Abstract
Two human Golli (for gene expressed in the oligodendrocyte lineage)-MBP (for myelin basic protein) cDNAs have been isolated from a human oligodendroglioma cell line. Analysis of these cDNAs has enabled us to determine the entire structure of the human Golli-MBP gene. The Golli-MBP gene, which encompasses the MBP transcription unit, is approximately 179 kb in length and consists of 10 exons, seven of which constitute the MBP gene. The human Golli-MBP gene contains two transcription start sites, each of which gives rise to a family of alternatively spliced transcripts. At least two Golli-MBP transcripts, containing the first three exons of the gene and one or more MBP exons, are produced from the first transcription start site. The second family of transcripts contains only MBP exons and produces the well-known MBPs. In humans, RNA blot analysis revealed that Golli-MBP transcripts were expressed in fetal thymus, spleen, and human B-cell and macrophage cell lines, as well as in fetal spinal cord. These findings clearly link the expression of exons encoding the autoimmunogen/encephalitogen MBP in the central nervous system to cells and tissues of the immune system through normal expression of the Golli-MBP gene. They also establish that this genetic locus, which includes the MBP gene, is conserved among species, providing further evidence that the MBP transcription unit is an integral part of the Golli transcription unit and suggest that this structural arrangement is important for the genetic function and/or regulation of these genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegretta M., Nicklas J. A., Sriram S., Albertini R. J. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990 Feb 9;247(4943):718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- Bell R. B., Steinman L. Trimolecular interactions in experimental autoimmune demyelinating disease and prospects for immunotherapy. Semin Immunol. 1991 Jul;3(4):237–245. [PubMed] [Google Scholar]
