Abstract
Background:
Low serum 25-hydroxyvitamin D [25(OH)D] and osteoarthritis (OA) are commonly found in patients followed up in a primary care office. Clear evidence to support the link between 25-hydroxyvitamin D levels and OA is lacking.
Aim:
To describe the association of serum 25-hydroxyvitamin D status in patients with OA in the primary care office.
Materials and Methods:
We reviewed the records of 1,455 patients seen in our primary care office between November 2013 and October 2014. All patients were older than 18 years and had a diagnosis of OA. Demographic characteristics as well as 25(OH)D levels and comorbidities were analyzed.
Results:
Levels of 25(OH)D were available in 1,222 patients with OA. Fifty-one percent of the patients had a low 25(OH)D level. Patients with OA and low 25(OH)D were on an average 5 years younger than patients with OA and normal 25(OH)D (P < 0.001). African Americans (71.7%) and Hispanics (63.1%) had a higher prevalence of low 25(OH)D compared to Whites (42.9%) and other races (49.1%) (P < 0.001). There were significantly more smokers (15.4%) and patients with type 2 diabetes (27.6%) in the group of patients with osteoarthritis and low 25(OH)D (P < 0.001). A lower prevalence of hypothyroidism (18.5% versus 27.4%) and higher body mass index (BMI) were also noted in the group of interest.
Conclusion:
Patients with low levels of 25(OH)D and OA are younger than their counterparts with low 25(OH)D level. Future studies are needed to clarify the relationship between 25(OH)D level and OA.
Keywords: Low serum 25-hydroxyvitamin D [25(OH) D], osteoarthritis (OA), primary care office
Introduction
The associations between low serum 25-hydroxyvitamin D [25(OH)D] level and various disorders have been assessed in a large and rapidly expanding literature.[1] Several national and international systematic reviews have been published exploring the associations between low 25(OH)D level and osteoarthritis (OA).[2] However, the results remain conflicting.[3] OA is the most common form of arthritis, which affects approximately 15% of the population. Among all patients who suffer from OA, about 65% are aged 60 years and above. The high incidence of this illness is rather disturbing at this time because its frequency increases gradually with the aging of the population.[4] Low serum 25(OH)D has been widely reported in the North American population. There are studies, which suggest a rising trend of prevalence in the United States.[5] Because 25(OH)D influences bone quality, it is hypothesized that 25(OH)D status has an effect on the risk of the development or progression of OA although clear evidence to support this association is yet to be determined.[6]
A significant number of patients present in the primary care offices with an established diagnosis of OA and a low serum 25(OH)D defined as a serum 25(OH)D level of less than 30 ng/mL. The purpose of our study was to demonstrate the association of low serum 25(OH)D level in patients with OA in our suburban primary care office.
Materials and Methods
Study selection
Our study was a retrospective study. We reviewed the electronic medical records to find any association between serum 25(OH)D level and OA. Patients who were seen between November 1, 2014 and October 31, 2014 were included in this study. The study was reviewed and approved by the Institutional Review Board of the Cooper Health System, Camden, New Jersey, USA.
We included subjects who were adult patients aged 18 years and above with an established diagnosis of OA and who had an estimation of serum 25(OH)D level. The exclusion criteria were patients under the age of 18 years, patients who had no radiographic evidence of OA, and patients who did not have a serum 25(OH)D test. The sample size was determined as 1,315 in order to achieve the largest sample that would provide 80% power to the study hypotheses.
Data collection
We collected the following data for each patient: Age, gender, race, OA type (spine, knees, hips, and shoulders), serum 25(OH)D level, vitamin D replacement therapy, bone mineral density (osteopenia, osteoporosis, or normal), body mass index (BMI), associated medical conditions (such as hypertension, diabetes, hypothyroidism, chronic kidney disease, systemic lupus erythematosus, rheumatoid arthritis, scleroderma, undifferentiated mixed connective disease, small intestinal disease, and small intestinal surgeries), smoking history, alcohol use, steroid use, and diuretic use.
Statistical analysis
We entered the patients’ data in Microsoft Excel (2013, Redmond, Washington, USA) spreadsheet. Statistical analysis was done using Statistical Package for the Social Sciences (SPSS) version 15.01 (IBM, Armonk, New York, USA). The collected data were divided into two groups: Group 1 represented the patients with OA and normal serum 25(OH)D level and Group 2 represented patients with OA and low serum 25(OH)D level. We used independent t-test to compare the continuous variables, such as age and BMI, between the two groups, and compared the continuous variables between the different locations of OA (spine, knees, hips, and shoulders).
Pearson's chi-squared test and Fisher's exact test were used to compare the categorical variables between the two groups, and to compare the categorical variables in the location of OA between the normal and low serum 25(OH)D groups. Pearson's chi-squared test was used to compare the categorical variables (such as gender and race) between the groups. One-way analysis of variance (ANOVA) was used to determine whether there were any significant differences in the means of OA locations and continuous variables [such as age, BMI, and serum 25(OH)D level].
