Abstract
BACKGROUND
The Pulmonary-specific Quality-of-Life Scale (PQLS) was developed to measure quality of life (QoL) among patients awaiting lung transplant. The objective of this study was to determine the psychometric properties of the PQLS, identify empirically derived sub-scales, and examine ability to detect changes in pulmonary-specific QoL scores after lung transplantation.
METHODS
Data were derived from the INSPIRE trial, a dual-site randomized controlled trial of coping skills training in 389 lung transplant candidates (obstructive [48.3%], restrictive [24.2%], cystic fibrosis [13.6%], and other [13.9%]). Cronbach alpha was calculated to assess the internal reliability of the PQLS (n = 388). Test-retest reliability was assessed with correlation coefficients between baseline and 12-week post-baseline scores for the usual care control condition (n = 140). Convergent validity was assessed with correlation coefficients between the PQLS and established measures of QoL and emotional distress, 6-minute walk test distance, forced expiratory volume in 1 second, and use of supplemental oxygen at rest (n = 388). Change from baseline to 6 months post-transplantation was assessed with repeated measures analysis of variance (n = 133).
RESULTS
The PQLS was internally reliable and stable across 12 weeks. The PQLS correlated strongly with QoL measures (e.g., Shortness of Breath Questionnaire, r = 0.78, p < 0.0001), moderately with mood and anxiety (e.g., Beck Depression Inventory-II, r = 0.59, p < 0.0001), and modestly with lung disease severity (e.g., 6-minute walk test, r = −0.41, p < 0.0001). PQLS scores improved by nearly 2 SDs after transplant.
CONCLUSIONS
These results demonstrated the reliability, validity, and sensitivity to change of the PQLS for measuring pulmonary QoL among patients with advanced lung disease and the responsiveness of the PQLS to changes in QoL after lung transplantation.
Keywords: lung transplant, quality of life, cystic fibrosis, pulmonary fibrosis, COPD
For many patients with end-stage lung disease, lung transplantation is the only treatment option that offers hope for improved longevity. However, lung transplant candidates typically face a prolonged and arduous evaluation process, and after transplantation, lung transplant recipients experience high rates of diabetes, renal dysfunction, hypertension, malignancy, and negative side effects from immunosuppressant medication.1 In recognition of the high costs (financial and human) associated with lung transplantation and the high frequency of significant post-transplant complications, quality of life (QoL) has become an increasingly important clinical end-point to evaluate treatment effectiveness.2
In longitudinal studies, health-related QoL tends to improve significantly in the first 6 months after lung transplantation and may continue to improve through the first year. For example, among 112 lung transplant recipients followed from 2 to 12 months post-transplant, QoL as measured by the Short Form General Health Survey (SF-36) improved between post-transplant months 2 and 6 and stabilized from 6 to 12 months.3 Among 61 patients followed from before transplant to 12 months post-transplant, QoL as measured by the SF-36, the Quality of Life Profile for Chronic Diseases, and the Saint George’s Respiratory Questionnaire revealed considerable improvement from before transplant to 6 months post-transplant and stable scores for most domains from 6 to 12 months.4 Post-transplant QoL results are mixed after the first year, with some studies showing stable scores and some studies showing declines in QoL associated with comorbidities or bronchiolitis obliterans syndrome.5
Various instruments have been used to assess generic QoL, but few are specific to patients with advanced lung disease. The widely used SF-366 and the Nottingham Health Profile7 have been used to assess QoL in lung transplant candidates.8,9 However, generic QoL instruments such as these may not be sensitive to the unique effects of lung disease on QoL, such as the physical and emotional toll associated with dyspnea and the use of supplemental oxygen. The St. George’s Respiratory Questionnaire10,11 is a widely used respiratory distress questionnaire that yields a total QoL score and 3 sub-scale scores.12 However, this instrument was designed to measure the impact of breathlessness from mild chronic obstructive pulmonary disease and asthma and may not adequately measure the impact of more severe lung disease (e.g., the use of high-volume supplemental oxygen) or other lung diseases such as cystic fibrosis (CF) or idiopathic pulmonary fibrosis. The University of California, San Diego Shortness of Breath Questionnaire (SOBQ)13 was designed to measure the effects of dyspnea on QoL in patients with moderate-to-severe chronic lung disease. However, the SOBQ is a unidimensional measure that does not assess multiple dimensions of QoL.
The Pulmonary-specific Quality-of-Life Scale (PQLS)14 was specifically developed to measure multidimensional pulmonary QoL among patients with advanced lung disease who are awaiting lung transplantation. However, psychometric evidence for the PQLS is lacking. The aim of the present study was to evaluate the psychometric properties of the PQLS. First, we conducted a factor analysis of the PQLS to identify sub-scales. Next, we examined internal consistency and test-retest reliability. We then explored convergent validity with regard to generic and disease-specific measures of QoL, measures of emotional distress, and measures of lung disease severity. Finally, we considered the sensitivity of the PQLS to change after lung transplantation.
