization is, however, important because these are different pathologies with differing aetiologies.
Mineral deficiency without scale or bone resorption
In this study, a specific staining for osteoclast detection, such as tartrate‐resistant acid phosphatase (TRAP) staining was not used, but several studies show that S. salar osteoclasts are typical multinucleated giant cells that create Howship's lacunae. Activated, multinucleated S. salar osteoclasts have been found to be even larger than human osteoclasts (Kacem et al., 1998; Witten & Hall, 2003; Domon et al., 2004). An increase of osteoclastic resorption would thus have been observed with standard histology. These observations match data presented by Fjelldal et al. (2013) who analysed changes of alkaline phosphatase (ALP) and TRAP plasma values as indirect evidence for bone formation and resorption, respectively. Fish that received a low P diet had decreased levels of plasma TRAP activity, suggesting that these animals did not increase bone resorption. Given the sudden stop of bone matrix mineralization, it is remarkable that the induced P deficiency neither triggered bone nor scale resorption. Scale resorption could have been expected in view of studies that show that mineral deficiency primarily triggers scale resorption (Persson et al., 1999; Metz et al., 2014). Wild S. salar resorb scales in both sexes during the upstream spawning run (Jones, 1959; Shearer, 1992). The lack of resorption observed here and by Fjelldal et al. (2013) warrants a discussion about the assumption that S. salar exposed to low dietary P supply must mobilize skeletal phosphates as an attempt to maintain normal physiological functions (1998, 1998; Witten & Huysseune, 2009). For fish in this experiment, it was apparently sufficient to use the low amount of available P from the diet for soft tissue processes by stopping the mineralization of new skeletal matrices. Albrektsen et al. (2009) pointed out that vertebrae ash and P content are sensitive indicators for P utilization in terrestrial vertebrates and also highly useful to determine the dietary P requirement of fishes. It is shown here, based on chemical and morphological analysis, that scales are also good indicators for determining the animals' mineral status. The morphological findings are also in agreement with the results of the biochemical studies on scales reported by Skonberg et al. (1997). The authors conclude that scales were most responsive to dietary phosphorus concentrations, both in magnitude and speed of response. The data from this study are in agreement with this conclusion and provide an insight into how these changes have occurred. The histological examinations indicate that the cause of the low skeletal mineral content was not because minerals have been mobilized from the skeleton, but because large parts of the bone and scale matrix remained non‐mineralized.
A sudden stop of mineralization but growth continues
From 1977 onwards, S. salar skeletal mineralization was shown to depend on dietary phosphorus intake (Lall & Bishop, 1977; 1998, 1998). In contrast, S. salar and other teleosts can obtain calcium from the food or from the water. Highly efficient ion pumps located in the gill epithelium ensure sufficient calcium uptake even in calcium‐poor fresh waters (Guerreiro et al., 2002; Perry et al., 2003). The present observed abrupt stop of bone mineralization shows how much S. salar depends on dietary phosphorus intake for mineralization of the skeleton. Wild S. salar encounters long periods of repeated P deficiency as a part of its regular life cycle. Dietary P being the animals' principal P resource, the starvation periods prior to and after spawning are considered as periods of P deficiency (Witten & Hall, 2003; Witten & Huysseune, 2009). Different from the well‐fed fish of this experiment, starving S. salar in nature stop somatic growth. Interestingly, mineralization and somatic growth must not be linked, as shown on the example of starvation‐related stop of tooth formation in wild S. salar. Teeth that remain attached to the jaws continue to mineralize during the starvation period (Witten et al., 2005). In this study, the uncoupling of somatic growth and mineralization is also encountered, but the other way round: growth continues but mineralization stops. The continuation of growth and bone formation under conditions of P deficiency is remarkable given that phosphorus is also required for growth (Sugiura et al., 2004). For humans, the effect of P deficiency on growth is not well established, other than for growth retardation related to genetic disorders such as X‐linked hypophosphaetemia (Santos et al., 2013). In this study and others, P‐deficient fishes showed a growth reduction (Ketola, 1975; Albrektsen et al., 2009). No growth reduction was observed in several experiments that tested the effect of P‐deficient diets on farmed S. salar (Åsgård & Shearer, 1997; 1998, 1998; Helland et al., 2006; Fjelldal et al., 2009, 2012) and Nile tilapia Oreochromis niloticus (L. 1758) (Lu et al., 2013). Experimental feeding periods of these trials were 14, 63, 119, 77 and 150 days, respectively. From the perspective of this study, it can be speculated that in the aforementioned experiments there was sufficient P for soft tissue metabolism and growth and that the formation of bone matrix continued accordingly.
