Abstract
Background
Published prognostic models for overall survival after liver resection for intrahepatic cholangiocarcinoma (ICC) require external validation before use in clinical practice.
Study Design
From January 1993 to May 2013, consecutive patients who underwent resection of ICC were identified from a prospective database. The Wang nomogram was derived in an Asian cohort (n=367) and included clinicopathological variables and preoperative CEA and CA 19-9 levels. The Hyder nomogram was derived in a Eastern and Western multicenter cohort (n=514) using clinicopathological variables only. The AJCC Staging System 7th Edition (AJCC) and the preoperative Fudan risk score were also evaluated. Prognostic performance was assessed in terms of discrimination, calibration and stratification.
Results
One hundred eighty eight patients were included with a median follow-up of 41 months. Median OS was 48.7 months and estimated 3-year and 5-year OS were 59% and 45%, respectively. OS prediction accuracy, according to concordance index calculation (C-index), was respectively 0.72 in the Wang nomogram, 0.66 with the Hyder nomogram, 0.63 with the AJCC and 0.55 using the Fudan score. Both nomograms provided also effective patient stratification in distinct survival groups.
Conclusion
Both Wang and Hyder nomograms provided accurate patient prognosis estimation after liver resection for ICC and may be useful for decision making regarding adjuvant therapy.
The Wang nomogram appears to be more appropriate in patients undergoing formal portal lymphadenectomy and requires preoperative CEA and CA19-9 levels for optimal performance. Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy, with an incidence in the USA of about 1 per 100,000 (1). While ICC is much less common than hepatocellular carcinoma (HCC), its age-adjusted incidence has risen by 165%, from 0.32 per 100,000 to 0.85 per 100,000 over the last 30 years (2, 3). The only potentially curative treatment is complete resection, which offers a median overall survival of about 30 months (4–7). Adjuvant or neoadjuvant therapy might improve survival after resection, although this hypothesis is mainly based on extrapolation of data from two randomized controlled trials for biliary cancers in the palliative setting (8–10). Prognostic models could potentially optimize identification of patients most likely to benefit from such treatment.
The 7th edition of the American Joint Committee on Cancer (AJCC) staging system introduced a separate TNM classification for ICC, whereas earlier versions did not differentiate between hepatocellular cancer and intrahepatic cholangiocarcinoma (2). Factors included in the AJCC staging for ICC are the number of tumors, vascular invasion, direct invasion of extrahepatic structures, periductal invasion (versus mass-forming lesions), lymph node metastasis, and distant metastasis. Several studies found additional prognostic factors, including a positive surgical margin, tumor size, tumor differentiation, and patient age (11–13). Prognostic nomograms including such additional variables may, therefore, be more accurate than the conventional AJCC staging system for predicting outcome (14).
Recently, one preoperative prognostic score and two prognostic nomograms have been published (7, 15, 16), but none of these models has been externally validated. The aim of this study was to evaluate and validate the existing prognostic scores for overall survival after resection of ICC in a large, single center cohort.
Methods
Study population
The Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSKCC) approved this study. All included patients underwent a liver resection, and ICC was confirmed at pathological evaluation of the resected specimen. Lymphadenectomy was performed at the discretion of the surgeon, either as a formal peripancreatic and portocaval lymph node (LN) dissection or as a targeted excision according to preoperative imaging and intraoperative findings. Resections were extended to extrahepatic structures when required to achieve a macroscopically complete resection.
Perioperative data
Clinical preoperative variables included demographics, preoperative tumor markers (CEA, CA 19-9, and AFP), and the time interval between diagnosis and resection. The number of liver lesions and the diameter of the largest tumor were evaluated using preoperative CT, MRI, and intraoperative ultrasonography (US). Tumor boundary type, as defined and adopted in the Fudan score, was assessed on preoperative cross-sectional imaging (15).
Pathologic Assessment
Pathologic variables included size and number of tumors, differentiation grade, resection margin status, vascular invasion, perineural invasion, number, sites and involvement of harvested LN, and histology of nontumoral liver parenchyma (2). Extrahepatic involvement (EHI) was defined as direct invasion of any extrahepatic organs excluding the gallbladder (pT3). Morphological subtype was defined as mass-forming (MF), periductal infiltrating (PI) and mixed (MFPI) (17, 18).
Follow-Up
Clinical and radiographic monitoring was performed every 4–6 months. Adjuvant therapy was offered, at the discretion of the multidisciplinary team, to patients at high risk of recurrence, especially in case of lymph node positive disease, vascular invasion or R1 resection.
Nomograms and risk scores validation
The Wang nomogram was published in 2013 on a derivation cohort of 367 ICC patients resected at a single Asian center (7). Preoperative CEA and CA19-9 levels, as well as tumor size, were included as linear continuous variables. Additional dichotomous variables were vascular invasion (No/Yes), nodal status (pN0/pN1), and direct invasion or local metastasis (No/Yes). The nomogram concordance-index (C-index) was 0.75 (95% CI, 0.68–0.83) in a subsequent validation cohort (n=82) from the same center.
