Abstract
Objective:
Nontraumatic spinal cord injuries (NTSCIs) form a heterogeneous group of diseases, which may evolve into a life-threatening condition. We sought to characterize spectrum, causes of admission and predictors of death in patients with NTSCI treated at the neurological intensive care unit (NICU).
Methods:
We performed a retrospective observational analysis of NTSCI cases treated at a tertiary care center between 2001 and 2013. Among the 3937 NICU admissions were 93 patients with NTSCI (2.4%). Using multivariate logistic regression analysis, we examined predictors of mortality including demographics, etiology, reasons for admission and GCS/SAPS (Glasgow Coma Scale/Simplified Acute Physiology Score) scores.
Results:
Infectious and inflammatory/autoimmune causes made up 50% of the NTSCI cases. The most common reasons for NICU admission were rapidly progressing paresis (49.5%) and abundance of respiratory insufficiency (26.9%). The mortality rate was 22.6% and 2.5-fold higher than in the cohort of all other patients treated at the NICU. Respiratory insufficiency as the reason for NICU admission [odds ratio (OR) 4.97, 95% confidence interval (CI) 1.38–17.9; p < 0.01], high initial SAPS scores (OR 1.04; 95% CI 1.003–1.08; p = 0.04), and the development of acute kidney injury throughout the stay (OR 7.25, 1.9–27.5; p = 0.004) were independent risk factors for NICU death.
Conclusions:
Patients with NTSCI account for a subset of patients admitted to the NICU and are at risk for adverse outcome. A better understanding of predisposing conditions and further knowledge of management of critically ill patients with NTSCI is mandatory.
Keywords: intensive care, mortality, nontraumatic spinal cord injury, treatment
Introduction
Spinal cord injury (SCI), regardless of traumatic or nontraumatic origin, results in high burden of impairment and devastating outcomes. A systematic review revealed that overall mortality of patients with SCI is up to three times higher than in the general population when analyzing standardized mortality rates [Van Den Berg et al. 2010]. Notably, survival rates are lower in individuals with nontraumatic SCI (NTSCI) compared with those with traumatic SCI. Lower survival rates were associated with older age, higher neurological levels, and completeness of SCI. Causes of death stemmed from secondary complications, with failure of the respiratory system being the leading cause. Runner-ups were disorders of the heart and the circulatory system. The epidemiology of SCIs has undergone substantial changes during recent years. The incidence of NTSCIs is increasing as it occurs more commonly in older age groups [Sebastia-Alcacer et al. 2014]. Given global ageing, the incidence of NTSCIs may even surpass their traumatic counterparts within a few decades [New and Sundararajan, 2008; Noonan et al. 2012].
Leading causes of NTSCI are tumors and degenerative diseases of the spinal column followed by vascular and autoimmune disorders [New et al. 2015]. A prospective study of 585 adult patients with spastic paraparesis or tetraparesis in Northern England provided further insights to the relative distribution of different causes [Moore and Blumhardt, 1997]. The most frequent underlying condition was cervical spondylotic myelopathy (24%), followed by extrinsic neoplastic or developmental tumor (16%) and multiple sclerosis (18%) and motor neuron disease (MND, 4%). A further 15 other diagnoses accounted for the remainder, whereas the diagnosis remained uncertain in 27.4%. With the lack of evidence-based guidelines, management is adapted to the specific circumstances of individual patients and conditions, resources and experiences of the treating physicians [Sellner et al. 2010; Scott et al. 2011; Andersen et al. 2012; Savage et al. 2014; Leveque et al. 2015; Readdy et al. 2015]. Independent of the underlying pathology, patients with SCIs frequently require admission to an intensive care unit due to life-threatening conditions arising during the acute or chronic course of the disease. While several studies characterized the population of critically ill patients with traumatic SCI, there is scant knowledge about patients with NTSCI who require admission to an ICU [Zach et al. 1976; Hachen, 1977; Tator et al. 1984; Ryken et al. 2013]. Intensive cardiopulmonary support is the mainstay of ICU treatment due to traumatic SCI due to the occurrence of neurogenic shock and ventilatory insufficiency and in an attempt to reduce secondary injury [Casha and Christie, 2011]. Yet, reasons for admission may differ between traumatic SCIs and NTSCIs in various aspects including the frequency of comorbid conditions, physiologic reserve and disease course, as well as intensive care management [New et al. 2002].
Here, we aimed to characterize a cohort of critically ill patients with NTSCI and analyzed their disease course while treated on a neurological intensive care unit (NICU). In addition, we sought to identify independent risk factors to predict NICU mortality.
Methods
Patients
We conducted a retrospective observational study of NTSCI patients admitted to the NICU of a tertiary care center. The study period was 2001–2013, and the bed capacity was eight until January 2012 and nine thereafter.
We used the electronic database of the hospital and reviewed the medical charts for patients with a diagnosis of NTSCI at NICU admission. The definition of NTSCI was the presence of a nontraumatic condition causing spinal cord dysfunction. We classified the etiology of NTSCI according to the International Spinal Cord Injury Society (ISCoS) consensus [New and Marshall, 2014]. Patients in need of immediate surgical intervention were also included in the analysis. Age <18 years, pregnancy, and any traumatic cause of SCI including iatrogenic conditions were considered as exclusion criteria. When a repeat NICU admission took place, only data from their first stay were included in the statistical analysis.