Methods
Patients
Data were derived from the INSPIRE study, a randomized, controlled trial of patients awaiting lung transplant. A detailed description of the methods and results is published elsewhere.15 The INSPIRE study was approved by the institutional review boards of Duke University Medical Center and Washington University. Briefly, all patients listed for lung transplantation at Duke University Medical Center and Washington University between September 2000 and August 2004 were eligible to participate and were contacted. The study enrolled 389 lung transplant candidates, who were randomly assigned to 12 weeks of either a coping skills training program or usual medical care. Participants completed assessments at baseline and at 12 weeks. Participants who went on to receive a lung transplant also completed assessments at 6 months post-transplant. Figure 1 presents a detailed description of participant recruitment for the study and retention for the 6-month post-transplant follow-up assessment.
Figure 1.
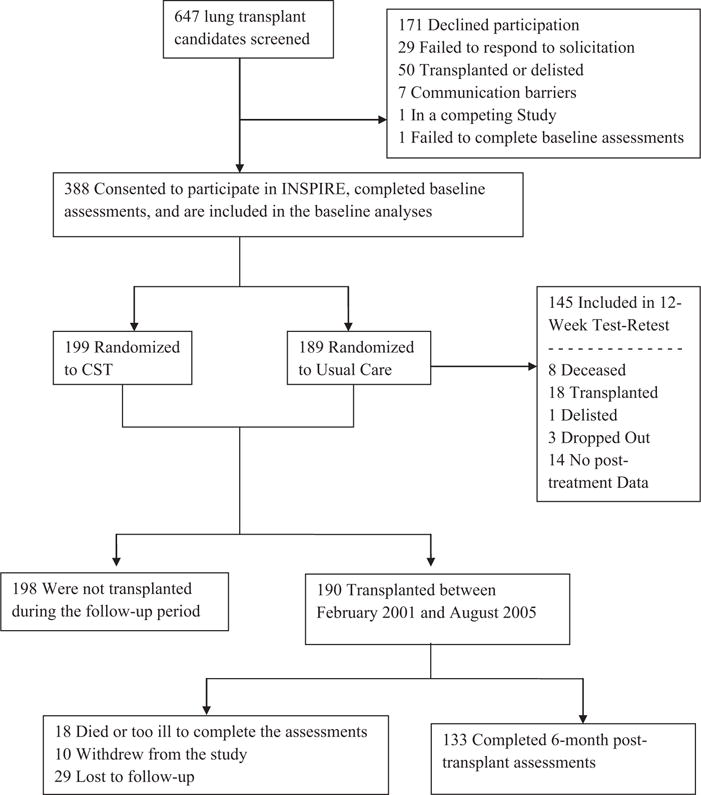
Patient enrollment and retention. CST, coping skills training.
Demographics and medical information
Patients were asked to self-report their age, gender, ethnicity, years of completed education, medical diagnosis, and supplemental oxygen use at rest in liters per minute.
Procedures
PQLS
The 25-item PQLS14 was designed to assess health-related QoL across 7 domains during the past month in patients with pulmonary disease using a 5-point Likert-type scale. The PQLS yields a Total Score, calculated by reverse scoring 10 items and then summing all 25 items. The PQLS was also designed to produce 7 sub-scales: physical functioning, psychological/emotional status, functional status/activities of daily living, social activities, intimacy/relationships/sexuality, occupational functioning, and view of self. However, these 7 sub-scales were conceptually derived and consequently of questionable validity. One of the goals of the present study is to derive data-driven sub-scales. In the event of missing data, we scored the PQLS by inserting the mean score for the data-derived sub-scale.
The PQLS was created with considerable input from patients, consistent with patient reported outcome standards for “patient centeredness.”16,17 Patients who were listed for lung transplantation were contacted through their medical providers and asked to participate in a questionnaire development study. Consenting patients were interviewed in focus groups and on a one-to-one basis. They were asked broad questions (e.g., “how has your pulmonary disease affected your life?”) and specific questions about the effects of pulmonary disease on QoL domains identified through literature review. Responses from patients were used to compile the questions for the PQLS. In addition, items from the Duke Activity Status Index18 were adapted to tap activities of daily living and physical functioning. The resulting instrument was administered to participants for feedback. The instrument took about 10 minutes to complete, and participants generally found it to be well tolerated. Participants complained about 1 question, which they found confusing and difficult to answer and which did not correlate well with the overall measure. This item was removed. The PQLS was used in a pilot intervention study14 before being used in the INSPIRE clinical trial.15
Sample items from the PQLS include “I am able to walk up a flight of stairs without getting winded”; “Because of my lung disease, I have had to limit my household activities (e.g., cooking and cleaning)”; “With my lung disease, I am able to go on trips (e.g., away for the weekend)”; “Projects or tasks at work take longer to finish than they used to”; and “I have been self-conscious about my heavy breathing.” The Total Score can vary between 25 and 125; lower PQLS scores indicate better health-related QoL. See Appendix A for the PQLS measure with scoring instructions.