The Hyder nomogram was published in 2014 from a multi-institutional cohort of 514 patients from 13 Western and Eastern centers (16). This nomogram included age, tumor size, number of lesions, nodal status, vascular invasion and underlying cirrhotic liver parenchyma. Continuous variables for age and tumor size were included after cubic splines transformation, and the number of tumors was transformed to a binary variable (solitary versus multiple). Categorical variables were defined as follows: nodal status (pNx/pN0/pN1), vascular invasion (none/microvascular/macrovascular) and underlying cirrhotic liver (No/Yes). The nomogram C-index in the derivation cohort was 0.69 (95% CI, 0.62–0.76), and bootstrap validation showed minimal evidence of overfit. The study did not include a validation cohort.
The Fudan score (15) was derived from an Asian single center cohort (n=344) in 2011, using only prognostic factors that are preoperatively available: alkaline phosphatase (ALP), CA 19-9, number of tumors, tumor size and tumor boundary type. All variables were transformed into binary variables. Tumor boundary type was categorized on imaging as distinct versus obscure. Distinct boundary was defined as a regular border of thin ring-like iso-attenuation or arterial enhancement relative to the liver. Conversely, obscure boundary on imaging was described as an ill-defined border and represented a worse prognosis. These five prognostic factors resulted in a simple risk score in which one point was assigned for each factor. Patients were stratified in risk groups for death after resection: low risk for 0 points, intermediate risk for 1 point, high risk for 2–3 points, and extremely high risk for 4–5 points.
Statistical analysis
Overall survival (OS) and recurrence free survival (RFS) were calculated from the time of surgical resection until time of death (for OS), or until first relapse or death (for PFS). Recurrence was defined as tumor relapse either biopsy proven or newly detected tumor on 2 consecutive radiologic images, with or without elevation of tumor markers. Patient who did not experience the event of interest by the end of the study were censored at the time of the last available follow-up. OS and PFS were estimated using the Kaplan-Meier method and compared between clinico/pathological characteristics using the log-rank test.
Performance of the nomograms was validated using the MSKCC patients in terms of discrimination and calibration. Discrimination was quantified with the C-index using Harrell’s method (19). The C-index provides the probability that, in a randomly selected pair of patients, in which one patient dies before the other, the patient who died first had the worse predicted outcome from the nomogram. Calibration consisted of grouping patients in quartiles according to their nomogram-predicted probabilities, and comparing the mean of the group with the observed Kaplan-Meier OS curves. Results from calibration are presented as a calibration plot. To compare the two nomograms, a significance test was conducted using the bootstrap (20). Specifically, bootstrap sample was drawn from our data set and the c-index for both nomograms as well as their difference was estimated. The process was repeated 1000 times and the differences obtained were ranked from smallest to largest. The p-value is twice the rank of the observation nearest to 0 (representing no difference between the two nomograms).
All p values were based on two-tailed statistical analysis and a p value <0.05 was considered to indicate statistical significance. All analyses were performed with SAS v. 9.2 statistical software (SAS Institute Inc, Cary, North Carolina), SPSS software, version 22.0 for Windows (SPSS Inc., Chicago, IL) and R software, version 3.1.1.
Results
Descriptive Data
From January 1993 to May 2013, 199 consecutive patients underwent liver resection for ICC at MSKCC. Patients with mixed-type primary liver tumors (n=5), distant metastatic disease at the time of resection (n=1, peritoneal carcinosis) or postoperative death within 90 days after surgery (n=5) were excluded. The remaining 188 patients were included in this study. The preoperative, operative and pathologic characteristics are listed in Table 1 for both the MSKCC cohort and the three cohorts for whom the prognostic models are considered for validation. Patients from the MSKCC cohort were older (median age at surgery, 65.8 years), and were more often female (59.8%). The hepatitis B virus (HBV) infection rate was lower in MSKCC’s patient set. All cohorts were comparable in terms of preoperative biomarkers.
Table 1.
Descriptive Data in MSKCC Cohort and Nomograms and Fudan Score Cohorts
| Description | MSKCC (n=188) |
Hyder nomogram (n=514) |
Wang cohort (n=367) |