Results
A total of 1,455 electronic medical records of patients with OA were reviewed. Of them, 140 (9.6%) patient were excluded as they did not have radiographic evidence of OA. Among the rest of the 1,315 patients who had radiographic evidence of OA, 93 (7.1%) patients did not have serum 25(OH)D level checked; hence, they were excluded. In all of the remaining 1,222 patients (92.9%), 51% had low serum 25(OH)D and 49% had normal serum 25(OH)D. The difference was not statistically significant [Figure 1].
Figure 1.
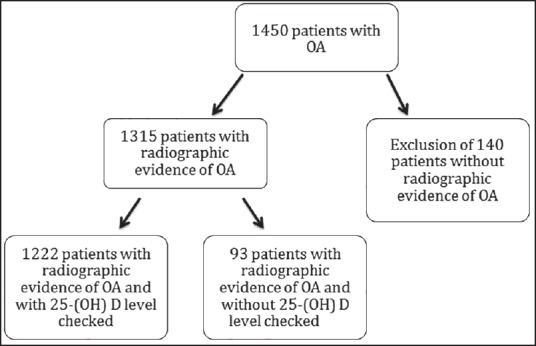
Study flow diagram [OA = Osteoarthritis, 25(OH)D = 25-hydroxyvitamin D]
Baseline characteristics
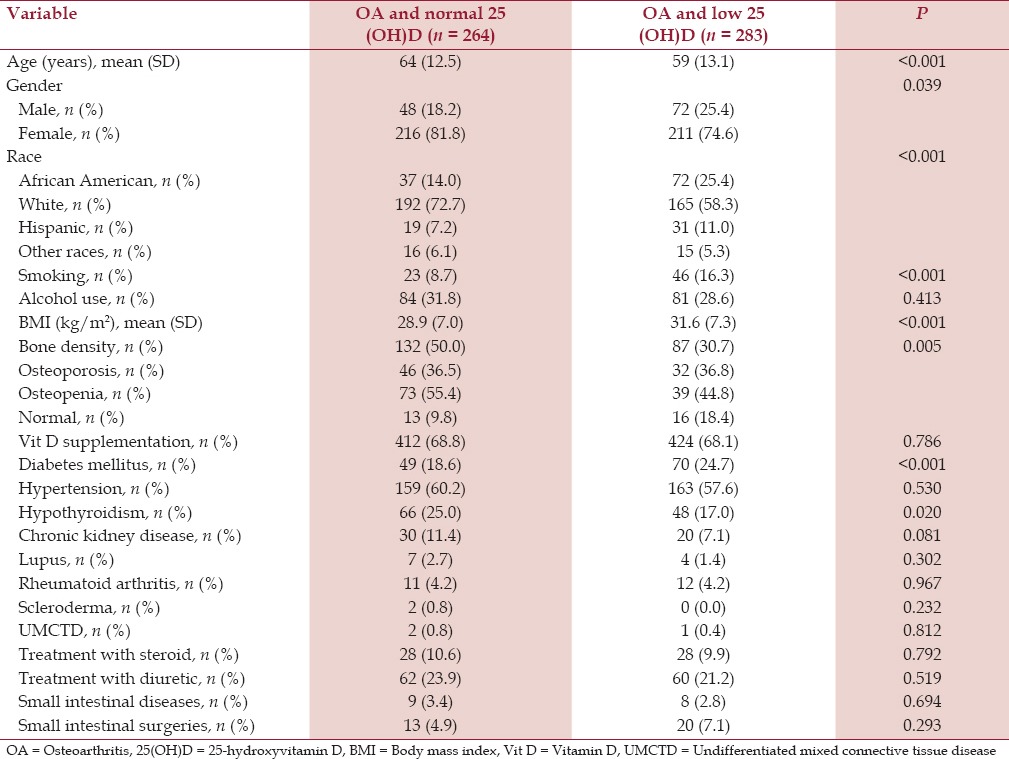
The mean age of the patients in the OA group and low serum 25(OH)D group was 61 years (SD 12.5) and in the OA group and normal serum 25(OH)D group was 66 years (SD 11.7). The mean age difference was statistically significant (P < 0.001) [Table 1]. Both the groups had more females than males in a comparable female-to-male ratio of 4:1 [Table 1]. Analysis of the racial prevalence showed a significantly higher prevalence of low serum 25(OH)D in the African Americans (71.7%) and the Hispanics (63.1%) compared to the Whites (42.9%) and other races (49.1%) (P < 0.001) [Figure 2]. Patients with OA and low serum 25(OH)D had a significantly higher prevalence of cigarette smoking (15.4%) compared to the patients with OA and normal serum 25(OH)D (8.5%) (P < 0.005). Although about one-third of all patients had a history of alcohol intake, patients with OA and low serum 25(OH)D had a significantly lower prevalence of alcohol intake (31.3%) compared to the patients with OA and normal serum 25(OH)D (39.4%) (P < 0.005) [Table 1, Figure 3].
Table 1.