Data collection
We extracted information on the following covariates from the medical charts: demographic data, comorbidities, pre-existent NTSCI, reason for NICU admission, level of SCI, and Glasgow Coma Scale (GCS) items. The Simplified Acute Physiology Score (SAPS) II was calculated on admission [Le Gall et al. 1993]. In addition, we recorded need for and type of spinal surgery, need for mechanical ventilation, tracheostomy, invasive hemodynamic monitoring, pharmacological circulatory support, blood transfusion, extracorporeal therapies, as well as the incidence of acute kidney injury and NICU-related complications (urinary tract infection, ventilator-associated pneumonia, hemorrhage, central-line-associated bloodstream infection, thromboembolic complications). We used the definitions from the Kidney Disease Improving Global Outcomes consensus paper for acute kidney injury [Kellum and Lameire, 2013]. At NICU discharge, we recorded changes in neurological function compared to NICU admission, transfer destination, length of NICU stay, need for NICU readmission, NICU mortality, and causes of death.
Ethics approval
The Medical Ethics Committee of the County of Salzburg reviewed the protocol and waived the requirement for ethical review for this retrospective study (415-EP/73/539-2015).
Statistical analysis
We used IBM SPSS Statistics software package for statistical analysis (IBM SPSS Statistics 20; Erlangen, Germany). Comparisons between different NTSCI subgroups were performed with a one-way analysis of variance (ANOVA) or the χ2 test, as appropriate. In order to identify independent risk factors predicting NICU mortality, bivariate correlation analyses were calculated. All variables which were significantly correlated with NICU mortality at a p-value <0.05 qualified for inclusion into a multivariate model in which NICU mortality served as the dependent variable. Before entering variables into this model, interaction relationships were evaluated and variables showing a Spearman rank correlation coefficient >0.5 were excluded. The binary logistic regression model was then calculated using stepwise forward inclusion. Results of the regression analysis are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). In all analyses, p-values were two-tailed, and a p-value <0.05 was considered to indicate statistical significance. All data are presented as median values with absolute ranges, if not otherwise indicated.
Results
Identification of patients with NTSCI
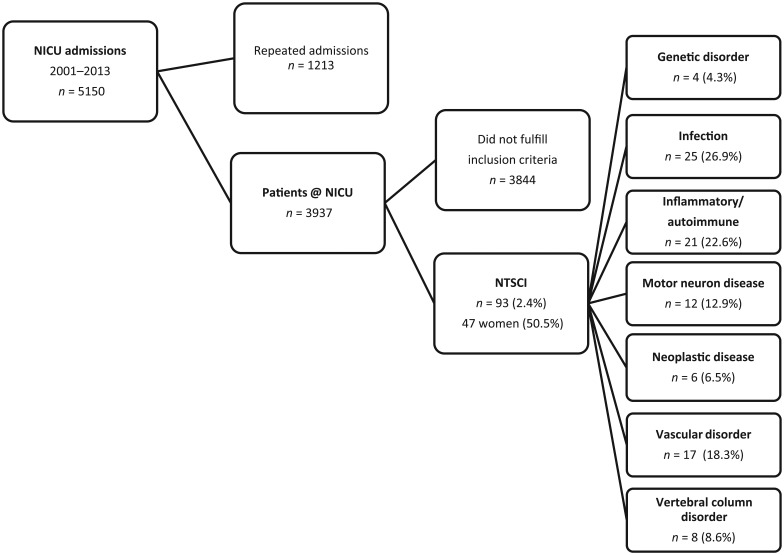
We identified 5150 patients who were treated at the NICU during the study period. Of these, 1213 patients required repeated admissions resulting in 3937 primary NICU admissions. We found 93 patients (2.4%), which had a NTSCI as underlying condition. The selection process and the frequency of different etiologies are shown in Figure 1. Infectious (spinal epidural abscess, n = 23; tetanus, n = 2) and inflammatory/autoimmune causes (ADEM, n = 3; transverse myelitis n=8; multiple sclerosis, n = 10) made up 50% of the cases. Six patients with myelitis fulfilled the radiological criteria for longitudinally extensive transverse myelitis. Further etiologies included MND (n = 12), vascular disorders (spinal cord infarction, n = 12; intraspinal hemorrhage: arteriovenous malformation, n = 3; cavernoma, n = 2) and disorders of the vertebral column (cervical myelopathy, n = 8). Less frequent were genetic disorders (spinal muscular atrophy type II, n = 2; spinal muscular atrophy type IV, n = 1; Friedreich’s ataxia, n = 1) and neoplastic causes (primary intraspinal tumors, n = 2; metastases n = 4).
Figure 1.
NTSCI on NICU algorithm. Distribution of different subgroups and their diagnoses are indicated. ADEM, acute disseminated encephalomyelitis; AVM, arteriovenous malformation; NTSCI, nontraumatic spinal cord injury; NICU, neurological intensive care unit.
