Abstract
Background
Resistance to second-line anti-tuberculosis drugs (SLD) severely compromises treatment options of drug-resistant tuberculosis (TB). We assessed the association between acquisition of resistance (AR) to second-line injectable drugs (SLI) or fluoroquinolones (FQ) and mortality among TB cases confirmed by positive culture results with available initial and final drug susceptibility test (DST) results.
Methods
We analyzed data from U.S. National TB Surveillance System, 1993–2008. Acquired resistance was defined as drug susceptibility at initial DST but resistance to the same drug at final DST. We compared survival with Kaplan-Meier curves and analyzed the association between AR and mortality using a univariate extended Cox proportional hazards model adjusted for age.
Results
Of 2,329 cases with both initial and final DST to SLI, 49 (2.1%) acquired resistance; 13/49 (26.5%) had treatment terminated by death versus 222 (10.0%) of those without AR to SLI (P<0.001). Of 1,187 cases with both initial and final DST to FQ, 32 (2.8%) acquired resistance; 12/32 (37.5%) had treatment terminated by death versus 121 (10.9%) of those without AR to FQ (P=0.001). Controlling for age, mortality was significantly greater among cases with AR to SLD than among cases without AR (adjusted hazard ratio (aHR)[SLI], 2.8; 95% confidence interval (CI),1.4–5.4; aHR[FQ], 1.9; 95% CI,1.0–3.5). MDR TB at treatment initiation, positive HIV status, and extrapulmonary disease were also significantly associated with mortality.
Conclusion
Mortality was significantly greater among TB cases with AR to SLD. Providers should consider AR to SLD early in treatment, monitor DST results, and avoid premature deaths.
Keywords: Tuberculosis, acquired drug resistance
Introduction
In 2013, the World Health Organization (WHO) reported approximately 4% of new tuberculosis (TB) cases and 20% of previously treated TB cases globally had multidrug-resistant (MDR) TB, defined as TB resistant to at least isoniazid and rifampicin. Among all MDR-TB cases globally, about 10% also had resistance to at least one injectable second-line drug and a fluoroquinolone, i.e., extensively drug-resistant (XDR) TB [1]. The acquisition of resistance (AR) to second-line drugs (SLD) presents a serious challenge to treating patients with drug-resistant TB worldwide.
Acquired drug resistance can be attributed to several factors, such as poor adherence to treatment, poor clinical management, and inadequate or unstable drug supply [2]. Treatment of drug-resistant tuberculosis takes longer, is more toxic, more expensive and less effective than treatment of pan-sensitive TB [3–4]. The Global Plan to Stop TB 2011–2015 estimates that $900 million would have been needed in 2013 to address MDR TB worldwide, including up to $300 million for second-line drugs alone [5].
The acquisition of resistance to second-line anti-TB drugs during treatment can lead to XDR TB [6]. Treatment outcomes among patients with XDR TB are poor; only 33% have treatment success, and 26% die from TB [1, 5–9]. Despite the decreasing number of TB cases and low prevalence of MDR TB, acquisition of resistance to second-line anti-TB drugs during treatment still occurs in the United States [6, 10–11, 13–14]. Understanding the consequences of AR to SLD is important for prognosis and development of strategies for improving outcomes among patients with drug-resistant forms of TB.
The objective of our study was to assess the effect of AR to key SLD on mortality among the subset of TB cases with repeated drug susceptibility tests (DST) for second-line drugs in the United States.
Methods
We analyzed data from the National TB Surveillance System (NTSS) at the U.S. Centers for Disease Control and Prevention (CDC) for the years 1993–2008. Each record in NTSS represents one case of TB. Variables in NTSS include demographic and clinical characteristics, initial drug regimen, length of treatment, and conventional phenotypic DST results [11]. While testing and reporting DST results for isoniazid, rifampicin and ethambutol is routine for the initial positive culture in the United States, second-line DST and repeated DST are performed only when indicated. There are no standard guidelines for conducting second-line DST testing in the United States. Each state follows their own algorithm which is typically based on individual physician practices. Possible indications for SLD DST may be a combination of demonstrated resistance to the first-line drugs, high index of suspicion for MDR TB (e.g., born in region with a high prevalence of drug resistance, previous episode or incomplete TB treatment) or poor treatment response during the current TB episode. DST to SLD usually implies that a physician has considered initiating second-line anti-tuberculosis treatment.
A subset of culture-confirmed TB cases with both initial and final DST results to second-line anti-TB drugs were included in the analysis and described elsewhere [13]. To understand the effect of AR to SLD on mortality during treatment, we compared death rates among TB cases with and without AR to SLD. For all individual drugs tested the initial DST was defined as the first reported DST result, and final DST was defined as the last reported DST result. For this analysis SLD included injectable second-line drugs (SLI) specifically: amikacin, kanamycin, capreomycin; and fluoroquinolones (FQ) including: ofloxacin and ciprofloxacin. AR was defined as drug susceptibility at initial DST but resistance to the same drug at final DST.
