Abstract
Hypertrophic cardiomyopathy (HCM) is defined as myocardial hypertrophy in the absence of another cardiac or systemic disease capable of producing the magnitude of present hypertrophy. In about 70% of patients with HCM, there is left ventricular outflow tract (LVOT) obstruction (LVOTO) and this is known as obstructive type of hypertrophic cardiomyopathy (HOCM). Cases refractory to medical treatment have had two options either surgical septal myectomy or alcohol septal ablation (ASA) to alleviate LVOT gradient. ASA may cause some life-threatening complications including conduction disturbances and complete heart block, hemodynamic compromise, ventricular arrhythmias, distant and massive myocardial necrosis. Glue septal ablation (GSA) is a promising technique for the treatment of HOCM. Glue seems to be superior to alcohol due to some intrinsic advantageous properties of glue such as immediate polymerization which prevents the leak into the left anterior descending coronary artery and it is particularly useful in patients with collaterals to the right coronary artery in whom alcohol ablation is contraindicated. In our experience, GSA is effective and also a safe technique without significant complications. GSA decreases LVOT gradient immediately after the procedure and this reduction persists during 12 months of follow-up. It improves New York Heart Association functional capacity and decrease interventricular septal wall thickness. Further studies are needed in order to assess the long-term efficacy and safety of this technique.
Keywords: Cyanoacrylate, hypertrophic cardiomyopathy, septal ablation
Introduction
Hypertrophic cardiomyopathy (HCM) is defined as myocardial hypertrophy in the absence of another cardiac or systemic disease capable of producing the magnitude of present hypertrophy.1,2 The prevalence of HCM is estimated to be one case per 500 people.1,2 In about 70% of patients with HCM, there is left ventricular outflow tract (LVOT) obstruction (LVOTO) and constitutes the obstructive type (HOCM). LVOTO causes an increase in left ventricular systolic pressure leading to a complex interaction of abnormalities that include prolongation of ventricular relaxation, increased left ventricular diastolic pressure, myocardial ischemia, decreased cardiac output and mitral regurgitation due to systolic anterior motion (SAM) of the mitral valve.1,2 While most patients with HOCM remain asymptomatic throughout life, LVOTO may cause symptoms such as exertional dyspnea, chest pain, fatigue and syncope. Initial management for HOCM includes pharmacological agents.1,2 However, approximately 10% of patients remain symptomatic despite maximal medical therapy. These patients are candidates for septal myectomy or non-surgical septal reduction therapy (NSRT).3
After its first introduction in 1995, alcohol septal ablation (ASA) has become the most widely used method of NSRT in symptomatic patients who are refractory to medical therapy for the treatment of HOCM.4 It has been shown that ASA decreases rest and provokes LVOT gradient that is accompanied by reduction in basal septal diameter, improvement in NYHA Class and increase in exercise capacity.5 However, ASA may cause some life-threatening complications including conduction disturbances and complete heart block, hemodynamic compromise, ventricular arrhythmias, distant and massive myocardial necrosis.5–7 Therefore, various alternative modalities as a substitute of alcohol have been tried in an attempt to reduce the complication rates of ASA. Coils,8 polyvinyl alcohol particles,9 radiofrequency ablation,10 n-butyl cyanoacrylate septal ablation (GSA) and gelatin particles11 are the other possible options. As an alternative method, we previously published the first ever use of glue (n-butyl cyanoacrylate) GSA).12,13 In this paper, we aimed to summarize the rationale and patient selection in GSA; then we will overview the technique and outcomes after GSA.
Methods
A literature search was undertaken on MEDLINE, EMBASE, Cochrane Library, and Web of Science for other NSRT methods septal from 1995 to 2015. The search was limited to English language and studies conducted on humans. Additional data were gathered from the author’s database.