Patient characteristics
Table 1 summarizes the clinical characteristics of the cohort. A total of 28 (30.1%) patients suffered acute exacerbation of a pre-existent NTSCI. Differences between NTSCI subgroups were observed for age, frequency of pre-existent neuromuscular diseases and malignancy, pre-existent NTSCI, level of SCI, reason for NICU admission, need for spinal surgery, and incidence of neurological improvement at NICU discharge. The most common reasons for NICU admission were rapidly progressing paresis (49.5%) and abundance of respiratory insufficiency (26.9%). The rate of patients with improved neurological function at discharge was 36.6%. None of the patients with MND, neoplasia or genetic disorders improved over the NICU stay. The overall mortality rate for NTSCI was 22.6%. The overall NICU mortality for all other patients admitted to the NICU during the study period was 9.0% (annual range 6.1–19.2%). Thus, risk for death at the NICU was 2.5-fold higher for patients with NTSCI. Significant death rates were present in patients with genetic disorders (50%) and MND (41.7%).
Table 1.
Characteristics of the study cohort. Demographics of all patients with NTSCI and their subgroups.
| Total | Infection | Inflammatory disorders | Vascular disorders | Motor neuron disease | Degenerative disorders | Neoplastic | Genetic disorders | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| n (%) | 93 (100) | 25 (26.9) | 21 (22.6) | 17 (18.3) | 12 (12.9) | 8 (8.6) | 6 (6.5) | 4 (4.3) | |
| Age (years) | 61 (17) | 68 (12) | 48 (22) | 67 (14) | 65 (10) | 67 (13) | 59 (15) | 53 (19) | 0.001* |
| Male gender (n/%) | 46 (49.5) | 10 (40) | 6 (28.6) | 12 (70.6) | 6 (50) | 5 (62.5) | 4 (66.7) | 3 (75) | 0.13 |
| No comorbid condition (n/%) | 22 (23.7) | 9 (36) | 5 (23.8) | 4 (23.5) | 1 (8.3) | 1 (12.5) | 1 (16.7) | 1 (25) | 0.62 |
| Comorbid conditions (n/%) | |||||||||
| cardiovascular disease | 29 (31.2) | 7 (28) | 5 (23.8) | 10 (58.8) | 3 (25) | 2 (25) | 0 (0) | 2 (50) | 0.22 |
| pulmonary disease | 9 (9.7) | 3 (12) | 1 (4.8) | 2 (11.8) | 1 (8.3) | 1 (12.5) | 0 (0) | 1 (25) | 0.85 |
| diabetes mellitus | 11 (11.8) | 2 (8) | 2 (9.5) | 4 (23.5) | 1 (8.3) | 0 (0) | 1 (16.7) | 1 (25) | 0.79 |
| neuromuscular disease | 21 (22.6) | 2 (8) | 9 (42.9) | 0 (0) | 9 (75) | 0 (0) | 0 (0) | 1 (25) | <0.001* |
| malignancy | 16 (17.2) | 4 (16) | 2 (9.5) | 2 (11.8) | 1 (8.3) | 1 (12.5) | 6 (100) | 0 (0) | 0.001* |
| Pre-existent NTSCI (n/%) | 28 (30.1) | 0 (0) | 9 (42.9) | 1 (5.9) | 9 (75) | 1 (12.5) | 4 (66.7) | 4 (100) | <0.001* |
| Spinal cord injury level (n/%) | <0.001* | ||||||||
| cervical | 37 (39.8) | 9 (36) | 9 (42.9) | 9 (52.9) | 0 (0) | 8 (100) | 2 (33.3) | 0 (0) | |
| thoracic | 12 (12.9) | 4 (16) | 1 (4.8) | 4 (23.5) | 0 (0) | 0 (0) | 3 (50) | 0 (0) | |
| thoracolumbar | 12 (12.9) | 8 (32) | 0 (0) | 4 (23.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| diffuse | 32 (34.4) | 4 (16) | 11 (52.4) | 0 (0) | 12 (100) | 0 (0) | 1 (16.7) | 4 (100) | |
| Reason for NICU admission (n/%) | <0.001* | ||||||||
| rapidly progressing paresis | 46 (49.5) | 12 (48) | 14 (66.7) | 11 (64.7) | 0 (0) | 4 (50) | 2 (33.3) | 0 (0) | |
| respiratory insufficiency | 25 (26.9) | 2 (8) | 2 (9.5) | 4 (23.5) | 10 (83.3) | 1 (12.5) | 2 (33.3) | 4 (100) | |
| impaired consciousness | 8 (8.6) | 1 (4) | 3 (14.3) | 1 (5.9) | 1 (8.3) | 2 (25) | 2 (33.3) | 0 (0) | |
| sepsis | 6 (6.5) | 7 (28) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| intractable pain | 4 (4.3) | 2 (8) | 1 (4.8) | 0 (0) | 0 (0) | 1 (12.5) | 0 (0) | 0 (0) | |
| cardiac arrest | 2 (2.2) | 0 (0) | 1 (4.8) | 0 (0) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | |
| seizures | 2 (2.2) | 1 (4) | 0 (0) | 1 (5.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Need for spinal surgery (n/%) | 22 (23.7) | 15 (60) | 0 (0) | 2 (11.8) | 0 (0) | 4 (50) | 1 (16.7) | 0 (0) | <0.001* |
| laminectomy and decompression | 19 | 15 | 0 | 0 | 0 | 4 | 0 | 0 | |
| embolization | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | |
| tumor resection | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Improved neurology at discharge (n/%) | 34 (36.6) | 15 (60) | 11 (52.4) | 5 (29.4) | 0 (0) | 3 (37.5) | 0 (0) | 0 (0) | 0.002* |
| NICU length of stay (days) | 25 (1–225) | 13 (1–48) | 41 (5–225) | 12 (1–86) | 33 (1–172) | 31 (1–127) | 27 (1–152) | 30 (4–61) | 0.035 |
| NICU mortality (n/%) | 21 (22.6) | 4 (16) | 4 (19) | 4 (23.5) | 5 (41.7) | 1 (12.5) | 1 (16.7) | 2 (50) | 0.47 |
| NICU readmission (n/%) | 15 (16.1) | 6 (24) | 7 (33.3) | 1 (5.9) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0.09 |
SAPS, Simplified Acute Physiology Score; GCS, Glasgow Coma Scale; NICU, neurological intensive care unit; NTSCI, nontraumatic spinal cord injury.