The authors thank I. Rusten and colleagues at the Skretting ARC Lerang fish station, Forsand, Norway, for their help in conducting the feeding experiment.
References
References
- Albrektsen, S. , Hope, B. & Aksnes, A. (2009). Phosphorus (P) deficiency due to low P availability in fishmeal produced from blue whiting (Micromesistius poutassou) in feed for under‐yearling Atlantic salmon (Salmo salar) smolt. Aquaculture 296, 318–328. [Google Scholar]
- AOAC (1995). Official methods of analysis of AOAC international. 16th ed., Arlington, VA: Association of Official Analytical Chemists. [Google Scholar]
- Arratia, G. & Schultze, H.‐P. (1992). Reevaluation of the caudal skeleton of certain actinopterygian fishes. III. Salmonidae. Homologization of caudal skeletal structures. Journal of Morphology 214, 187–249. [DOI] [PubMed] [Google Scholar]
- Arratia, G. , Schultze, H. P. & Casciotta, J. (2001). Vertebral column and associated elements in dipnoans and comparison with other fishes: development and homology. Journal of Morphology 250, 101–172. [DOI] [PubMed] [Google Scholar]
- Åsgård, T. & Shearer, K. D. (1997). Dietary phosphorus requirement of juvenile Atlantic salmon, Salmo salar L. Aquaculture Nutrition 3, 17–23. [Google Scholar]
- Baeverfjord, G. , Åsgaård, T. & Shearer, K. D. (1998). Development and detection of phosphorus deficiency in Atlantic salmon, Salmo salar L., parr and post‐smolts. Aquaculture Nutrition 4, 1–11. [Google Scholar]
- Baeverfjord, G. , Fjelldal, P. G. , Albrektsen, S. , Hatlen, B. , Denstadli, V. , Ytteborg, E. , Takle, H. , Lock, E.‐J. , Berntssen, M. H. G. , Lundebye, A. K. , Åsgård, T. & Waagbø, R. (2012). Mineral nutrition and the impact on bone development and skeletal deformities – a review. Nofima Report 37/2012, 1–91.
- Beresford, W. A. (1981). Chondroid Bone, Secondary Cartilage and Metaplasia. Baltimore, MD and Munich: Urban & Schwarzenberg. [Google Scholar]
- Bonucci, E. (2009). The osteocyte: the underestimated conductor of the bone orchestra. Rendiconti Lincei 20, 237–254. [Google Scholar]
- Burger, E. H. , Klein‐Nulend, J. & Smit, T. H. (2003). Strain‐derived canalicular fluid flow regulates osteoclast activity in a remodelling osteon – a proposal. Journal of Biomechanics 36, 1453–1459. [DOI] [PubMed] [Google Scholar]
- Carroll, R. L. (1988). Vertebrate Paleontology and Evolution. New York, NY: W.H. Freeman and Company. [Google Scholar]
- Cordell, D. , Rosemarin, A. , Schröder, J. J. & Smit, A. L. (2011). Towards global phosphorus security: a systems framework for phosphorus recovery and reuse options. Chemosphere 84, 747–758. [DOI] [PubMed] [Google Scholar]
- Currey, J. D. (1999). What determines the bending strength of compact bone? Journal of Experimental Biology 202, 2495–2503. [DOI] [PubMed] [Google Scholar]
- Currey, J. D. (2003). Role of collagen and other organics in the mechanical properties of bone. Osteoporosis International 14, S29–S36. [DOI] [PubMed] [Google Scholar]
- De Clercq, A. , Vandenplas, S. & Huysseune, A. (2014). A comparison of the larval and juvenile dentition in Polypterus senegalus . Journal of Applied Ichthyology 30, 790–795. [Google Scholar]
