Abstract
Objectives:
Sarcopenia increases falls and fracture risk. Sarcopenia clinical trials require robust quantitative tools to evaluate muscle function; jumping mechanography (JM) is likely one such tool. However, US data comparing JM with traditional tests across the lifespan is limited. This study evaluated the effect of age and sex on JM compared with traditional function tests and lean mass.
Methods:
US adults (213 women/119 men; mean age 65.4 years, range 27-96) performed functional tests including JM, Short Physical Performance Battery (SPPB) and grip strength (GS). Appendicular lean mass (ALM) was measured using DXA.
Results:
Men had higher relative jump power [mean (SD) 28.5 (10.52) vs. 21.9 (7.11) W/kg], GS [35.5 (9.84) vs. 22.7 (6.98) kg] and ALM/ht2 [8.25 (1.35) vs. 6.99 (1.38) kg/m2] (all p<0.0001); no difference was observed for SPPB components. JM parameters were more strongly correlated with age than traditional tests (R2=0.38-0.61 vs. R2=0.01-0.28) and weakly with GS and chair rise time (R2=0.30-0.36).
Conclusion:
JM parameters are correlated with GS and chair rise time and demonstrate stronger correlations with age. JM shows promise as a valuable tool to evaluate and monitor interventions for sarcopenia as it could potentially detect change in muscle function more precisely than existing tools.
Keywords: Aging, Skeletal Muscle, Sarcopenia, Jumping Mechanography, Muscle Function Tests
Introduction
Sarcopenia, the age-related decline in muscle mass and function[1] is common and is associated with increased risk for falls, hip fractures, decreased mobility and reduced quality of life[2,3]. Poor performance in physical function tests, such as gait speed and chair rise, predict increased risk for falls and fracture[4-6]. As muscle function predicts disability and mortality more strongly than muscle mass[7-9], recent consensus sarcopenia definitions include both mass and function measurement[10-12]. However, traditional methodologies to assess muscular function have important limitations[13,14]. These limitations include yes/no determinations, timing variability resulting from tester subjectivity, requiring some level of disability to be considered abnormal, or inability to be performed by those with substantial disability (e.g. SPPB balance, gait speed, chair rise test)[13]. Due to these limitations, there is growing interest in alternative methods of measuring muscle function such as jumping mechanography[13-15].
Jumping mechanography (JM) uses countermovement jumps performed on a force platform to assess jumping power and height. JM is reproducible and safe in older adults[13-19] and can differentiate sarcopenic from non-sarcopenic individuals when measures of leg strength cannot[20]. Furthermore, leg power has been shown to decline at an earlier age than leg strength[21,22]. Lastly, limited data find JM to be well correlated with traditional muscle and physical function tests[14,17]. JM is a physiologically complex task that allows for a high level of intensity, thus combining two desirable characteristics of muscle function tests[23]. Despite this, the countermovement jump is a natural movement that does not necessitate extensive training and has no significant learning effect[17]. JM does not require any external weights or loading, enabling the participant to determine maximum effort, thus potentially reducing injury risk. Importantly, for those with substantial impairment, this computerized method can detect even small displacements of the center of gravity in people as long as they can they can lift-off from the force plate. Based on these potential advantages, JM may prove more useful than traditional tests to quantitatively measure muscular function in older adults.
However, current JM data have limitations: while data exist for European countries[16-19,24] and Japan[25,26], none exists for the US. We, and others, have published data on small groups of middle-aged and older US adults[14,15,20]. However, a cohort that covers the entire adult lifespan and is large enough to be able to compare differences between men and women is needed. A cohort consisting of US community-dwelling adults may prove useful for comparison for future studies. Furthermore, available studies comparing JM parameters to other muscle and physical function tests are weakened by small study size, limited age range and small number of muscle/physical function tests and muscle mass assessments included[13,14,17,24,25]. Lastly, sex differences among muscle/physical function tests are not always well defined[2,13,14,24,27]. As such, additional data are needed to fill these knowledge gaps.
The primary purposes of this study were to evaluate the effect of age and sex on JM parameters, and compare these data with traditional muscle and physical function tests in a US cohort. We hypothesized that both JM parameters and traditional tests are lower in older adults, lower in women, and that JM parameters will correlate with muscle and physical function tests.
Methods
Subjects
This study consisted of four cohorts, one at the University of Pittsburgh, the other three at the University of Wisconsin-Madison, all of which performed jumping mechanography on the Leonardo force platform (Novotec, Germany) and the same functional tests. All participants were community-dwelling adults above age 25; each study had slightly different inclusion/exclusion criteria as noted below. All studies were approved by the institutional review board at their respective university.