We used Kaplan-Meier curves to visually compare unadjusted survival rates among TB cases with and without AR, testing the difference between strata with the log-rank test. Cox proportional hazards modeling was used to assess the effect of AR to SLD on survival time during treatment. We graphed log (−log [Survival Probability]) versus log (time) to test the proportional hazards assumption. We excluded cases with initial resistance to these specific SLD since they were not at risk for acquired resistance. We calculated survival time using the difference between the treatment start date and treatment end date. The Wilcoxon test was used to compare median time to death among TB cases with and without AR to SLD.
In NTSS the reasons for stopping treatment included completion of treatment, moved, refused, lost to follow-up, died, or other/unknown; no data are available for cause of death. Thus we used death as the study endpoint, with date treatment stopped presumed to be the date of death. Otherwise cases were censored at the treatment end date, where the reasons for stopping treatment included completion of treatment, moved, refused, or lost to follow-up. Hazard ratios adjusted for age (aHR) and 95% confidence intervals (CI) were used to measure the magnitude and precision of the association of AR with the probability of death during treatment. We used an extended Cox proportional hazards model with time-dependent coefficients to assess the effect of AR to SLI since the Kaplan-Meier curves of those with and without AR to SLI intersected at the 8 month point in time. We investigated other possible demographic and clinical predictors of death, including sex, illicit drug use, HIV status, sites of disease, presence of MDR TB at diagnosis, and use of any SLD in the initial regimen. Illicit drug use was defined as reported use of injection or non-injection illicit drugs within the year prior to diagnosis. The HIV variable was recoded to include two subgroups: positive and not-positive cases. We divided anatomic site of disease into two groups: pulmonary only versus any extrapulmonary disease. A p-value ≤0.05 was considered statistically significant. Analysis was conducted utilizing SAS software version 9.3 (SAS Institute, Cary, North Carolina).
Because TB is a reportable disease in all U.S. jurisdictions, and the data for NTSS are collected as part of routine public health practice, this project was determined at CDC to not be human subjects research. The researchers obtained permission for the use of NTSS data following specific established procedure and training assuring the confidentiality of the data.
Results
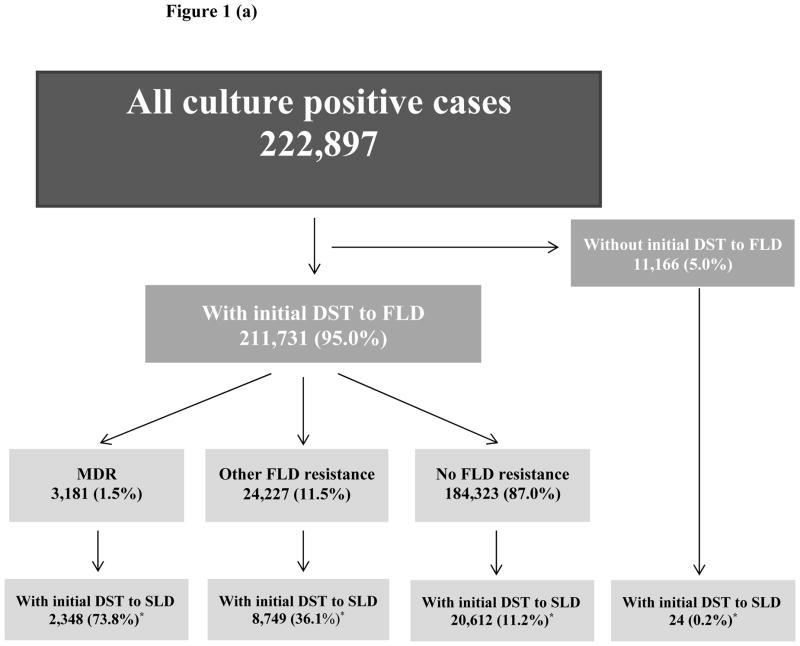
During 1993–2008, a total of 222,897 TB cases confirmed by positive culture results were reported to CDC including 211,731 (95%) cases with initial DST to first line anti-TB drugs and 31,733 (14%) cases with DST results to any second line drugs (Figure 1a). DST results to second line anti-TB drugs were reported for 74% of cases with initial multi-drug resistance, and for 11% of cases without any resistance to first line drugs (Figure 1a). Among all cases with initial DST to first line drugs, cases with and without initial DST to second line drugs had similar gender distribution (64.5% and 63.9% respectively were males, p=0.03), mean age (48 years old, p=0.02), site of disease (75.6% and 74.8% respectively had pulmonary TB, p<0.0001) and HIV status (11.0% and 11.4% respectively reported HIV positive status, p<0.0001). Among individuals with initial DST to SLD 6.6% were previously diagnosed with TB disease versus 4.9% among those without initial DST to SLD (p<0.0001).
Figure 1.