Baseline characteristics of patients with osteoarthritis and serum 25-hydroxyvitamin D level
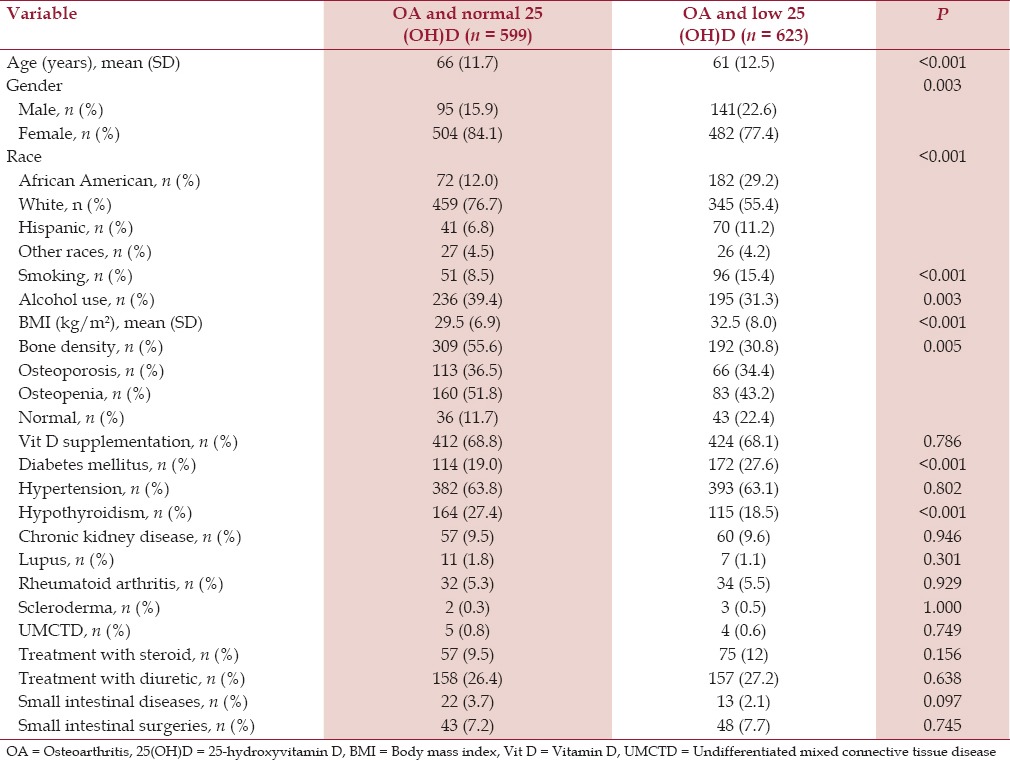
Figure 2.
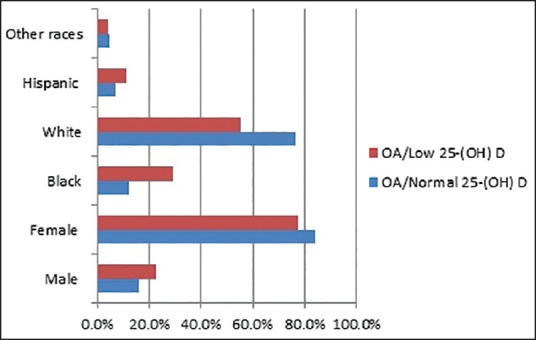
Gender and race distribution [OA= Osteoarthritis, 25(OH) D = 25-hydroxyvitamin D]
Figure 3.
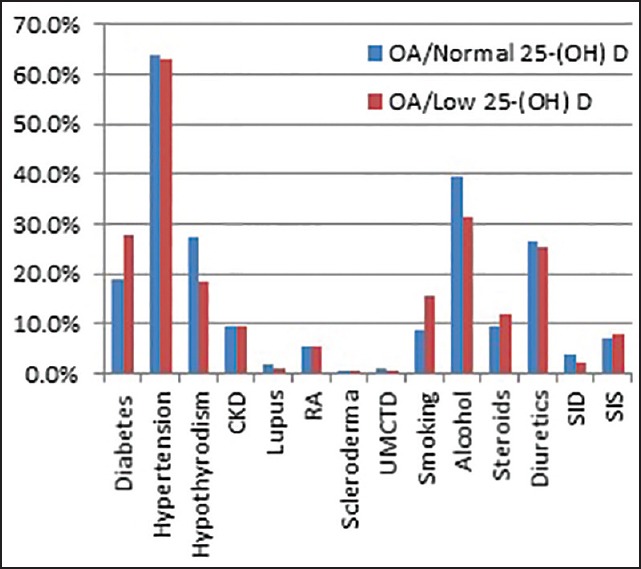
Prevalence of comorbid conditions [OA= Osteoarthritis, 25(OH)D = 25-hydroxyvitamin D, T2DM = type 2 diabetes mellitus, CKD = Chronic kidney disease, RA = Rheumatoid arthritis, UMCTD = Undifferentiated mixed connective tissue disease, SID = Small intestinal diseases, SIS = Small intestinal surgeries]
Metabolic and bone effects
We found that patients with OA and low serum 25(OH)D had a significantly higher mean BMI (kg/m2 ) [32.5, standard deviation (SD) 8.0] compared to the patients with OA and normal serum 25(OH)D (29.5, SD 6.9) (P < 0.001). About half of the patients in the normal serum 25(OH)D group had a bone density test available, out of which 88.3% had low bone mineral density while one-third of the patients in the low serum 25(OH)D group had a bone density test available, out of which 77.6% had low bone mineral density. The difference was not statistically significant [Table 1]. More than two-third of the patients in both the groups were taking supplemental vitamin D therapy [Table 1, Figure 3].
