Abstract
Background and Aims:
Myoclonus is a major side-effect following etomidate injection requiring use of medical intervention.
Material and Methods:
In this double-blinded clinical trial, 50 consecutive patients, randomly received sufentanil 0.2 μg/kg or midazolam 0.015 mg/kg, 90 s before induction of anesthesia with etomidate (0.3 mg/kg). Then, the patients were monitored for any myoclonic movements during anesthesia.
Results:
The incidence of myoclonus was 28% in the sufentanil group and 84% in the midazolam group. The frequency and intensity of myoclonus were significantly higher in the midazolam group, compared to the sufentanil group (P < 0.001). Myoclonus duration in the sufentanil and midazolam groups were 5.8 ± 13.2 and 69 ± 47.8 s, respectively (P < 0.0010).
Conclusion:
The frequency, intensity and duration of myoclonus in the midazolam group, were significantly more prevalent than the sufentanil group.
Keywords: Etomidate, midazolam, myoclonus, sufentanil
Introduction
Etomidate is a popular anesthetic agent since it is associated with hemodynamic stability,[1] minimal respiratory depression,[2] cerebral protection,[3,4] and rapid recovery after either a single dose or a continuous infusion.[5] However, this agent is associated with a frequent incidence of myoclonic movements.[6]
The mechanism of myoclonus is not clear and does not seem to be associated with seizure-like electroencephalographic activities.[7] However, myoclonus is believed to be the result of activities either in the brainstem or deep cerebral structures. The incidence of myoclonus is highly variable (0-80%) and can be problematic in patients with open globe injuries or those at high risk of aspiration.[8]
The incidence of myoclonus can be reduced after premedication with either opioid or benzodiazepine.[9,10,11,12] Among the evaluated medications, both midazolam, and sufentanil, as pretreatment agents, have been reported to effectively reduce the myoclonus. However, a comparison between midazolam and sufentanil has not been made so far, and it is not clear which agent is a better option for reducing myoclonus after etomidate injection.
Therefore, we conducted a prospective, randomized, double-blind, clinical trial to compare the efficacy of midazolam and sufentanil in reducing the incidence and severity of myoclonus after etomidate injection in patients, undergoing elective ophthalmic surgery with general anesthesia.
Material and Methods
The current study was approved by the ethics committee of our university. All patients signed a written informed consent before participation. Fifty adult patients with American Society of Anesthesiologists class II or III, who were scheduled for elective eye surgery under general anesthesia, were selected for the study.
The exclusion criteria were allergy to benzodiazepine or opioid, neuropsychological or neuromuscular diseases, adrenal dysfunction or epilepsy, addiction; and analgesic or sedatives administration within 24 h before the procedure.
Patients did not receive premedication and were randomly assigned to one of the two groups (n = 25 per group) Group M consisted of patients receiving midazolam (0.015 mg/kg) and group S consisted of patients receiving sufentanil (0.2 μ/kg). Randomization was performed using a computer-generated table. An anesthesiologist, who was blinded to the study and was not involved in anesthesia induction, prepared and coded syringes containing a total volume of 4 ml of medications. Patient monitoring included electrocardiography, pulse oximetry, noninvasive blood pressure (BP) monitoring, and capnography.
Patients received isotonic saline solution (5 mL/kg) over 10 min at room temperature and were preoxygenated with 100% oxygen; then, the medication was injected over 30 s. Ninety seconds after pretreatment with the medication, etomidate (0.3 mg/kg) was intravenously injected within 20 s. Two minutes after intravenous (IV) administration of etomidate, anesthesia was completed by atracurium (0.5 mg/kg) and fentanyl (2 μ/kg) to guarantee adequate anesthetic induction.
Patients were continuously monitored during anesthesia by one single physician, blinded to treatment procedures. Heart rate (HR), BP and mean arterial pressure (MAP) in pre- and post-induction (90 s after administration) were recorded.