Figure 1(a). Selection of cases with initial DST to any SLD (31,733)
Notes: DST: drug susceptibility test, SLD: second-line drugs, FLD: first-line drugs, MDR: multidrug resistance, *: percentage calculated from the number in the above box
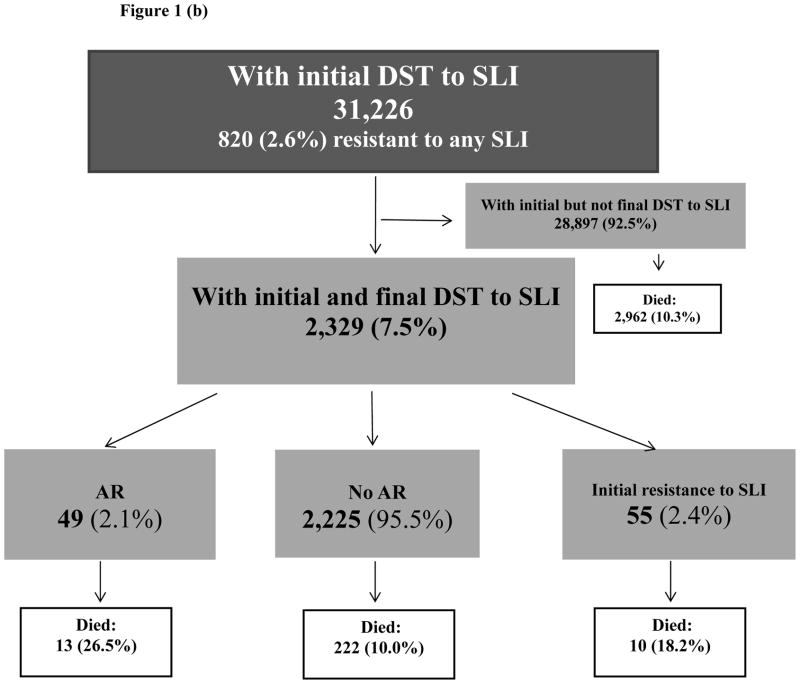
Figure 1(b). Selection of study population: second-line injectable drugs
Notes: DST: drug susceptibility test, SLI: second-line injectable drugs, AR: acquired resistance
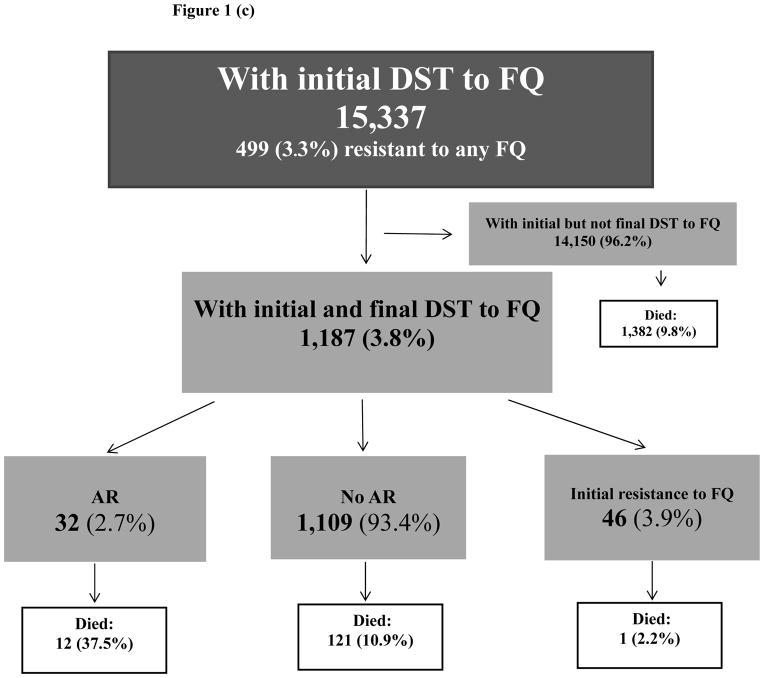
Figure 1(c). Selection of study population: fluoroquinolones
Notes: DST: drug susceptibility test, FQ: fluoroquinolones, AR: acquired resistance
Of 31,733 cases with initial DST results to any SLD, 2,329 cases had both initial and final DST results to SLI and 1,187 cases had both initial and final DST results to FQ (Figure 1b, 1c). Demographic and clinical characteristics of cases with AR to SLI and AR to FQ were described previously [13].
SLI analytic group
Out of 31,226 cases with initial DST results to SLI, 820 (2.6%) had reported resistance to any SLI. Both initial and final DST results to SLI were reported in 2,329 cases (SLI analytic group). Compared to 28,897 cases with only initial DST to SLI, cases in the SLI analytic group were significantly younger (mean age 46 years old versus 48, p<0.0001), were more likely to have HIV (15% versus 11%, p<0.0001) and pulmonary disease (87% versus 75%, p<0.0001); 61% were males (versus 64%, p=0.001). Among individuals with initial DST to SLI 10.3% died during treatment (Figure 1b).
