Abstract
Background
Malaria elimination requires reducing both the potential of mosquitoes to transmit parasites to humans and humans to transmit parasites to mosquitoes. To achieve this goal in Southern province, Zambia a mass test and treat (MTAT) campaign was conducted from 2011–2013 to complement high coverage of long-lasting insecticide-treated nets (LLIN). To identify factors likely to increase campaign effectiveness, a modelling approach was applied to investigate the simulated effect of alternative operational strategies for parasite clearance in southern province.
Methods
OpenMalaria, a discrete-time, individual-based stochastic model of malaria, was parameterized for the study area to simulate anti-malarial drug administration for interruption of transmission. Simulations were run for scenarios with a range of artemisinin-combination therapies, proportion of the population reached by the campaign, targeted age groups, time between campaign rounds, Plasmodium falciparum test protocols, and the addition of drugs aimed at preventing onward transmission. A sensitivity analysis was conducted to assess uncertainty of simulation results. Scenarios were evaluated based on the reduction in all-age parasite prevalence during the peak transmission month one year following the campaign, compared to the currently-implemented strategy of MTAT 19 % population coverage at pilot and 40 % coverage during the first year of implementation in the presence of 56 % LLIN use and 18 % indoor residual spray coverage.
Results
Simulation results suggest the most important determinant of success in reducing prevalence is the population coverage achieved in the campaign, which would require more than 1 year of campaign implementation for elimination. The inclusion of single low-dose primaquine, which acts as a gametocytocide, or ivermectin, which acts as an endectocide, to the drug regimen did not further reduce parasite prevalence one year following the campaign compared to the currently-implemented strategy. Simulation results indicate a high proportion of low-density infections were missed by rapid diagnostic tests that would be treated and cleared with mass drug administration (MDA).
Conclusions
The optimal implementation strategy for MTAT or MDA will vary by background level of prevalence, by rate of infections imported to the area, and by ability to operationally achieve high population coverage. Overall success with new parasite clearance strategies depends on continued coverage of vector control interventions to ensure sustained gains in reduction of disease burden.
Keywords: Malaria, Modelling, Elimination, Plasmodium falciparum, Primaquine, Ivermectin, Zambia
Background
Malaria elimination requires reducing both the potential of mosquitoes to transmit parasites to humans and humans to transmit parasites to mosquitoes, including reaching humans with asymptomatic infections. With the backdrop of several years of high coverage of long-lasting insecticide-treated mosquito nets (LLINs), many countries are looking to reorient their malaria programmes from control to elimination, including the adoption of additional strategies for programme implementation and surveillance.
Zambia has a long history of malaria control efforts dating back to the 1920s with the mining sector in the copperbelt [1]. With the advent of the Roll Back Malaria initiative in 1998, Zambia began to introduce additional insecticide-treated bed nets (ITNs) as part of small-scale delivery programmes targeting vulnerable populations. Following initiation of malaria funding from the Global Fund for AIDS, Tuberculosis and Malaria and other partners, a scaling up of key malaria interventions, including mass distribution of ITNs, renewed efforts to increase IRS coverage, artemisinin-combination therapy (ACT) for anti-malarial treatment, and rapid diagnostic tests for case management began to reach communities by 2005. This scaling up for impact (SUFI) approach contributed to an observed 63 % decrease in Plasmodium falciparum prevalence in ages 2–9 and a 22 % decrease in malaria deaths [2]. In response to the need to plan next steps to accelerate the goal of malaria elimination the Zambia national malaria control centre (NMCC) in partnership with PATH MACEPA is conducting an evaluation of strategies for anti-malarial drug campaigns. These strategies are designed to complement the existing high coverage of vector control interventions and aim to contribute to a marked reduction of community level malaria. In addition to the 2011–2013 implementation strategy of mass test and treat (MTAT) targeting all ages with three rounds of artemether-lumefantrine (AL) [3] an additional set of operational and chemotherapeutic options are currently under evaluation [4]. These changes to the design include replacing AL with another ACT, dihydroartemisinin + piperaquine (DHAP), which clears asexual blood stages of current infections and has a longer prophylactic period against future malaria infection [5], as well as use of single low-dose (SLD) primaquine [6] and/or ivermectin [7] to stop onward transmission to humans.
Primaquine is a gametocytocidal drug that reduces the duration of late-stage P. falciparum gametocytes, the transmissible stage of the parasite from human to mosquito, and therefore has the potential to reduce malaria transmission [8]. The World Health Organization (WHO) currently recommends the use of SLD primaquine in addition to an ACT for all patients with parasitologically-confirmed P. falciparum malaria to aid in malaria elimination and to contain the spread of artemisinin resistance [9]. However, this use of primaquine is not currently widespread due to the limited availability of appropriate dosing regimens for young children and some residual confusion regarding the safety of SLD versus standard higher dosing of primaquine in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. Clinical trials to determine the lowest possible dose effective at clearing gametocytes are in process in a number of locations considering the inclusion of the drug in elimination strategies including Uganda [10], The Gambia [11], and Mali [12].
Ivermectin is an anthelmintic drug currently used for control of onchocerciasis and lymphatic filariasis. It also has endectocidal properties reducing the sporogony [13] and survivorship [14] of mosquitoes that take a blood meal from humans with the drug in their system. Studies indicate that mass drug administration (MDA) with ivermectin has the potential to assist in the interruption of malaria transmission [15, 16], and has the potential to combine malaria elimination efforts with other helminth-based diseases considered for elimination.
OpenMalaria is a stochastic individual-based model of malaria that has been used to address questions relevant to malaria epidemiology, vaccine development [17], cost-effectiveness of different combinations of malaria control interventions [18], and vector control technologies [19]. In order to assist the National Malaria Control Programme and implementing partners in Zambia with model-based guidance on community-based treatment strategies, OpenMalaria was employed to simulate the effectiveness of changing the operational strategy of the malaria testing and treatment campaign. This paper presents the simulation results of MTAT with AL, MTAT with DHAP (with or without ivermectin), and MDA with DHAP (with or without ivermectin and/or SLD primaquine).
Methods
OpenMalaria model
Alternative strategies and drug interventions against malaria parasites and the mosquito vector were evaluated using OpenMalaria. OpenMalaria combines stochastic individual-based simulation models for malaria in humans with a periodically-forced deterministic model for malaria in mosquitoes [20–23]. The models have been fitted to multiple field data sets and the models for malaria in humans include demography, acquired immunity and super-infection, variations in parasite densities and infectiousness to mosquitoes and the clinical effects of malaria. The model for malaria in mosquitoes includes multiple mosquito species, varying vector behaviour and non-human hosts [20–23]. The model platform consists of an ensemble of fourteen model variants that allow different assumptions such as heterogeneity in exposure and decay of immunity to help quantify the effects of uncertainty in model formulation [23]. Details of the model and simulation set up can be found in the references above.