Other self-report questionnaires
The SOBQ13 is a 24-item questionnaire that assesses dyspnea with activities of daily living in patients with moderate-to-severe chronic lung disease. Sample items from the SOBQ include “Over the last year, I have coughed…”; “How long did the worst attack of chest trouble last?”; and “If you have a wheeze, is it worse in the morning?” Scores can range from 0 to 120, and higher scores indicate greater overall shortness of breath. A 5-unit change has been recommended as the minimally clinically important difference.19
The SF-366 is a 36-item questionnaire that measures generic health-related QoL across 8 life domains and produces scores in 2 higher order domains: Physical Component Score (PCS) and Mental Component Score (MCS). Scores on these 2 higher order domain scales can range from 0 to 100, and higher scores indicate better health-related QoL and functioning. A 5.0-unit change has been recommended for the minimally clinically important difference for the PCS and MCS20.
The Beck Depression Inventory-II (BDI-II)21 is a 21-item measure assessing cognitive, affective, and somatic symptoms of depression. Scores can vary from 0 to 63, and higher scores indicate higher levels of depressive symptoms. A 5-unit change has been recommended as the minimally clinically important difference.22
The State Trait Anxiety Inventory–State Form (STAI-S)23 is a 20-item survey that assesses current levels of anxiety. Scores can range from 20 to 80, and higher scores indicate a greater state anxiety.
The Perceived Social Support Scale (PSSS)24 is a 24-item measure assessing perceived support from family, friends, and significant others that uses a 5-point Likert-type scale. Representative items include “I get the help and support I need from my friends,” “I receive invitations to be with others,” and “My family really tries to help me.” Scores can vary from 12 to 84, and higher scores indicate better perceived social support.
Measures of functional capacity and lung function
The 6-minute walk test (6MWT)25 is a standard protocol determining exercise tolerance by measuring the distance that patients are able to walk within 6 minutes. Patients were asked to cover as much distance as possible at a self-selected pace and were provided with enough oxygen to maintain saturations of ≥90%.
Forced expiratory volume in 1 second (FEV1) is the volume of air that can forcibly be blown out in 1 second. FEV1 is a standard measure in spirometry. Predicted normal values were calculated per Hankinson et al.26
See Appendix B for scatterplots showing the relationship between the PQLS and the other primary variables.
Statistical analysis
Lung diseases were grouped into 4 categories: obstructive lung disease (e.g., chronic obstructive pulmonary disease/emphysema, bronchiectasis), CF, restrictive lung disease (e.g., idiopathic pulmonary fibrosis), and other (e.g., pulmonary vascular disease, idiopathic pulmonary arterial hypertension, sarcoidosis). Continuous variables were examined for outliers and divergence from normality. Supplemental oxygen use at rest was highly skewed and consequently was recoded from a continuous variable into an ordinal predictor (i.e., no supplemental oxygen use at rest; 1–2 liters/min; ≥3 liters/min).
To identify PQLS sub-scales for analysis, we used principal axis factor analysis to group PQLS items based on empirical and conceptual criteria. A Scree test was used to determine the total number of factors retained for analysis, all of which had eigenvalues >1.0. A minimum loading of 0.40 was required, and Promax rotation was used. Alternative factor solutions were explored, although a 3-factor solution was found to provide the best fit and interpretability.
Internal reliability for the PQLS was assessed by calculating Cronbach alpha for the Total Score and for each of the sub-scales. Cronbach alpha is a number between 0 and 1.0 that represents the degree to which items in a measure correlate with one another. A higher Cronbach alpha suggests that the items are measuring 1 construct. Test-retest reliability was examined by calculating Pearson correlation coefficients between PQLS scores from baseline to 3 months post-baseline assessment for the sub-group of INSPIRE participants in the usual medical care control group. Convergent validity was assessed by examining Pearson correlation coefficients between the PQLS Total Score and the scores on the general and disease-specific QoL instruments (i.e., SF-36 PCS and MCS and the SOBQ), functional measures of lung disease severity (i.e., 6MWT, FEV1), and measures of mood and anxiety (e.g., BDI-II, STAI-S, PSSS).
Sensitivity to change after transplantation was evaluated with repeated measures analysis of variance (ANOVA). PQLS Total Score served as the repeating dependent variable. Predictors included gender and treatment group assignment. Because age is confounded with native disease (i.e., patients with CF are considerably younger than patients with chronic obstructive pulmonary disease and pulmonary fibrosis), age was not entered as a covariate. To evaluate the effect of native disease on change in PQLS score after transplant, we repeated the primary analysis using a limited data set excluding the 4 post-transplant patients with diagnoses in the “Other” category and added native disease diagnosis as a categorical predictor. Finally, we repeated the primary analysis using the PQLS sub-scales.