- Deschamps, M.‐H. , Poirier Stewart, N. , Demanche, A. & Vandenberg, G. W. (2014). Preliminary study for phenotypic description of vertebral abnormalities in triploid trout subjected to prolonged deficiency in phosphorus. Journal of Applied Ichthyology 30, 833–839. [Google Scholar]
- Domon, T. , Fukui, A. , Taniguchi, Y. , Suzuki, R. , Takahashi, S. , Yamamoto, T. & Wakita, M. (2004). Odontoclasts in the Chinook salmon differ from mammalian odontoclasts by exhibiting a great proportion of cells with high nuclei number. Anatomy and Embryology 209, 119–128. [DOI] [PubMed] [Google Scholar]
- Fiaz, A. W. , Léon‐Kloosterziel, K. M. , Gort, G. , Schulte‐Merker, S. , van Leeuwen, J. L. & Kranenbarg, S. (2012). Swim‐training changes the spatio‐temporal dynamics of skeletogenesis in zebrafish larvae (Danio rerio). PLoS One 7, e34072;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaz, A. W. , Leon‐Kloosterziel, K. M. , Schulte‐Merker, S. , van Leeuwen, J. L. & Kranenbarg, S. (2014). Exploring the molecular link between swim‐training and caudal fin development in zebrafish (Danio rerio) larvae. Journal of Applied Ichthyology 30, 728–739. [Google Scholar]
- Fjelldal, P. G. , Grotmol, S. , Kryvi, H. , Gjerdet, N. R. , Taranger, G. L. , Hansen, T. , Porter, M. J. R. & Totland, G. K. (2004). Pinealectomy induces malformation of the spine and reduces the mechanical strength of the vertebrae in Atlantic salmon, Salmo salar . Journal of Pineal Research 36, 132–139. [DOI] [PubMed] [Google Scholar]
- Fjelldal, P. G. , Nordgarden, U. & Hansen, T. (2007). The mineral content affects vertebral morphology in underyearling smolt of Atlantic salmon (Salmo salar L.). Aquaculture 270, 231–239. [Google Scholar]
- Fjelldal, P. G. , Hansen, T. , Breck, O. , Sandvik, R. , Waagbø, R. , Berg, A. & Ørnsrud, R. (2009). Supplementation of dietary minerals during the early seawater phase increase vertebral strength and reduce the prevalence of vertebral deformities in fast‐growing under‐yearling Atlantic salmon (Salmo salar L.) smolt. Aquaculture Nutrition 15, 366–378. [Google Scholar]
- Fjelldal, P. G. , Hansen, T. & Albrektsen, S. (2012). Inadequate phosphorus nutrition in juvenile Atlantic salmon has a negative effect on long‐term bone health. Aquaculture 334–337, 117–123. [Google Scholar]
- Fjelldal, P. G. , Lock, E. J. , Hansen, T. , Waagbø, R. , Wargelius, A. , Gil‐Martens, L. , El‐Mowafi, A. & Ørnsrud, R. (2013). Continuous light induces bone resorption and affects vertebral morphology in Atlantic salmon (Salmo salar L.) fed a phosphorus deficient diet. Aquaculture Nutrition 18, 610–619. [Google Scholar]
- Franz‐Odendaal, T. , Hall, B. K. & Witten, P. E. (2006). Buried alive: how osteoblasts become osteocytes? Developmental Dynamics 235, 176–190. [DOI] [PubMed] [Google Scholar]
- Guerreiro, P. M. , Fuentes, J. M. , Canario, A. V. & Power, D. M. (2002). Calcium balance in sea bream (Sparus aurata): the effect of oestradiol‐17ß. Journal of Endocrinology 173, 377–385. [DOI] [PubMed] [Google Scholar]
- Gunter, H. & Meyer, A. (2014). Molecular investigation of mechanical strain‐induced phenotypic plasticity in the ecologically important pharyngeal jaws of cichlid fish. Journal of Applied Ichthyology 30, 630–635. [Google Scholar]
- Hall, B. K. & Witten, P. E. (2007). The origin and plasticity of skeletal tissues in vertebrate evolution and development In Major Transitions in Vertebrate Evolution (Anderson J. S. & Sues H.‐D., eds), pp. 13–56. Bloomington, IN: Indiana University Press. [Google Scholar]