Baseline scenario parameterization
The baseline scenario parameterization incorporated published literature and available data from field studies to represent the context of malaria in Zambia’s Southern province, where elimination strategies in the country were initiated. The seasonal pattern of malaria transmission corresponds to the majority of infections occurring from January to May with Anopheles gambiae and A. funestus as the primary vectors [24, 25]. The baseline case management system assumes 21.8 % of fever cases access an anti-malarial, either the recommended first line anti-malarial drug (AL) introduced in 2006 [26] or sulfadoxine–pyrimethamine (SP), the second most commonly-used anti-malarial [25]. 81 % of the population is assumed to fully adhere to AL [27], and 39.4 % to adhere to SP [28]. Non-adherers to AL receive a reduced probability of clearing the infection, while all non-adherers to SP are assumed to not clear infections. Existing malaria control interventions described in Fig. 1. Coverage of any given intervention is assumed to be independent of coverage of any other intervention. Simulations assume 71.8 % of the population in the study area own an LLIN, 41.3 % of the population slept under a net the previous night [29], and 18 % of households received indoor residual spraying (IRS). Coverage of MTAT interventions were chosen based on the preliminary results from the MTAT pilot in 2011 and 2012. Overall, 19 % of the simulated population received an MTAT intervention in December 2011; in each of the 2012 dry season rounds, 40 % of the simulated individuals received an MTAT intervention so that over three rounds approximately 78 % of the simulated population received at least one malaria test.
Fig. 1.

Schematic of malaria control interventions existing in Southern province, Zambia from 2006 to 2012 that served to inform the baseline scenario used for this experiment. The blue circles correspond to months with no active deployment of malaria control interventions. The green circles corresponds to months where vector control interventions were deployed and the open red circles correspond to months were drug interventions were deployed
This baseline scenario was simulated on a population of 10,000 individuals with Zambia’s population age structure [30]. Simulation results from the baseline scenario were validated against observed rapid diagnostic test (RDT) positivity rates by facility catchment area following the scale up of the MTAT programme using AL [29]. Results were stratified by baseline parasite prevalence, taking into account variations in transmission intensity.
Experiment design and analysis
To investigate the effects of operational considerations of MTAT with AL on health outcomes, assuming the baseline MTAT implementation as illustrated in Fig. 1, the scenarios described in Table 1 were included in the experiment and altered one at a time. These include varying the age groups targeted by the campaign, varying the time between campaign delivery rounds, and varying the proportion of the population reached by the campaign. MTAT campaign coverage numbers were chosen to correspond to the preliminary findings of coverage achieved by the 2011–2012 intervention implementation. Age groups were chosen to correspond to the groups with the highest parasite prevalence rates. To investigate the effects of changing the drug regimen on health outcomes of the MTAT intervention, scenarios were simulated with the longer-acting DHAP, both alone and paired with ivermectin (Table 2). To investigate the effects of removing the RDT and implementing an MDA campaign, scenarios were simulated with DHAP alone, DHAP + SLD primaquine, DHAP + ivermectin, and the combination of all three drugs (Table 2).
Table 1.
Experiment design for mass test and treat (MTAT) with artemether-lumefantrine (AL)
| Variable | Simulation levels |
|---|---|
| Target ages receiving the campaign | All ages, children under 5, children 5–14, adults 25–49 |
| Time between MTAT delivery rounds (total of three rounds) | 2, 3, 4, 8 weeks |
| Proportion of the population reached per round by the MTAT campaign | 0, 40, 72, 100 % |
| Proportion of the population who fully adhere to the AL drug regimen | 50, 75, 100 % |
Baseline values indicated in italics
Table 2.
Experiment design for campaign implementation with dihydroartemisinin–piperaquine (DHAP)
| Drug combination | Population reached by MTAT campaign | Population reached by MDA campaign | Ivermectin duration of effectiveness | Single low-dose (SLD) primaquine half-life |
|---|---|---|---|---|
| Campaign implementation with DHAP | 40, 72 % | 40, 72 % | ||
| DHAP + ivermectin | 40, 72 %a | 40, 72 % | 2, 4, 7 days | |
| DHAP + SLD primaquine | 40, 72 % | 12, 48, 72 h | ||
| DHAP + ivermectin + SLD primaquine | 40, 72 % |
Baseline values indicated in italics
aIn this scenario ivermectin is given to every individual reached by the campaign, regardless of the result of their malaria test
A sensitivity analysis was conducted to investigate the sensitivity of the intervention to changes in factors likely to influence the effect of the specific intervention or strategy. This includes the proportion of the population who fully adhere to the AL drug regimen (Table 1), and the duration of the effects of ivermectin and SLD primaquine (Table 2). In addition, each of the scenarios described above were simulated over fourteen model variants with varied assumptions about malaria epidemiology to account for model uncertainty [23], and three random seeds to account for the effects of stochasticity.
Intervention scenarios were evaluated based on the simulated reduction in mean all-age parasite prevalence taken over the 14 model variants and three random seeds (mean parasite prevalence) in the peak month of malaria transmission one year following the intervention compared to the baseline scenario.
Intervention assumptions
Drugs
AL is assumed to clear 100 % of asexual blood stage infections [31] in individuals with full adherence to the drug regimen with a prophylactic period of 25 days [32]. DHAP is assumed to have an equivalent effect on clearing existing asexual blood stage infections as AL, but goes on to have a prophylaxis benefit for an additional 10 days [32] during which new infective mosquito bites do not go on to transmit infection. Ivermectin is given as a single dose with an 85 % killing effect on mosquitoes; killing time is varied to be either 2, 4 or 7 days according to the dose. Primaquine is given as a single low dose formulation and is assumed to immediately kill all sexual stage 5 parasites with a half-life of 2 days. However, all remaining earlier stage parasites and those arising from an incompletely cleared infection become stage 5 parasites and are able to be transmitted. Adherence rate for both AL and DHAP is assumed to be 75 % in the baseline simulations but is varied in the sensitivity analysis as described above. Because missing the final dose has been reported to be the most common reason for non-adherence [33] leading to a lower mean plasma lumefantrine level in individuals treated with AL [34], the probability of infection clearance is reduced by 15 % for non-adherers. Adherence to SLD primaquine and ivermectin is assumed to be 100 % given their administration at the start of the drug regimen and therefore likelihood of direct observation. Assumptions about the decay of effectiveness of the range of drugs simulated in this experiment are summarized in Fig. 2.
Fig. 2.