University of Pittsburgh – Developmental Epidemiologic Cohort Study (DECOS)
Sixty-eight community dwelling men and women age 70+ were recruited from the Pittsburgh area using the Pittsburgh Claude D. Pepper Older Americans Independence Center Research Registry for a study designed to assess ways to measure physical activity, fatigue and muscle power[28]. Volunteers were excluded for serious illness, recent surgery, recent cardiovascular events, cognitive impairment, and inability to walk across a room without severe pain.
University of Wisconsin – Jumping reproducibility study
This study evaluated jumping mechanography reproducibility in community dwelling men and women age 70+[13,14]. Volunteers were excluded if they were unable to stand without assistance, had recent surgeries impacting their ambulation, recent cardiovascular events or serious illness, or a bone mineral density (BMD) T-score below -3.5 plus a prior fragility fracture.
University of Wisconsin – MIDUS
MIDUS is the national survey of Midlife in the United States; it was designed to study health and well being in mid-life[29]. Participants were identified through a nationally representative random-digit-dial sample of non-institutionalized, English-speaking adults, aged 25-74, in the coterminous United States. This analysis uses a sub-sample of individuals from the MIDUS Refresher cohort who participated in biomarker data collection at the University of Wisconsin.
University of Wisconsin – Vitamin D study
Participants in this study were postmenopausal Caucasian women, ages 47-83 years, with low serum 25(OH)D levels. Volunteers were excluded from the study if they had 25(OH)D levels >30 ng/mL, serious illness, or recent cancer.
Sarcopenia definitions
Currently there are several proposed consensus definitions for sarcopenia. We elected to use three of these to analyze this population. The three definitions vary by factors included and cut-offs used as noted below.
FNIH definition
The Foundation of the NIH proposed sarcopenia definition includes ALM corrected for BMI and grip strength. The cut-off for ALM/BMI for males is <0.789 and for females is <0.512. The cut-off for grip strength for males is <26 kg and for females is <16 kg[11].
European definition
The European working group proposed definition includes grip strength, gait speed, and height-corrected ALM. The cut-off for grip strength for males is <30 kg and for females is <20 kg. The cut-off for gait speed is ≤0.8 m/s. The cut-off for ALM/ht2 for males is <7.26 kg/m2 and females is <5.45 kg/m2 [10].
International definition
The international working group proposed definition uses gait speed and height-corrected ALM. The cut-off for gait speed is <1.0 m/s. The cut-off for ALM/ht2 for males is ≤7.23 kg/m2 and for females is ≤5.67 kg/m2 [12].
Muscle and physical function tests
Jumping mechanography
Jumping mechanography has been described in detail elsewhere[15]. In brief, participants perform two-leg maximal countermovement jumps on a force plate (Leonardo, Novotec, Pforzheim, Germany). Maximal jump height [m], relative power [W/kg], and velocity [m/s] were calculated using Leonardo software version 4.2. The jump with maximal height was chosen for analysis and those trials without a jump height were excluded.
Muscle function test
Grip strength was the only test that strictly measured maximal muscle force and was assessed using a Jamar handheld dynamometer according to an established protocol[30]. Both hands were measured three times each and the maximal grip of all measurements was used for analyses.
Physical function tests
Physical function assessment consisted of the Short Physical Performance Battery (SPPB) components. The SPPB was completed using standard methodology and includes a balance assessment, gait speed, and timed chair rise[31,32]; the balance component was not collected in the MIDUS cohort, and as such, the total SPPB score could not be calculated.
Lean mass assessment
Lean mass was measured using total body dual-energy x-ray absorptiometry (DXA) (GE Healthcare iDXA, Madison, WI, software version 13.6; Hologic, QDR 4500A, Bedford, MA). The appendicular lean mass (ALM) to height ratio (ALM/height2 in kg/m2) was calculated in all cohorts.
Statistics
Statistical analyses were performed using JMP software (SAS Cary, NC). Tukey-Kramer HSD analyses were performed to explore differences between the cohorts. Linear regression analyses, multivariate regressions and T-tests were utilized to compare performance of men versus women as well as performance in relation to age. Differences in relative jump power between sarcopenic and non-sarcopenic individuals were examined using T-tests. As the Total SPPB score is not a continuous variable and the distribution of this variable was not normal in our cohort, an ordinal logistic regression model was used to assess the relationship to age and other muscle function tests. To be able to present these results in a similar fashion as the results from the linear regression we decided to use “pseudo-R2” values, which are also known as Nagelkerke R2 or Craig and Uhler R2. Although these values have limitations and cannot be directly compared to R2-values from linear regression we felt it was the best option available.