Comorbid medical conditions
Hypertension was the most common associated comorbid medical condition in both the groups. Patients with OA and low serum 25(OH)D had a significantly higher prevalence of type 2 diabetes mellitus (27.6%) compared to the patients with OA and normal serum 25(OH)D (19%) (P < 0.005) [Table 1]. Patients with OA and low serum 25(OH)D had a lower prevalence of hypothyroidism (18.5%) compared to the patients with OA and normal serum 25(OH)D (27.4%) (P < 0.005) [Figure 3]. There was no significant difference in the prevalence of hypertension and chronic kidney disease. Similarly, there was no significant difference in the prevalence of rheumatoid arthritis, systemic lupus erythematosus, scleroderma, and undifferentiated mixed connective tissue disease, which affected less than 10% of all the patients in each group [Table 1].
Analysis by specific joint region
The majority of the patients had spine OA followed by knee OA in patients with normal serum 25(OH)D level (44.2% and 39.2%, respectively) and in patients with low serum 25(OH)D level (45.5% and 39.5%, respectively) [Table 2] Less than one-fifth of all the patients had OA of the hip or OA of the shoulder in both the groups [normal serum 25(OH)D level: 7.8% and 8.8%, respectively; low serum 25(OH)D level: 8.3% and 6.7%, respectively] [Tables 5 and 6]. The differences were not statistically significant.
Table 2.
Distribution of osteoarthritis in diff erent joint region
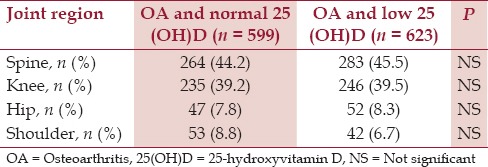
Table 5.
Baseline characteristics of patients with hip osteoarthritis and serum 25-hydroxyvitamin D level
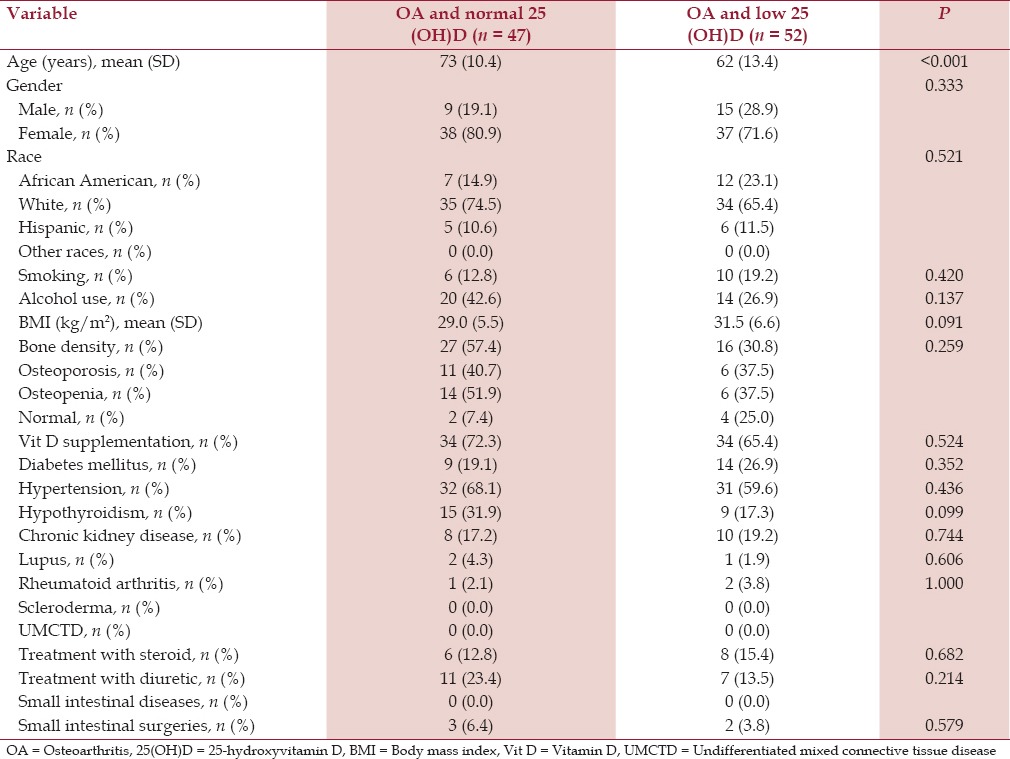
Table 6.