Results
There were 389 lung transplant candidates enrolled in the INSPIRE study. Of these, 1 patient did not complete baseline QoL measures and was excluded from further analyses. With regard to the PQLS, 96% of participants completed the entire measure, and no participant completed the PQLS with more than 1 item missing. Baseline characteristics for the 388 lung transplant candidates included in these analyses are summarized in Table 1. Participants included men and women (43.7% male) and were largely white (87.7%) and well educated (63% attended at least some college). As expected, age varied by native disease; patients with CF were younger on average than patients with obstructive or restrictive lung diseases. The most common lung disease category was obstructive (48.3%), followed by restrictive (24.2%), CF (13.6%), and other (13.9%). The PQLS Total Score was not related to age (r = 0.10, p > 0.05) or gender (t = 0.26; p > 0.05). Histograms of the distribution of the PQLS Total Score and sub-scale scores were examined, and no evidence for a ceiling or floor effect was observed.
Table 1.
Characteristics of Study Participants
| Mean (SD) or n, %
|
|||||
|---|---|---|---|---|---|
| All (n = 388, 100%) | Obstructive (n = 188, 48%) | Restrictive (n = 94, 24%) | CF (n = 53, 14%) | Other (n = 54, 14%) | |
| Demographics | |||||
| Age (years) | 49.4 (11.8) | 55.1 (6.7) | 52.7 (9.4) | 30.1 (8.6) | 42.0 (10.1) |
| Male gender | 170, 43.7% | 85, 45% | 57, 61% | 18, 33% | 11, 20% |
| White ethnicity | 341, 87.7% | 175, 93% | 81, 86% | 51, 94% | 35, 65% |
| Education ≥12 years | 339, 84.5% | 153, 81% | 84, 93% | 50, 93% | 50, 93% |
| Pulmonary function | |||||
| FEV1% | 35.2 (31.6) | 21.0 (7.1) | 53.1 (18.4) | 26.9 (7.2) | 63.0 (22.5) |
| 6MWT (feet) | 1,070 (399) | 950 (355) | 1,168 (420) | 1,302 (326) | 1,083 (429) |
| Oxygen use at rest | |||||
| None | 156, 41% | 52, 29% | 39, 42% | 31, 58% | 34, 65% |
| 1–2 liters/min | 145, 48% | 88, 49% | 32, 35% | 15, 28% | 10, 19% |
| ≥3 liters/min | 77, 20% | 41, 23% | 21, 23% | 7, 13% | 8, 15% |
| Mood | |||||
| BDI-II | 11.8 (7.5) | 12.4 (8.1) | 11.1 (6.8) | 10.6 (6.4) | 12.1 (7.2) |
| STAI-S | 37.2 (11.6) | 38.2 (12.8) | 36.5 (10.0) | 35.2 (8.9) | 37.2 (11.8) |
BDI-II, Beck Depression Inventory-II; CF, cystic fibrosis; FEV1%, percent of predicted normal value for forced expiratory volume in 1 second; 6MWT, 6-minute walk test; STAI-S, State Trait Anxiety Inventory—State Form.
Factor analysis
The Scree plot suggested that a 3-factor solution provided the best fit for the observed data. The first factor, labeled “Task Interference,” consisted of items 9, 11, 12, 13, 15, 16, 21, and 22 (i.e., most of the items that constituted the original Functional Status, Social Activities, and Occupational Functioning sub-scales; eigenvalue = 6.3). The second factor, labeled “Psychological,” consisted of items 5, 6, 7, 20, 23, 24, and 25 (i.e., most of the items that constituted the original Psychological/Emotional Status and View of Self sub-scales; eigenvalue = 2.0). The third factor comprised the original Physical Function factor (items 1–4; eigenvalue = 1.2). Items 8, 10, 14, 17, 18, and 19 (including most of the original Intimacy sub-scale items) did not load on any factors.
Reliability of PQLS
Internal reliability statistics for the PQLS are summarized in Table 2. The coefficient alphas for the PQLS Total Score were ≥0.86 for the sample as a whole and for each disease category. Among the PQLS sub-scales, coefficient alphas were generally > 0.80.
Table 2.
Pulmonary-specific Quality-of-Life Scale Internal Reliability
| Coefficient alpha
|
|||||
|---|---|---|---|---|---|
| All (n = 388) | Obstructive (n = 188) | Restrictive (n = 93) | CF (n = 53) | Other (n = 54) | |
| PQLS total | 0.88 | 0.86 | 0.88 | 0.89 | 0.89 |
| Sub-scales | |||||
| Physical | 0.80 | 0.83 | 0.80 | 0.82 | 0.66 |
| Psychological | 0.83 | 0.84 | 0.83 | 0.83 | 0.82 |
| Task Interference | 0.84 | 0.79 | 0.86 | 0.83 | 0.89 |
CF, cystic fibrosis; PQLS, Pulmonary-specific Quality-of-Life Scale.