- Helland, S. , Denstadli, V. , Witten, P. E. , Hjelde, K. , Storebakken, T. & Baeverfjord, G. (2006). Occurrence of hyper dense vertebrae in Atlantic salmon (Salmo salar L.) fed diets with graded levels of phytic acid. Aquaculture 261, 603–614. [Google Scholar]
- Huysseune, A. , Sire, J.‐Y. & Meunier, F. J. (1994). Comparative study of lower pharyngeal jaw structure in two phenotypes of Astatoreochromis alluaudi (Teleostei: Cichlidae). Journal of Morphology 221, 25–43. [DOI] [PubMed] [Google Scholar]
- Jones, J. W. (1959). The Salmon. New York, NY: Harper & Brothers. [Google Scholar]
- Kacem, A. , Meunier, F. J. & Baglinière, J. L. (1998). A quantitative study of morphological and histological changes in the skeleton of Salmo salar during its anadromous migration. Journal of Fish Biology 53, 1096–1109. [Google Scholar]
- Ketola, H. G. (1975). Requirement of Atlantic salmon for dietary phosphorus. Transactions of the American Fisheries Society 104, 548–551. [Google Scholar]
- Kyle, H. M. (1927). Über die Entstehung und Bildung der Hartsubstanz bei Fischen. Zeitschrift für Mikroskopisch‐Anatomische Forschung 9, 317–384. [Google Scholar]
- Lall, S. P. & Bishop, F. J. (1977). Studies on mineral and protein utilization by Atlantic salmon growth in sea water. Fisheries and Marine Service Technical Report No. 688. Halifax, NS: Department of Fisheries and Environment.
- Le Luyer, J. , Deschamps, M.‐H. , Proulx, E. , Poirier Stewart, N. , Robert, C. & Vandenberg, G. W. (2014). Responses of different body compartments to acute dietary phosphorus deficiency in juvenile triploid rainbow trout (Oncorhynchus mykiss, Walbaum). Journal of Applied Ichthyology 30, 826–833. [Google Scholar]
- Lu, J. , Yoshizaki, G. , Endo, M. & Takeuchi, T. (2013). Effect of dietary high amount of calcium and phosphorus on reducing the prevalence of morphological deformities in GH‐transgenic Nile tilapia. Fisheries Science 79, 647–658. [Google Scholar]
- Metz, J. R. , Leeuwis, R. H. J. , Zethof, J. & Flik, G. (2014). Zebrafish (Danio rerio) in calcium poor water mobilise calcium and phosphorus from scales. Journal of Applied Ichthyology 30, 671–677. [Google Scholar]
- Naylor, R. L. , Hardy, R. W. , Bureau, D. P. , Chiu, A. , Elliott, M. , Farrell, A. P. I. , Forster Gatlin, D. M. , Goldburg, R. J. , Hua, K. & Nichols, P. D. (2009). Feeding aquaculture in an era of finite resources. Proceedings of the National Academy of Sciences of the United States of America 106, 15103–15110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordvik, K. , Kryvi, H. , Totland, G. K. & Grotmol, S. (2005). The salmon vertebral body develops through mineralization of two preformed tissues that are encompassed by two layers of bone. Journal of Anatomy 206, 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (2011). Nutrient Requirements of Fish. Washington, DC: National Academy Press. [Google Scholar]
- Owen, M. A. G. , Eynon, B. , Woodgate, S. , Davies, S. J. & Fox, S. (2012). Increased water current induces micro‐architectural changes to the vertebral bone of juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 344, 141–146. [Google Scholar]