Correlation coefficients and their confidence intervals of the various muscle/physical function tests with age were used to examine whether correlations significantly differed from each other. Linear regression analyses assessed correlations between muscle and physical function tests and lean mass in the entire cohort and those over 65 years of age. Least square multivariate regressions, which included demographic (e.g. age, sex, BMI) and muscle/physical function tests, were used to examine which factors independently are associated with relative jump power.
Results
Study participants
The analyses include 332 participants, 213 females and 119 males. Their mean age was 65.4±17.4 years (range 27-96) and average BMI was 28.2±6.6 kg/m2 (range 13-59). For details regarding the differences between cohorts, see [Supplemental Table 1].
Supplemental Table 1.
Demographics by cohort.
| Age (years) | BMI (kg/m2) | ALM/Ht2 (kg/m2) | Grip (kg) | Gait (m/s) | Chair Rise (s) | Jump Height (m) | Relative Power (W/kg) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | ||
| University of Pittsburgh | n = 62 | 78.4$≠ (5.61) | 26.7$ (4.04) | 6.94$ (1.13) | 24.8$ (9.08) | 1.16 (0.192) | 12.2$≠ (2.99) | 0.18$ (0.079) | 20.1$ (6.22) | ||||||||
| (F) = 36 (M) = 26 | 78.6 (4.81) | 78.0 (6.65) | 26.6 (4.67) | 27.0 (3.05) | 6.35* (0.955) | 7.72 (0.839) | 20.1* (7.07) | 31.4 (7.37) | 1.10* (0.163) | 1.23 (3.01) | 12.6 (2.95) | 11.6 (3.02) | 0.16* (0.063) | 0.22 (0.084) | 17.6* (4.77) | 23.5 (6.42) | |
| MIDUS | n = 122 | 46.1+#≠ (10.23) | 31.0+# (8.16) | 8.35+#≠ (1.63) | 31.6+#≠ (11.44) | 1.17# (0.231) | 8.85+#≠ (3.35) | 0.29+#≠ (0.100) | 31.1+#≠ (9.89) | ||||||||
| (F) = 77 (M) = 45 | 46.0 (10.34) | 46.1 (10.14) | 31.7 (8.55) | 29.7 (7.36) | 7.91* (1.52) | 9.08 (1.57) | 25.9* (7.36) | 41.2 (10.74) | 1.17 (0.234) | 1.18 (0.226) | 8.99 (2.98) | 8.62 (3.92) | 0.24* (0.074) | 0.36 (0.096) | 27.0* (7.16) | 38.1 (9.99) | |
| UW Jumping Validation | n = 96 | 80.7$≠ (5.87) | 25.5$≠ (4.11) | 6.97$ (1.15) | 25.3$ (9.43) | 1.09 (0.204) | 13.7$≠ (4.25) | 0.18$ (0.056) | 19.8$ (4.88) | ||||||||
| (F) = 48 (M) = 48 | 80.1 (5.45) | 81.3 (6.27) | 25.0 (4.25) | 26.1 (3.93) | 6.19* (0.806) | 7.75 (0.894) | 18.3* (4.78) | 32.4 (7.41) | 1.10 (0.212) | 1.09 (0.198) | 14.0 (4.08) | 13.5 (4.44) | 0.15* (0.046) | 0.20 (0.056) | 17.5* (4.23) | 22.2 (4.37) | |
| UW Vitamin D | n(F) = 52 | 67.0+$# (8.32) | 28.5# (6.35) | 6.79$ (0.983) | 23.7$ (5.11) | 1.20# (0.183) | 11.7+$# (2.95) | 0.19$ (0.55) | 21.6$ (5.59) | ||||||||
Different from Pittsburgh, p<0.05;
Different from MIDUS, p<0.05;
Different from Jumping Validation, p<0.05;
Different from Vitamin D, p<0.05
Different from Male, p<0.05
Sex differences
Men had higher jump height, relative jump power, and grip strength compared to women (p<0.0001). No gender differences were present in gait speed, timed chair rise and total SPPB score (see Table 1).
Table 1.
Total demographics by sex.