The intensity of myoclonus was clinically graded as follows: 0 = no myoclonus, 1 = mild myoclonus (short movement of a body segment), 2 = moderate myoclonus (mild movements of two different muscles), and 3 = severe myoclonus (intense clonic movements in two or more muscle groups).[10]
Statistical considerations
Based on previous studies, a minimum number of 23 patients were required in each group (with a significance level of 90% and a power of 80%).[9,10] Hence, 25 patients in each group were included in this study. Statistical analyses were performed using SPSS version 16 (SPSS Inc., Chicago, IL. US). T-test and Chi-square were performed to compare patients' characteristics, and comparisons between the groups were performed by Mann-Whitney test. P < 0.05 was considered statistically significant.
Results
Demographic information of the patients is shown in Table 1; there was no significant difference between the two groups in terms of age, although they were different regarding sex distribution (P = 0.047) [Table 1].
Table 1.
Demographic characteristics of the patients

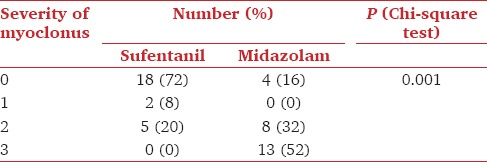
The incidence of myoclonus was significantly different between the two groups; it was 28% (7 patients) in the sufentanil group and 84% (21 patients) in the midazolam group (P = 0.001). The intensity of myoclonus was also significantly more severe in midazolam groups compare to sufentanil group [Table 2].
Table 2.
Severity of myoclonus in sufentanil and midazolam groups
The mean duration of myoclonus was significantly less in sufentanil group than in midazolam group (5.8 ± 13.2 s vs. 69 ± 47.8 s; P < 0.001).
There was a significant difference in HR pre- and post-anesthesia induction in the sufentanil group. The mean HR was 83.96 ± 18.1 before the induction and 71.8 ± 15.2 after the induction in this group (P < 0.001). However, the difference in HR before and after the induction in the midazolam group was not significant (77.24 ± 15.1 vs. 76.2 ± 13) (P = 0.705).
Mean arterial pressure was significantly different before and after the induction in the sufentanil group; it was 120.46 ± 15.4 mmHg before the induction and 109.5 ± 18.8 mmHg after the induction (P = 0.0001). However, MAP was not significantly different before and after the induction in the midazolam group; it was 123.12 ± 19 mmHg before the induction and 116.2 ± 25.5 mmHg after the induction (P = 0.098).
The mean HR before and after the induction was significantly different between the two groups (P = 0.002). The mean difference in HR before and after the induction was 12.16 ± 10.3 in the sufentanil group and 1.04 ± 13.5 in the midazolam group.
The mean of MAP before and after the induction was not significantly different between the two groups (P = 0.406). The mean difference in MAP before and after the induction was 10.09 ± 12.6 mmHg in the sufentanil group and 6.9 ± 20 mmHg in the midazolam group.
Discussion
The current study showed that pretreatment with sufentanil 0.2 μg/kg significantly prevents the occurrence and reduces the severity of myoclonus during anesthesia induction with etomidate. The mean duration of myoclonus in the sufentanil group was significantly less than that of the midazolam group.
The mean of variations in HR before and after the induction between the two groups was significantly different, but the mean of MAP before and after the induction was not significantly different between the two groups.
Application of different medications, as pretreatment agents, has been investigated for the reduction of myoclonus after etomidate injection. Benzodiazepine has been shown to reduce etomidate-associated myoclonus.[11] However, diazepam (0.0625-0.0125 mg/kg IV) or flunitrazepam (0.01 mg/kg IV) failed to reduce myoclonic movements.[12,13] On the other hand, Doenicke et al. reported a significant reduction in the incidence of myoclonus when 0.1 mg/kg of diazepam was administrated 5 min before etomidate.[14]
Schwarzkopf et al. reported that the incidence of myoclonic movements was significantly lower in patients, who were pretreated with midazolam 0.015 mg/kg (20%), compared to the placebo group (90%).[9] In this study, all patients in the study groups were premeditated with oral midazolam; however, in our study, patients did not receive premedication.