Baseline characteristics of patients with shoulder osteoarthritis and serum 25-hydroxyvitamin D level
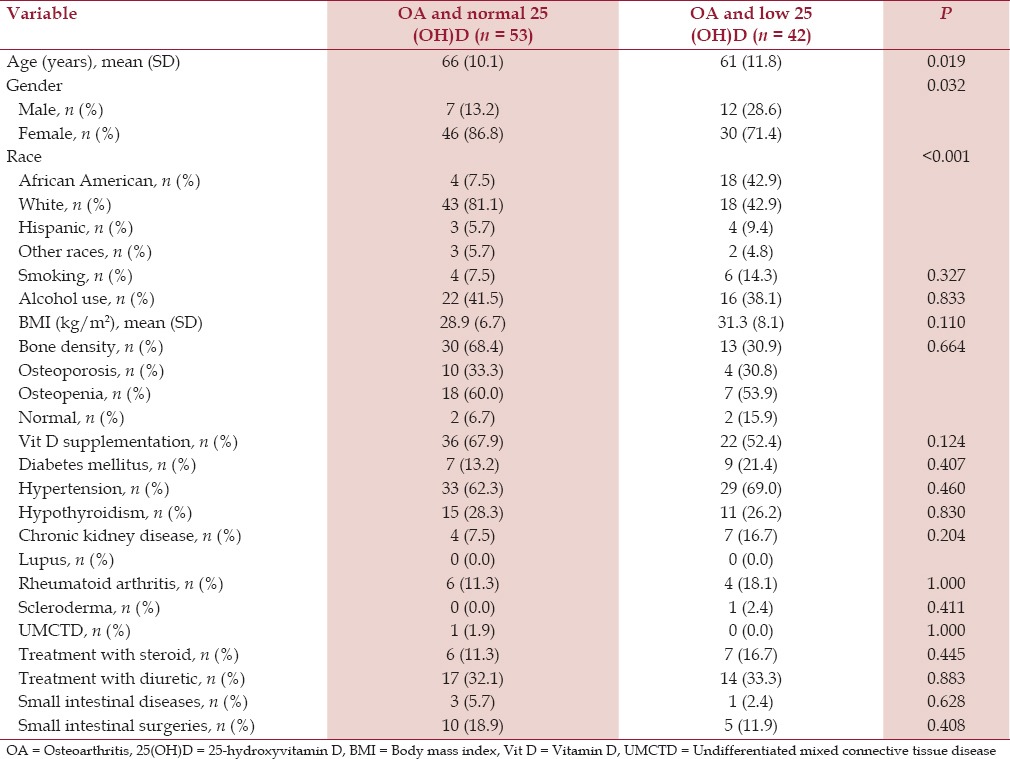
Patients who had OA of the spine, hip, and knee, and low serum 25(OH)D were younger (mean ages 59 years (SD 13.1), 62 years (SD 13.4), and 62 years (SD 11.2), respectively) compared to the patients who had OA of the spine, hip, and knee and normal serum 25(OH)D (mean ages 64 years (SD 12.5), 73 years (SD 10.4), and 67 years (SD 10.7), respectively). All the differences were statistically significant (P < 0.001) [Tables 3–5].
Table 3.
Baseline characteristics of patients with spine osteoarthritis and serum 25-hydroxyvitamin D level
Patients with spine OA and low serum 25(OH)D level had a significantly higher prevalence of cigarette smoking (16.3%) compared to the patients with spine OA and normal serum 25(OH)D (8.7%) (P < 0.001). Patients with spine OA and low serum 25(OH)D had a significantly higher mean BMI (kg/m2 ) (31.6 SD 7.3) compared to the patients with spine OA and normal serum 25(OH)D (28.9, SD 7.0) (P < 0.001). Similarly, patients with spine OA and low serum 25(OH)D had a higher prevalence of type 2 diabetes mellitus (24.7%) compared to the patients with spine OA and normal serum 25(OH)D (18.6%) (P < 0.001) [Table 3].