Rationale and patient selection
N-Butyl cyanoacrylate, a cyanoacrylate ester, is a butyl ester of 2-cyano-2-propenoic acid. It is a clear colorless and water-insoluble liquid.12,13 N-Butyl cyanoacrylate (in monomer form) polymerizes rapidly in the presence of ionic substances like moisture, blood or tissue fluids.12–14 N-Butyl cyanoacrylate has been used as an intravascular embolic agent for a long time in the treatment of vascular malformations and hemorrhage. Evolution in the use of n-butyl cyanoacrylate in HOCM started with the experimental models. Matos et al.14 performed a feasibility study for the use of n-butyl cyanoacrylate for controlled myocardial infarction in dogs. They demonstrated that the glue does not escape from the target artery through capillaries or small collateral vessels, and produces sharply demarcated scar confined to the supply zone of the injected vessel. On the other hand, it has been known that ethanol instillation causes tissue injury by direct necrotizing effect, acute dehydration and fixation of the surrounding tissues.12,13 Due to the in vivo fixation with alcohol, necrotic tissue lacks the infiltration and phagocytosis by leukocytes and macrophages, and does not transform into granulation tissue. The result of ASA is patchy necrosis and scar tissue in the interventricular septum with unpredictable size and irregular border. Moreover, it has been shown that monomorphic ventricular tachycardia may be induced by programmed ventricular stimulation in a swine model of alcohol injection.12,13 However, monomorphic ventricular tachycardia could not be induced in dogs after myocardial infarction created by the injection of glue.12,13 Taking into account the above factors, n-butyl cyanoacrylate seems to have important advantages in NSRT.
Moreover, alcohol is a liquid chemical agent with low viscosity and may leak into the left anterior descending coronary artery (LAD) in case of incomplete balloon inflation or balloon rupture.15 Alcohol flow through septal artery connections to other coronary branches may induce distant myocardial infarction.7 From this point of view, glue seems to be superior to alcohol due to immediate polymerization which prevents the leak into the LAD. GSA is particularly useful in patients with collaterals to the RCA in whom alcohol ablation is merely contraindicated.13
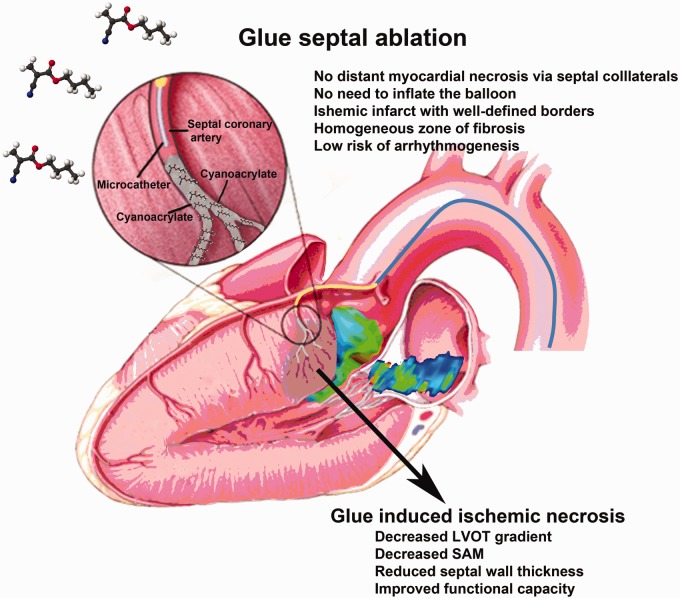
The main principles of the GSA procedure are similar to the ASA (Figure 1). Reducing the interventricular septal thickness and dynamic LVOTO by embolizing the septal arterial branches via trans-catheter route is the main aim of GSA. Asymmetric septal hypertrophy of the basal interventricular septum and SAM of the anterior mitral leaflet are the two most important contributors of the LVOTO. The anterior mitral leaflet to septal contact zone is the main area of interest and the septal artery supplying blood to this zone is the prime target for GSA.16 The septal perforators from the LAD and the RCA supply the anterior two-thirds and posterior one-third of the basal septum, respectively.16 There is extensive collateral circulation between the septal branches of these arteries. Usually, the largest proximal septal branch is the target for the ASA. Sometimes, there are multiple small proximal septal branches and more than one artery may need to be targeted to achieve clinical success for NSRT.16
Figure 1.