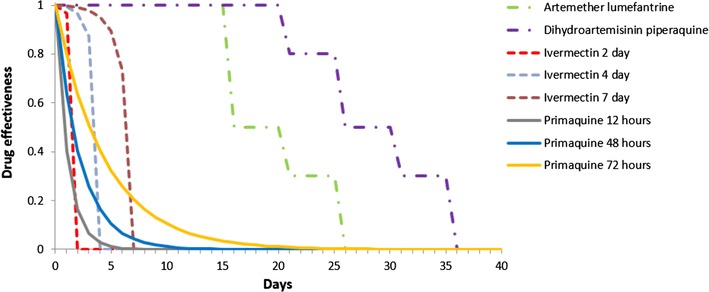
Assumptions about the decay in drug effectiveness. ACT (dashed/dotted lines) decay in a step-wise fashion with AL (green) lasting 25 days and DHAP (purple) 35 days. PQ (solid line) has an exponential decay with varying half-life of effectiveness of 12 (gray), 48 (blue), and 72 h (orange). Ivermectin (dashed lines) smoothly decays to efficacy of zero after 2 (red), 4 (grey), and 7 days (brown)
Delivery method
With MTAT implementation, an ACT is only given to individuals with a positive RDT result; thus, the sensitivity and specificity of the RDT establish the likely proportion of which individuals will test positive according to parasite density of the infection, and which individuals will be infected but test negative. These will typically be individuals with lower parasite density who may also have a lower likelihood of transmitting to mosquitoes. In scenarios including ivermectin with MTAT, while ACT is only given to individuals who test positive, ivermectin is given to every individual reached by the campaign, regardless of the result of their malaria test. This is because ivermectin is not a therapeutic drug for malaria treatment, but rather acts as an endectocide that is toxic to mosquitoes that bite an individual with the drug in their system. With MDA implementation, the drugs are given to everyone. However, this is moderated by the population coverage of the intervention; some people are reached and others are not, and an individual has the same independent likelihood of receiving the intervention in each delivery round.
Vector control
LLIN deterrency and pre- and post-prandial killing of malaria vector mosquitoes were parameterized using experimental hut data, as described in Briët et al. [19]. LLINs are assumed to decay over time at different rates for their physical state, chemical state, and attrition from circulation. IRS deterrency and post-prandial killing are assumed to decay over time with an exponential function and a half-life of 4 months. Both LLINs and IRS are assumed to only affect the proportion of indoor biting and resting mosquitoes. Additional details of the model description and parameterization of vector control interventions are described elsewhere [19, 35].
Results
MTAT with AL strategy used in 2011–2013
The model was able to simulate the range of observed prevalence of health facility catchment areas at each of the three MTAT deployment rounds in each of the three parasite prevalence strata at the time of the December 2011 pilot survey (Fig. 3). Both the observed and simulated data show the variance in prevalence between health facility catchment areas decreasing through the season. However, for the health facility catchment areas representing the highest parasite prevalence rate, the simulation runs under-estimated the peak parasite prevalence rate (no estimates were greater than 35 %) for the June/July intervention round when the observed prevalence rates reached as high as 48 % (Fig. 3) [28]. This trend was also observed in subsequent intervention rounds (Fig. 3).
Fig. 3.
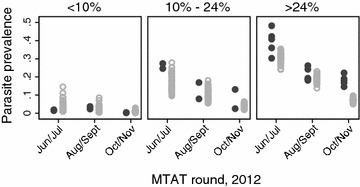
Validation of the baseline scenario parameterization: simulated versus observed effect of three rounds of MTAT using AL, by parasite prevalence at pilot conducted in December 2011 [29]. Black circles represent observed all-age mean parasite prevalence rates in health facility catchment areas. Grey circles represent simulated mean parasite prevalence rates
Results suggest a substantial proportion of infections in the population exist with parasite densities of fewer than 100 parasites per microlitre, the RDT detection limit assumed by the model. Simulations indicate the median proportion of infections that are sub patent is 20 % in scenarios with a baseline of less than 10 % mean parasite prevalence at the 2011 survey, dropping to 10 % in scenarios with baseline mean parasite prevalence in the two higher prevalence categories (Fig. 4).
Fig. 4.
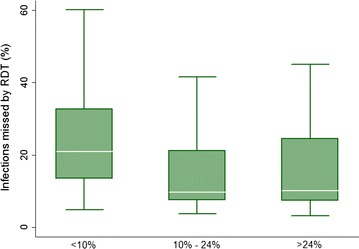
Prevalence of subpatent infections. The percentage of simulated infections in the population with parasitaemia of fewer than 100 parasites per microlitre, the simulated detection limit of the RDT used in the MTAT campaign. White lines represent the simulated median value, green boxes represent the inter-quartile range, and capped bars represent the upper and lower adjacent values for simulated results using an ensemble of 14 model variants and three random seeds for populations in each category of mean parasite prevalence
Changing operational strategy of MTAT campaign
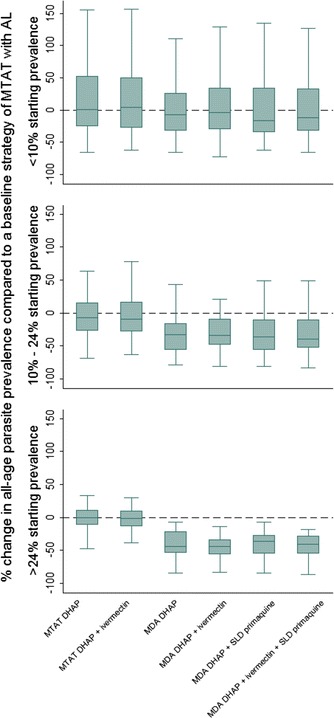
Changes in the proportion of the population reached by the campaign, the number of days between screen and treat rounds, and varying the anti-malarial drug used in the campaign had the greatest effect in areas with a starting mean parasite prevalence of greater than 24 %, and the least effect in areas with a starting mean parasite prevalence of less than 10 % (Fig. 5). A contributing factor to this effect could be the greater variation in simulated results in this lowest-prevalence category. For example, when simulating the parasite prevalence given 72 % coverage of MTAT with AL, the standard deviation for the <10 versus >24 % prevalence groups were ±62.5 and ±13.8, respectively (Table 3; Fig. 5).
Fig. 5.