Significant differences between groups.
Data are given as median values with minimum and maximum, if not indicated otherwise.
Variables related to the NICU stay
Table 2 depicts characteristics of the NICU stay and related interventions. Almost half of the patients required mechanical ventilation, and duration of mechanical ventilation showed great variability. A tracheostomy was performed in 21.5%, some patients required therapeutic plasma exchange. Renal failure was observed in 29%. Vasopressor therapy and blood transfusion was performed in 40.9% and 21.5%, respectively. Urinary tract infection and ventilator-associated pneumonia made up more than 50% of the NICU-related complications. The most frequent causes of NICU death were respiratory failure (7.5%) and multiple organ dysfunction (9.7%). ICU survivors were transferred to the neurological ward (61.5%), another ICU (7.5%), a long-term care facility (7.5%), or home (2.2%).
Table 2.
Clinical data and NICU-related interventions of NTSCI patients.
| Total | Infection | Inflammatory Disorders | Vascular Disorders | Motor Neuron Disease | Degenerative Disorders | Neoplastic | Genetic Disorders | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| n | 93 | 25 | 21 | 17 | 12 | 8 | 6 | 4 | |
| SAPS II (points) | 29.5 (16–77) | 26 (13–58) | 16 (5–64) | 23 (7–65) | 40 (22–77) | 31 (13–70) | 37 (29–48) | 32 (6–44) | 0.003* |
| GCS at NICU admission (points) | 11 (3–15) | 14 (3–15) | 15 (3–15) | 14 (3–15) | 7 (3–15) | 13 (3–16) | 9 (3–15) | 6 (6–15) | 0.002* |
| Need for mechanical ventilation (n/%) | 44 (47.3) | 10 (40) | 10 (47.6) | 5 (29.4) | 11 (91.7) | 3 (37.5) | 2 (33.3) | 3 (75) | 0.03* |
| noninvasive ventilation | 35 (37.6) | 9 (36) | 7 (33.3) | 3 (17.6) | 11 (91.7) | 2 (25) | 1 (16.7) | 2 (50) | 0.003* |
| noninvasive ventilation failure | 30 (85.7) | 8 (88.9) | 7 (100) | 3 (100) | 8 (72.7) | 2 (100) | 1 (100) | 1 (50) | 0.44 |
| invasive ventilation | 39 (41.9) | 9 (36) | 10 (47.6) | 5 (29.4) | 8 (66.7) | 3 (37.5) | 2 (33.3) | 2 (50) | 0.53 |
| Duration of mechanical ventilation (days) | 12 (1–137) | 4 (1–37) | 12 (5–62) | 34 (12–81) | 25 (1–90) | 50 (44–56) | 137 (137) | 38 (35–40) | 0.001* |
| Need for tracheostomy (n/%) | 20 (21.5) | 3 (12) | 5 (23.8) | 1 (5.9) | 6 (50) | 2 (25) | 1 (16.7) | 2 (50) | 0.07 |
| Hemodynamic interventions (n/%) | |||||||||
| invasive arterial monitoring | 84 (90.3) | 25 (100) | 17 (81) | 15 (88.2) | 9 (75) | 8 (100) | 6 (100) | 4 (100) | 0.12 |
| advanced hemodynamic monitoring | 3 (3.2) | 1 (4) | 1 (4.8) | 1 (5.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.95 |
| need for vasopressor therapy | 38 (40.9) | 11 (44) | 8 (38.1) | 6 (35.3) | 5 (41.7) | 3 (37.5) | 2 (33.3) | 3 (75) | 0.87 |
| need for blood transfusion | 20 (21.5) | 7 (28) | 4 (19) | 3 (17.6) | 2 (16.7) | 4 (50) | 0 (0) | 0 (0) | 0.27 |
| Acute kidney injury (n/%) | 27 (29) | 11 (44) | 5 (23.8) | 4 (23.5) | 1 (8.3) | 4 (50) | 1 (16.7) | 1 (25) | 0.24 |
| Need for extracorporeal therapies (n/%) | |||||||||
| continuous veno-venous hemofiltration | 5 (5.4) | 1 (4) | 3 (14.3) | 1 (5.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.53 |
| therapeutic plasma exchange | 17 (18.3) | 1 (4) | 15 (71.4) | 0 (0) | 0 (0) | 0 (0) | 1 (16.7) | 0 (0) | <0.001* |
| NICU-related complications (n/%) | |||||||||
| urinary tract infection | 25 (26.9) | 5 (20) | 9 (42.9) | 2 (11.8) | 3 (25) | 3 (37.5) | 1 (16.7) | 2 (50) | 0.3 |
| ventilator-associated pneumonia | 23 (24.7) | 5 (20) | 7 (33.3) | 2 (11.8) | 4 (33.3) | 2 (25) | 1 (16.7) | 2 (50) | 0.58 |
| hemorrhage | 10 (10.8) | 1 (4) | 3 (14.3) | 3 (17.6) | 1 (8.3) | 2 (25) | 0 (0) | 0 (0) | 0.51 |
| central line-associated bloodstream infection | 6 (6.5) | 1 (4) | 1 (4.8) | 0 (0) | 1 (8.3) | 2 (25) | 0 (0) | 1 (25) | 0.18 |
| thromboembolic complication | 5 (5.4) | 2 (8) | 2 (9.5) | 1 (5.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.84 |