Fudan score (n=344) |
|---|---|---|---|---|
| Age at surgery, y | ||||
| Median (range) | 65.8 (19–89) | 53 (23–78) | ||
| Median (IQR) | 65.7 (57.5–74.3) | 59.2 (50–69) | ||
| > 65 y, n (%) | 96 (51) | 87 (25.3) | ||
| Female sex, n (%) | 110 (59.8) | 241 (46.1) | 121 (33) | 145 (42.2) |
| Ethnicity, n (%) | NR | NR | ||
| White | 135 (71.8) | 314 (61.1) | ||
| Black | 9 (4.8) | 16 (3.1) | ||
| Asian | 44 (23.4) | 184 (35.8) | ||
| Hepatitis, n (%) | 18 (9.5) | |||
| HBV | 9 (4.8) | 173 (33.4) | 187 (51) | |
| HCV | 9 (4.8) | 19 (3.7) | 8 (2.2) | 96 (27.9) |
| Albumin, g/L, | NR | |||
| Median (range) | 4.2 (2.1–6.3) | 4.2 (3–6.5) | ||
| <3.5 g/dL, n (%) | 8 (4.2) | 22 (6.4) | ||
| Total Bilirubin, mg/L | NR | |||
| Median (range) | 0.6 (0.2–28.5) | 0.8 (0.3–18.5) | ||
| >17.1 mmol/L, n (%) | 30 (15.9) | 88 (25.6) | ||
| CA19-9, U/mL | ||||
| Median (range) | 43 (1–53902) | 41.2 (0.4–1000) | ||
| Median (IQR) | 43 (14.4–135.5) | 25.9 (1–145) | ||
| >37 U/mL, n (%) | 66 (35.1) | 200 (58.1) | ||
| CEA, µg/L | ||||
| Median (range) | 2.3 (0.1–410) | 2.5 (0.1–809.6) | ||
| Median (IQR) | 2.3 (1.3–3.4) | 1.3 (0.3–3) | ||
| >5µg/L, n (%) | 5 (2.7) | 69 (23) | ||
| Tumor Size, cm | ||||
| Median (range) | 6 (1–24) | 5.5 (0.4–22) | ||
| Median (IQR) | 6 (4–9) | 6 (4–8.6) | ||
| ≥10, n (%) | 39 (20.7) | 56 (16.3) | ||
| Solitary lesion, n (%) | 134 (71.3) | 384 (74.7) | 258 (75) | |
| Multiple lesions, n (%) | 54 (28.7) | 130 (25.3) | 86 (25) | |
| 2–3 | 19 (10.1) | |||
| ≥3 | 35 (18.6) | 42 (11.4) | ||
| <3 | 153 (81.4) | 325 (88.6) | ||
| Underlying liver, n (%) | NR | |||
| Steatosis | 65 (35.9) | NR | NR | |
| Cirrhosis | 9 (4.8) | 44 (8.6) | 78 (21.3) | |
| LVI, n (%) | 68 (36.2) | 124 (24.1) | 57 (16.6) | |
| Microvascular | 46 (24.5) | 68 (13.2) | 54 (14.7) | |
| Macrovascular | 22 (11.7) | 56 (10.9) | 37 (10.1) | |
| pN stage, n (%) | NR | |||
| pNx | 96 (51.3) | 262 (60) | 74 (20.2) | |
| pN0 | 71 (37.6) | 162 (31.5) | 211 (57.5) | |
| pN1 | 21 (11.1) | 90 (17.5) | 82 (22.3) | |
| AJCC 7th Stage, n (%) | NR | NR | ||
| I | 75 (39.9) | 129 (37.5) | ||
| II | 49 (26) | 75 (21.8) | ||
| III | 31 (16.5) | 23 (6.7) | ||
| IV | 33 (17.6) | 117 (34) | ||
| Extrahepatic invasion*, n (%) | 36 (19.1) | 14 (2.7) | 35 (9.5) | NR |
| Morphological type, n (%) | NR | NR | ||
| Mass-Forming | 175 (93) | 345(94) | ||
| Periductal invasion | 13 (7) | 20 (5.5) | ||
| Adjuvant Therapy, n (%) | 51 (27.1) | 122 (23.7) | NR | NR |
Gallbladder excluded
MSKCC, Memorial Sloan Kettering Cancer Center; CA19-9, carcinogen antigen 19-9; CEA, carcinoembryonic antigen; GB, gallbladder; HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; LVI, lymphovascular invasion; NR, not reported in the original publications; AJCC, American Joint Committee on Cancer.
Liver resection consisted of a major hepatectomy (three or more segments) in 125 patients (66.1%), achieving complete (R0) resection in 152 patients (80.4%). Extrahepatic resection was performed in 36 patients (19.1%) for direct invasion of diaphragm (n=6), inferior vena cava (n=4, with right adrenal gland resection in 3), stomach (n=2) and hilar structures (n=21, e.g. biliary only, n=14; vascular only, n=2; combined biliary and vascular, n=5). Lymph node dissection or sampling was performed in 92 patients (48.7%) and lymph node involvement was found in 21 patients (11.1%). Tumor size and number were comparable across the different studies, but vascular invasion and extrahepatic organ invasion were more frequently observed in the MSKCC cohort. Thirteen patients (6.9%) received neoadjuvant treatment for initial local unresectability including systemic chemotherapy alone (n=10) and intra-arterial floxuridine (FUDR) (n=3) using a hepatic arterial infusion pump. Postoperatively, fifty-one patients (27.1%) received adjuvant chemotherapy including gemcitabine-based therapy (n=36, of which 4 receiving gemcitabine + oxaliplatine and 4 receiving gemcitabine + cisplatine), fluorouracil-based therapy (n=9) and platinum compounds only (n=1). Five patients received adjuvant intra-arterial floxuridine (FUDR) using a hepatic arterial infusion pump.
Survival Data
With the median follow up of 42.5 months (range, 5–192), we observed 98 deaths. The median OS was 47.8 months (95% CI, 37.6–68.9 months). The 1-, 3-, and 5-year OS rates were 91% (95%CI: 86%–95%), 59% (95%CI: 51%–67%) and 45% (95%CI: 37%–53%), respectively. After primary resection, median RFS was 21 months (95% CI: 11.8–30.1 months). Recurrence occurred in 110 patients (58.5%). Palliative systemic chemotherapy was offered after diagnosis of recurrence in 103 patients, combined with metastasectomy (n=12), local ablation (n=9), radiation therapy (n=15) and liver-directed therapy (hepatic artery embolization or hepatic arterial infusion, n=11).