Test-retest reliability was calculated using the baseline and 12-week post-baseline data for participants who were randomly assigned to the usual control condition (n = 145). The mean (SD) of the time between baseline and the 12-week assessment was 125 (35) days. Correlation coefficients were r = 0.81 for the PQLS Total Score, 0.52 for the Physical Function subscale, 0.78 for the Task Interference subscale, and 0.71 for the Psychological sub-scale (all p < 0.0001). Because the medical condition of patients with advanced lung disease may vary over a 12-week period, a second test-retest analysis was conducted comparing participants in the usual care control condition who used the same amount of supplemental oxygen at both time points (n = 82) with participants who used different amounts of supplemental oxygen at both time points (n = 63). The correlation coefficients were similar between participants with stable and unstable supplemental oxygen use for the PQLS Total Score (r = 0.82 and 0.78, respectively) and for the Psychological subscale (r = 0.68 and r = 0.74, respectively). However, correlation coefficients were higher for participants with stable supplemental oxygen use compared with participants with unstable oxygen use for the Physical Function sub-scale (r = 0.64 and 0.33, respectively) and for the Task Interference sub-scale (r = 0.84 and 0.63, respectively).
Convergent validity of the PQLS with measures of mood and QoL
Table 3 summarizes the relationships between the PQLS Total Score and clinical and other QoL measures. The PQLS was generally associated with other QoL measures. Higher PQLS Total Scores (i.e., worse QoL) were associated with worse dyspnea-related QoL as measured by the SOBQ (r = 0.78), worse physical and mental QoL as measured by the SF-36 (r = −0.71 and −0.62, respectively), greater depressive symptoms as measured by the BDI-II (r = 0.59), and greater anxiety as measured by the STAI-S (r = 0.45) (all p < 0.0001). Also, higher (worse) PQLS Total Scores were associated with lower levels of perceived social support (PSSS) (r = −0.17; p < 0.001), although this relationship was quite modest. Some evidence for curvilinear relationships between the PQLS and the BDI-II, STAI-S, and SF-36 MCS was found in exploratory linear regression analyses, suggesting that the relationships between PQLS and the other measures are slightly stronger when scores are worse. However, the addition of a curvilinear term explained only a very small amount (1%–3%) of the variance in the relationship between the PQLS and other measures of emotional distress.
Table 3.
Relationships Between PQLS and Existing Measures of QoL, Pulmonary Function, and Mood
| Obstructive (n = 188) | Restrictive (n = 93) | CF (n = 53) | Other (n = 54) | |
|---|---|---|---|---|
| QoL | ||||
| SF-36 PCS | −0.66a | −0.79a | −0.67a | −0.73a |
| SF-36 MCS | −0.63a | −0.70a | −0.60a | −0.55a |
| SOBQ | 0.76a | 0.81a | 0.77a | 0.76a |
| Pulmonary function | ||||
| FEV1% | −0.10 | −0.37b | −0.01 | −0.15 |
| 6MWT | −0.27b | −0.52b | −0.37c | −0.35d |
| Mood | ||||
| BDI-II | 0.68a | 0.50b | 0.47b | 0.54a |
| STAI-S | 0.46a | 0.45b | 0.40c | 0.42c |
| PSSS | −0.25b | −0.13 | −0.05 | −0.11 |
BDI-II, Beck Depression Inventory-II; CF, cystic fibrosis; FEV1%, percent of predicted normal value for forced expiratory volume in 1 second; PQLS, Pulmonary-specific Quality-of-Life Scale; PSSS, Perceived Social Support Scale; QoL, quality of life; SF-36 PCS, Short Form General Health Survey Physical Component Scale; SF-36 MCS, Short Form General Health Survey Mental Component Scale; 6MWT, 6-minute walk test; SOBQ, Shortness of Breath Questionnaire; STAI-S, State Trait Anxiety Inventory—State Form.
p < 0.0001.
p < 0.001.
p < 0.01.
p < 0.05.
The relationships between the PQLS Psychological subscale and the other measures of psychological function and QoL were particularly robust. Worse QoL as measured by the PQLS Psychological subscale correlated strongly with worse scores on the SF-36 MCS (r = −0.66), the BDI-II (r = 0.71), and the STAI-S (r = 0.59) and modestly with the PSSS (r = −0.28; all p < 0.0001).
Convergent validity of the PQLS with measures of disease severity and physical function
PQLS Total Score was associated with measures of disease severity, with higher (worse) PQLS scores associated with shorter 6MWT distance (r = −0.41, p < 0.0001) and lower FEV1 (r = −0.21, p < 0.001). We examined these relationships by disease type and observed that the relationships between PQLS total score and 6MWT distance were statistically significant across all 4 disease type categories (r = −0.27 to −0.52), whereas the relationship between PQLS and FEV1 was significant for the restrictive lung disease category (r = −0.37, p < 0.001) but small and nonsignificant for the other diagnostic categories (r = −0.01 to −0.15).