Results of changing operational strategy for mass test and treat using artemether-lumefantrine. Change (%) in the simulated mean parasite prevalence compared to the simulated mean parasite prevalence measured 1 year after implementation of the existing intervention strategy, as a result of varying the operational strategy of the intervention. Categories represented in the columns include the proportion of the population reached by the campaign (coverage), the number of days between screen and treat rounds (time between rounds), the ages reached by the intervention (target ages), and varying the anti-malarial drug used in the campaign (change drug to DHAP). The existing strategy consists of three rounds of mass test and treat with AL covering 40 % per round of all ages in the population with 60 days between treatment rounds. The rows represent results stratified by the pre-intervention mean parasite prevalence in the population as measured by RDT. Centre solid lines represent the simulated median value, blue boxes represent the inter-quartile range, and capped bars represent the upper and lower adjacent values for simulated results using an ensemble of 14 model variants and three random seeds
Table 3.
Effect of changing operational strategy compared to a baseline strategy of three rounds mass test and treat with artemether-lumefantrine at 40 % coverage per round of all ages of the population with 60 days between rounds
| % Change in median parasite prevalence (IQR), by pre-intervention mean parasite prevalence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Coverage (%) | Frequency (days) | Ages | Change drug (%) | |||||||
| 0 | 72 | 100 | 15 | 20 | 30 | Under 5 | 5–14 | 25–49 | DHAP 40 | DHAP 72 | |
| <10 | 0.0 (−23.7, 107.4) |
−6.1 (−32.6, 44.7) |
−8.7 (−31.6, 43.4) |
0 (−28.9, 56.3) |
−2.4 (−30.0, 42.6) |
−2.9 (−26.3, 48.9) |
5.5 (−24.7, 112.6) |
7.4 (−23.2, 86.1) |
5.8 (−23.9, 111.3) |
1.6 (−28.9, 64.2) |
−5.5 (−30.0, 43.2) |
| 10–24 | 65.8 (17.5, 107.1) |
−19.7
(−38.8, −1.0) |
−37.6
(−53.2, −17.8) |
−15.1 (−32.5, 6.7) |
−18.6
(−43.2, −4.4) |
−26.2
(−40.8, −2.8) |
60.8 (20.6, 95.2) |
43.9 (4.1, 84.4) |
40.9 (−2.9, 74.3) |
−0.8 (−20.1, 28.1) |
−25.5
(−41.4, −7.4) |
| >24 | 47.5 (42.7, 56.3) |
−25.6
(−33.1, −16.8) |
−44.2
(−49.9, −36.6) |
−18.2
(−27.5, −11.1) |
−21.4
(−31.8, −13.6) |
−25.3
(−33.3, −17.2) |
46.3 (37.2, 51.34) |
36.8 (29.1, 49.7) |
29.8 (19.4, 38.1) |
2.9 (−3.1, 9.8) |
−25.7
(−34.5, −17.2) |
Italics indicate results that perform better than the baseline strategy
Targeting specific age groups for the MTAT intervention, as opposed to reaching all-ages as in the current MTAT strategy, resulted in an increase in prevalence (reduced efficacy) in all prevalence categories. This is because a smaller proportion of the population is reached, even though this proportion of the population may have a higher mean parasite prevalence. Unlike other operational strategy changes, changing the time between campaign rounds to 30 days or less showed a greater simulated benefit (reduction of mean parasite prevalence) in the higher-prevalence areas, although this benefit decreases as the prevalence level in the population decreases.
From MTAT to MDA
Simulation results suggest that the most important determinant of success in reducing prevalence is campaign coverage (Fig. 5; Table 3), with a greater effect in areas of higher all-age parasite prevalence. However, in these simulated scenarios, even when achieving high coverage with MDA in areas with a pre-intervention all-age parasite prevalence of less than 10 %, complete elimination will require more than 1 year of campaign implementation. Indeed, preliminary findings from the Southern province MDA intervention trials suggest these strategies are able to reduce parasite prevalence in areas of 8–0.5 %; not elimination, but close enough to warrant moving on to surveillance-based interventions.
Changing drug
Changing the intervention drug to DHAP did not result in a simulated reduction in mean parasite prevalence when compared to simulated results using AL. When compared to implementation with AL, adding either SLD primaquine or ivermectin to DHAP did not result in further simulated reduction of mean parasite prevalence 1 year after intervention implementation in either the MTAT or MDA delivery strategies (Fig. 6; Table 4).
Fig. 6.
Results of changing drug and testing protocol for anti-malarial drug distribution. Change (%) in the simulated mean parasite prevalence compared to the simulated mean parasite prevalence measured 1 year after implementation of a baseline strategy, for different combinations and testing protocols of DHAP, SLD primaquine, and ivermectin. Rows represent different coverage levels achieved by the simulated intervention. For drug combinations at 40 % coverage per round, the baseline includes the existing intervention strategy of three rounds of mass test and treat with AL covering 40 % per round of all ages in the population with 60 days between treatment rounds. For drug combinations at 72 % coverage per round, the baseline includes three rounds of mass test and treat with AL covering 72 % per round of all ages in the population with 60 days between treatment rounds. Rows represent results stratified by the pre-intervention mean parasite prevalence in the population as measured by RDT. Centre solid lines represent the simulated median value, green boxes represent the inter-quartile range, and capped bars represent the upper and lower adjacent values for simulated results using an ensemble of 14 model variants and three random seeds
Table 4.
Results of changing drug and testing protocol for anti-malarial drug distribution compared to a strategy of three rounds mass test and treat with artemether-lumefantrine at 72 % coverage per round of all ages of the population with 60 days between rounds
| % Change in median parasite prevalence (IQR) compared to baseline strategy, by pre-intervention mean parasite prevalence | |||||||
|---|---|---|---|---|---|---|---|
| % | Baseline intervention strategy | MTAT with DHAP | MTAT with DHAP + ivermectin | MDA with DHAP | MDA with DHAP + ivermectin | MDA with DHAP + SLD primaquine | MDA with DHAP + ivermectin + SLD primaquine |
| <10 | MTAT with AL, 72 % coverage, 60 day frequency between rounds, targeting all ages | 0.56 (−25.5, 52.4) |
4.5 (−28.0, 50.10 |
−7.6 (−32.2, 25.5) |
−3.6 (−30.5, 33.9) |
−17.1 (−35.0, 33.6) |
−12.3 (−32.8, 32.2) |
| 10–24 | −7.2 (−27.0, 15.3) |
−8.8 (−28.6, 16.3) |
−33.0
(−56.0, −16.0) |
−33.5
(−48.1, −9.5) |
−35.7
(−56.7, −10.8) |
−39.0
(−53.5, −10.8) |
|
| >24 | −0.2 (−11.9, 11.4) |
−1.1 (−13.6, 9.7) |
−44.1
(−53.6, −21.9) |
−43.6
(−56.1, −33.7) |
−36.4
(−55.4, −27.2) |
−40.4
(−55.1, −28.4) |
|
Italics indicate results that perform better than the baseline strategy
Sensitivity analysis
Altering the proportion of the population adhering to the drug regimen, altering the half-life of PQ, and altering the duration of effectiveness of ivermectin did not contribute greatly to the overall uncertainty in model predictions (Fig. 7). The range seen in simulation results are therefore more likely to be due to stochasticity and model uncertainty rather than variations in drug half-life/duration of effectiveness or adherence.