Predictive performances
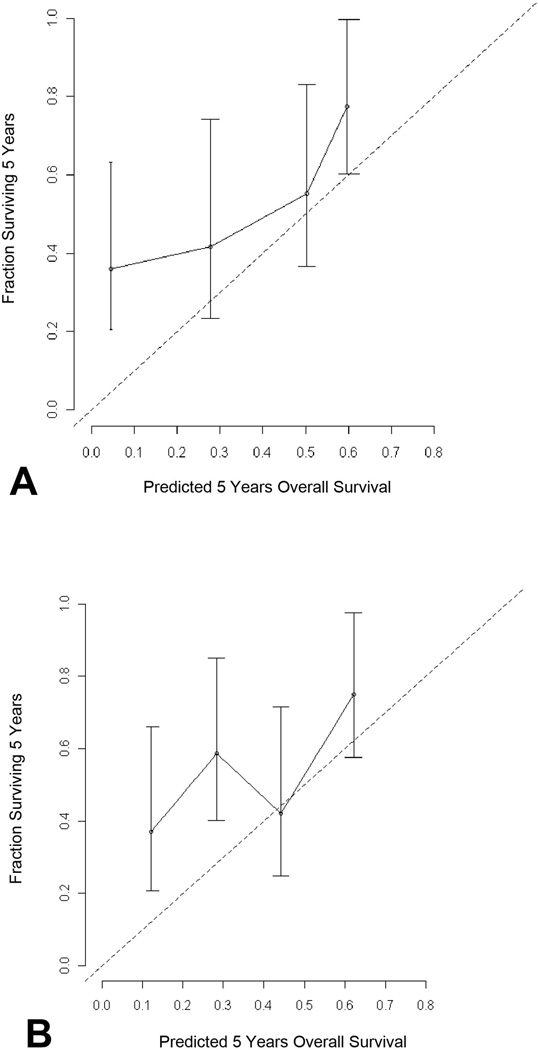
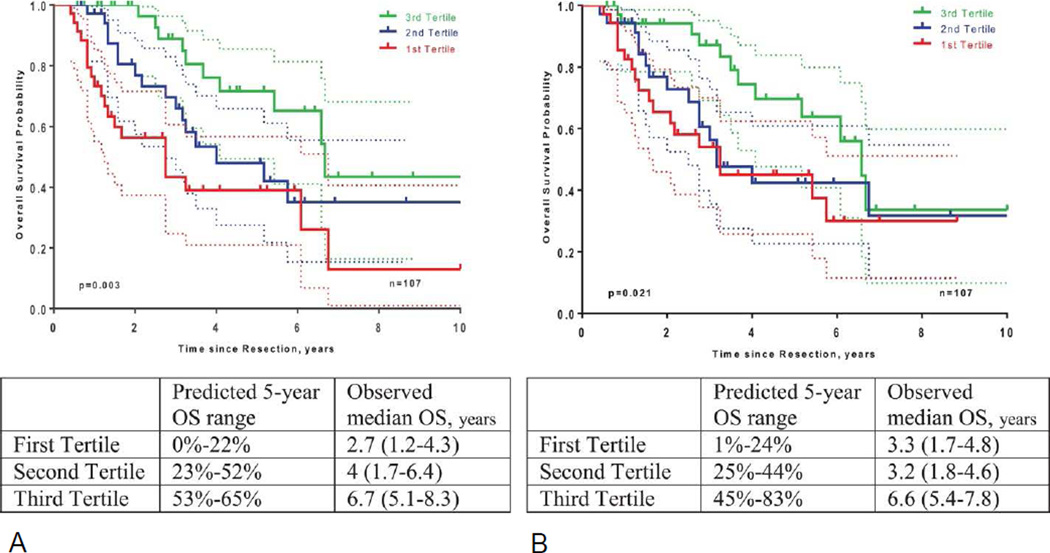
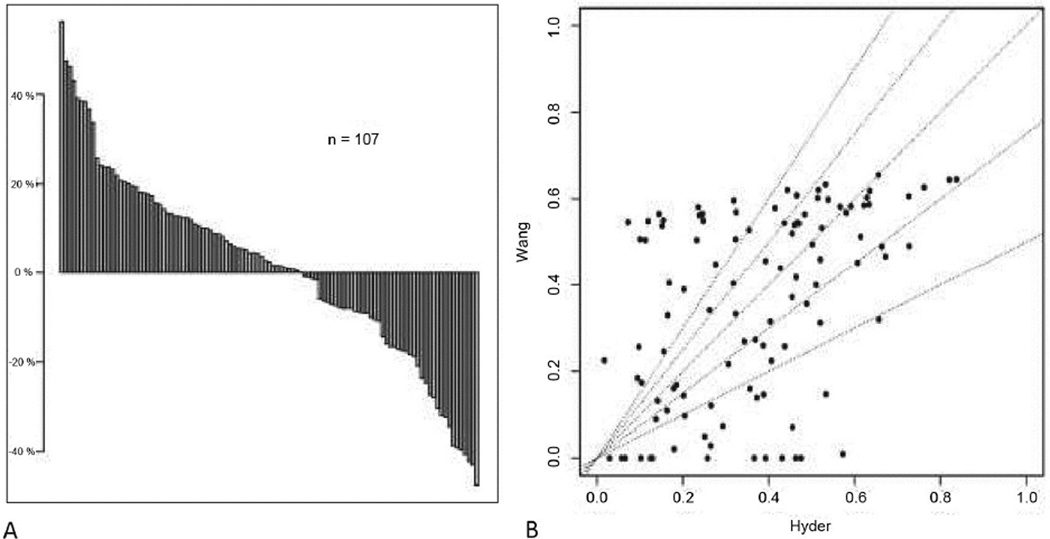
Variables required for both nomograms are summarized in Table 2. In the MSKCC cohort, 81 patients had missing values for the CEA and/or CA19-9 levels. After excluding patients with missing tumor markers levels, the C-index for the nomogram from Wang et al was 0.72 (95%CI: 0.64–0.80) in this 107 patient cohort. The nomogram from Hyder et al. had a C-index of 0.63 (95%CI: 0.57–0.69) in the whole cohort (n=188) and 0.66 (95%CI: 0.56–0.74) in the cohort used for Wang nomogram assessment (n=107). Nomogram calibration plots are displayed in Figure 1. Patient stratification using prediction tertiles was optimal with the Wang nomogram (p=0.003) and the Hyder nomogram (p=0.021) (Figure 2). Different 5-year OS prediction ranges in each tertile corresponded to distinct median OS observed in our cohort (n=107). Stratification appeared more distinct between worse risk patients (1st and 2nd tertiles) with the Wang nomogram.. Figure 3 shows how inconsistent the survival predictions from both nomograms are in some patients.