| Causes of death (n/%) | 0.07 | ||||||||
| multiple organ dysfunction | 7 (7.5) | 2 (8) | 1 (4.8) | 2 (11.8) | 0 (0) | 0 (0) | 0 (0) | 2 (50) | |
| respiratory failure | 9 (9.7) | 2 (8) | 0 (0) | 1 (5.9) | 2 (8) | 1 (12.5) | 1 (16.7) | 0 (0) | |
| electromechanical dissociation | 2 (2.2) | 0 (0) | 0 (0) | 1 (5.9) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | |
| intracranial hypertension | 2 (2.2) | 0 (0) | 2 (9.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
SAPS, Simplified Acute Physiology Score; GCS, Glasgow Coma Scale; NICU, neurological intensive care unit; NTSCI, nontraumatic spinal cord injury.
Significant differences between groups.
Data are given as median values with minimum and maximum, if not indicated otherwise.
Predictors of NICU death
The NICU admission SAPS II count, NICU admission GCS, need and duration for mechanical ventilation, rate of noninvasive ventilation, as well as the need for therapeutic plasma exchange differed between NTSCI subgroups. A cervical level of injury, respiratory insufficiency as the reason for NICU admission, the SAPS II, and development of acute kidney injury during the NICU stay independently predicted NICU mortality in a multivariate analysis (Table 3).
Table 3.
Bivariate and multivariate analysis to identify independent risk factors for intensive care unit mortality. All variables which were significantly associated with NICU mortality qualified for inclusion in a multivariate analysis.
| Variable |
Bivariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| Spearman rank correlation coefficient | p-value | OR | 95% CI | p-value | |
| Age (years) | 0.258 | 0.01 | |||
| Simplified Acute Physiology Score II (points) | 0.313 | 0.002 | 1.04 | 1.003–1.08 | 0.04 |
| Cervical injury level (binary) | −0.281 | 0.006 | 0.13 | 0.03–0.64 | 0.01 |
| Diffuse injury level (binary) | 0.313 | 0.002 | |||
| Respiratory insufficiency as NICU admission cause (binary) | 0.369 | <0.001 | 4.97 | 1.38–17.9 | 0.01 |
| Need for mechanical ventilation (binary) | 0.364 | <0.001 | |||
| Need for norepinephrine support (binary) | 0.336 | 0.001 | |||
| Development of acute kidney injury (binary) | 0.278 | 0.007 | 7.25 | 1.9–27.5 | 0.004 |
| Need for continuous veno-venous hemofiltration (binary) | 0.213 | 0.04 | |||
NICU, neurological intensive care unit; OR, odds ratio; CI, confidence interval.
Discussion
In this retrospective study, we identified 2.4% of a NICU population to suffer from NTSCI. Infection, inflammatory and vascular disorders as well as MND were the most frequent causes of NTSCI. All-cause NICU mortality was 22.6% with multiple organ dysfunction and respiratory failure being the leading causes of death. NTSCI subgroups differed in age, pre-existent diseases, level of SCI, reason for NICU admission, as well as the need for surgery and specific NICU interventions. A cervical level of injury, respiratory insufficiency as reason for NICU admission, SAPS II, and development of acute renal failure were independent risk factors for mortality. Although the percentage of NICU patients with NTSCI appears small, the frequent need for mechanical ventilation, prolonged NICU stay, as well as a comparatively high readmission rate and NICU mortality suggest that these patients require special attention and specific knowledge of the neuro-intensivist. With a significant variation between NTSCI subgroups, the spinal cord lesion involved the cervical level in two thirds of patients. Patients with acute episodes of NTSCI suffered from infection, degenerative, vascular or inflammatory disorders and predominantly required NICU admission because of rapidly progressing paresis. On the other hand, patients with pre-existent NTSCI, typically those with multiple sclerosis, MND, neoplastic or genetic disorders, were admitted to the NICU because of medical complications such as respiratory insufficiency or impaired consciousness.