Of 2,329 cases in SLI analytic group, 49 (2.1%) acquired resistance to at least one SLI during treatment, while 55 (2.4%) cases had initial resistance to SLI (Figure 1b). Among 49 cases with AR to SLI, 35 (71%) acquired resistance to capreomycin, 16 (33%) to kanamycin, and 7 (14%) to amikacin. Two persons acquired resistance to all three SLI. Median time between initial and final DST was 6 months among cases with AR to SLI, 3 months among cases with initial resistance to SLI and 2 months among cases without any resistance. Initial resistance to any FLD was reported in 98% (48/49) cases with AR to SLI versus 45% (1008/2225) among cases without AR to SLI (Table 1).
Table 1.
Initial resistance to first-line anti-TB drugs among TB cases with and without acquired resistance to second-line drugs, United States, 1993 – 2008
| Initial resistance to FLD | Second-line injectable drugs | Fluoroquinolones | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| AR (N=49) n (%) |
No AR (N=2,225) n (%) |
Initial res. to SLI (N=55) n (%) |
AR† (N=32) n (%) |
No AR (N=1,109) n (%) |
Initial res. to FQ (N=46) n (%) |
|
|
| ||||||
| MDR | 34 (69.4%) | 479 (21.5%) | 48 (87.3%) | 24 (75.0%) | 350 (31.6%) | 31 (67.4%) |
| EMB resistance | 23 (67.7%) | 275 (57.4%) | 41 (85.4%) | 19 (79.2%) | 213 (60.9%) | 23 (74.2%) |
| Non MDR | 15 (30.6%) | 1,746 (78.5%) | 7 (12.7%) | 8 (25.0%) | 759 (68.4%) | 15 (32.6%) |
| INH and RIF suscep. | 1 (6.7%) | 1211 (69.4%) | 3 (42.9%) | 2 (25.0%) | 399 (52.6%) | 7 (46.7%) |
| INH mono-resistance | 10 (66.7%) | 453 (26.0%) | 3 (42.9%) | 5 (62.5%) | 320 (42.2%) | 8 (53.3%) |
| RIF mono-resistance | 4 (26.6%) | 76 (4.4%) | 1 (14.2%) | 1 (12.5%) | 38 (5.0%) | 0 |
| EMB resistance | 2 (13.3%) | 57 (3.3%) | 4 (57.1%) | 4 (50%) | 44 (5.8%) | 3 (20%) |
| Missing 1st line DST | 0 | 6 (0.2%) | 0 | 0 | 2 (0.2%) | 0 |
Notes: FLD: first-line drugs, AR: acquired resistance, SLI: second-line injectable drugs, MDR: multidrug resistance, INH: isoniazid, RIF: rifampicin, EMB: ethambutol, DST: drug susceptibility test
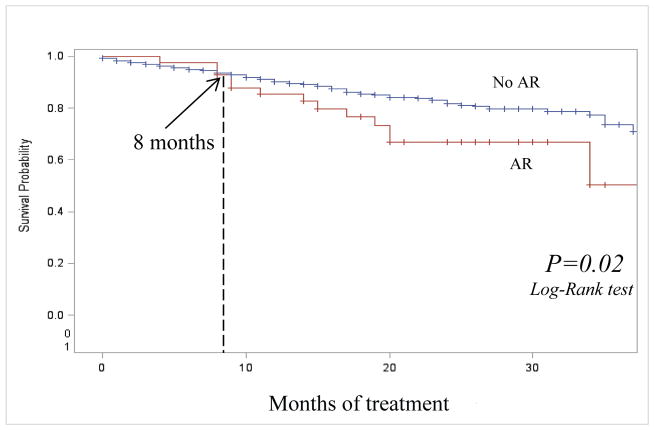
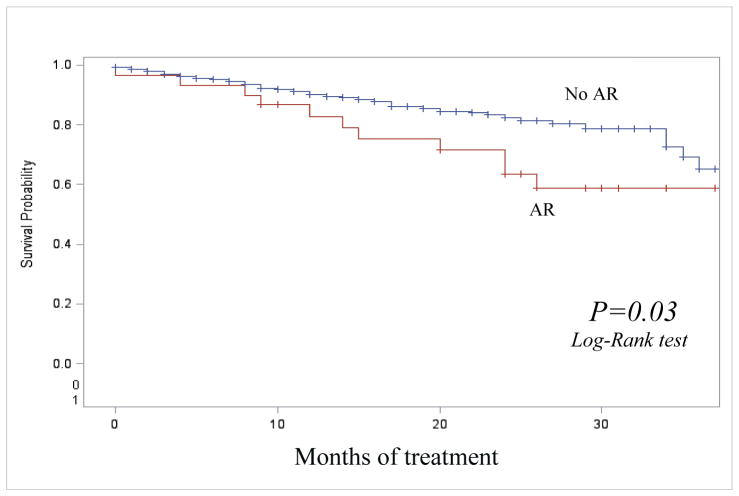
Of 49 individuals with AR, 13 (26.5%) died during treatment versus 18.2% (10/55) with initial resistance and 10.0% (222/2,225) who did not acquire resistance. Among 2,329 cases in the SLI analytic group, 561 (24.1%) were MDR TB at treatment initiation (Table 1). Of 34 MDR TB individuals at treatment initiation who acquired resistance to SLI, 11 (32.4%) died during treatment, versus 20.8% (10/48) among those with MDR and initial resistance to any SLI and 17.6% (83/479) among individuals with MDR who did not acquire resistance during treatment. We compared unadjusted survival rates using Kaplan-Meyer curves of 2,274 cases with and without AR to SLI during treatment, having excluded 55 cases with initial resistance to SLI. After 8 months of treatment, the survival was significantly worse among the cases with AR compared to cases without AR (p=0.02) (Figure 2). The median time to death among those with AR to SLI who died during treatment was 14.5 months (IQR:9–19) versus 9.2 months (IQR:3–13) among individuals without AR to SLI who died during treatment (p=0.02).