| Entire Cohort | Females (213) | Males (119) | Females vs. Males p-value | |
|---|---|---|---|---|
| Age (years) | 65.4 (17.38) | 64.4 (16.73) | 67.3 (18.42) | 0.1522 |
| Weight (kg) | 78.8 (20.53) | 75.8 (20.27) | 84.3 (19.95) | 0.0003 |
| Height (cm) | 166.8 (8.45) | 162.7 (6.14) | 174.2 (6.86) | <0.0001 |
| BMI (kg/m2) | 28.2 (6.61) | 28.6 (7.12) | 27.7 (5.57) | 0.1988 |
| Grip Strength (kg) | 27.3 (10.2) | 22.7 (6.98) | 35.5 (9.84) | <0.0001 |
| Repeated Chair Rise Time (s) | 11.3 (4.04) | 11.4 (3.78) | 11.2 (4.50) | 0.7763 |
| Gait Speed (m/s) | 1.15 (0.21) | 1.15 (0.21) | 1.15 (0.22) | 0.8698 |
| Total SPPB | 10.5 (1.52) | 10.6 (1.48) | 10.4 (1.59) | 0.3717 |
| ALM/ht2 (kg/m2) | 7.44 (1.49) | 6.99 (1.38) | 8.25 (1.35) | <0.0001 |
| Jump Height (m) | 0.22 (0.09) | 0.20 (0.07) | 0.27 (0.11) | <0.0001 |
| Relative Power (W/kg) | 24.3 (9.04) | 21.9 (7.11) | 28.5 (10.52) | <0.0001 |
| Velocity (m/s) | 1.61 (0.42) | 1.49 (0.36) | 1.81 (0.44) | <0.0001 |
Data shown as mean (standard deviation).
Correlations with age, muscle, physical function tests and lean mass
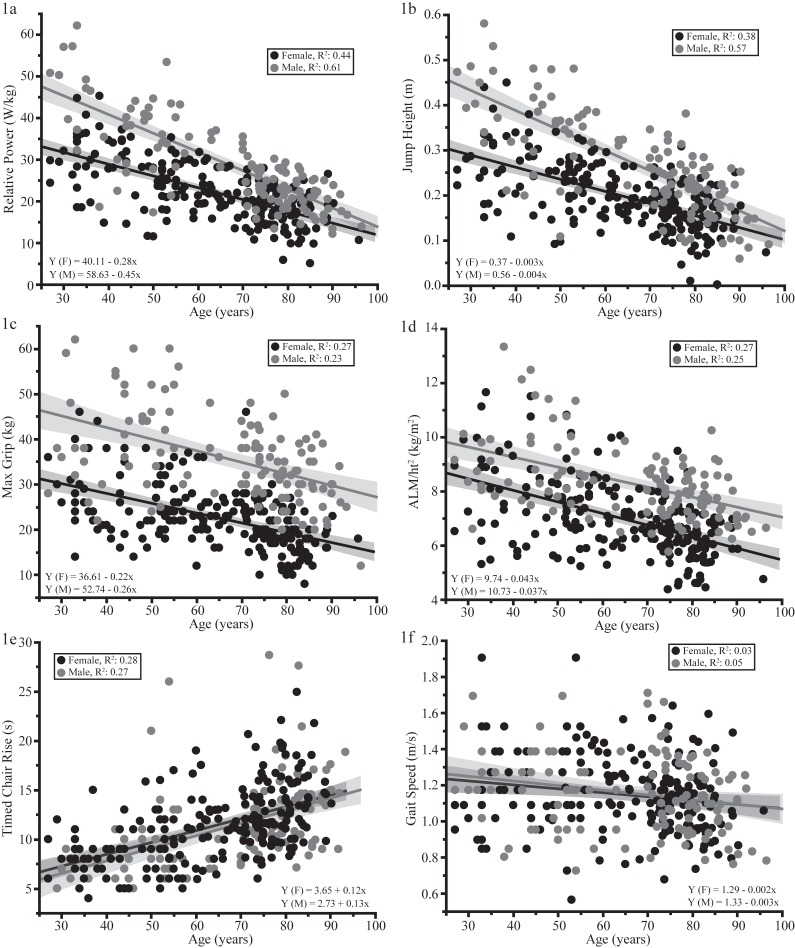
Age was negatively associated with performance in all muscle and physical function tests and ALM/height2 (Figure 1 a-f) irrespective of sex (all p-values ≤0.0005). Jumping mechanography parameters had the numerically highest R2 values with age ranging from 0.38 to 0.61. Gait speed had the numerically lowest relationship with age (R2=0.04) while chair rise time, grip strength, and ALM/height2 were weak (R2 values 0.12-0.27). Total SPPB score was also only weakly correlated with age using a logistic regression model (R2=0.14). Analysis of the correlation coefficients showed that the confidence intervals of jumping parameters and timed chair rise were higher and did not overlap with those of grip strength or gait speed. Additionally, grip strength confidence intervals were higher than and did not overlap with those of gait speed (data not shown).
Figure 1.
(a-f). Linear regression analysis of age and various JM parameters, traditional muscle and physical function tests, and ALM/ht2. For both males (gray) and females (black) JM parameters had the highest R2 values (Figure 1a-b). Gait speed had the lowest values, whereas grip strength, chair rise time, and ALM/ht2 R2 values fell in-between (Figure c-f). JM, grip strength and ALM/ht2 parameters had sex differences; males had higher values compared to females. This difference was not seen for chair rise time or gait speed.