When compared with a population of NTSCI admitted to a spinal rehabilitation unit, our critically ill NTSCI patient population included a higher percentage of patients with infection, inflammatory and vascular disorders, but a lower rate of patients with degenerative or neoplastic spinal conditions [New et al. 2015]. This difference may be explained by the typically acute onset of infectious, inflammatory and vascular disorders as well as their higher rate of neurological improvement compared with the other NTSCI subgroups [Sellner et al. 2009; Heldner et al. 2012]. On the other hand, we cannot exclude that some NTSCI patients with degenerative or neoplastic NTSCI were transferred directly to a medical or neurosurgical ICU, or were not admitted due to palliative care. The latter is further supported by a prospective study on survival in NTSCI patients with malignant spinal cord compression. The authors reported a median survival of only 2.9 months [Loblaw et al. 2003]. It also needs to be noted that the case mix of admissions may be driven by the relative frequencies of NTSCI in the region. For instance, a hospital-based study of frequencies of NTSCI at a tertiary care center in Yaounde, Cameroon reported that etiologies were dominated by primary and secondary spinal tumors including prostate carcinoma, lymphoma and liver carcinoma and each accounted for 24.5% of cases [Lekoubou Looti et al. 2010]. Their case series also comprised patients with spinal tuberculosis (12.9%), tropical spastic paraparesis (4.8%), and HIV myelopathy (1.4%). Spinal tuberculosis and Pott’s disease may be prone to NICU treatment.
In contrast to patients with traumatic SCI, critically ill patients with NTSCI are older, have a more balanced male-to-female ratio and suffer from comorbid diseases in the majority of cases [New et al. 2011; Jain et al. 2015; Majdan et al. 2015]. Another difference to traumatic SCIs is the rate of neurologic improvement during the NICU stay, which was observed in 36.6% of all patients, or even in 43.1% when analyzing only NTSCI patients with acute NTSCI.
The most frequent interventions in the NICU were mechanical ventilation, invasive hemodynamic monitoring and pharmacological circulatory support. While approximately 50% of NTSCI patients required mechanical ventilation, the percentage was highest in patients with MND and those with genetic disorders. In most cases requiring mechanical ventilator support, noninvasive ventilation was attempted initially, but resulted in a high failure rate independent of the NTSCI subgroup. One potential reason for this high failure rate of noninvasive ventilation could be the prolonged requirement of mechanical ventilatory support, which was especially the case in NTSCI patients with vascular and degenerative disorders as well as in those with MND. The fact that almost half of NTSCI patients admitted to the NICU required pharmacological support to preserve hemodynamic function is due to affection of sympathetic circulatory regulation by the spinal cord lesion. Advanced hemodynamic monitoring may be indicated in selected patients with cardiovascular instability to balance fluid loading and catecholamine therapy. While renal replacement therapy was rarely necessary, plasma exchange was used as a therapeutic intervention in NTSCI patients with inflammatory disorders. Spinal surgery was performed only in selected NTSCI cases such as patients with infection or degenerative disorders.
Compared with other NICU patients, the mortality rate of our NTSCI cases is high. The main causes of death were multiple organ dysfunction and respiratory failure occurring most frequently in NTSCI patients with MND and genetic disorders. Respiratory insufficiency and rapidly progressing paresis accounted for 76.4% of admissions. These two reasons for admission differed in terms of associated mortality. We found a 44% mortality for patients with presence of respiratory insufficiency, whereas only 13% with progressing paresis. It is imaginable that the reason why patients with rapidly progressing paresis were admitted to the NICU was imminent respiratory insufficiency. Indeed, the inability to protect the airway and impaired central respiratory drive requires early recognition and admission to the ICU [Markandaya et al. 2012]. Clinical differences among NTSCI subgroups, especially the level of consciousness, is also reflected by the significantly lower GCS count of patients suffering from MND compared with NTSCI patients with inflammatory or infectious disorders. Patients with respiratory insufficiency also had a significantly higher SAPS II count compared with patients admitted to the NICU because of rapidly progressing paresis.
The multivariate logistic regression analysis revealed that a SAPS II count, respiratory insufficiency as the reason for NICU admission and development of acute kidney injury during the NICU stay were independent predictors of NICU mortality. As in other populations of critically ill patients, a high systemic disease severity, as indicated by SAPS II count, as well as the development of organ dysfunctions, such as respiratory failure or acute kidney injury, are associated with mortality in NTSCI patients too [Sakr et al. 2012; Sekulic et al. 2015]. Similarly, respiratory insufficiency has been shown to be a major indicator for morbidity and mortality after acute traumatic SCI [Reines and Harris, 1987]. Interestingly, a cervical level of NTSCI appeared to be a protective factor. As these patients were typically admitted to the NICU before respiratory insufficiency developed, it can be hypothesized that NICU admission of NTSCI patients before development of organ dysfunction, in particular respiratory failure, is associated with improved survival.