Glue septal ablation, mechanisms. and major advantages.
Appropriate selection of the patient remains as a cornerstone for the success of NSRT. Patient selection for NSRT is based on the evaluation of individual clinical and echocardiographic parameters (septal hypertrophy was defined as a thickness greater than 15 mm, and a ratio of septal to posterior wall thickness of greater than 1.7/1).13,17 According to the current recommendations, ASA should be proposed to patients who have substantial impairment in functional capacity (New York Heart Association [NYHA] class III or IV), refractory to maximal medical therapy and the presence of significant LVOTO (defined as ≥50 mmHg at rest or with provocation).17 Therefore, based on these recommendations, we have performed GSA in patients with ASA indications, especially those patients with septal collaterals to the RCA in whom ASA is absolutely contraindicated. Patients with primary mitral valve disease or anomalous papillary muscle insertion are more suited for myectomy as they may require concomitant mitral valve surgery. Patients with concomitant coronary artery disease may be considered for coronary artery by-pass grafting (CABG) and septal myectomy.
GSA technique
Details of the technique are reported elsewhere.12,13 Femoral access is the route for GSA. A coronary angiography is performed both for the evaluation of anatomy and septal arteries. A temporary pacemaker electrode is introduced through the right femoral vein to the right ventricular apex. The septum is avoided as the pacing site, because fluctuation in pacing threshold may occur during the procedure due to the proximity of this region to the target area for GSA. Baseline left ventriculography is performed to assess the mitral regurgitation and the site and extent of hypertrophy. Dynamic obstruction can be unmasked by an extrastimulus, amyl nitrate or Valsalva's maneuver. Intravenous heparin is used to prevent thromboembolic complications. After the cannulation of LAD by 6–8 F guiding catheter, a 0.014-inch guide wire is introduced through the catheter, and advanced into the septal branch. This septal artery is selectively cannulated with a 4 F catheter over the guide wire (GlideCath Vertebral, Terumo Europe N.V. Leuven, Belgium). Selective angiography of the septal artery is routinely performed to show the anatomy and collateral branches to other coronary arteries (Figure 2(a)). Contrast echocardiography is performed to make sure that the pertinent septal artery is the target vessel supplying the hypertrophied septum. A microcatheter (Excelsior SL-10, Boston Scientific, Fremond, CA, USA) is then advanced deep enough into the septal artery through the 4 F catheter. Approximately, 0.5 ml n-butyl cyanoacrylate (Liquiband, MedLogic Global Ltd., Plymouth, Devon, UK) is mixed with 2.5 ml of Lipiodol which yields 1/6 diluted glue (17%). Cyanoacrylate and contrast agent mixture is instilled through the microcatheter into the septal artery slowly by watching persistence of contrast staining in the septum (Figure 2(b)). There is no need to inflate the balloon at the proximal portion of septal artery because of immediate polymerization of glue and low risk of backflow into the LAD. Microcatheter is then pulled back into the 4 F catheter to prevent the remaining glue adhered to the tip of microcatheter to escape into the LAD, and whole system is withdrawn together. If the immediate transthoracic echocardiography shows that the drop in LVOT gradient is insufficient, the second septal artery may be targeted for ablation with the same technique after having verification by contrast echocardiography that the particular branch also supplies the hypertrophied septum. Contrast echocardiography might be optionally performed with intracoronary injection of 1–2 ml Albunex (manufactured by Nycomed AS, Oslo, Norway; 400 million air-filled albumin microspheres per ml, mean diameter 4 ± 1 microns).