Correlations among muscle/physical function tests and lean mass are presented in [Table 2]. As expected, jumping mechanography parameters in the whole group were closely correlated with each other (R2=0.87-0.94). Additionally, jump power and velocity were well correlated with grip strength and jump power with ALM/ht2. The relationship of JM parameters with gait speed and total SPPB score was generally weak. The multivariate regression analysis showed that relative jump power was independently associated with age, BMI, ALM, jump velocity, jump force, and timed chair rise. Traditional tests were weakly correlated with each other, and had a minimal or no significant association with ALM/ht2. Similar relationships were observed when the cohort was limited to those ≥65 years old (data not shown).
Table 2.
Muscle and physical function correlations.
| Jump Relative Power (W/kg) | Velocity (m/s) | ALM/ht2 (kg/m2) | Grip Strength (kg) | Chair Rise Time (s) | Gait Speed (m/s) | Total SPPB | |
|---|---|---|---|---|---|---|---|
| Jump Height (m) | 0.87 | 0.91 | 0.14 | 0.32 | 0.30 | 0.10 | 0.27 |
| Jump Relative Power (W/kg) | 0.94 | 0.14 | 0.32 | 0.33 | 0.10 | 0.27 | |
| Jump Velocity (m/s) | 0.14 | 0.34 | 0.36 | 0.12 | 0.28 | ||
| ALM/ht2 (kg/m2) | 0.25 | 0.05 | NS | 0.01 | |||
| R2≤0.31 | Grip Strength (kg) | 0.10 | 0.08 | 0.08 | |||
| 0.32≤R2≤0.66 | Repeated Chair Rise Time (s) | 0.13 | 0.67 | ||||
| R2>0.66 | Gait Speed (m/s) | 0.26 | |||||
Sarcopenia prevalence and relationship to relative jump power
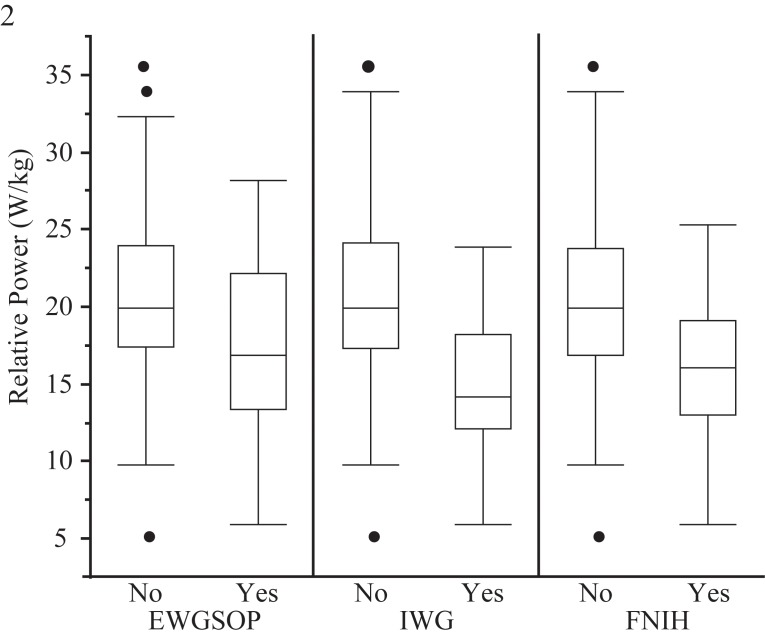
To further characterize our study population, we determined sarcopenia prevalence using three commonly accepted definitions, those of the Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project (FNIH)[11], the European working group on sarcopenia in older people (EWGSOP)[10], and the International Working Group on Sarcopenia (IWG)[12]. Prevalence varied by definition; 15.5% (males 20.3%, females 12.4%) met the EWGSOP definition, while prevalence was numerically lower using IWG (combined sexes 7.4%, males 9.3%, females 6.1%) and FNIH definitions (combined sexes 8.5%, males 10.8%, females 7.0%). The comparison of relative jump power between those with and without sarcopenia showed significantly lower jump power in those with sarcopenia regardless of sarcopenia definition applied (Figure 2, all p<0.05). Individuals without sarcopenia had an average relative jump power between 20.3-20.4 W/kg. The difference in relative jump power between sarcopenic and non-sarcopenic participants ranged from 16.1% for EWGSOP, and 23.5% for FNIH, to 31.5% for IWG (Figure 2). Values for sarcopenic individuals (regardless of definition) were also significantly lower for jump height, velocity and force but not relative force (data not shown).
Figure 2.
Differences in relative jump power between sarcopenic and non-sarcopenic individuals. The figure illustrates that relative jump power was lower in sarcopenic individuals irrespective of which sarcopenia definition was used.