In the study by Hwang et al., IV administration of midazolam 2 min before etomidate injection reduced the incidence of myoclonus up to 17%.[15] In the current study, the incidence of myoclonus in the midazolam-treated group was 84%, which was significantly higher than that observed in the study by Hwang. In the study by Hwang, etomidate was administered 2 min after midazolam (0.05 mg/kg); the higher dosage and longer interval in the study by Hwang may account for the lower incidence of myoclonus, following midazolam administration in their study, compared to ours.
Several opioids with different doses have been investigated as pretreatment medications to prevent myoclonus. Pretreatment with 100 μg of fentanyl reduced the incidence of myoclonus up to 8%.[6] Use of higher fentanyl doses (500 μg) significantly reduced myoclonic movements; however, the incidence of apnea increased during induction.[16]
In a study by Fassoulaki et al., they found no change in myoclonic occurrence when fentanyl 100 μg was administered before anesthesia induction with etomidate.[11]
Remifentanil (1 μg/kg), administered 2 min before etomidate injection, was reported to reduce the incidence of myoclonus to 7%, without any clinically relevant side-effects.[17] In another study, pretreatment with remifentanil (with the same dose) was associated with a 17% incidence of myoclonus, which was higher than that observed in the study by Kelsaka et al. (7%). Furthermore, 10% of the remifentanil-treated patients experienced clinically relevant side-effects such as chest muscle rigidity and bradycardia. Therefore, their results suggested that remifentanil may not be a good option for routine pretreatment during etomidate injection.[15]
Other investigators have recommended sufentanil (0.3 μg/kg) as a pretreatment agent before anesthesia induction with etomidate. In fact, this medication has been shown to completely prevent myoclonus without causing any side-effects.[10] Studies with other opioids such as alfentanil and buprenorphine have not shown higher efficiencies.[18,19]
In our study, pretreatment with sufentanil (0.2 μg/kg) was associated with a significantly reduced incidence and severity of myoclonus; however, pretreatment with midazolam resulted in a higher incidence and severity of myoclonus (including moderate and severe grades of myoclonus). The duration of myoclonus in the sufentanil group was also less than that of the midazolam group. Due to the findings of the current study, in the sufentanil group, HR reduced after induction, but the mean difference in MAP was not clinically significant after sufentanil administration.
Limitations
The sample of our study was small so further studies are required for comparing different doses of opioids (e.g., sufentanil) and determining other adverse effects.
Conclusion
According to the results of this study, it seems using sufentanil as a pretherapeutic agent might reduce the risk of development of etomidate-induced myoclonus.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bennett JM, Ehrenfeld JM, Markham L, Eagle SS. Anesthetic management and outcomes for patients with pulmonary hypertension and intracardiac shunts and Eisenmenger syndrome: A review of institutional experience. J Clin Anesth. 2014;26:286–93. doi: 10.1016/j.jclinane.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 2.Li YW, Ma L, Sui B, Cao CH, Liu XD. Etomidate with or without flumazenil anesthesia for stem cell transplantation in autistic children. Drug Metabol Drug Interact. 2014;29:47–51. doi: 10.1515/dmdi-2013-0043. [DOI] [PubMed] [Google Scholar]