Fig. 7.
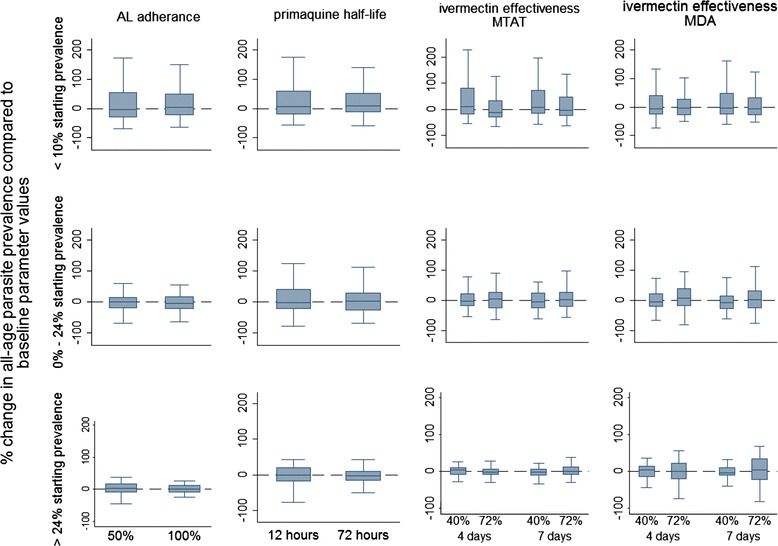
Sensitivity analysis: artemether-lumefantrine (AL) adherence, single low dose primaquine half-life, and ivermectin duration of effectiveness. Change (%) in the simulated mean parasite prevalence compared to the simulated mean parasite prevalence after 1 year of implementation of a baseline strategy, as a result of varying the operational strategy of the intervention. Categories represented in the columns include proportion of the population adhering to the artemether-lumefantrine (AL) regimen, the half-life of single low dose primaquine, and duration of effectiveness at different coverage levels for testing strategies of ivermectin paired with AL. The rows represent results stratified by the pre-intervention mean parasite prevalence in the population as measured by RDT. For all categories, the baseline includes the existing intervention strategy of three rounds of mass test and treat with AL covering 72 % per round of all ages in the population with 60 days between treatment rounds. Baseline AL adherence is 75 %, baseline primaquine half-life is 48 h, and baseline ivermectin duration of effectiveness is 2 days. Centre solid lines represent the simulated median value, blue boxes represent the inter-quartile range, and capped bars represent the upper and lower adjacent values for simulated results using an ensemble of 14 model variants and three random seeds
Discussion
In an era where vector control strategies have been able to greatly reduce disease burden across the malaria endemic world, many countries are now evaluating how to transition programmes from control to elimination. Mass distribution of anti-malarials, with or without a diagnostic, are under consideration in many settings to markedly reduce the human parasite reservoir to allow a shift to case detection via surveillance as an intervention. Results of this study are consistent with both modelling and empirical evidence that has shown implementation of drug-based strategies to be effective within a transmission season in settings where sufficient coverage of the population can be achieved [36]. Yet, recent studies on screen and treat for strategies targeting the whole community [37] and specific risk groups [38] have been unable to show sustained impact. This study examined how the OpenMalaria platform, parameterized according to the malaria transmissions dynamics in southern Zambia, can assist the Zambia NMCC to increase the effectiveness of these planned interventions through changes in implementation design.
Results suggest the optimal implementation strategy for deployment of community-based anti-malarial treatments requires a consistent approach addressing the variation in baseline malaria prevalence, rate of imported infections, and the achievement of high coverage in the population. Simulations conducted for this study suggest that an area such as Southern province with heterogeneous transmission and an uncertain infection importation rate, drug-based interventions based on detecting infections using current RDTs will not be sufficient to interrupt transmission. Indeed, as observed in the field study, there was no difference detected at follow-up in parasite prevalence between MTAT intervention and control in either high or low transmission areas [3]. In this setting, a high proportion of low-density infections will be missed by RDTs that would be treated and cleared with MDA.
Simulation results suggest the most important determinant of success in reducing prevalence is the coverage of the population achieved in the campaign. The Southern province field study was designed to show how much impact would be achieved after 1 year of campaign implementation in order to move towards surveillance as an intervention by focal MDA (fMDA) and case investigation. However, even with high coverage with MDA in areas with a pre-intervention all-age parasite prevalence of less than 10 %, simulations suggest that elimination will require more than 1 year of campaign implementation. The inclusion of SLD primaquine (a gametocytocide), and ivermectin (an endectocide), to the drug regimen did not further reduce parasite prevalence within this 1 year post-campaign. It is important to note that mean parasite prevalence measured at earlier time points will yield different results, as the greatest effects will be seen several months post-campaign. Simulation of ACT regimens in a generic setting have been investigated elsewhere, both with and without the option of including SLD primaquine and/or ivermectin [39, 40]. The simulation results presented here are less optimistic about the additional added benefit of PQ and ivermectin in interrupting transmission, although, importantly, the results are evaluated at different time points.
Success of anti-malarial drug administration campaigns depends on the sustained high coverage of vector control interventions. Zambia has been able to achieve high rates of household LLIN ownership and has steadily increased its within-household availability of LLINs through successive mass distribution campaigns. However, LLINs must be used or at least deployed in the household for their full impact to be attributable. Improving simulation estimates by including more direct measures of full coverage and usage of LLINs may yield greater overall reductions in predicted parasite prevalence in combination with the anti-malarial campaigns. Similarly with IRS, improved strategies to target the application of insecticides could be evaluated for specific benefits. Ensuring that transmitting mosquitoes are susceptible to IRS chemicals, that IRS is prioritized to transmission areas, and that full coverage of targeted areas is achieved will maximize the contribution of this intervention where drug strategies are attempted.
Planning for elimination scenarios: limitations and considerations
In the process of this simulation, we have identified key aspects of both the intervention tools and their delivery; improved data from the field may help in the future to improve the model and its ability to predict outcomes. As noted above, intervention coverage is a critical variable for these population-wide treatment efforts and intervention programming should improve on the quality of the coverage data. For example, most coverage estimates only examine the coverage of the household members in households that were reached; a more accurate estimation of the population denominator and coverage could perhaps be achieved by comparing satellite imaging of houses to actual field geo-position data of which houses were reached by a campaign.