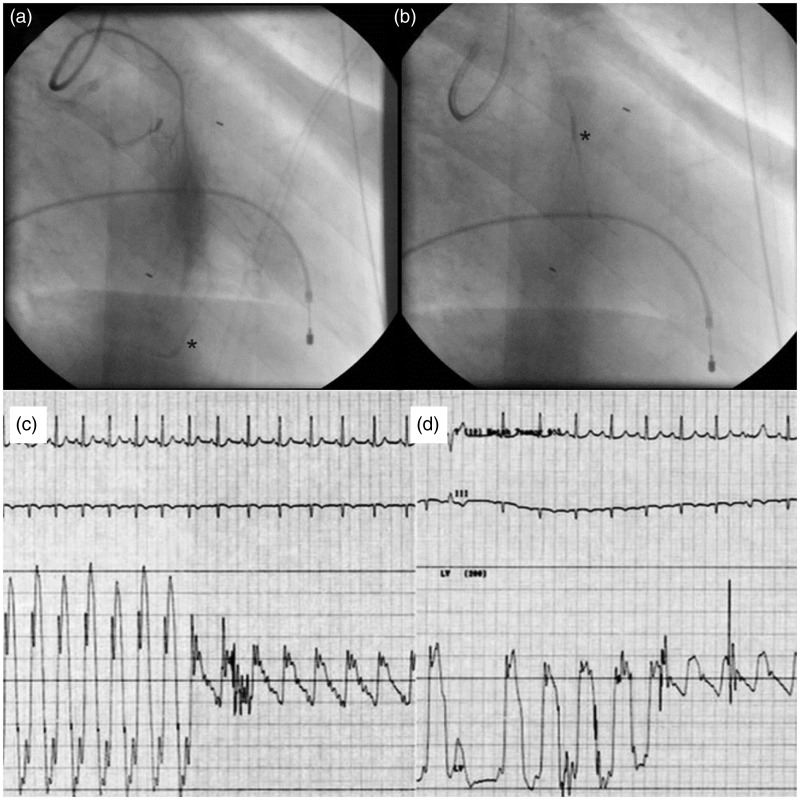
Figure 2.
Selective angiography of septal artery disclosed collateral branches to the right coronary artery (a), the ablated septal artery was filled with glue and contrast medium (b). The gradient across left ventricular outflow tract (c) falls immediately after GSA (d).
Intravenous analgesia may be required for the transient chest pain that accompanies the procedure. The gradient across LVOT usually falls immediately after GSA (Figure 2(c) and (d)). Post-GSA angiography is done to verify the occlusion of septal artery and integrity of the LAD.
Most of the complications related to the use of glue are avoidable by using proper technique. Early polymerization of glue can occur in the microcatheter leading to the occlusion of lumen and the potential loss of access.18 If this occurs, we recommend to replace the microcatheter. Excessive reflux around the microcatheter tip should be avoided. Reflux of polymerized glue around the microcatheter may adhere to its tip, increasing the risk of nontarget embolization or catheter retention.18 As mentioned before, GSA did not require balloon inflation and inadvertently inflated balloon as done in ASA might cause adherence of glue to the balloon and thus create a giant plug in the vessel.
All patients should be monitored in the intensive care unit or coronary care unit for at least 48 h after the procedure. Cardiac enzymes [myoglobin, creatinin kinase–MB (CK-MB) and troponin T] should be checked every 4 h until the CK-MB peak was reached. In our experience, median hospitalization time is 48 h (interquartile range 48–72 h).
Histological changes following ablation
As mentioned above, glue produces sharply demarcated scar confined to the supply zone of the injected vessel. Histologic examination of the glue-induced ischemic infarct shows a homogeneous zone of fibrosis with well-defined borders, and chronic granulomatous inflammation with foreign body multinucleated giant cell reaction in the vessel wall and adjacent tissues.14 The long-term results of this perivascular inflammation have not been known yet.