- Campagnoni A. T. Molecular biology of myelin proteins from the central nervous system. J Neurochem. 1988 Jul;51(1):1–14. doi: 10.1111/j.1471-4159.1988.tb04827.x. [DOI] [PubMed] [Google Scholar]
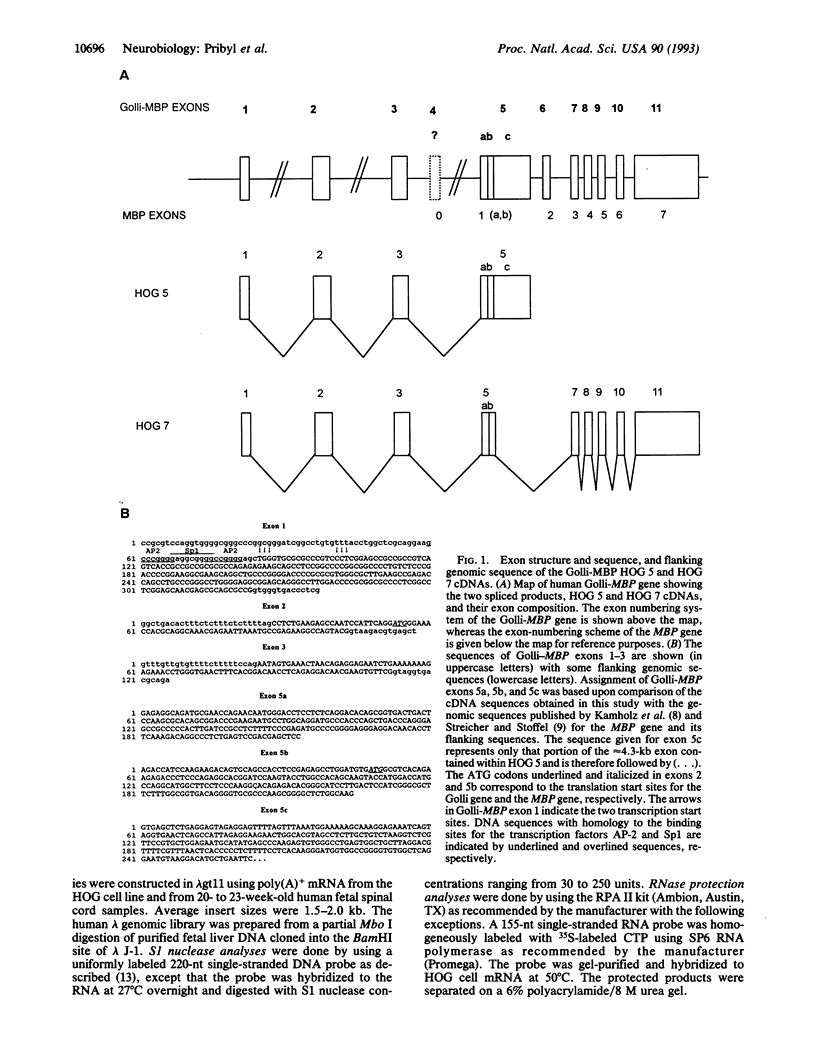
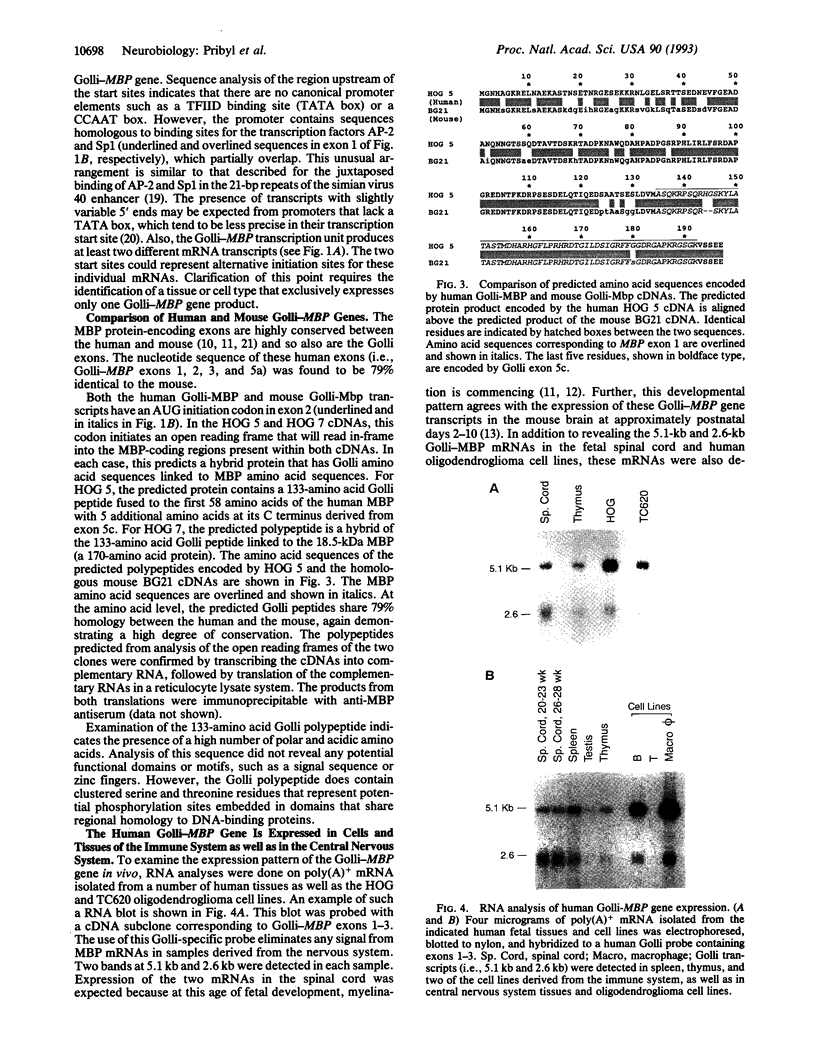
- Campagnoni A. T., Pribyl T. M., Campagnoni C. W., Kampf K., Amur-Umarjee S., Landry C. F., Handley V. W., Newman S. L., Garbay B., Kitamura K. Structure and developmental regulation of Golli-mbp, a 105-kilobase gene that encompasses the myelin basic protein gene and is expressed in cells in the oligodendrocyte lineage in the brain. J Biol Chem. 1993 Mar 5;268(7):4930–4938. [PubMed] [Google Scholar]
- González-Crespo S., Boronat A. Expression of the rat growth hormone-releasing hormone gene in placenta is directed by an alternative promoter. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8749–8753. doi: 10.1073/pnas.88.19.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler D. A., Benjamin D. S., Burks J., Weiner H. L. Myelin basic protein and proteolipid protein reactivity of brain- and cerebrospinal fluid-derived T cell clones in multiple sclerosis and postinfectious encephalomyelitis. J Immunol. 1987 Jul 1;139(1):68–72. [PubMed] [Google Scholar]
- Kamholz J., Toffenetti J., Lazzarini R. A. Organization and expression of the human myelin basic protein gene. J Neurosci Res. 1988 Sep;21(1):62–70. doi: 10.1002/jnr.490210110. [DOI] [PubMed] [Google Scholar]
- Kamholz J., de Ferra F., Puckett C., Lazzarini R. Identification of three forms of human myelin basic protein by cDNA cloning. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4962–4966. doi: 10.1073/pnas.83.13.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima T., Tiu S. N., Merrill J. E., Vinters H. V., Dawson G., Campagnoni A. T. Expression of oligodendrocyte-associated genes in cell lines derived from human gliomas and neuroblastomas. Cancer Res. 1993 Jan 1;53(1):170–175. [PubMed] [Google Scholar]
- Kitamura K., Newman S. L., Campagnoni C. W., Verdi J. M., Mohandas T., Handley V. W., Campagnoni A. T. Expression of a novel transcript of the myelin basic protein gene. J Neurochem. 1990 Jun;54(6):2032–2041. doi: 10.1111/j.1471-4159.1990.tb04908.x. [DOI] [PubMed] [Google Scholar]