When interpreting the results of this study, relevant limitations need to be acknowledged. Since study data were collected only at one center, our results cannot be extrapolated to other hospitals and settings as differences in NICU admission policy, therapeutic management and end-of-life decisions may exist [Aslakson et al. 2014; Fowler et al. 2015]. Some NTSCI subgroups, such as patients with degenerative, neoplastic or genetic disorders, comprised only few cases. Therefore, it is likely that the results for these subgroups would have been different had more patients been included. Given the comparatively low incidence of these NTSCI subgroups and the long observation period of our study, it seems that only a multicenter approach can render more valid data. Finally, we used a retrospective study design, which per se carries the disadvantage of missing values and incomplete datasets in some study patients. Although NTSCI patients make up a small part of NICU patients, they pose specific challenges to the neuro-intensivist. Further research on the management of critically ill patients with NTSCI is warranted.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Grassner reports no disclosures. Dr Marschallinger reports no disclosures. Dr Dünser reports no disclosures. Dr Novak has received speakers’ honoraria from Astellas and CSC Angelini Pharmaceuticals, He has no specific conflicts relevant to this work. Dr Zerbs reports no disclosures. Dr Aigner reports no disclosures. Dr Trinka has received research funding from UCB Pharma, Biogen-Idec, Red Bull, Merck, the European Union, FWF Österreichischer Fond zur Wissenschaftsförderung, and Bun-desministerium für Wissenschaft und Forschung and has acted as a paid consultant to Eisai, Takeda, Ever Neuropharma, Biogen, Bial, Sunovion, and UCB, and has received speakers’ honoraria from Bial, Eisai, GL Lannacher, GlaxoSmithKline, Boehringer, Sunovion, Newbridge Pharma, and UCB Pharma. He has no specific conflicts relevant to this work. Dr Sellner received research funding from the Paracelsus Medical University, Bayer, Biogen-Idec, Merck and Novartis, has acted as paid consultant to Novartis and Genzyme, and has received speakers’ honoraria from Biogen-Idec, Ever Neuropharma, Genzyme, Novartis and Teva-Ratiopharm. He has no specific conflicts relevant to this work
Contributor Information
Lukas Grassner, Center for Spinal Cord Injuries, BG Trauma Center Murnau, Germany Institute of Molecular Regenerative Medicine, Paracelsus Medical University, Salzburg, Austria; Spinal Cord Injury and Tissue Regeneration Center, Paracelsus Medical University, Salzburg, Austria.
Julia Marschallinger, Institute of Molecular Regenerative Medicine, Paracelsus Medical University, Salzburg, Austria; Spinal Cord Injury and Tissue Regeneration Center, Paracelsus Medical University, Salzburg, Austria.
Martin W. Dünser, Department of Anesthesiology, Perioperative Medicine and General Intensive Care Medicine, Salzburg University Hospital, Paracelsus Medical University, Salzburg, Austria
Helmut F. Novak, Department of Neurology, Christian Doppler Medical Center, Paracelsus Medical University, Salzburg, Austria
Alexander Zerbs, Department of Neurology, Christian Doppler Medical Center, Paracelsus Medical University, Salzburg, Austria.
Ludwig Aigner, Institute of Molecular Regenerative Medicine, Paracelsus Medical University, Salzburg, Austria; Spinal Cord Injury and Tissue Regeneration Center, Paracelsus Medical University, Salzburg, Austria.
Eugen Trinka, Department of Neurology, Christian Doppler Medical Center, Paracelsus Medical University, Salzburg, Austria; Spinal Cord Injury and Tissue Regeneration Center, Paracelsus Medical University, Salzburg, Austria.
Johann Sellner, Department of Neurology, Christian Doppler Medical Center, Paracelsus Medical University, Ignaz-Harrer-Str. 79, A-5020 Salzburg, Austria; Department of Neurology, Klinikum rechts der Isar, Technische Universität München, Germany.
References
- Andersen P., Abrahams S., Borasio G., De Carvalho M., Chio A., Van Damme P., et al. for the EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis (2012) EFNS Guidelines on the Clinical Management of Amyotrophic Lateral Sclerosis (MALS) - revised report of an EFNS Task Force. Eur J Neurol 19: 360–375. [DOI] [PubMed] [Google Scholar]
- Aslakson R., Curtis J., Nelson J. (2014) The changing role of palliative care in the ICU. Crit Care Med 42: 2418–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casha S., Christie S. (2011) A systematic review of intensive cardiopulmonary management after spinal cord injury. J Neurotrauma 28: 1479–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler R., Abdelmalik P., Wood G., Foster D., Gibney N., Bandrauk N., et al. (2015) Critical care capacity in Canada: results of a national cross-sectional study. Crit Care 19: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachen H. (1977) Idealized care of the acutely injured spinal cord in Switzerland. J Trauma 17: 931–936. [DOI] [PubMed] [Google Scholar]
- Heldner M., Arnold M., Nedeltchev K., Gralla J., Beck J., Fischer U. (2012) Vascular diseases of the spinal cord: a review. Curr Treat Options Neurol 14: 509–520. [DOI] [PubMed] [Google Scholar]
- Jain N., Ayers G., Peterson E., Harris M., Morse L., O’Connor K., et al. (2015) Traumatic spinal cord injury in the United States, 1993–2012. JAMA 313: 2236–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum J., Lameire N. for the KDIGO AKI Guideline Work Group (2013) Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (part 1). Crit Care 17: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall J., Lemeshow S., Saulnier F. (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270: 2957–2963. [DOI] [PubMed] [Google Scholar]