Figure 2. Survival during treatment among TB cases with and without acquired resistance to second-line injectable drugs, United States, 1993 – 2008.
Notes: AR: acquired resistance
After controlling for age, the mortality rate was significantly higher among cases with AR to SLI after 8 months of treatment (aHR, 2.8; 95% CI, 1.4–5.4) compared to cases without AR (Table 2). Higher mortality rate was also associated with illicit drug use (aHR, 2.0; 95% CI, 1.4–2.9), positive HIV status (aHR, 10.9; 95% CI, 7.5–15.8), MDR TB at treatment initiation (aHR, 1.6; 95% CI, 1.2–2.1), extrapulmonary disease (aHR, 2.6; 95% CI, 1.9–3.4) and Directly Observed Therapy (aHR, 2.5; 95% CI, 1.9–3.4).
Table 2.
Acquired resistance to second-line injectable drugs and other predictors of death*
| Characteristic | Hazard Ratio | 95% Confidence Intervals | Age Adjusted Hazard Ratio | 95% Confidence Intervals |
|---|---|---|---|---|
|
| ||||
| AR to SLI (<8 months of treatment) | ||||
| Yes | 1.1 | 0.4 – 3.4 | 1.4 | 0.4 – 4.5 |
| No | Reference | Reference | ||
|
| ||||
| AR to SLI (>8 months of treatment) | ||||
| Yes | 2.4 | 1.3 – 4.7 | 2.8 | 1.4 – 5.4 |
| No | Reference | Reference | ||
|
| ||||
| Age groups | ||||
| 0 – 24 | 0.6 | 0.3 – 1.3 | ||
| 25 – 44 | 1.5 | 1.1 – 2.1 | N/A | N/A |
| 45 – 64 | Reference | |||
| > 64 | 3.5 | 2.4 – 5.1 | ||
|
| ||||
| Gender | ||||
| Male | 1.2 | 0.9 – 1.6 | 1.1 | 0.8 -1.5 |
| Female | Reference | Reference | ||
|
| ||||
| Illicit drugs use | ||||
| Yes | 1.6 | 1.1 – 2.3 | 2.0 | 1.4 – 2.9 |
| No | Reference | Reference | ||
|
| ||||
| HIV status | ||||
| Positive | 5.0 | 3.9 – 6.5 | 8.8 | 6.5 – 11.9 |
| Not positive | Reference | Reference | ||
|
| ||||
| MDR TB | ||||
| Yes | 1.3 | 1.0 – 1.8 | 1.6 | 1.2 – 2.1 |
| No | Reference | Reference | ||
|
| ||||
| Site of disease | ||||
| Any Extrapulmonary | 2.3 | 1.8 – 3.1 | 2.6 | 1.9 – 3.4 |
| Pulmonary only | Reference | Reference | ||
|
| ||||
| Initial regimen | ||||
| Any SLD | 1.5 | 1.1 – 2.0 | 1.7 | 1.2 – 2.2 |
| No SLD | Reference | Reference | ||
|
| ||||
| DOT | ||||
| Self-administrated | 2.8 | 2.1 – 3.7 | 2.5 | 1.9 – 3.4 |
| Directly observed | Reference | Reference | ||
Notes: AR: acquired resistance, SLI: second-line injectable drugs, N/A: not available, HIV: human immunodeficiency virus, MDR TB: multidrug-resistant tuberculosis, SLD: second-line drugs, DOT: Directly Observed Therapy.