Clinical outcome of GSA
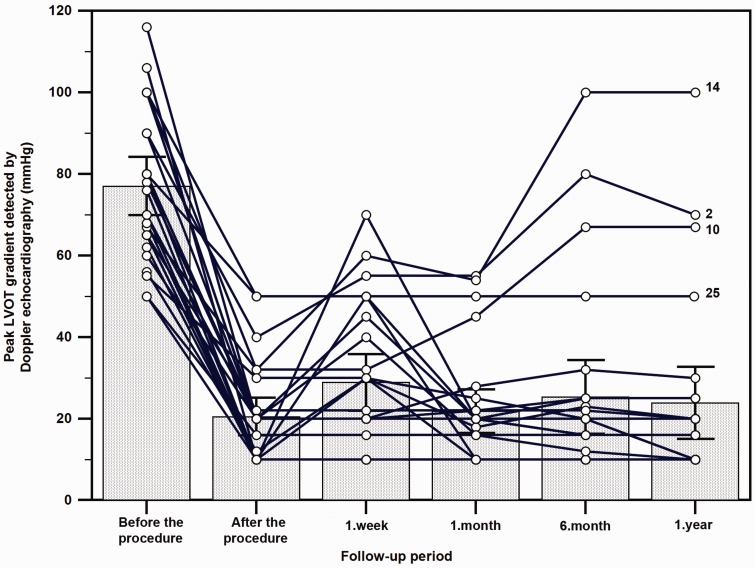
We performed GSA in 27 patients (mean age = 44.3 ± 16.1; 16 males, 11 females). Median follow-up period was 17 months (interquartile range, 12–21 months). Among the study population, 13 (48.1%) were on beta blockers, 14 (51.9%) on verapamil, and 4 (14.8%) were on diltiazem (four patients were using combinations because of severe symptoms). In 19 patients, we ablated only 1 septal artery, but in eight (29.6%) patients, GSA was needed in two septal arteries. Among 27 patients, 10 patients (37%) had septal collateral branches to the RCA. After the procedure, peak LVOT gradient was reduced significantly both in cardiac catheterization (67.1 ± 13.2 vs. 15.7 ± 6.7 mmHg, p < 0.001) and Doppler echocardiographic measurements (77.8 ± 18.0 vs. 20.5 ± 11.7 mmHg, p < 0.001). During 12 months of follow-up, reduction of peak LVOT gradient which was detected by Doppler echocardiography persisted (Figure 3). Post procedure peak serum CK- MB fraction concentration was 41.8 ± 20.9 ng/ml, myoglobin was 88.4 ± 44.0 ng/ml, and peak serum troponin T concentration was 1 ng/ml (IQR, 0.5–1.1).
Figure 3.
Peak LVOT (left ventricular outflow) gradient detected by Doppler echocardiography during one year of follow-up.
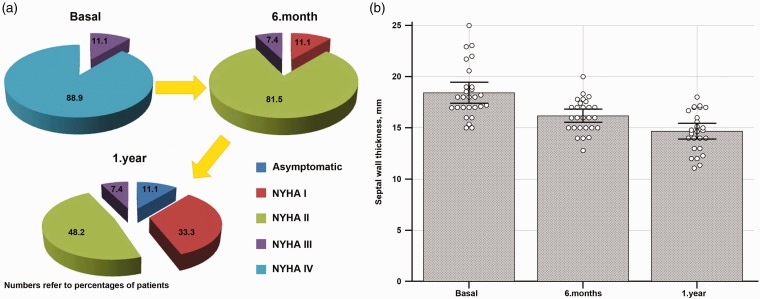
NYHA functional class improved (3.0 ± 0.3 vs. 1.5 ± 0.8, p < 0.001) and interventricular septum thickness decreased (18.4 ± 2.6 vs. 14.7 ± 1.9, p < 0.001) significantly during one year of follow-up (Figure 4). Only four (14.8%) patients had the peak LVOT gradient ≥50 mmHg and two (7.4%) patients had NYHA class III symptoms.
Figure 4.
(a) Improvement of New York Heart Association (NYHA) functional class during one year of follow-up after GSA: (b) reduction in septal wall thickness during one year of follow-up after GSA.