ANOVA showed that worse PQLS was associated with greater participant supplemental oxygen use. PQLS Total Scores were lower (better) for participants who used no supplemental oxygen at rest (mean [SD] 74.1 [17.6]) compared with participants who used 1 to 2 liters/min (mean [SD] 82.7 [13.8]) and patients who used ≥3 liters/min (mean [SD] 87.2 [12.7]) (F = 22.4, p < 0.0001). Planned contrasts revealed that PQLS scores were higher (worse) for participants who used supplemental oxygen at rest compared with participants who did not (p < 0.0001) and higher (worse) for participants who used ≥3 liters/min compared with participants who used 1 to 2 liters/min (p < 0.05).
The PQLS Task Interference sub-scale was associated with other measures of disease severity, with a strength of association similar to the PQLS Total Score (e.g., Task Interference and 6MWT, r = −0.37; Task Interference and FEV1, r = −0.23; both p < 0.0001). The Physical sub-scale was associated with other measures of disease severity, although the relationships were not as strong compared with the PQLS Total Score (e.g., PQLS Physical sub-scale and 6MWT, r = −0.27).
Sensitivity to change in PQLS after transplantation
Of the 388 participants who provided baseline data and were included in these analyses, 190 underwent transplantation between February 2001 and August 2005, and 133 (70%) completed assessments at the 6-month post-transplant clinic visit (Figure 1). Baseline PQLS scores were similar for the 133 patients who completed the 6-month assessments (mean [SD] 80.2 [15.9]) compared with the 57 patients who did not (mean [SD] = 78.8 [16.1]) (p = 0.57). Also, the frequency of missing data was similar between the CF (26%), obstructive (22%), and restrictive (36%) diagnostic groups (p = 0.20). The median time between baseline and transplant was 384 days (interquartile range = 138–671 days), and the median time between transplantation and 6-month post-transplant assessments was 192 days (interquartile range = 185–210 days).
ANOVA was conducted to examine change in QoL before and after transplantation, with PQLS Total Score entered as the within-subjects repeating dependent variable and gender and group entered as predictors (Table 4). PQLS Total Scores improved from a baseline average of 80.2 (SD = 15.9) to a post-transplant average of 51.4 (SD = 15.0) (p < 0.0001).
Table 4.
Repeated-Measures Analysis of Variance for Change in QoL Before and After Transplant
| Time
|
Time * gender F | Time * group F | ||||
|---|---|---|---|---|---|---|
| Baseline (M, SD) | 6 months post-transplant (M, SD) | Effect size | F | |||
| PQLS Total Score | 80.2 (15.9) | 51.4 (15.0) | 1.9 | 422.12a | 2.00 | 0.18 |
| Physical | 16.8 (3.8) | 9.1 (3.9) | 2.0 | 327.90a | 0.16 | 1.48 |
| Psychological | 19.0 (6.2) | 12.3 (4.6) | 1.2 | 185.55a | 6.12b | 4.72b |
| Task Interference | 29.3 (6.7) | 18.5 (6.9) | 1.6 | 264.13a | 1.18 | 0.00 |
PQLS, Pulmonary-specific Quality-of-Life Scale.
Effect size is change, as measured in SD.
p < 0.0001.
p < 0.05.
To evaluate the effects of lung disease diagnosis on change in PQLS scores after transplant, we performed a repeated measures ANOVA in which PQLS Total Score served as the repeating dependent variable, and gender, group, and diagnosis were entered as predictors. Because the “other” diagnostic group was very small (n = 5), data for this group were eliminated from this analysis. ANOVA revealed significant main effects for time (p < 0.0001) and for diagnosis (p = 0.027). Patients in the CF group had better (lower) PQLS Total Scores before and after transplantation compared with participants in the other diagnostic categories. Neither other main effects nor interactions were significant. In a post hoc sensitivity analysis, the inclusion of transplant center as a predictor was not significant and did not alter the primary outcomes.
Finally, we repeated the repeated measures ANOVA for each of the PQLS sub-scales (Table 4). The main effect for time was significant in each analysis (p < 0.0001). Improvement was large for all sub-scales, including Physical, Psychological, and Task Interference (2.0, 1.2, and 1.6 SD improvement). There were significant time-by-gender and time-by-group interactions for the ANOVA predicting the Psychological subscale (both p < .05); female participants exhibited worse baseline PQLS Psychological scores compared with male participants (mean [SD] 20.4 [5.9] vs 17.8 [6.2]), and participants who were assigned to the coping skills training condition exhibited worse baseline PQLS Psychological scores compared with participants who were assigned to the usual medical care control condition (mean [SD] = 20.1 [6.3] vs 18 [5.9]). Post-transplant scores were similar for female participants compared with male participants (mean [SD] = 12.4 [4.2] and 12.2 [5.0], respectively), and for participants assigned to coping skills training compared with participants assigned to usual medical care (mean [SD] = 12.3 [4.9] and 12.3 [4.3], respectively).