Results received using Cox Proportional Hazard regression
FQ analytic group
Of 15,337 cases with initial DST to FQ, 499 (3.3%) had reported resistance to any FQ. Both initial and final DST results to FQ were reported in 1,187 cases (FQ analytic group). Compared to 14,150 cases with only initial DST to FQ, cases in the FQ analytic group were older (mean age 45 years old versus 43 years old, p<0.0001), were more likely to have HIV (19% versus 14%, p<0.0001) and pulmonary disease (85% versus 71%, p<0.0001); 68% were males (versus 63%, p=0.001). Among individuals with initial DST to FQ 9.8% died during treatment (Figure 1c).
Of 1,187 cases in FQ analytic group, 32 (2.7%) acquired resistance to at least one FQ during treatment, while 46 (3.9%) cases had initial resistance to FQ (Figure 1c). Among 32 cases with AR to FQ, 20 (63%) acquired resistance to ciprofloxacin and 14 (44%) to ofloxacin. Two persons acquired resistance to both FQ. Median time between initial and final DST was 5 months among cases with AR to FQ, 3 months among cases with initial resistance to FQ and 2 months among cases without any resistance. Initial resistance to any FLD was reported in 94% (30/32) cases with AR to FQ versus 64% (708/1109) among cases without AR to FQ (Table 1).
Of 32 individuals with AR to FQ, 12 (37.5%) died during treatment versus 2.2% (1/46) with initial resistance and 10.9% (121/1,109) who did not acquire resistance. Among 1,187 cases in the FQ analytic group, 405 (34.1%) were MDR TB at treatment initiation (Table 1). Of 24 individuals with MDR TB at treatment initiation who acquired resistance to FQ, 10 (41.7%) died during treatment, compared to 3.2% (1/31) among those with MDR and initial resistance to any FQ; and 18.0% (63/350) among those with MDR at treatment initiation who did not acquire resistance during treatment. Similar to the SLI group, the survival in the FQ analytic group was significantly lower among cases with AR compared to cases without AR (p=0.03) (Figure 3), excluding 46 cases with initial resistance to FQ. The median time to death among those with AR to FQ who died during treatment was 18.4 months (IQR:8.5–24) versus 9.8 months (IQR:3–15) among individuals without AR to FQ who died during treatment (p=0.047).
Figure 3. Survival during treatment among TB cases with and without acquired resistance to fluoroquinolones, United States, 1993 – 2008.
Notes: AR: acquired resistance
In the FQ analytic group, after adjusting for age, cases with AR to FQ had almost 2-fold greater mortality rate than cases without AR to FQ (aHR, 1.9; 95% CI, 1.0–3.6) (Table 3). A greater mortality rate in this group was also associated with illicit drug use (aHR, 2.4; 95% CI, 1.6–3.7), positive HIV status (aHR, 11.5; 95% CI, 6.7–19.5), MDR TB at treatment initiation (aHR, 1.8; 95% CI, 1.2–2.5), extrapulmonary disease (aHR, 3.6; 95% CI, 2.5–5.1) and Directly Observed Therapy (aHR, 3.1; 95% CI, 2.1–4.5).
Table 3.
Acquired resistance to fluoroquinolones and other predictors of death*
| Characteristic | Hazard Ratio | 95% Confidence Intervals | Age Adjusted Hazard Ratio | 95% Confidence Intervals |
|---|---|---|---|---|
|
| ||||
| AR to FQ | ||||
| Yes | 2.0 | 1.1 – 3.6 | 1.9 | 1.0 – 3.5 |
| No | Reference | Reference | ||
|
| ||||
| Age groups | ||||
| 0 – 24 | 0.7 | 0.3 – 1.5 | ||
| 25 – 44 | 1.5 | 1.0 – 2.3 | N/A | N/A |
| 45 – 64 | Reference | |||
| > 64 | 3.6 | 2.2 – 6.1 | ||
|
| ||||
| Sex | ||||
| Male | 1.6 | 1.1 – 2.3 | 1.4 | 0.9 – 2.1 |
| Female | Reference | Reference | ||
|
| ||||
| Illicit drugs use | ||||
| Yes | 2.1 | 1.4 – 3.3 | 2.4 | 1.6 – 3.7 |
| No | Reference | Reference | ||
|
| ||||
| HIV status | ||||
| Positive | 6.5 | 4.6 – 9.2 | 9.8 | 6.6 -14.6 |
| Not positive | Reference | Reference | ||
|
| ||||
| MDR TB | ||||
| Yes | 1.6 | 1.1 – 2.3 | 1.8 | 1.2 – 2.5 |
| No | Reference | Reference | ||
|
| ||||
| Site of disease | ||||
| Any Extrapulmonary | 3.3 | 2.3 – 4.7 | 3.6 | 2.5 – 5.1 |
| Pulmonary only | Reference | Reference | ||
|
| ||||
| Initial regimen | ||||
| Any SLD | 1.5 | 1.0 – 2.3 | 1.6 | 1.1 – 2.4 |
| No SLD | Reference | Reference | ||
|
| ||||
| DOT | ||||
| Self-administrated | 3.2 | 2.2 – 4.7 | 3.1 | 2.1 – 4.5 |
| Directly observed | Reference | Reference | ||
Notes: AR: acquired resistance, FQ: fluoroquinolones, N/A: not available, HIV: human immunodeficiency virus, MDR TB: multidrug-resistant tuberculosis, SLD: second-line drugs, DOT: Directly Observed Therapy.