As shown in Figure 3, LVOT gradient following GSA shows a triphasic response. This response is very similar to LVOT gradient reduction after ASA.6,19 In the first phase, there is immediate decrease in the gradient following GSA. This has been attributed to the stunning of the septum. In the second phase, there might be some increase in the LVOTO because of peri-infarct edema. This stage lasts for 7–14 days after the procedure. In the third phase, the LVOTO gradually decreases, as the scar forms over weeks and months and the septum becomes thinner gradually.6,19
When patients with collaterals to RCA were compared with those without collaterals, there was no statistically significant difference between pre-procedural gradient, post-procedural gradient, and post-procedural peak levels of cardiac biomarkers. Patients with recurrent gradient after 12 months of follow-up had lower post-procedural CK-MB levels (51.5 ± 20.4 ng/ml vs. 20.3 ± 13.1 ng/ml, p = 0.028) than those without significant gradient.
There was no major complication (complete heart block, ventricular arrhythmias and distant infarction) during and after the procedure. There was no sustained ventricular tachycardia or complete heart block (CHB) on ambulatory ECG monitorization during the follow-up. In five (18.5%) patients, right bundle branch block, and in three (11.1%) patients, left axis deviation were observed. Recurrent gradient was present only in four (15%) patients whose post-procedural CK-MB and troponin T levels were lower than those without gradient. This might be explained by smaller infarct area which resulted with recurrence of LVOT gradient.
Each therapeutic option available for LVOTO reduction offers unique advantages and disadvantages (Table 1). ASA has a principal role in the current management of drug-refractory HCM patients with LVOTO and became the treatment of choice in most patients. However, ASA may cause some life-threatening complications including conduction disturbances and CHB, hemodynamic compromise, ventricular arrhythmias, and distant and massive myocardial necrosis. GSA is a promising, safe, and effective technique for the treatment of HOCM. In the future, larger studies, ideally with a randomized comparison between GSA and ASA or surgical myectomy, are warranted to evaluate the precise role of this new percutaneous technique in symptomatic patients with HOCM. The use of intracardiac echocardiography to provide continuous intraprocedural imaging of the treated segment of the septum and facilitation of septal artery cannulation by magnetic navigation will further increase the success rates of ASA and GSA.
Table 1.
Comparison of surgical myectomy, ASA and GSA.
| GSA | ASA | Surgical myectomy | |
|---|---|---|---|
| Advantages | • Less invasive • Avoidance of sternotomy • Shorter hospital stay and recovery time • Possible in high-risk patients • Ability to treat CAD requiring PCI • Lower incidence of CHB • Lower risk of distant and/or massive necrosis • Lower risk arrhythmogenesis | • Less invasive • Avoidance of sternotomy • Shorter hospital stay and recovery time • Lower cost • Wider availability • Possible in high-risk patients • Lower risk of stroke in older patients • Ability to treat CAD requiring PCI | • Ability to treat concomitant conditions (mitral valve disease, CAD requiring CABG, myocardial bridges) • Lower incidence of CHB |
| Disadvantages | • Requires suitable coronary anatomy • Further studies are needed in order to assess the long-term efficacy and safety | • Requires suitable coronary anatomy • Higher incidence of CHB • High rate of RBBB • Potential for arrhythmogenicity | • Open heart surgery with cardiopulmonary bypass • Longer hospital stay • Higher cost • Limited availability of expertise • High rate of LBBB |
| Procedural success | 85.2% | 83–89% | 78–94% |
| Complete heart block | 0 | ∼10–14% | 1–10% |
| Mortality | 0 | 0.6–1.8% | <1% |
ASA, alcohol septal ablation; CABG, coronary artery by-pass grafting; CAD, coronary artery disease, CHB, complete heart block; GSA, glue septal ablation; LBBB, left bundle branch block; PCI, percutaneous coronary intervention, RBBB, right bundle branch block.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
None.
Guarantor
SO is the guarantor for all the content presented in this paper.
Contributorship
All authors were involved in the design phase, the analysis and the interpretation of the results. SO drafted the manuscript, and all authors approved the final version.