Discussion
This is the largest US group in which jumping mechanography data are reported. The main findings in our cohort were that jumping mechanography parameters were more highly correlated with age than were traditional muscle/physical function tests and DXA-measured lean mass. Furthermore, a sex difference exists for muscle function tests such as grip strength and jumping mechanography but not for physical function tests such as gait speed. Lastly, jumping mechanography parameters were more highly correlated with grip strength and DXA measured lean mass than traditional physical function tests.
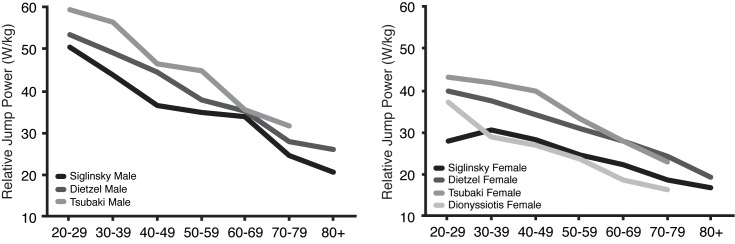
The observation that jumping mechanography was more highly correlated with age than other tests was also reported in another study that included JM and other muscle function tests[27]. The relationship of relative jump power with age in this analysis was similar to other data from Europe and Japan, although no direct statistical comparison was possible (Supplemental Figure)[16,24,26]. Values in this cohort were numerically lower compared to most of the other groups, which is likely due to inclusion and exclusion criteria of the different studies. For example, Dietzel and colleagues excluded individuals with joint replacements and/or walking aids[24]. The criteria that Tsubaki and others used were even more stringent; the authors excluded individuals with any impairment of activities of daily living, those who were unable to walk 800m unaided, those who were unable to climb a standard staircase, and those with a chair rise time above 10 seconds[26]. Furthermore, we had a sarcopenia prevalence of 7.4% to 15.5% depending which definition was applied. As such, it is not surprising that relative jump power in our cohort was numerically lower because our study represented a larger portion of community-dwelling adults by using less restrictive criteria. Our data might prove useful for future studies that examine individuals with muscle/physical function impairments, including those with sarcopenia. This is highlighted by the fact that we demonstrated that jumping mechanography parameters can distinguish between sarcopenic and non-sarcopenic individuals regardless of which definition is applied.
Supplement Figure 1.
Relative power by decade compared to past publication. The relationship of relative jump power and age in this cohort was essentially identical to other cohorts.
As expected, men had greater muscle function (e.g. grip strength, JM) than women, however, no sex difference was observed in physical function tests (e.g. gait speed and chair rise time). There is conflicting data regarding sex differences and physical function tests[32-34]. Despite potential sex differences, the SPPB uses the same cutoffs regardless of sex[32]. Additionally, older adults had lower muscle/physical function values than younger individuals; these age differences were more prominent for JM parameters than gait speed. Gait speed had the poorest relationship with age, a somewhat unexpected finding as many prospective studies demonstrate that gait speed predicts adverse health outcomes in older adults including falls, fractures and death[5,35,36].
To our knowledge, only one other group has compared jumping mechanography parameters with traditional function assessments[17]. The authors reported higher associations than those found in this cohort. This might be due to fact that Rittweger and colleagues recruited 26 very healthy individuals. As such, it is possible that there was less variation in performance among participants. Our data suggest that jumping mechanography parameters are better correlated with measures of maximal force (i.e. grip strength) and muscle mass than are traditional physical function tests. This likely reflects the fact that jumping mechanography and grip strength require greater intensity and muscle mass correlates better with maximal force/power than with velocity which many is assessed by many traditional functional tests[23].
The large study size allowed examination of relationships between parameters in the entire cohort and also among only older adults. In those age 65+ (similar to observations across the lifespan) jumping mechanography parameters were best correlated with age. As such, it is possible that jumping mechanography may be superior to traditional tests at quantifying muscular function in studies of older adults because of its higher correlation with age and good correlation with established function tests and DXA measured lean mass. This could potentially lead to better validity when diagnosing low muscle function and greater sensitivity to change when monitoring sarcopenia interventions.
The correlation between usual gait speed and age in our study is lower than reported by others[37]. Evaluating the individuals included in this analysis to find a possible explanation for this difference, it became apparent that our young individuals had lower, and our older individuals had higher, gait speed values than reported elsewhere[38,39]. This led to a linear regression curve having less decrement with age. Additionally, the relative jump power values in our young cohort were lower overall but had comparable R2 values and slopes to other convenience samples reported in the literature (Supplemental Figure)[16,24,26]. This could potentially reflect a shift muscle/physical function of younger US individuals as many sources note greater obesity and lower activity than in prior generations[40] or may be the characteristics of our specific sample. Population based studies are needed to further define the effect of age on JM parameters.