Discussion
This study demonstrated the reliability, validity, and ability of the PQLS to detect changes in pulmonary-specific QoL among patients with advanced lung disease. The PQLS Total Score was internally reliable across a wide variety of patients with advanced lung diseases with Cronbach alpha>40.85, suggesting that this instrument is measuring a cohesive underlying construct. Also, the PQLS Total Score appeared to be relatively stable over a 12-week period, particularly when the analysis was limited to participants who used the same amount of supplemental oxygen at rest over the 12-week period. Convergent validity was established through strong correlations between the PQLS Total Score and established measures of QoL—general (SF-36) and disease-specific (SOBQ)—through moderate correlations in the expected direction with measures of depression and anxiety and through modest correlations in the expected direction with measures of disease severity (6MWT, use of supplemental oxygen, FEV1 for patients with restrictive lung disease). The PQLS Total Score was highly sensitive to the effects of lung transplant on disease-specific QoL. On average, QoL was nearly 2 SDs better after lung transplant compared with before transplant.
Through factor analysis, 3 empirically derived sub-scales of the PQLS were identified, which we labeled Physical, Psychological, and Task Interference. Psychometric findings for these PQLS sub-scales were generally strong. The subscales were observed to be internally reliable, and the strong relationships between the PQLS Psychological subscale and measures of mood and anxiety demonstrate convergent validity. In addition, all sub-scales were sensitive to change in QoL after lung transplantation.
Although all PQLS sub-scales improved after lung transplantation, the effects were most pronounced for the Physical and Task Interference sub-scales compared with the Psychological sub-scale. Similar findings were reported by Finlen Copeland et al2 in a secondary analysis of QoL data, gathered before and after lung transplantation, among a cohort of 131 patients enrolled in a cytomegalovirus prevention trial. In that study, the SF-36 PCS scores improved considerably after lung transplantation, but the SF-36 MCS scores did not. These data suggest that improvement in psychological QoL may lag behind improvement in physical QoL. In addition, although the findings reported by Finlen-Copeland et al suggest that general psychological QoL may not improve after transplantation, findings from the INSPIRE study suggest that pulmonary-specific psychological QoL may improve substantially after lung transplantation. Perhaps this discrepancy reflects a greater sensitivity of the PQLS to pulmonary-specific psychological QoL compared with the SF-36. In a qualitative review of QoL after lung transplant, Singer et al5 noted that findings for post-transplant improvement in physical QoL are generally robust, whereas findings for post-transplant emotional or mental QoL are heterogeneous and often small.
Greater improvement in PQLS after lung transplantation was observed among patients with a diagnosis of CF compared with patients with a diagnosis of obstructive or restrictive lung disease. Similarly, the qualitative review by Singer et al5 concluded that lung transplantation may result in greater improvement among patients with CF compared with patients with other diagnoses. Although the present study was not designed to assess the value of lung transplantation for different diagnostic groups, the present findings nevertheless support the conclusions of Singer et al.5
This study has some limitations. First, data were gathered in the pre-lung allocation score era, and the findings may not generalize to a modern lung transplant cohort. Second, the cohort in the present study was well educated and almost entirely white, and it is unclear that these findings would extend to minority patients. However, the sample was representative of the lung transplant population, which typically includes relatively few minorities. Third, post-transplant findings were based only on the 70% of transplant recipients who survived and completed the post-transplant assessments. Although pre-transplant PQLS scores were similar for the 70% of participants who completed post-transplant assessments compared with the 30% who did not, we cannot rule out the possibility of a selective attrition bias. Fourth, in the test-retest reliability analyses, we used supplemental oxygen use as an indication of medical stability. Supplemental oxygen use alone paints an incomplete picture of disease severity. Fifth, no attempt was made in this study to determine the minimal clinically important difference for the PQLS, although this is an important next step.16,17 Sixth, the PQLS has not been validated in other languages. Seventh, data were collected over a relatively limited follow-up period. Finally, the PQLS sub-scales identified through factor analysis should be considered preliminary until confirmed in future studies.
In conclusion, the PQLS is a brief self-report questionnaire that was specifically developed to measure QoL among patients awaiting lung transplant. The present findings demonstrated the reliability, validity, and responsiveness to transplantation of the PQLS. The sub-scales provide insight into the effects of lung disease across multiple dimensions of QoL. Additional research is needed to confirm the present findings and to clarify some of the possible limitations in reliability and validity of the PQLS.
Supplementary Material
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute Grant No. HL 065503.
Footnotes
Disclosure statement
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of the presented manuscript or other conflicts of interest to disclose.