References
- 1.Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2008; 29: 270–276. [DOI] [PubMed] [Google Scholar]
- 2.Prinz C, Farr M, Hering D, et al. The diagnosis and treatment of hypertrophic cardiomyopathy. Dtsch Arztebl Int 2011; 108: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durand E, Mousseaux E, Coste P, et al. Non-surgical septal myocardial reduction by coil embolization for hypertrophic obstructive cardiomyopathy: early and 6 months follow-up. Eur Heart J 2008; 29: 348–355. [DOI] [PubMed] [Google Scholar]
- 4.Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 1995; 346: 211–214. [DOI] [PubMed] [Google Scholar]
- 5.Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J Interv Cardiol 2006; 19: 319–327. [DOI] [PubMed] [Google Scholar]
- 6.Yoerger DM, Picard MH, Palacios IF, et al. Time course of pressure gradient response after first alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am J Cardiol 2006; 97: 1511–1514. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal SC, Purcell IF, Furniss SS. Apical myocardial injury caused by collateralisation of a septal artery during ethanol septal ablation. Heart 2005; 91: e2–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafont A, Durand E, Brasselet C, et al. Percutaneous transluminal septal coil embolisation as an alternative to alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Heart 2005; 91: 92–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross CM, Schulz-Menger J, Kramer J, et al. Percutaneous transluminal septal artery ablation using polyvinyl alcohol foam particles for septal hypertrophy in patients with hypertrophic obstructive cardiomyopathy: acute and 3-year outcomes. J Endovasc Ther 2004; 11: 705–711. [DOI] [PubMed] [Google Scholar]
- 10.Lawrenz T, Kuhn H. Endocardial radiofrequency ablation of septal hypertrophy. A new catheter-based modality of gradient reduction in hypertrophic obstructive cardiomyopathy. Z Kardiol 2004; 93: 493–499. [DOI] [PubMed] [Google Scholar]
- 11.Llamas-Esperon GA, Sandoval-Navarrete S. Percutaneous septal ablation with absorbable gelatin sponge in hypertrophic obstructive cardiomyopathy. Catheter Cardiovasc Interv 2007; 69: 231–235. [DOI] [PubMed] [Google Scholar]
- 12.Oto A, Aytemir K, Deniz A. New approach to septal ablation: glue (cyanoacrylate) septal ablation. Catheter Cardiovasc Interv 2007; 69: 1021–1025. [DOI] [PubMed] [Google Scholar]
- 13.Oto A, Aytemir K, Okutucu S, et al. Cyanoacrylate for septal ablation in hypertrophic cardiomyopathy. J Interv Cardiol 2011; 24: 77–84. [DOI] [PubMed] [Google Scholar]
- 14.Matos GF, Hammadeh R, Francois C, et al. Controlled myocardial infarction induced by intracoronary injection of n-butyl cyanoacrylatein dogs: a feasibility study. Catheter Cardiovasc Interv 2005; 66: 244–253. [DOI] [PubMed] [Google Scholar]
- 15.Holmes DR, Jr., Valeti US, Nishimura RA. Alcohol septal ablation for hypertrophic cardiomyopathy: indications and technique. Catheter Cardiovasc Interv 2005; 66: 375–389. [DOI] [PubMed] [Google Scholar]
- 16.Angelini P. The “1st septal unit” in hypertrophic obstructive cardiomyopathy: a newly recognized anatomo-functional entity, identified during recent alcohol septal ablation experience. Tex Heart Inst J 2007; 34: 336–346. [PMC free article] [PubMed] [Google Scholar]
- 17.Maron BJ, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol 2003; 42: 1687–1713. [DOI] [PubMed] [Google Scholar]
- 18.Tokuda T, Tanigawa N, Shomura Y, et al. Transcatheter embolization for peripheral pseudoaneurysms with n-butyl cyanoacrylate. Minim Invasive Ther Allied Technol 2009; 18: 361–365. [DOI] [PubMed] [Google Scholar]
- 19.Lakkis NM, Nagueh SF, Dunn JK, et al. Nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy: one-year follow-up. J Am Coll Cardiol 2000; 36: 852–855. [DOI] [PubMed] [Google Scholar]