Study strengths include large sample size and variety of muscle and physical function assessments performed in addition to ALM/ht2. However, this study has limitations. The young end of this cohort is small, which could introduce bias. In addition, our older population of community dwelling individuals had remarkably few medical conditions, which may not be indicative of the general population. Additionally, is important to note that ALM was calculated differently between the Pittsburgh and Wisconsin groups. Although GE Healthcare and Hologic densitometers generate similar mass measurement relative to each other, the actual reported mass in units are higher on GE units, which will impact sarcopenia categorization[41-43]. Many of the regression analyses were statistically significant, but R2 values were often only modest. Additional weaknesses include cross-sectional design; longitudinal data are needed to validate the observations reported here; and lack of other potential evaluations such as: 6-minute walk, fastest walking speed, stair climb, timed up and go, and dynamometer-tested lower extremity strength.
In conclusion, jumping mechanography parameters are more closely correlated with age than traditional muscle/physical function tests. This study highlights jumping mechanography as a promising tool for assessing muscle function and sarcopenia due to its potential ability to detect change in function over traditional tests. However, further research is needed to examine which combination of tests should be used in the clinical and research evaluation of sarcopenia, and whether JM is associated with geriatric outcomes such as falls and fractures.
Acknowledgements
The MIDUS research is supported by a grant from the National Institute on Aging (P01-AG020166). The research was further supported by the General Clinical Research Centers Program and UL1TR000427 (UW) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health”. Jump Validation and Vitamin D studies at the University of Wisconsin sponsored by an investigator initiated research grant from Merck & Co., Inc.
Footnotes
Edited by: F. Rauch
References
- 1.Cruz-Jentoft AJ, Landi F. Sarcopenia. Clin Med (Northfield Il) 2014;14:183–6. doi: 10.7861/clinmedicine.14-2-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper C, Dere W, Evans W, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012;23:1839–48. doi: 10.1007/s00198-012-1913-1. [DOI] [PubMed] [Google Scholar]
- 3.Sayer AA, Robinson SM, Patel HP, et al. New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing. 2013;42:145–50. doi: 10.1093/ageing/afs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 5.Cawthon PM, Fullman RL, Marshall L, et al. Physical performance and risk of hip fractures in older men. J Bone Miner Res. 2008;23:1037–44. doi: 10.1359/JBMR.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward RE, Leveille SG, Beauchamp MK, et al. Functional performance as a predictor of injurious falls in older adults. J Am Geriatr Soc. 2015;63:315–20. doi: 10.1111/jgs.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitations and physical disability: The Concord health and ageing in men project. J Am Geriatr Soc. 2010;58:2055–62. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 8.Cawthon PM, Fox KM, Gandra SR, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–9. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. The journals of gerontology Series A, Biological sciences and medical sciences. 2006;61:72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Studenski SA, Peters KW, Alley DE, et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69:547–58. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buehring B, Hind J, Fidler E, et al. Tongue strength is associated with jumping mechanography performance and handgrip strength but not with classic functional tests in older adults. J Am Geriatr Soc. 2013;61:418–22. doi: 10.1111/jgs.12124. [DOI] [PubMed] [Google Scholar]
- 14.Buehring B, Krueger D, Fidler E, et al. Reproducibility of jumping mechanography and traditional measures of physical and muscle function in older adults. Osteoporos Int. 2015;26:819–25. doi: 10.1007/s00198-014-2983-z. [DOI] [PubMed] [Google Scholar]
- 15.Buehring B, Krueger D, Binkley N. Jumping mechanography: a potential tool for sarcopenia evaluation in older individuals. J Clin Densitom. 2010;13:283–91. doi: 10.1016/j.jocd.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Dionyssiotis Y, Galanos A, Michas G, et al. Assessment of musculoskeletal system in women with jumping mechanography. International journal of women’s health. 2010;1:113–8. doi: 10.2147/ijwh.s5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rittweger J, Schiessl H, Felsenberg D, Runge M. Reproducibility of the jumping mechanography as a test of mechanical power output in physically competent adult and elderly subjects. J Am Geriatr Soc. 2004;52:128–31. doi: 10.1111/j.1532-5415.2004.52022.x. [DOI] [PubMed] [Google Scholar]