Table 2.
Variables Required for Nomograms, the Fudan Score and the AJCC 7th Edition
| Variable | Wang nomogram | Hyder nomogram | Fudan score | AJCC 7th Edition |
|---|---|---|---|---|
| Age | Continuous (transformed with cubic splines) | |||
| Alkaline Phosphatase | Binary (≤147 U/l/>147U/l) | |||
| Biomarkers (CA19-9, CEA) | Continuous (linear) | CA19-9 only Binary (≤37µg/l/>37 µg/l) | ||
| Tumor size | Continuous (linear) | Continuous (transformed with cubic splines) | Binary <10cm/≥10cm | |
| Tumor number | Ordinal variable Solitary/1–2 / ≥3 | Binary Solitary/Multiple | Binary Solitary/Multiple | Binary variable Solitary/Multiple |
| Tumor Boundary Type | Binary Distinct/Obscure | |||
| Lymphovascular invasion | Binary Yes/No | Categorical variable Micro/Macro/No | Binary variable Yes/No | |
| Direct extrahepatic invasion | Binary variable Yes/No | Binary variable Yes/No | ||
| Lymph node status | Binary variable Yes/No | Categorical variable pN0/pN1/pNx | Binary variable Yes/No | |
| Underlying cirrhosis | Binary variable Yes/No |
AJCC, American Joint Committee on Cancer; CA19-9, carcinogen antigen 19-9; CEA, carcinoembryonic antigen.
Figure 1.
Calibration plots of the (A) Wang nomogram and (B) the Hyder nomogram in 107 patients. The dashed line represents the reference line.
Figure 2.
Kaplan-Meier OS curves for patients stratified by predicted tertiles by the (A) Wang nomogram and the (B) Hyder nomogram.
Figure 3.
Differences in 5-year OS probability for each patient by Hyder and Wang nomograms. (A) The waterfall plot shows the inconsistency between both nomograms (Hyder – Wang). Patients with a difference in 5-year OS probability above 0% had better predicted 5-year OS probability by Hyder nomogram. Patients with a difference in 5-year OS probability below 0% had better predicted 5-year OS probability by Wang nomogram. This inconsistency between both nomograms is completely random, without any correlation, as shown on the (B) cloud plot
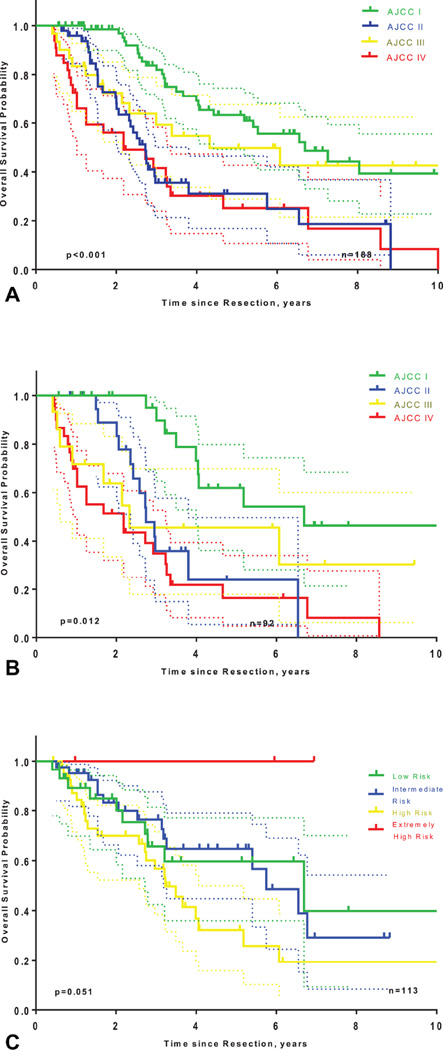
The AJCC 7th Edition staging system had a C-index of 0.63 (95%CI: 0.58–0.67). Survival in patients stratified by AJCC stages (7th Edition), is represented in Figure 2. As shown in Figure 4A, tumor stage according to the AJCC 7th Edition was a significant predictive factor for OS in the whole cohort (p<0.001), but separation of the curves was poor (median OS for stage II = 32.7 months; median OS for stage III patients = 51.9 months; median OS for stage IV = 25.2 months). Excluding patients with unknown nodal status (pNx) improved predictive accuracy (c-index= 0.68; 95%CI: 0.61–0.75). Still, patients stratification remained poor, with unclear survival differences between stage II, III, and IV patients, as displayed on Figure 4B (median OS for stage II = 32.9 months; median OS for stage III patients = 28 months; median OS for stage IV = 26.9 months).