Results received using Cox Proportional Hazard regression
Of 1,155 culture-positive cases with initial and final DST results to both SLI and FQ, 72 were excluded due to initial resistance to any SLDs. Of the remaining 1,083 cases, 5 (0.5%) acquired resistance to both SLI and FQ. All five had MDR TB at the initial DST, thus they got XDR TB. All five cases were HIV positive and occurred before 2001. One person from 5 acquired XDR TB cases completed therapy while four others died during treatment.
Discussion
This study describes the mortality associated with AR to two crucial classes of anti-TB medications for treating MDR TB, the injectable agents and fluoroquinolones. During 1993–2008, 49 cases acquired resistance to SLI and 13 of the patients (26.5%) died during treatment. Among the 32 individuals with acquired resistance to FQ, 12 (37.5%) died during treatment. Mortality among individuals without acquired resistance to SLI or FQ was less than 11% in both groups. Although the survival rate was significantly lower among individuals with AR to SLD compared to those without AR, the median time to death among individuals with AR was longer than among those without AR. Biologically this might be explained by the fall and rise phenomenon frequently experienced by patients who receive a failing treatment regimen. This is the period of time when the majority of bacilli that are drug-susceptible are eliminated by chemotherapy, while drug-resistant bacilli continue to proliferate eventually replacing drug-susceptible bacilli as the dominant population [15]. In addition, the longer time to death in individuals acquiring AR may partly be artificial, in that patients who die earlier have less time for AR to develop and be documented. Greater mortality was significantly associated with AR to SLD in both analytic groups; it was 2.8-fold greater among individuals with acquired resistance to at least one SLI and 1.9-fold greater among individuals with acquired resistance to at least one FQ, compared to those without any AR.
To our knowledge, data are limited on the consequences of AR to SLD during treatment, particularly the impact of AR on mortality. A recent individual patient data (IPD) meta-analysis assessed the impact of initial resistance to FQ and SLI on outcomes of MDR TB patients worldwide [9]. Similar to this IPD meta-analysis our results indicated treatment success was less likely in patients who had more advanced disease, HIV infection, or an initial TB regiment including SLD. Several research groups have reported the development of FQ-resistant TB and XDR TB during treatment [3, 6, 9, 16], but not the consequences of this acquisition of resistance. FQ exposure before TB diagnosis was associated with an increased risk of death among TB patients reported in Tennessee, USA during 2007–2009 [16].
Our study had several limitations. In the United States, initial DST for first-line drugs is performed routinely, but DST for second-line drugs and final DST are performed only when requested by the physician; wherein standard nation-wide guidelines for second-line DST testing are not available in the US. Therefore, most of the cases did not have final DST or DST results for SLD. Thus these results may not be generalizable to all cases confirmed by positive culture results, reported in the U.S. during the study period. The calculated mortality rate was similar between individuals with only initial DST to SLD and those with both, initial and follow-up DST to SLD without acquired resistance to SLD in both analytic groups (Figure 1b and 1c), however. This suggests that selection bias might be negligible. Also, TB genotyping was not available during most of the study period [17], so we were unable to assess the potential of mixed infection, cross-contamination, and re-infection with both drug-susceptible and drug-resistant strains. Thus there was a risk of misclassification for AR; however we believe that this risk was minimal, as the prevalence of mixed infection and the likelihood of reinfection are low in the United States [18]. In addition, some TB patients initially susceptible to SLD died or were lost to follow up before the second set of susceptibility testing was done. Thus, this study underestimates the real number of cases with acquired SLD resistance in the United States, but it is based on the most complete national data currently available. Finally, only the initial treatment regimen is reported in NTSS, and important changes to therapy, especially for drug-resistant TB, could be made during treatment. Accordingly, we did not have detailed information regarding TB treatment for this analysis. We also could not estimate association between risk of death and side-effects of drugs or comorbidities during TB treatment because correspondent data were not available in the NTSS. However CDC has planed to establish an MDR/XDR TB registry that collects information on treatment regimens, drugs adverse effects and other comorbidities with the aim to further investigate this issue [19, 20]. Additionally, the relatively small number of patients with AR did not allow us to adjust estimates for AR for factors other than age in the multivariable regression model.
In conclusion, the probability of death during TB treatment was significantly greater among TB cases with AR to SLD than among cases with baseline resistance to the same drugs and cases without resistance to those drugs, indicating the need for more vigilant clinical monitoring and timely repeat DST during the course of treatment. Providers should consider possibility of emergence of AR to SLD and monitor DST results in cases with increased risk of AR to SLD, including individuals with MDR TB, positive HIV status or extrapulmonary disease.