Analysis was conducted with the end-point measurement of reduction in parasite prevalence. This is, indeed, an easily-measured quantity in the field. However, future applications of the OpenMalaria model in the study area should also include an end-point measurement that corresponds to potential vector control interventions, including ivermectin. The goal of ivermectin is not to reduce prevalence, but to reduce transmission. Further analysis measuring reduction in EIR or probability of interrupting transmission would be a more useful way of evaluating the impact of this intervention. In addition to the clear need for direct application of field-measurable quantities, it would be helpful to have a standard definition for defining interruption of transmission for model outputs that is operationally relevant to programme settings. Ideally this would include identifying the number of cases per week or per month in a given health facility catchment area that the health system would be able to handle.
The model has numerous limitations that are inherent in the introduction of many assumptions required for model parameterization. For example, the modelled analysis included a sensitivity analysis for different levels of adherence to AL with the assumption that the majority of non-adherers missed the final dose in the six-dose regimen, with results showing little impact on overall effectiveness. However, non-adherence would have a far greater impact on the cure rate of DHAP if a single dose of this three-dose regimen is missed [41], something not included in this experiment.
The field implementation targeted health facility catchment areas for complete campaign coverage, while the simulation coverage parameterization and analysis is applied to the general population without explicit geographical limits. While model parameterization assumes an individual has the same independent likelihood of receiving the intervention, there are some groups that have a higher risk of being infected. These groups, including adults aged 25–49 that are more highly mobile than the rest of the population, were targeted in the simulation study design by varying the age groups targeted by the campaign. However, because these groups may be less likely to be reached during a general campaign, this higher risk is not captured in the baseline scenario of the model and may affect the impact of the simulated interventions even if a certain level of coverage is reached for the population as a whole.
The model parameterization assumes a conservative value for AL and DHAP prophylaxis based on modelling pharmacokinetics [41]. However, a recent publication of post-treatment prophylaxis of anti-malarials suggests both a shorter duration of protection and a greater difference between the duration of protection between AL and DHAP in people over 5 years of age [42]. This greater difference in prophylaxis benefit would affect the simulation results by increasing the additional transmission prevented by DHAP as compared to AL.
For the ivermectin analysis, the model assumes a common effect of ivermectin no matter the age of mosquito post-emergence, which has been shown to be different in trial settings [14]. Several studies [15] have found a higher mortality rate in mosquitoes exposed to ivermectin than the 85 % assumed in this experiment; this could indicate the experiment design underestimates the effect.
The model assumes a constant rate of yearly case importation (20 cases per 1000 individuals); this rate will undoubtedly vary by season and from location to location even within southern province, and data is not currently available to be able to parameterize this value correctly. Because the OpenMalaria simulations assumed an ongoing rate of imported infections, it is not straightforward from model outputs to distinguish between cases arising from imported infections and cases that were a result of local transmission. This parameter is likely a key driver of the differences in the simulated versus observed results of the model validation shown in Fig. 3. If possible, future iterations of field work could improve the ascertainment of mobility and likely imported versus locally transmitted cases. This includes methods such as parasite genotyping or using human movement via mobile phone data as a proxy of parasite flow [43]. Understanding these dynamics will aid not only in developing more relevant baseline model parameterization, but understanding thresholds for importation in a population on the effect of intervention mixes. Combined with transmission estimates, these metrics can provide a more granular view of what intervention mix to deploy, and where.
Demonstrating elimination, and evaluating the ability of interventions to interrupt transmission, requires metrics that are practical in operational settings. The reproductive number under control settings, Rc, has been proposed as a metric to determine whether an area has achieved elimination [44] by calculating the ratio of local cases to imported infections. However, the operational scale at which this should be applied is unclear, as is the geographical definition of infection importation in this context. For example, should importation from higher-transmission zones in the same province be considered as part of the definition, or only importation from a neighbouring country? The area in Southern province currently evaluating anti-malarial administration strategies is bound by the vast Lake Kariba to the east and south, and much lower malaria transmission areas to the north and west, making it an ideal setting to test elimination strategies. A country level metric is likely unsuitable for a country like Zambia which has highly heterogeneous endemicity in different parts of the country. On the other hand, applying such a metric at a more granular administrative level would not confer recognition of elimination by the WHO. Country-specific elimination strategic plans should address how progress will be tracked at both national and sub-national levels.
Other simulation studies and study data suggest the proportion of sub-patent infections missed by RDTs is higher in areas of lower transmission [37, 45, 46], consistent with the simulation results from this study (Fig. 4). Transmission due to asymptomatic infections becomes increasingly important (in relative terms) as cases in a country become fewer and national control programmes approach elimination and prevention of reintroduction. As such, further validation and calibration of models to the prevalence of sub-patent transmissible infections in different epidemiological settings needs to be conducted as new study data becomes available.
Finally, an important limitation for interpreting results is the non-spatially explicit structure of the OpenMalaria model. Focal test and treat and focal MDA strategies that involve targeting specific households, geographic areas or risk groups, and reactive case detection are all implementation strategies in the study area but are not evaluated in the context of this experiment. In addition, there are options for anti-malarial drug administration intervention design that will be relevant in the Southern province that are not explored in this study. Future analyses could examine the potential impact of this larger package of intervention strategies as they are implemented in the same or adjacent geographic areas; such analyses could further contribute to the future programme design by the NMCC.
Conclusion
The optimal strategy for implementation of community-based interventions involving anti-malarial distribution will vary by background level of malaria prevalence and by rate of infections imported to the area. The ability to achieve high coverage of the population is critical to the success of anti-malarial distribution campaigns. This is reflected in the simulation results showing MDA to be more effective than MTAT at reducing mean parasite prevalence 1 year after intervention implementation. While there was a simulated added benefit of the longer prophylactic effect of DHAP compared to AL, the addition of SLD primaquine and ivermectin, both alone and in combination, did not lead to any appreciable reduction in mean parasite prevalence within the year after implementation. Continued coverage and use of optimized vector control interventions is necessary to maximize the gains in reduction of disease burden. OpenMalaria can be helpful in assisting malaria control and elimination programmes to understand what intervention strategies are most appropriate in different settings, and under which conditions these strategies will be the most effective.
Authors’ contributions
EMS designed the experiments, performed the literature review for model parameterization, analysed results and drafted the manuscript. EMS, JMM and ML parameterized the model and contributed to the modelling study design. RS and JMM designed the field studies. JMM supervised and conducted the intervention implementation. RS, EMS and NC conceived and designed the modeling study. All authors participated in designing the experiments and interpreting the results. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank the study participants and communities of Southern province, and the Zambia Ministry of Health and the National Malaria Control Centre at national and province levels, for their support and cooperation. We acknowledge colleagues at the Swiss Tropical and Public Health Institute and PATH MACEPA for helpful discussions. We also acknowledge the MalariaControl.net volunteers for donating computing resources to run simulations.