Figure 4.
Kaplan-Meier OS curves for patients stratified according to the AJCC 7th Edition Staging System, (A) in the MSKCC cohort, (B) in only patients with pathologic lymph node status, and (C) according to the Fudan score.
One hundred thirteen patients had available data for Fudan score assessment (75 patients had missing values for the ALP and/or CA19-9 levels). Discrimination was acceptable (C-index= 0.55; 95%CI: 0.47–0.64) and prognostic stratification tended to be significant (p=0.051), but stratification with respect to the different risk score strata was not optimal (Figure 4C).
Impact of Adjuvant Therapy
Patients who received adjuvant therapy were mostly those with a predicted poor prognosis by both nomograms (Wang 1st tertile = 59.4%, p<0.001; Hyder 1st tertile = 43.8%, p=0.17) or with more advanced disease (AJCC stage III–IV=51%, p=0.001; high risk or extremely high risk Fudan score = 57.6%, p=0.001). Overall, 51 patients (27.1%) received adjuvant therapy that was a negative prognostic variable in univariate analysis (HR=5.728; p=0.017).
Discussion
This study is the first to externally validate two recently published nomograms and one risk score for outcome after resection of ICC. Both nomograms showed an optimal discrimination and calibration and provided an accurate patient stratification. In contrast, the Fudan score failed to achieve optimal discrimination and correct stratification in the current study.
Both nomograms included different variables. Notably, the Wang nomogram included the tumor markers CA 19-9 and CEA. Although those biomarkers are not considered in other models or in the AJCC staging system, they have previously been reported as important prognostic variables (21, 22). Additionally, direct extrahepatic invasion, already included in the AJCC staging system, was included in the Wang nomogram and may reflect adverse tumor biology. Conversely, the Hyder nomogram did not include either of these variables while incorporating age and underlying cirrhosis as prognostic factors. Although underlying cirrhosis has been reported as a poor prognostic factor in ICC, no studies other than the Hyder nomogram have related it to overall survival after resection of ICC (3). In our cohort, the underlying cirrhosis rate was low (4.8%), resulting in a low additive predictive capacity. Another noteworthy difference between both nomograms is related to the lymph node status. Hyder et al. coded this variable as a categorical variable (pN0, pN1 or pNx), whereas patients with missing lymph node status (Nx) were considered node-negative (N0) in the Wang nomogram. Some studies have suggested that no suspicion of nodal disease based on preoperative imaging and intraoperative assessment may act as a reasonable surrogate to routine LN dissection and reported similar outcomes in ICC patients with pN0 and pNx disease (23, 24). However, portal lymph node involvement is an independent prognostic factor in ICC and such a nodal status classification may be inappropriate, as several studies advocate a routine lymphadenectomy for accurate staging (25–27). Other variables were included in both nomograms but differently modeled. Tumor size had been removed from the T-stage in the 7th edition of the AJCC staging system but was included as a variable in both Wang and Hyder, modeled as a continuous variable in the former. Estimating the clinical meaning of continuous variables using a linear model might be inaccurate. In contrast, Hyder et al. observed in their study that the actual effect of tumor size on the risk of death was linear up to nearly 7 cm, whereas this effect leveled off over 7 cm; hence, tumor size was transformed in a non linear continuous variable using restricted cubic splines in this model. The number of liver lesions was also modeled differently for both nomograms. The multiplicity of lesions appears to represent strong evidence of unfavorable tumor biology. Wang et al. considered the number of lesions as an ordinal variable, stratifying patients as solitary tumor, multiple tumors but less than three, or three and more. Although multiplicity of lesions may represent aggressive tumor biology, such impact on overall survival regarding differential tumor number has never been reported. Similarly, vascular invasion is a poor prognostic factor and a recurrence risk factor, and differentiating microvascular and macrovascular invasion has been suggested as an important distinction in staging ICC which was only considered in the Hyder nomogram (28). Taken altogether, these differences might account for the inconsistency in 5-year OS prediction by between both nomograms (Figure 3).
Despite these discrepancies, both nomograms provided optimal discrimination and stratification when compared on the same patient population. Patients’ stratification using predicted 5 year-OS tertiles appeared slightly more accurate with the Wang nomogram (Figure 2 and Table 3). The concordance index tended to show a more accurate discrimination with the Wang nomogram (C-index = 0.72; 95% CI: 0.64–0.80) than with the Hyder nomogram (C-index = 0.66; 95% CI: 0.56–0.74). This difference was not significant statistically (p=0.17), which is not surprising, given that large sample sizes are typically required to establish statistical significance between c-indices. Accordingly, while one can not formally endorse the Wang nomogram for use in clinical practice, it is interesting to note that this prognostic model was developed in an Eastern population but fit well to our Western cohort, providing the most clear-cut patient stratification in distinct survival groups. The Wang nomogram requires both routine preoperative CA19-9 and CEA levels and routine lymph node dissection for being applicable. In contrast, the Hyder nomogram might have a broader clinical applicability by obviating routine preoperative tumor marker assessment and routine formal portal lymphadenectomy and being developed from an international cohort.