- 3.Liberman MY, Ching S, Chemali J, Brown EN. A closed-loop anesthetic delivery system for real-time control of burst suppression. J Neural Eng. 2013;10:046004. doi: 10.1088/1741-2560/10/4/046004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TK, Park IS. Comparative Study of Brain Protection Effect between Thiopental and Etomidate Using Bispectral Index during Temporary Arterial Occlusion. J Korean Neurosurg Soc. 2011;50:497–502. doi: 10.3340/jkns.2011.50.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dela Cruz JE, Sullivan DN, Varboncouer E, Milbrandt JC, Duong M, Burdette S, et al. Comparison of procedural sedation for the reduction of dislocated total hip arthroplasty. West J Emerg Med. 2014;15:76–80. doi: 10.5811/westjem.2013.7.15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyman Y, von Hofsten K, Ritzmo C, Eksborg S, Lönnqvist PA. Effect of a small priming dose on myoclonic movements after intravenous anaesthesia induction with Etomidate-Lipuro in children. Br J Anaesth. 2011;107:225–8. doi: 10.1093/bja/aer129. [DOI] [PubMed] [Google Scholar]
- 7.Hoyer C, Kranaster L, Janke C, Sartorius A. Impact of the anesthetic agents ketamine, etomidate, thiopental, and propofol on seizure parameters and seizure quality in electroconvulsive therapy: A retrospective study. Eur Arch Psychiatry Clin Neurosci. 2014;264:255–61. doi: 10.1007/s00406-013-0420-5. [DOI] [PubMed] [Google Scholar]
- 8.Bahn EL, Holt KR. Procedural sedation and analgesia: A review and new concepts. Emerg Med Clin North Am. 2005;23:503–17. doi: 10.1016/j.emc.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Schwarzkopf KR, Hueter L, Simon M, Fritz HG. Midazolam pretreatment reduces etomidate-induced myoclonic movements. Anaesth Intensive Care. 2003;31:18–20. doi: 10.1177/0310057X0303100103. [DOI] [PubMed] [Google Scholar]
- 10.Hueter L, Schwarzkopf K, Simon M, Bredle D, Fritz H. Pretreatment with sufentanil reduces myoclonus after etomidate. Acta Anaesthesiol Scand. 2003;47:482–4. doi: 10.1034/j.1399-6576.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 11.Fassoulaki A, Pateras C, Kaniaris P. Fentanyl in the prevention of etomidate-induced myoclonus. Cah Anesthesiol. 1987;35:201–2. [PubMed] [Google Scholar]
- 12.Korttila K, Tammisto T, Aromaa U. Comparison of etomidate in combination with fentanyl or diazepam, with thiopentone as an induction agent for general anaesthesia. Br J Anaesth. 1979;51:1151–7. doi: 10.1093/bja/51.12.1151. [DOI] [PubMed] [Google Scholar]
- 13.Castillo Monsegur J, Villalonga Morales A, Nalda Felipe MA. Pharmacological prevention of myoclonias during anesthesia induction with etomidate. Comparative study of fentanyl, flunitrazepam and pancuronium. Rev Esp Anestesiol Reanim. 1987;34:270–2. [PubMed] [Google Scholar]
- 14.Doenicke A, Kugler J, Penzel G, Laub M, Kalmar L, Killian I, et al. Cerebral function under etomidate, a new non-barbiturate i.v. hypnotic (author's transl) Anaesthesist. 1973;22:357–66. [PubMed] [Google Scholar]
- 15.Hwang JY, Kim JH, Oh AY, Do SH, Jeon YT, Han SH. A comparison of midazolam with remifentanil for the prevention of myoclonic movements following etomidate injection. J Int Med Res. 2008;36:17–22. doi: 10.1177/147323000803600103. [DOI] [PubMed] [Google Scholar]
- 16.Stockham RJ, Stanley TH, Pace NL, Gillmor S, Groen F, Hilkens P. Fentanyl pretreatment modifies anaesthetic induction with etomidate. Anaesth Intensive Care. 1988;16:171–6. doi: 10.1177/0310057X8801600207. [DOI] [PubMed] [Google Scholar]
- 17.Kelsaka E, Karakaya D, Sarihasan B, Baris S. Remifentanil pretreatment reduces myoclonus after etomidate. J Clin Anesth. 2006;18:83–6. doi: 10.1016/j.jclinane.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Khalil SN, Lawson KS, Hanis CL, Lemak NA, Ruiz RS. Alfentanil decreases myoclonus caused by etomidate. Middle East J Anaesthesiol. 1999;15:185–92. [PubMed] [Google Scholar]
- 19.Klausen NO, Johansen SH, Janstrup F, Hansen JG. Preoperative buprenorphin does not prevent myoclonia seen after etomidate. Br J Anaesth. 1982;54:475. doi: 10.1093/bja/54.4.475. [DOI] [PubMed] [Google Scholar]