- 18.Caserotti P, Aagaard P, Larsen JB, Puggaard L. Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports. 2008;18:773–82. doi: 10.1111/j.1600-0838.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 19.Caserotti P, Aagaard P, Simonsen EB, Puggaard L. Contraction-specific differences in maximal muscle power during stretch-shortening cycle movements in elderly males and females. Eur J Appl Physiol. 2001;84:206–12. doi: 10.1007/s004210170006. [DOI] [PubMed] [Google Scholar]
- 20.Singh H, Kim D, Kim E, et al. Jump test performance and sarcopenia status in men and women, 55 to 75 years of age. J Geriatr Phys Ther. 2014;37:76–82. doi: 10.1519/JPT.0b013e3182a51b11. [DOI] [PubMed] [Google Scholar]
- 21.Strollo SE, Caserotti P, Ward RE, et al. A review of the relationship between leg power and selected chronic disease in older adults. J Nutr Health Aging. 2015;19:240–8. doi: 10.1007/s12603-014-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojanen T, Rauhala T, Hakkinen K. Strength and power profiles of the lower and upper extremities in master throwers at different ages. J Strength Cond Res. 2007;21:216–22. doi: 10.1519/00124278-200702000-00039. [DOI] [PubMed] [Google Scholar]
- 23.Edwards MH, Buehring B. Novel Approaches to the Diagnosis of Sarcopenia. J Clin Densitom. 2015;18:472–7. doi: 10.1016/j.jocd.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Dietzel R, Gast U, Heine T, et al. Cross-sectional assessment of neuromuscular function using mechanography in women and men aged 20-85 years. J Musculoskelet Neuronal Interact. 2013;13:312–9. [PubMed] [Google Scholar]
- 25.Tsubaki A, Kubo M, Kobayashi R, et al. Age-Related Changes in Physical Function in Community-Dwelling People Aged 50-79 Years. Journal of Physical Therapy Science. 2010;22:23–7. [Google Scholar]
- 26.Tsubaki A, Kubo M, Kobayashi R, et al. Normative values for maximum power during motor function assessment of jumping among physically active Japanese. J Musculoskelet Neuronal Interact. 2009;9:263–7. [PubMed] [Google Scholar]
- 27.Runge M, Rittweger J, Russo CR, et al. Is muscle power output a key factor in the age-related decline in physical performance? A comparison of muscle cross section, chair-rising test and jumping power. Clin Physiol Funct Imaging. 2004;24:335–40. doi: 10.1111/j.1475-097X.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- 28.Lange-Maia BS, Newman AB, Strotmeyer ES, et al. Performance on fast- and usual-paced 400-m walk tests in older adults: are they comparable? Aging Clin Exp Res. 2015;27:309–14. doi: 10.1007/s40520-014-0287-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radler BT. The Midlife in the United States (MIDUS) Series: A National Longitudinal Study of Health and Well-being. Open health data. 2014:2. doi: 10.5334/ohd.ai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadsworth CT, Krishnan R, Sear M, et al. Intrarater reliability of manual muscle testing and hand-held dynametric muscle testing. Phys Ther. 1987;67:1342–7. doi: 10.1093/ptj/67.9.1342. [DOI] [PubMed] [Google Scholar]
- 31.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. The journals of gerontology Series A, Biological sciences and medical sciences. 2000;55:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 32.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 33.Cooper R, Hardy R, Aihie Sayer A, et al. Age and gender differences in physical capability levels from mid-life onwards: the harmonisation and meta-analysis of data from eight UK cohort studies. PLoS One. 2011;6:e27899. doi: 10.1371/journal.pone.0027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fragala MS, Clark MH, Walsh SJ, et al. Gender differences in anthropometric predictors of physical performance in older adults. Gend Med. 2012;9:445–56. doi: 10.1016/j.genm.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bohannon RW. Population representative gait speed and its determinants. J Geriatr Phys Ther. 2008;31:49–52. doi: 10.1519/00139143-200831020-00002. [DOI] [PubMed] [Google Scholar]
- 38.Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26:15–9. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 39.Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. 2011;97:182–9. doi: 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. J Am Med Assoc. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kistorp CN, Svendsen OL. Body composition analysis by dual energy X-ray absorptiometry in female diabetics differ between manufacturers. Eur J Clin Nutr. 1997;51:449–54. doi: 10.1038/sj.ejcn.1600424. [DOI] [PubMed] [Google Scholar]
- 42.Gillette-Guyonnet S, Andrieu S, Nourhashemi F, et al. Comparison of bone mineral density and body composition measurements in women obtained from two DXA instruments. Mech Ageing Dev. 2003;124:317–21. doi: 10.1016/s0047-6374(02)00199-9. [DOI] [PubMed] [Google Scholar]
- 43.Soriano JM, Ioannidou E, Wang J, et al. Pencil-beam vs fan-beam dual-energy X-ray absorptiometry comparisons across four systems: body composition and bone mineral. J Clin Densitom. 2004;7:281–9. doi: 10.1385/jcd:7:3:281. [DOI] [PubMed] [Google Scholar]