The Fudan score provided moderate but acceptable discrimination (C-index>0.5) (19). However, stratification using the four risk levels of this score was not optimal (Figure 4C). These findings are likely the result of several factors. First, tumor boundary type is a very subjective variable, resulting in significant interobserver variability. Second, preoperative evaluation of the number of tumors may be inaccurate, given that detecting small satellite nodules on preoperative imaging can be challenging (17). Additionally, serum alkaline phosphatase level was a binary variable in this score but that has never been reported as a prognostic factor in ICC. Moreover, serum alkaline phosphatase activity is increased in many pathologic conditions, associated to an increased all-cause mortality risk and reported to have different baseline levels between Eastern and Western populations (29). Thus, using this variable may limit the applicability of this score developed in an Eastern population to a Western population. Unlike the AJCC staging system and both nomograms that are pathology-based prognostic tools, the Fudan score allows a preoperative prognostic estimation. Such prognostic tools can help to identify preoperatively patients with poor prognostic and improve their management.
The AJCC staging system provided a correct discrimination (0.63 (95%CI: 0.58–0.67) but a suboptimal stratification with a worse median OS for stage II patients (32.7 months) than for stage III patients (51.9 months). AJCC staging system discrimination was improved when the staging system was applied only to the 92 patients who underwent LN dissection (C-index = 0.68; 95%CI: 0.61–0.75). Similarly, patient stratification was slightly more accurate with median OS in stage II and III of 32.9 and 28 months, respectively but curves separation remained poor (Figures 4A, 4B). Consequently, the AJCC Staging System may not be appropriate for prognostic estimations in patients without lymph node dissection. Furthermore, the lack of stratification between stages II and III may be due to the definition of pathologic T stage. Stage II encompasses pT2 tumors (T2a: solitary tumor with vascular invasion and T2b: multiple tumors with or without vascular invasion) and stage III defines tumor perforating the visceral peritoneum or directly involving the local extrahepatic structures. The clinical significance of multiple tumors (pT2b), meaning intrahepatic metastases or satellite lesions, is likely underestimated by this definition and would explain the overlapping survival curves of stage II patients (pT2b), who had inferior survival compared to stage III patients with solitary tumors, regardless of size.
Adjuvant therapy is usually recommended for patient with worrisome tumor features (positive margin, node-positive disease, vascular invasion) and its positive impact on prognosis is based on findings from randomized trials in non resected patients with advanced or metastatic biliary neoplasms and retrospective series (9, 10, 30). In the current study, adjuvant therapy was delivered to high risk patients and did not modify significantly patient stratification. Survival was worse in patients receiving adjuvant therapy in the whole cohort and in stratified patient’s subsets. This finding, however, should be considered carefully given the small proportion of patients receiving adjuvant therapy (27.1% in the whole cohort and 30.3% in the 107 patients used for nomograms validation).
The present study has several limitations. First, missing data on tumor markers considerably reduced the number of patients available for validation of the Wang et al. nomogram. Second, the validation cohort was similar to the derivation cohorts of the published nomograms regarding key prognostic factors identified in a recent meta-analysis (Figure 1) but, regarding the underlying liver disease potentially driving tumor biology, a large proportion of patients in the current study had non-alcoholic steatosis in the non-tumorous liver, much higher than previously reported in Western studies (31–33). Similarly, HBV (4.8%) and cirrhosis (4.8%) rates were lower in the current study. Additionally, in the current cohort, patients who underwent resection after neoadjuvant therapy (n=13) might represent a patient category not appropriate for these prognostic models.
In conclusion, both nomograms may be useful for patient prognosis estimation and recommendation for adjuvant therapy after liver resection for ICC. The nomogram proposed by Wang et al appeared to have the best overall prognostic accuracy in the present cohort but requires routine portal lymph node dissection and preoperative tumor markers (CEA and CA19-9), in addition to the variables necessary for the AJCC staging system, for optimal performance. By contrast, the Fudan score and AJCC staging system were of limited utility in this data set. Further investigations are needed in wider series to define the most appropriate prognostic nomogram for clinical practice.