- Lekoubou Looti A., Kengne A., Djientcheu Vde P., Kuate C., Njamnshi A. (2010) Patterns of non-traumatic myelopathies in Yaounde (Cameroon): a hospital based study. J Neurol Neurosurg Psychiatry 81: 768–770. [DOI] [PubMed] [Google Scholar]
- Leveque J., Marong-Ceesay B., Cooper T., Howe C. (2015) Diagnosis and treatment of cervical radiculopathy and myelopathy. Phys Med Rehabil Clin N Am 26: 491–511. [DOI] [PubMed] [Google Scholar]
- Loblaw D., Laperriere N., Mackillop W. (2003) A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol) 15: 211–217. [DOI] [PubMed] [Google Scholar]
- Majdan M., Brazinova A., Mauritz W. (2015) Epidemiology of traumatic spinal cord injuries in Austria 2002–2012. Eur Spine J, in press. [DOI] [PubMed] [Google Scholar]
- Markandaya M., Stein D., Menaker J. (2012) Acute treatment options for spinal cord injury. Curr Treat Options Neurol 14: 175–187. [DOI] [PubMed] [Google Scholar]
- Moore A., Blumhardt L. (1997) A prospective survey of the causes of non-traumatic spastic paraparesis and tetraparesis in 585 patients. Spinal Cord 35: 361–367. [DOI] [PubMed] [Google Scholar]
- New P., Marshall R. (2014) International spinal cord injury data sets for non-traumatic spinal cord injury. Spinal Cord 52: 123–132. [DOI] [PubMed] [Google Scholar]
- New P., Rawicki H., Bailey M. (2002) Nontraumatic spinal cord injury: demographic characteristics and complications. Arch Phys Med Rehabil 83: 996–1001. [DOI] [PubMed] [Google Scholar]
- New P., Reeves R., Smith E., Townson A., Eriks-Hoogland I., Gupta A., et al. (2015) International retrospective comparison of inpatient rehabilitation for patients with spinal cord dysfunction epidemiology and clinical outcomes. Arch Phys Med Rehabil 96: 1080–1087. [DOI] [PubMed] [Google Scholar]
- New P., Simmonds F., Stevermuer T. (2011) A population-based study comparing traumatic spinal cord injury and non-traumatic spinal cord injury using a national rehabilitation database. Spinal Cord 49: 397–403. [DOI] [PubMed] [Google Scholar]
- New P., Sundararajan V. (2008) Incidence of non-traumatic spinal cord injury in Victoria, Australia: a population-based study and literature review. Spinal Cord 46: 406–411. [DOI] [PubMed] [Google Scholar]
- Noonan V., Fingas M., Farry A., Baxter D., Singh A., Fehlings M., et al. (2012) Incidence and prevalence of spinal cord injury in Canada: a national perspective. Neuroepidemiology 38: 219–226. [DOI] [PubMed] [Google Scholar]
- Readdy W., Chan A., Matijakovich D., Dhall S. (2015) A review and update on the guidelines for the acute non-operative management of cervical spinal cord injury. J Neurosurg Sci 59: 119–128. [PubMed] [Google Scholar]
- Reines H., Harris R. (1987) Pulmonary complications of acute spinal cord injuries. Neurosurgery 21: 193–196. [DOI] [PubMed] [Google Scholar]
- Ryken T., Hurlbert R., Hadley M., Aarabi B., Dhall S., Gelb D., et al. (2013) The acute cardiopulmonary management of patients with cervical spinal cord injuries. Neurosurgery 72(Suppl. 2): 84–92. [DOI] [PubMed] [Google Scholar]
- Sakr Y., Lobo S., Moreno R., Gerlach H., Ranieri V., Michalopoulos A., et al. (2012) Patterns and early evolution of organ failure in the intensive care unit and their relation to outcome. Crit Care 16: R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage P., Sharkey R., Kua T., Schofield L., Richardson D., Panchmatia N., et al. (2014) Malignant spinal cord compression: NICE guidance, improvements and challenges. QJM 107: 277–282. [DOI] [PubMed] [Google Scholar]
- Scott T., Frohman E., De Seze J., Gronseth G., Weinshenker B. for the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology (2011) Evidence-based guideline: clinical evaluation and treatment of transverse myelitis. Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 77: 2128–2134. [DOI] [PubMed] [Google Scholar]
- Sebastia-Alcacer V., Alcanyis-Alberola M., Giner-Pascual M., Gomez-Pajares F. (2014) Are the characteristics of the patient with a spinal cord injury changing? Spinal Cord 52: 29–33. [DOI] [PubMed] [Google Scholar]
- Sekulic A., Trpkovic S., Pavlovic A., Marinkovic O., Ilic A. (2015) Scoring systems in assessing survival of critically ill ICU patients. Med Sci Monit 21: 2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellner J., Boggild M., Clanet M., Hintzen R., Illes Z., Montalban X., et al. (2010) EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol 17: 1019–1032. [DOI] [PubMed] [Google Scholar]
- Sellner J., Luthi N., Schupbach W., Gebhardt A., Findling O., Schroth G., et al. (2009) Diagnostic workup of patients with acute transverse myelitis: spectrum of clinical presentation, neuroimaging and laboratory findings. Spinal Cord 47: 312–317. [DOI] [PubMed] [Google Scholar]
- Tator C., Rowed D., Schwartz M., Gertzbein S., Bharatwal N., Barkin M., et al. (1984) Management of acute spinal cord injuries. Can J Surg 27: 289–293, 296. [PubMed] [Google Scholar]
- Van Den Berg M., Castellote J., De Pedro-Cuesta J., Mahillo-Fernandez I. (2010) Survival after spinal cord injury: a systematic review. J Neurotrauma 27: 1517–1528. [DOI] [PubMed] [Google Scholar]
- Zach G., Seiler W., Dollfus P. (1976) Treatment results of spinal cord injuries in the Swiss Paraplegic Centre of Basle. Paraplegia 14: 58–65. [DOI] [PubMed] [Google Scholar]