- Penido, M. G. & Alo, U. S. (2012). Phosphate homeostasis and its role in bone health. Pediatric Nephrology 27, 2039–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, S. F. , Shahsavarani, A. , Georgalis, T. , Baya, A. M. , Furimsky, M. & Thomas, S. L. Y. (2003). Channels, pumps, and exchangers in the gill and kidney of freshwater fishes: their role in ionic and acid‐base regulation. Journal of Experimental Zoology A 300, 53–62. [DOI] [PubMed] [Google Scholar]
- Persson, P. , Björnsson, B. T. & Takagi, Y. (1999). Characterisation of morphology and physiological actions of scale osteoclasts in the rainbow trout. Journal of Fish Biology 54, 669–684. [Google Scholar]
- Poirier Stewart, N. , Deschamps, M.‐H. , Witten, P. E. , Le Luyer, J. , Proulx, E. , Huysseune, A. , Bureau, D. P. & Vandenberg, G. W. (2014). X‐ray‐based morphometrics: an approach to diagnose vertebral abnormalities in under‐mineralized vertebrae of juvenile triploid all‐female rainbow trout (Oncorhynchus mykiss) fed with a phosphorus deficient diet. Journal of Applied Ichthyology 30, 796–803. [Google Scholar]
- Presnell, J. K. & Schreibman, M. P. (1998). Humason's Animal Tissue Techniques, 5th edn. Baltimore, MD: The John Hopkins University Press. [Google Scholar]
- Risbud, M. V. & Shapiro, I. M. (2011). Notochordal cells in the adult intervertebral disc: new perspective on an old question. Critical Reviews in Eukaryotic Gene Expression 21, 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, P. K. , Witten, P. E. , Hall, B. K. & Lall, S. P. (2002). Effects of dietary phosphorus on bone growth and mineralisation of vertebrae in haddock (Melanogrammus aeglefinus L.). Fish Physiology and Biochemistry 27, 35–48. [Google Scholar]
- Santos, F. , Fuente, R. , Mejia, N. , Mantecon, L. , Gil‐Peña, H. & Ordoñez, F. A. (2013). Hypophosphatemia and growth. Pediatric Nephrology 28, 595–603. [DOI] [PubMed] [Google Scholar]
- Shanthanagouda, A. H. , Guo, B.‐S. , Ye, R. R. , Chao, L. , Chiang, M. W. L. , Singaram, G. , Cheung, N. K. M. , Zhang, G. E. & Au, D. W. T. (2014). Japanese medaka: a non‐mammalian vertebrate model for studying sex and age‐related bone metabolism in vivo . PLoS One 9, e88165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skonberg, D. I. , Yogeva, L. , Hardy, R. W. & Dong, F. M. (1997). Metabolic response to dietary phosphorus intake in rainbow trout (Oncorhynchus mykiss). Aquaculture 157, 11–24. [Google Scholar]
- Sugiura, S. H. , Hardy, R. W. & Roberts, R. H. (2004). The pathology of phosphorus deficiency in fish‐a review. Journal of Fish Diseases 27, 255–265. [DOI] [PubMed] [Google Scholar]
- Sullivan, M. , Reid, S. W. J. , Ternent, H. , Manchester, N. J. , Roberts, R. J. , Stone, D. A. J. & Hardy, R. W. (2007a). The aetiology of spinal deformity in Atlantic salmon, Salmo salar L.: influence of different commercial diets on the incidence and severity of the preclinical condition in salmon parr under two contrasting husbandry regimes. Journal of Fish Diseases 30, 759–767. [DOI] [PubMed] [Google Scholar]
- Sullivan, M. , Hammond, G. , Roberts, R. J. & Manchester, N. J. (2007b). Spinal deformation in commercially cultured Atlantic salmon, Salmo salar L.: a clinical and radiological study. Journal of Fish Diseases 30, 745–752. [DOI] [PubMed] [Google Scholar]