Funding and ethics statement
This work was supported by the Bill and Melinda Gates Foundation, under grants number OPP1032350 (Swiss TPH) and OPP1089412 (PATH MACEPA).
Competing interests
The experiment design, analysis, and manuscript writing was performed while EMS was employed by the Swiss Tropical and Public Health Institute. At the time of manuscript submission EMS is currently employed by the Bill & Melinda Gates Foundation, who provided funding for both PATH MACEPA and Swiss TPH.
Contributor Information
Erin M. Stuckey, Email: erin.stuckey@gatesfoundation.org
John M. Miller, Email: jmiller@path.org
Megan Littrell, Email: mlittrell@psi.org.
Nakul Chitnis, Email: Nakul.Chitnis@unibas.ch.
Rick Steketee, Email: rsteketee@path.org.
References
- 1.Utzinger J, Tozan Y, Doumani F, Singer BH. The economic payoffs of integrated malaria control in the Zambian copperbelt between 1930 and 1950. Trop Med Int Health. 2002;7:8. doi: 10.1046/j.1365-3156.2002.00916.x. [DOI] [PubMed] [Google Scholar]
- 2.Chanda E, Masaninga F, Coleman M, Sikaala C, Katebe C, MacDonald M, et al. Integrated vector management: the Zambian experience. Malar J. 2008;7:164. doi: 10.1186/1475-2875-7-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsen DA, Bennett A, Hamainza B, Yukich JO, Keating J, Littrell M, et al. Population-wide malaria testing and treatment with rapid diagnostic tests and artemether-lumefantrine in southern Zambia: a community randomized step-wedge control trial design. Am J Trop Med Hyg. 2015;92:913–921. doi: 10.4269/ajtmh.14-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisele TP, Silumbe K, Finn T, Chalwe V, Kamuliwo M, Hamainza B, et al. Assessing the effectiveness of household-level focal mass drug administration and community-wide mass drug administration for reducing malaria parasite infection prevalence and incidence in southern province, Zambia: study protocol for a community randomized controlled trial. Trials. 2015;16:347. doi: 10.1186/s13063-015-0862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lwin KM, Phyo AP, Tarning J, Hanpithakpong W, Ashley EA, Lee SJ, et al. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin–piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother. 2012;56:1571–1577. doi: 10.1128/AAC.05877-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutanto I, Suprijanto S, Kosasih A, Dahlan MS, Syafruddin D, Kusriastuti R, et al. The effect of primaquine on gametocyte development and clearance in the treatment of uncomplicated falciparum malaria with dihydroartemisinin–piperaquine in South sumatra, western Indonesia: an open-label, randomized, controlled trial. Clin Infect Dis. 2013;56:685–693. doi: 10.1093/cid/cis959. [DOI] [PubMed] [Google Scholar]
- 7.Foy BD, Kobylinski KC, da Silva IM, Rasgon JL, Sylla M. Endectocides for malaria control. Trends Parasitol. 2011;27:423–428. doi: 10.1016/j.pt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steketee RW, ter Kuile F. Single low-dose primaquine to reduce malaria transmission. Lancet Infect Dis. 2014;14:91–92. doi: 10.1016/S1473-3099(13)70288-3. [DOI] [PubMed] [Google Scholar]
- 9.WHO . Updated WHO policy recommendation October 2012: single dose primaquine as a gametocytocide in Plasmodium falciparum malaria. Geneva: World Health Organization; 2012. [Google Scholar]
- 10.Eziefula AC, Bousema T, Yeung S, Kamya M, Owaraganise A, Gabagaya G, et al. Single dose primaquine for clearance of Plasmodium falciparum gametocytes in children with uncomplicated malaria in Uganda: a randomised, controlled, double-blind, dose-ranging trial. Lancet Infect Dis. 2013;14:130–139. doi: 10.1016/S1473-3099(13)70268-8. [DOI] [PubMed] [Google Scholar]
- 11.Okebe J, Bousema T, Affara M, DiTanna G, Eziefula AC, Jawara M, et al. The gametocytocidal efficacy of primaquine in malaria asymptomatic carriers treated with dihydroartemisinin–piperaquine in The Gambia (PRINOGAM): study protocol for a randomised controlled trial. Trials. 2015;16:70. doi: 10.1186/s13063-015-0597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Determining a tolerable dose of primaquine in G6PD-deficient persons without malaria in Mali (PQSAFETY). ClinicalTrials.gov identifier: NCT02535767 https://clinicaltrials.gov/ct2/show/NCT02535767.
- 13.Sylla M, Kobylinski KC, Gray M, Chapman PL, Sarr MD, Rasgon JL, et al. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J. 2010;9:365. doi: 10.1186/1475-2875-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobylinski KC, Deus KM, Butters MP, Hongyu T, Gray M, da Silva IM, et al. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 2010;116:119–126. doi: 10.1016/j.actatropica.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobylinski KC, Sylla M, Chapman PL, Sarr MD, Foy BD. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am J Trop Med Hyg. 2011;85:3–5. doi: 10.4269/ajtmh.2011.11-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaccour CJ, Kobylinski KC, Bassat Q, Bousema T, Drakeley C, Alonso P, et al. Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination. Malar J. 2013;12:153. doi: 10.1186/1475-2875-12-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Penny MA, Maire N, Studer A, Schapira A, Smith TA. What should vaccine developers ask? Simulation of the effectiveness of malaria vaccines. PLoS One. 2008;3:e3193. doi: 10.1371/journal.pone.0003193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuckey EM, Stevenson J, Galactionova K, Baidjoe AY, Bousema T, Odongo W, et al. Modeling the cost effectiveness of malaria control interventions in the highlands of western Kenya. PLoS One. 2014;9:e107700. doi: 10.1371/journal.pone.0107700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Briet OJ, Hardy D, Smith TA. Importance of factors determining the effective lifetime of a mass, long-lasting, insecticidal net distribution: a sensitivity analysis. Malar J. 2012;11:20. doi: 10.1186/1475-2875-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.OpenMalaria [http://code.google.com/p/openmalaria/wiki/Start]. Accessed 25 Nov 2014.