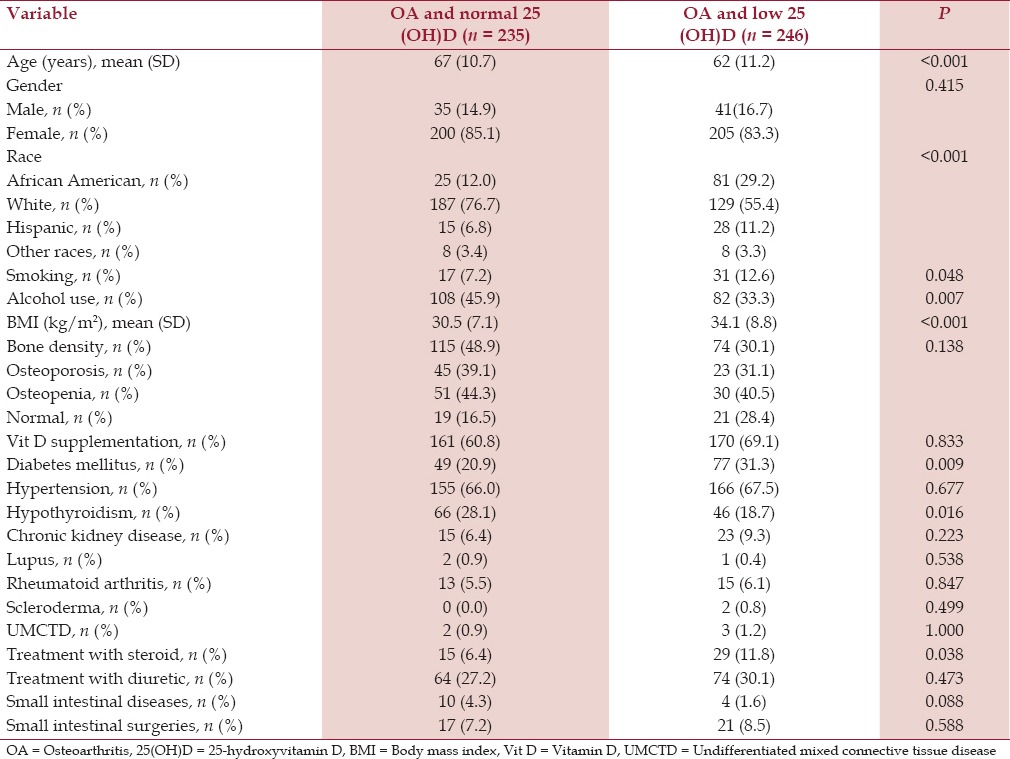
Patients with knee OA and low serum 25(OH)D had a significantly higher mean BMI (kg/m2 ) (34.1 SD 8.8) compared to the patients with knee OA and normal serum 25(OH)D (30.5, SD 7.1) (P < 0.001) [Table 4]. On the contrary, patients with hip OA and shoulder OA had comparable BMI in both the groups [Tables 5 and 6].
Table 4.
Baseline characteristics of patients with knee osteoarthritis and serum 25-hydroxyvitamin D level
Discussion
Our study provided six major observations. First, patients with low serum 25(OH)D had clinical manifestations of OA at a significantly younger age compared to the patients with normal serum 25(OH)D. Second, African American and Hispanic patients with OA had a significantly higher prevalence of low serum 25(OH)D compared to the Whites and other races. Third, the prevalence of cigarette smoking was significantly higher in patients with OA and low serum 25(OH)D.
Fourth, patients with OA and low serum 25(OH)D had a significantly higher mean BMI. Fifth, the prevalence of type 2 diabetes mellitus was significantly higher in patients with OA and low serum 25(OH)D. Sixth, patients with OA and low serum 25(OH)D had a significantly lower prevalence of hypothyroidism compared to the patients with normal serum 25(OH)D.
Although OA affects people of all ages, it is most commonly seen in people older than 65 years.[7] Our patients with OA and normal serum 259OH)D had a comparable mean age of 66 years but our patients with low serum 25(OH)D were diagnosed with OA at a significantly lower mean age of 61 years, which was 5 years younger. Bone metabolism is dependent on the key role played by vitamin D including its effect on the joint cells resulting in subchondral bone sclerosis and alterations in bone remodeling. Low serum 25(OH)D may promote such adverse joint changes at an early age. A younger mean age was consistently observed in patients with OA of the spine, knee, and hip, and with low serum 25(OH)D.
The 51% prevalence of low serum 25(OH)D in our patients with OA is comparable to the other studies that have found 41% prevalence of low serum 25(OH)D in outpatients aged 49-83 years,[8] and 57% in general medicine inpatients in the United States.[9]
In our study, women were significantly more affected with OA irrespective of serum 25(OH)D. Although many studies have shown that OA affects more women than men,[10] a higher prevalence of low 25(OH)D has been demonstrated among adult women with OA compared to men with OA.[11,12]
African American and Hispanic patients with OA had a significantly higher prevalence of low serum 25(OH)D compared to the Whites and other races. African American individuals synthesize less vitamin D per unit of sun exposure than White individuals because a darker complexion acts as a barrier to sunlight.[13,14] Furthermore, racial difference in variable; vitamin D receptor affinity for vitamin D metabolites may explain a similar variation in the prevalence of low vitamin D across other races with the observation of at least one vitamin D receptor polymorphism (Fokl), which is known to differ by race and ethnicity.[15,16,17]
Cigarette smoking is strongly associated with vitamin D deficiency in several studies and in our study.[18,19,20] In our study, the prevalence of cigarette smoking was significantly higher in patients with OA and low serum 25(OH)D compared to the patients with normal serum 25(OH)D.