- Kronquist K. E., Crandall B. F., Macklin W. B., Campagnoni A. T. Expression of myelin proteins in the developing human spinal cord: cloning and sequencing of human proteolipid protein cDNA. J Neurosci Res. 1987;18(3):395–401. doi: 10.1002/jnr.490180303. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Pette M., Fujita K., Kitze B., Whitaker J. N., Albert E., Kappos L., Wekerle H. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology. 1990 Nov;40(11):1770–1776. doi: 10.1212/wnl.40.11.1770. [DOI] [PubMed] [Google Scholar]
- Roth H. J., Kronquist K. E., Kerlero de Rosbo N., Crandall B. F., Campagnoni A. T. Evidence for the expression of four myelin basic protein variants in the developing human spinal cord through cDNA cloning. J Neurosci Res. 1987;17(4):321–328. doi: 10.1002/jnr.490170402. [DOI] [PubMed] [Google Scholar]
- Saruhan-Direskeneli G., Weber F., Meinl E., Pette M., Giegerich G., Hinkkanen A., Epplen J. T., Hohlfeld R., Wekerle H. Human T cell autoimmunity against myelin basic protein: CD4+ cells recognizing epitopes of the T cell receptor beta chain from a myelin basic protein-specific T cell clone. Eur J Immunol. 1993 Feb;23(2):530–536. doi: 10.1002/eji.1830230235. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Streicher R., Stoffel W. The organization of the human myelin basic protein gene. Comparison with the mouse gene. Biol Chem Hoppe Seyler. 1989 May;370(5):503–510. doi: 10.1515/bchm3.1989.370.1.503. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Mackin G. A., Matsui M., Orav E. J., Khoury S. J., Dawson D. M., Hafler D. A. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993 Feb 26;259(5099):1321–1324. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- de Ferra F., Engh H., Hudson L., Kamholz J., Puckett C., Molineaux S., Lazzarini R. A. Alternative splicing accounts for the four forms of myelin basic protein. Cell. 1985 Dec;43(3 Pt 2):721–727. doi: 10.1016/0092-8674(85)90245-4. [DOI] [PubMed] [Google Scholar]