- 21.Smith T, Maire N, Ross A, Penny M, Chitnis N, Schapira A, et al. Towards a comprehensive simulation model of malaria epidemiology and control. Parasitology. 2008;135:1507–1516. doi: 10.1017/S0031182008000371. [DOI] [PubMed] [Google Scholar]
- 22.Chitnis N, Hardy D, Smith T. A periodically-forced mathematical model for the seasonal dynamics of malaria in mosquitoes. Bull Math Biol. 2012;74:1098–1124. doi: 10.1007/s11538-011-9710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith T, Ross A, Maire N, Chitnis N, Studer A, Hardy D, et al. Ensemble modeling of the likely public health impact of a pre-erythrocytic malaria vaccine. PLoS Med. 2012;9:e1001157. doi: 10.1371/journal.pmed.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fornadel CM, Norris LC, Glass GE, Norris DE. Analysis of Anopheles arabiensis blood feeding behavior in southern Zambia during the two years after introduction of insecticide-treated bed nets. Am J Trop Med Hyg. 2010;83:848–853. doi: 10.4269/ajtmh.2010.10-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kent RJ, Thuma PE, Mharakurwa S, Norris DE. Seasonality, blood feeding behavior, and transmission of Plasmodium falciparum by Anopheles arabiensis after an extended drought in southern Zambia. Am J Trop Med Hyg. 2007;76:267–274. [PMC free article] [PubMed] [Google Scholar]
- 26.ZMOH. Zambia national malaria indicator survey 2010. Lusaka. 2011.
- 27.Chanda P, Hawela M. Assessment of patient compliance to Artemether-Lumefantrine in Zambia. Zambia National Malaria Control Centre ORO ed. 2005.
- 28.Depoortere E, Guthmann JP, Sipilanyambe N, Nkandu E, Fermon F, Balkan S, Legros D. Adherence to the combination of sulphadoxine–pyrimethamine and artesunate in the Maheba refugee settlement, Zambia. Trop Med Int Health. 2004;9:62–67. doi: 10.1046/j.1365-3156.2003.01157.x. [DOI] [PubMed] [Google Scholar]
- 29.PATH/MACEPA . Malaria test and treat campaign summary—NMCC briefing document. Lusaka: PATH/MACEPA; 2013. [Google Scholar]
- 30.Central Statistical Office ZMoH, Zambia Uo, Centre TDR, Inc. MI. Zambia demographic and health survey. 2009.
- 31.Hamainza BH, Hawela M, Chanda P, Chalwe V, Masaninga F, Kamuliwo M. Efficacy testing for artemether lumefantrine: Mansa site report. Lusaka: National Malaria Control Centre, Ministry of Health; 2011. [Google Scholar]
- 32.Winter K, Hastings IM. Development, evaluation, and application of an in silico model for antimalarial drug treatment and failure. Antimicrob Agents Chemother. 2011;55:3380–3392. doi: 10.1128/AAC.01712-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabanywanyi AM, Lengeler C, Kasim P, King’eng’ena S, Schlienger R, Mulure N, et al. Adherence to and acceptability of artemether-lumefantrine as first-line anti-malarial treatment: evidence from a rural community in Tanzania. Malar J. 2010;9:48. doi: 10.1186/1475-2875-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fogg C, Bajunirwe F, Piola P, Biraro S, Checchi F, Kiguli J, et al. Adherence to a six-dose regimen of artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Uganda. Am J Trop Med Hyg. 2004;71:525–530. [PubMed] [Google Scholar]
- 35.Chitnis N, Schapira A, Smith T, Steketee R. Comparing the effectiveness of malaria vector-control interventions through a mathematical model. Am J Trop Med Hyg. 2010;83:230–240. doi: 10.4269/ajtmh.2010.09-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poirot E, Sarbinski J, Sinclair D, Kachur SP, Slutsker L, Hwang J. Mass drug administration for malaria. Cochrane Database Syste Rev. 2013;12:CD008846. doi: 10.1002/14651858.CD008846.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tiono AB, Ouedraogo A, Ogutu B, Diarra A, Coulibaly S, Gansané A, et al. A controlled, parallel, cluster-randomized trial of community-wide screening and treatment of asymptomatic carriers of Plasmodium falciparum in Burkina Faso. Malar J. 2013;12:79. doi: 10.1186/1475-2875-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halliday KE, Okello G, Turner EL, Njagi K, Mcharo C, Kengo J, et al. Impact of intermittent screening and treatment for malaria among school children in Kenya: a cluster randomised trial. PLoS Med. 2014;11:e1001594. doi: 10.1371/journal.pmed.1001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerardin J, Eckhoff P, Wenger E. Mass campaigns with antimalarial drugs: a modelling comparison of artemether-lumefantrine and DHA-piperaquine with and without primaquine as tools for malaria control and elimination. BMC Infect Dis. 2015;15:144. doi: 10.1186/s12879-015-0887-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slater HC, Walker PG, Bousema T, Okell LC, Ghani AC. The potential impact of adding ivermectin to a mass treatment intervention to reduce malaria transmission: a modelling study. J Infect Dis. 2014;210:12. doi: 10.1093/infdis/jiu351. [DOI] [PubMed] [Google Scholar]
- 41.Hodel EM, Kay K, Hayes DJ, Terlouw DJ, Hastings IM. Optimizing the programmatic deployment of the anti-malarials artemether-lumefantrine and dihydroartemisinin–piperaquine using pharmacological modelling. Malar J. 2014;13:138. doi: 10.1186/1475-2875-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bretscher MT, Griffin J, Hugo P, Baker M, Ghani A, Okell L, et al. A comparison of the duration of post-treatment protection of artemether-lumefantrine, dihydroartemisinin–piperaquine and artesunate-amodiaquine for the treatment of uncomplicated malaria. Malar J. 2014;13(Suppl 1):P19. doi: 10.1186/1475-2875-13-S1-P19. [DOI] [Google Scholar]
- 43.Tatem AJ, Huang Z, Narib C, Kumar U, Kandula D, Pindolia DK, et al. Integrating rapid risk mapping and mobile phone call record data for strategic malaria elimination planning. Malar J. 2014;13:52. doi: 10.1186/1475-2875-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Churcher TS, Cohen JM, Novotny J, Ntshalintshali N, Kunene S, Cauchemez S. Measuring the path toward malaria elimination. Science. 2014;344:1230–1232. doi: 10.1126/science.1251449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okell LC, Bousema T, Griffin JT, Ouédraogo AL, Ghani AC, Drakeley CJ. Factors determining the occurrence of submicroscopic malaria infections and their relevance for control. Nat Commun. 2012;3:1237. doi: 10.1038/ncomms2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosha JF, Sturrock HJ, Greenhouse B, Greenwood B, Sutherland CJ, Gadalla N, et al. Epidemiology of subpatent Plasmodium falciparum infection: implications for detection of hotspots with imperfect diagnostics. Malar J. 2013;12:221. doi: 10.1186/1475-2875-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]