Summary.
This study provides an assessment of factors associated with mortality during treatment among TB patients with acquired resistance to key classes of second-line antituberculosis drugs. These data may be important for developing strategies for improving outcomes among patients with drug-resistant forms of TB.
Acknowledgments
Funding
U. S. Centers for Disease Control and Prevention
Footnotes
Disclaimer:
The views and opinions expressed in this article are those of the authors and do not necessarily represent an official position of the U.S. Centers for Disease Control and Prevention.
References
- 1.World Health Organization. Global Tuberculosis Control 2013. Geneva, Switzerland: WHO; 2013. Available at http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Caminero J. Multidrug-resistant tuberculosis: epidemiology, risk factors and case finding. Int J Tuberc Lung Dis. 2010 Apr;14(4):382–90. [PubMed] [Google Scholar]
- 3.Monedero I, Caminero J. Management of multidrug-resistant tuberculosis: an update. Ther Adv Respir Dis. 2010 Apr;4(2):117–27. doi: 10.1177/1753465810365884. [DOI] [PubMed] [Google Scholar]
- 4.Perri B, Proops D, Moonan P, et al. Mycobacterium tuberculosis cluster with developing drug resistance, New York, USA, 2003–2009. Emerg Infect Dis. 2011 Mar;17(3):372–8. doi: 10.3201/eid1703.101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. The Global Plan to Stop TB 2011–2015. Geneva, Switzerland: WHO; 2010. Available at http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf. [Google Scholar]
- 6.Shah N, Pratt R, Armstrong L, et al. Extensively drug-resistant tuberculosis in the United States, 1993–2007. JAMA. 2008 Nov 12;300(18):2153–60. doi: 10.1001/jama.300.18.2153. [DOI] [PubMed] [Google Scholar]
- 7.Brostrom R, Fred D, Heetderks A, et al. Islands of hope: building local capacity to manage an outbreak of multidrug-resistant tuberculosis in the Pacific. Am J Public Health. 2011 Jan;101(1):14–8. doi: 10.2105/AJPH.2009.177170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falzon D, Jaramillo E, Schünemann H, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011 Sep;38(3):516–28. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 9.Falzon D, Gandhi N, Migliori G, et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on MDR TB outcomes. Eur Respir J. 2012 Oct 25; doi: 10.1183/09031936.00134712. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Althomsons S, Cegielski J. Impact of second-line drug resistance on tuberculosis treatment outcomes in the United States: MDR TB is bad enough. Int J Tuberc Lung Dis. 2012 Oct;16(10):1331–4. doi: 10.5588/ijtld.11.0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, CDC; Oct, 2012. [Google Scholar]
- 12.World Health Organization. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: 2011 Update. Geneva, Switzerland: WHO; 2012. Available at http://whqlibdoc.who.int/publications/2011/9789241501583_eng.pdf. [PubMed] [Google Scholar]
- 13.Ershova J, Kurbatova E, Moonan P, et al. Acquired resistance to second-line drugs among persons with tuberculosis in the United States. Clin Infect Dis. 2012 Dec;55(12):1600–7. doi: 10.1093/cid/cis748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zignol M, van Gemert W, Falzon D, et al. Surveillance of anti-tuberculosis drug resistance in the world: an updated analysis, 2007–2010. Bull World Health Organ. 2012;90:111–9D. doi: 10.2471/BLT.11.092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinal M. What is the “rise and fall” phenomena and the “sequential regimen” mechanism? In: Friedan T, editor. Toman’s Tuberculosis, case detection, treatment and monitoring: questions and answers. Vol. 2. World Health Organization; Geneva: 2004. pp. 200–202. [Google Scholar]
- 16.Miramontes R, Lambert L, Haddad M, et al. Public health response to a multidrug-resistant tuberculosis outbreak among Guatemalans in Tennessee, 2007. South Med J. 2010 Sep;103(9):882–6. doi: 10.1097/SMJ.0b013e3181eba488. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, Moonan P, Cowan L, et al. Tuberculosis genotyping information management system: enhancing tuberculosis surveillance in the United States. Infect Genet Evol. 2011 doi: 10.1016/j.meegid.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Behr M. Tuberculosis due to multiple strains: a concern for the patient? A concern for tuberculosis control? Am J Respir Crit Care Med. 2004;169:554–555. doi: 10.1164/rccm.2401001. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Plan to Combat Extensively Drug-Resistant Tuberculosis Recommendations of the Federal Tuberculosis Task Force. MMWR. 2009 Feb 13;58(RR03):1–43. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5803a1.htm. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Report of Expert Consultations on Rapid Molecular Testing to Detect Drug-Resistant Tuberculosis in the United States. Atlanta, GA: U.S. Department of Health and Human Services, CDC; Sep, 2012. http://www.cdc.gov/tb/topic/laboratory/rapidmoleculartesting/default.htm. [Google Scholar]