Supplementary data
Supplementary data associated with this article can be found in the online version at www.jhltonline.org.
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2011 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2012. [Google Scholar]
- 2.Finlen Copeland CA, Vock DM, Pieper K, Mark DB, Palmer SM. Impact of lung transplantation on recipient quality of life: a serial, prospective, multicenter analysis through the first posttransplant year. Chest. 2013;143:744–50. doi: 10.1378/chest.12-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myaskovsky L, Dew MA, McNulty ML, et al. Trajectories of change in quality of life in 12-month survivors of lung or heart transplant. Am J Transplant. 2006;6:1939–47. doi: 10.1111/j.1600-6143.2006.01395.x. [DOI] [PubMed] [Google Scholar]
- 4.Kugler C, Strueber M, Tegtbur U, Neidermeyer J, Haverich A. Quality of life 1 year after lung transplantation. Prog Transplant. 2004;14:331–6. doi: 10.1177/152692480401400408. [DOI] [PubMed] [Google Scholar]
- 5.Singer JP, Chen J, Blanc PD, et al. A thematic analysis of quality of life in lung transplant: the existing evidence and implications for future directions. Am J Transplant. 2013;12:839–50. doi: 10.1111/ajt.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 7.Hunt SM, McEwen J. The development of a subjective health indicator. Sociol Health Illn. 1980;2:231–46. doi: 10.1111/1467-9566.ep11340686. [DOI] [PubMed] [Google Scholar]
- 8.Goetzmann L, Klaghofer R, Wagner-Huber R, et al. Quality of life and psychosocial situation before and after a lung, liver or an allogeneic bone marrow transplant. Swiss Med Wkly. 2006;136:281–90. doi: 10.4414/smw.2006.11362. [DOI] [PubMed] [Google Scholar]
- 9.Vermeulen KM, TenVergert EM, Verschuuren EA, Erasmus ME, van der Bij W. Pre-transplant quality of life does not predict survival after lung transplantation. J Heart Lung Transplant. 2008;27:623–7. doi: 10.1016/j.healun.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med. 1991;85(Suppl B):25–31. doi: 10.1016/s0954-6111(06)80166-6. discussion 33–7. [DOI] [PubMed] [Google Scholar]
- 11.Barr JT, Schumacher GE, Freeman S, LeMoine M, Bakst AW, Jones PW. American translation, modification, and validation of the St. George’s Respiratory Questionnaire. Clin Ther. 2000;22:1121–45. doi: 10.1016/S0149-2918(00)80089-2. [DOI] [PubMed] [Google Scholar]
- 12.Stavem K, Bjortuft O, Lund MB, Kongshaug K, Geiran O, Boe J. Health-related quality of life in lung transplant candidates and recipients. Respiration. 2000;67:159–65. doi: 10.1159/000029480. [DOI] [PubMed] [Google Scholar]
- 13.Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Chest. Vol. 113. University of California; San Diego: 1998. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire; pp. 619–24. [DOI] [PubMed] [Google Scholar]
- 14.Napolitano MA, Babyak MA, Palmer S, Tapson V, Davis RD, Blumenthal JA. Effects of a telephone-based psychosocial intervention for patients awaiting lung transplantation. Chest. 2002;122:1176–84. doi: 10.1378/chest.122.4.1176. [DOI] [PubMed] [Google Scholar]
- 15.Blumenthal JA, Babyak MA, Keefe FJ, et al. Telephone-based coping skills training for patients awaiting lung transplantation. J Consult Clin Psychol. 2006;74:535–44. doi: 10.1037/0022-006X.74.3.535. [DOI] [PubMed] [Google Scholar]
- 16.Methodology Committee of the Patient-Centered Outcomes Research Institute (PCORI) Methodological standards and patient-centeredness in comparative effectiveness research: the PCORI perspective. JAMA. 2012;307:1636–40. doi: 10.1001/jama.2012.466. [DOI] [PubMed] [Google Scholar]
- 17.Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res. 2013;22:1889–905. doi: 10.1007/s11136-012-0344-y. [DOI] [PubMed] [Google Scholar]
- 18.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–4. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 19.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD. 2005;2:105–10. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 20.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–92. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 22.Viljoen J, Iverson G, Griffiths S, Woodward T. Factor structure of the Beck Depression Inventory-II in a medical outpatient sample. J Clin Psychol Med Settings. 2003;10:289–91. [Google Scholar]
- 23.Spielberger CD. Manual for the State-Trait Anxiety Inventory STAI (Form Y) Palo Alto, CA: Mind Garden; 1983. [Google Scholar]
- 24.Blumenthal JA, Burg MM, Barefoot J, Williams RB, Haney T, Zimet G. Social support, type A behavior, and coronaryartery disease. Psychosom Med. 1987;49:331–40. doi: 10.1097/00006842-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 25.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. AST Statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