Acknowledgments
Support: Dr Doussot was supported by research fellowship grants from the French Association of Hepatobiliary Surgery and Transplantation (ACHBT) and the Universite de Bourgogne. This study was supported in part by NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
REFERENCES
- 1.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatol. Baltim. Md. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edge SB, editor. American Joint Committee on Cancer. AJCC cancer staging manual. 7th. New York: Springer; 2010. 7th ed. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology. 2009;49:116–123. doi: 10.1002/hep.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment .J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 5.Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann. Surg. 2011;254:824–829. doi: 10.1097/SLA.0b013e318236c21d. discussion 830. [DOI] [PubMed] [Google Scholar]
- 6.Ribero D. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch. Surg. 2012;147:1107. doi: 10.1001/archsurg.2012.1962. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Li J, Xia Y, et al. Prognostic Nomogram for Intrahepatic Cholangiocarcinoma After Partial Hepatectomy. J. Clin. Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 8.Zhu AX, Knox JJ. Adjuvant Therapy for Intrahepatic Cholangiocarcinoma: The Debate Continues. The Oncologist. 2012;17:1504–1507. doi: 10.1634/theoncologist.2012-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:1934–1940. doi: 10.1200/JCO.2011.40.5381. [DOI] [PubMed] [Google Scholar]
- 10.Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. ESMO. 2014;25:391–398. doi: 10.1093/annonc/mdt540. [DOI] [PubMed] [Google Scholar]
- 11.Endo I, Gonen M, Yopp AC, et al. Intrahepatic Cholangiocarcinoma: Rising Frequency, Improved Survival, and Determinants of Outcome After Resection. Ann. Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 12.Nathan H, Aloia TA, Vauthey J-N, et al. A Proposed Staging System for Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2009;16:14–22. doi: 10.1245/s10434-008-0180-z. [DOI] [PubMed] [Google Scholar]
- 13.Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014;149:565. doi: 10.1001/jamasurg.2013.5137. [DOI] [PubMed] [Google Scholar]
- 14.Cho CS, Gonen M, Shia J, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J. Am. Coll. Surg. 2008;206:281–291. doi: 10.1016/j.jamcollsurg.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Jiang W, Zeng Z-C, Tang Z-Y, et al. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann. Oncol. 2011;22:1644–1652. doi: 10.1093/annonc/mdq650. [DOI] [PubMed] [Google Scholar]
- 16.Hyder O, Marques H, Pulitano C, et al. A Nomogram to Predict Long-term Survival After Resection for Intrahepatic Cholangiocarcinoma: An Eastern and Western Experience. JAMA Surg. 2014;149:432. doi: 10.1001/jamasurg.2013.5168. [DOI] [PubMed] [Google Scholar]
- 17.Okabayashi T, Yamamoto J, Kosuge T, et al. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer. 2001;92:2374–2383. doi: 10.1002/1097-0142(20011101)92:9<2374::aid-cncr1585>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J. Hepatobiliary. Pancreat. Surg. 2003;10:288–291. doi: 10.1007/s00534-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA J. Am. Med. Assoc. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 20.Efron B. Monographs on statistics and applied probability. New York: Chapman & Hall; 1993. An introduction to the bootstrap. [Google Scholar]
- 21.Hatzaras I, Schmidt C, Muscarella P, et al. Elevated CA 19-9 portends poor prognosis in patients undergoing resection of biliary malignancies. HPB. 2010;12:134–138. doi: 10.1111/j.1477-2574.2009.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho SY, Park S-J, Kim SH, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann. Surg. Oncol. 2010;17:1823–1830. doi: 10.1245/s10434-010-0938-y. [DOI] [PubMed] [Google Scholar]
- 23.Choi S-B, Kim K-S, Choi J-Y, et al. The Prognosis and Survival Outcome of Intrahepatic Cholangiocarcinoma Following Surgical Resection: Association of Lymph Node Metastasis and Lymph Node Dissection with Survival. Ann. Surg. Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 24.Grobmyer SR, Wang L, Gonen M, et al. Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann. Surg. 2006;244:260–264. doi: 10.1097/01.sla.0000217606.59625.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farges O, Fuks D, Le Treut Y-P, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer. 2011;117:2170–2177. doi: 10.1002/cncr.25712. [DOI] [PubMed] [Google Scholar]
- 26.Li D-Y. Routine lymph node dissection may be not suitable for all intrahepatic cholangiocarcinoma patients: Results of a monocentric series. World J. Gastroenterol. 2013;19:9084. doi: 10.3748/wjg.v19.i47.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maithel SK, Gamblin TC, Kamel I, et al. Multidisciplinary approaches to intrahepatic cholangiocarcinoma: Intrahepatic Cholangiocarcinoma. Cancer. 2013;119:3929–3942. doi: 10.1002/cncr.28312. [DOI] [PubMed] [Google Scholar]
- 28.Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153:811–818. doi: 10.1016/j.surg.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunutsor SK, Apekey TA, Seddoh D, Walley J. Liver enzymes and risk of all-cause mortality in general populations: a systematic review and meta-analysis. Int. J. Epidemiol. 2014;43:187–201. doi: 10.1093/ije/dyt192. [DOI] [PubMed] [Google Scholar]
- 30.Sur MD, In H, Sharpe SM, et al. Defining the Benefit of Adjuvant Therapy Following Resection for Intrahepatic Cholangiocarcinoma. [Internet] [Accessed February 15, 2015];Ann. Surg. Oncol. [Internet] 2014 doi: 10.1245/s10434-014-4275-4. Available at: http://link.springer.com/10.1245/s10434-014-4275-4. [DOI] [PubMed] [Google Scholar]
- 31.Nkontchou G, Tran Van Nhieu J, Ziol M, et al. Peripheral intrahepatic cholangiocarcinoma occurring in patients without cirrhosis or chronic bile duct diseases: epidemiology and histopathology of distant nontumoral liver in 57 White patients. Eur. J. Gastroenterol. Hepatol. 2013;25:94–98. doi: 10.1097/MEG.0b013e328357cdd7. [DOI] [PubMed] [Google Scholar]
- 32.Reddy SK, Hyder O, Marsh JW, et al. Prevalence of Nonalcoholic Steatohepatitis Among Patients with Resectable Intrahepatic Cholangiocarcinoma. J. Gastrointest. Surg. 2013;17:748–755. doi: 10.1007/s11605-013-2149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexander J, Torbenson M, Wu T-T, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J. Gastroenterol. Hepatol. 2013;28:848–854. doi: 10.1111/jgh.12116. [DOI] [PubMed] [Google Scholar]