- Tarlo, L. B. H. (1964). The origin of bone In Bone and Tooth. Proceedings of the First European Bone and Tooth Symposium (Blackwood H. J. J., ed), pp. 3–15. Oxford: Pergamon Press. [Google Scholar]
- Taussky, H. H. & Shorr, E. (1953). A microcolorimetric method for the determination of inorganic phosphate. Journal of Biological Chemistry 202, 675–685. [PubMed] [Google Scholar]
- Vandervennet, E. , Wautier, K. , Verheyen, E. & Huysseune, A. (2006). From conical to spatulate: intra‐ and interspecific changes in tooth shape in closely related cichlids. Journal of Morphology 267, 516–525. [DOI] [PubMed] [Google Scholar]
- Vielma, J. & Lall, S. P. (1998. a). Control of phosphorus homeostasis of Atlantic salmon (Salmo salar) in fresh water. Fish Physiology and Biochemistry 19, 83–93. [Google Scholar]
- Vielma, J. & Lall, S. P. (1998. b). Phosphorus utilization by Atlantic salmon (Salmo salar) reared in freshwater is not influenced by higher dietary calcium intake. Aquaculture 160, 117–128. [Google Scholar]
- Viguet‐Carrin, S. , Garnero, P. & Delmas, P. D. (2006). The role of collagen in bone strength. Osteoporosis International 17, 319–336. [DOI] [PubMed] [Google Scholar]
- Witmer, L. M. (1995). The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils In Vertebrate Paleontology (Thomason J. J., ed.), pp. 19–33. Cambridge: Cambridge University Press. [Google Scholar]
- Witten, P. E. & Hall, B. K. (2003). Seasonal changes in the lower jaw skeleton in male Atlantic salmon (Salmo salar L.): remodelling and regression of the kype after spawning. Journal of Anatomy 203, 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten, P. E. & Huysseune, A. (2009). A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biological Reviews 84, 315–346. [DOI] [PubMed] [Google Scholar]
- Witten, P. E. , Hall, B. K. & Huysseune, A. (2005). Are breeding teeth in Atlantic salmon a component of the drastic alterations of the oral facial skeleton? Archives of Oral Biology 50, 213–217. [DOI] [PubMed] [Google Scholar]
- Witten, P. E. , Obach, A. , Huysseune, A. & Baeverfjord, G. (2006). Vertebrae fusion in Atlantic salmon (Salmo salar): development, aggravation and pathways of containment. Aquaculture 258, 164–172. [Google Scholar]
- Witten, P. E. , Gil‐Martens, L. , Huysseune, A. , Takle, H. & Hjelde, K. (2009). Towards a classification and an understanding of developmental relationships of vertebral body malformations in Atlantic salmon (Salmo salar L.). Aquaculture 295, 6–14. [Google Scholar]
- Wolff, J. (1892). Das Gesetz der Transfor mation der Knochen. Berlin: A. Hirschwald; [translation available as: Wolff, J. (1986). The Law of Bone Remodelling (translated by Maquet, P. & Furlong, R.), Berlin: Springer Verlag]. [Google Scholar]
- Ytteborg, E. , Torgersen, J. S. , Pedersen, M. E. , Baeverfjord, G. , Hannesson, K. O. & Takle, H. (2010). Remodeling of the notochord during development of vertebral fusions in Atlantic salmon (Salmo salar). Cell and Tissue Research 342, 363–376. [DOI] [PubMed] [Google Scholar]
Electronic Reference
- Shearer, W. M. (1992) Atlantic salmon scale reading guidelines. ICES Cooperative Research Report 188. Download available from The Flanders Marine Institute (Vlaams Instituut voor de Zee, VILZ) at www.vliz.be/imisdocs/publications/221120.pdf