It is postulated that smoking may alter vitamin D metabolism influencing the activity of 25-hydroxylase in the liver.[21] Cigarette smoke may also affect expression levels of the vitamin D receptor.[22]
Many studies have shown an association between decreasing serum 25(OH)D levels with increasing BMI as demonstrated in our study.[23,24,25] We found that the patients with OA and low serum 25(OH)D had a significantly higher mean BMI compared to patients with normal serum 25(OH)D. The effect of BMI on serum 25(OH)D may be explained by the fact that persons with high BMI usually have a high content of body fat, acting as a reservoir for lipid-soluble vitamin D, reducing its bioavailability.[23] Other contributing factors to the low 25(OH)D status among obese people might be lower than average exposure of large body areas to the sun and decreased amount of outdoor time because of limited mobility.[23,26] On the other hand, some studies have also found that higher BMI was not correlated with 25(OH)D levels.[11,27]
We found that the patients with OA and low serum 25(OH)D had a significantly higher prevalence of type 2 diabetes mellitus compared to the patients with normal serum 25(OH)D. This finding is consistent with other cross-sectional and prospective studies.[28,29]
The potential mechanisms include several effects, direct and indirect, of vitamin D on insulin action.[28]
Studies have shown a strong association between low serum 25(OH)D levels and hypothyroidism.[30] We found that 27.4% of our patients with OA and normal serum 25(OH)D had hypothyroidism, which was markedly lower than a study that found 72% association.[30]
Furthermore, we found a significantly lower prevalence of hypothyroidism in patients with OA and low serum 25(OH)D (18.5%) compared to the patients with normal serum 25(OH)D. This observation is unique and requires further research to understand the possible mechanism.
Our study had some limitations. A retrospective data review of the electronic health records might not have been able to document all laboratory results of serum 25(OH)D that were performed in the laboratories out of the hospital network, which perhaps got filed as paper documents. Many health insurance companies did not cover for the serum 25(OH)D test and hence, many patients with OA did not get the serum 25(OH)D test.
Our study had many strengths. A large sample size and strong inclusion criteria helped us study meticulously selected data for analysis. Our patients were established primary care patients who had a consistent follow-up record with clear documentation of the status of their OA over time. There has been an increase in research searching for the role of 25(OH)D in OA. Several clinical trials have ensued and are ongoing. To date, no strong evidence has been available to establish the role of 25(OH)D in OA, and the results are conflicting.[2,31,32,33] Our study provides unique observations in this field.
Conclusion
Patients with low serum levels of 25(OH)D and OA are younger than their counterparts with low 25(OH)D level. African American and Hispanic patients have a higher prevalence of low 25(OH)D. Patients with OA and low serum 25(OH)D have a higher prevalence of cigarette smoking and type 2 diabetes mellitus, a lower prevalence of hypothyroidism, and a higher BMI compared to the patients with OA and normal serum 25(OH)D. Future studies are needed to clarify the relationship between 25(OH)D level and OA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors thank Christine Rickette RN (study coordinator) for her contribution to this study.
References
- 1.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao Y, Winzenberg T, Nguo K, Lin J, Jones G, Ding C. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: A systematic review. Rheumatology (Oxford) 2013;52:1323–34. doi: 10.1093/rheumatology/ket132. [DOI] [PubMed] [Google Scholar]
- 3.Konstari S, Paananen M, Heliövaara M, Knekt P, Marniemi J, Impivaara O, et al. Association of 25-hydroxyvitamin D with the incidence of knee and hip osteoarthritis: A 22-year follow-up study. Scand J Rheumatol. 2012;41:124–31. doi: 10.3109/03009742.2011.617314. [DOI] [PubMed] [Google Scholar]
- 4.Mandi L. Epidemiology of osteoarthritis. In: Sharma L, Berenbaum F, editors. Osteoarthritis: A Companion to Rheumatology. Philadelphia: Mosby Elsevier; 2007. pp. 1–14. [Google Scholar]
- 5.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergink AP, Uitterlinden AG, Van Leeuwen JP, Buurman CJ, Hofman A, Verhaar JA, et al. Vitamin D status, bone mineral density, and the development of radiographic osteoarthritis of the knee: The Rotterdam Study. J Clin Rheumatol. 2009;15:230–7. doi: 10.1097/RHU.0b013e3181b08f20. [DOI] [PubMed] [Google Scholar]
- 7.Osteoarthritis. Atlanta, Georgia: Arthritis Foundation; 2015. [Accessed October 3, 2015]. at http://www.arthritis.org/about-arthritis/types/osteoarthritis/what-is-osteoarthritis.php . [Google Scholar]
- 8.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 9.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 10.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: Arthritis data from the Third National Health and Nutrition Examination Survey 1991-1994. J Rheumatol. 2006;33:2271–9. [PubMed] [Google Scholar]
- 11.Goula T, Kouskoukis A, Drosos G, Tselepis AS, Ververidis A, Valkanis C, et al. Vitamin D status in patients with knee or hip osteoarthritis in a Mediterranean country. J Orthop Traumatol. 2015;16:35–9. doi: 10.1007/s10195-014-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–81. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–6. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 14.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–32. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris SS, Eccleshall TR, Gross C, Dawson-Hughes B, Feldman D. The vitamin D receptor start codon polymorphism (FokI) and bone mineral density in premenopausal American black and white women. J Bone Miner Res. 1997;12:1043–8. doi: 10.1359/jbmr.1997.12.7.1043. [DOI] [PubMed] [Google Scholar]
- 16.Bid HK, Mishra DK, Mittal RD. Vitamin-D receptor (VDR) gene (Fok-I, Taq-I and Apa-I) polymorphisms in healthy individuals from north Indian population. Asian Pac J Cancer Prev. 2005;6:147–52. [PubMed] [Google Scholar]
- 17.Levin GP, Robinson-Cohen C, de Boer IH, Houston DK, Lohman K, Liu Y, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA. 2012;308:1898–905. doi: 10.1001/jama.2012.17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshimura N, Muraki S, Oka H, Morita M, Yamada H, Tanaka S, et al. Profiles of vitamin D insufficiency and deficiency in Japanese men and women: Association with biological, environmental, and nutritional factors and coexisting disorders: The ROAD study. Osteoporos Int. 2013;24:2775–87. doi: 10.1007/s00198-013-2372-z. [DOI] [PubMed] [Google Scholar]
- 19.Kassi EN, Stavropoulos S, Kokkoris P, Galanos A, Moutsatsou P, Dimas C, et al. Smoking is a significant determinant of low serum vitamin D in young and middle-aged healthy males. Hormones (Athens) 2015;14:245–50. doi: 10.14310/horm.2002.1521. [DOI] [PubMed] [Google Scholar]
- 20.Cutillas-Marco E, Fuertes-Prosper A, Grant WB, Morales-Suárez-Varela M. Vitamin D deficiency in South Europe: Effect of smoking and aging. Photodermatol Photoimmunol Photomed. 2012;28:159–61. doi: 10.1111/j.1600-0781.2012.00649.x. [DOI] [PubMed] [Google Scholar]
- 21.Need AG, Kemp A, Giles N, Morris HA, Horowitz M, Nordin BE. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos Int. 2002;13:83–8. doi: 10.1007/s198-002-8342-9. [DOI] [PubMed] [Google Scholar]
- 22.Haley KJ, Manoli SE, Tantisira KG, Litonjua AA, Nguyen P, Kobzik L, et al. Maternal smoking causes abnormal expression of the vitamin D receptor [abstract] Am J Respir Crit Care Med. 2009;179:A5874. [Google Scholar]
- 23.Lagunova Z, Porojnicu AC, Lindberg F, Hexeberg S, Moan J. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:3713–20. [PubMed] [Google Scholar]
- 24.Bischof MG, Heinze G, Vierhapper H. Vitamin D status and its relation to age and body mass index. Horm Res. 2006;66:211–5. doi: 10.1159/000094932. [DOI] [PubMed] [Google Scholar]
- 25.Arunabh S, Pollack S, Yeh J, Aloia JF. Body fat content and 25-hydroxyvitamin D levels in healthy women. J Clin Endocrinol Metab. 2003;88:157–61. doi: 10.1210/jc.2002-020978. [DOI] [PubMed] [Google Scholar]
- 26.Florez H, Martinez R, Chacra W, Strickman-Stein N, Levis S. Outdoor exercise reduces the risk of hypovitaminosis D in the obese. J Steroid Biochem Mol Biol. 2007;103:679–81. doi: 10.1016/j.jsbmb.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 27.Baraké R, Weiler H, Payette H, Gray-Donald K. Vitamin D status in healthy free-living elderly men and women living in Quebec, Canada. J Am Coll Nutr. 2010;29:25–30. doi: 10.1080/07315724.2010.10719813. [DOI] [PubMed] [Google Scholar]
- 28.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: A systematic review. Eur J Clin Nutr. 2011;65:1005–15. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kivity S, Agmon-Levin N, Zisappl M, Shapira Y, Barak V, Dankó K, et al. Thyroid disease and vitamin D deficiency. Ann Rheum Dis. 2010;69:A62. [Google Scholar]
- 31.Glover TL, Goodin BR, Horgas AL, Kindler LL, King CD, Sibille KT, et al. Vitamin D, race, and experimental pain sensitivity in older adults with knee osteoarthritis. Arthritis Rheum. 2012;64:3926–35. doi: 10.1002/art.37687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAlindon T, LaValley M, Schneider E, Nuite M, Lee JY, Price LL, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: A randomized controlled trial. JAMA. 2013;309:155–62. doi: 10.1001/jama.2012.164487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muraki S, Dennison E, Jameson K, Boucher BJ, Akune T, Yoshimura N, et al. Association of vitamin D status with knee pain and radiographic knee osteoarthritis. Osteoarthritis Cartilage. 2011;19:1301–6. doi: 10.1016/j.joca.2011.07.017. [DOI] [PubMed] [Google Scholar]


