Abstract
Staphylococcus aureus community-acquired (CA) MRSA strains are highly virulent and can cause infections in otherwise healthy individuals. The most important mechanism of the host for clearing S. aureus is phagocytosis by neutrophils and subsequent killing of the pathogen. Especially CA-MRSA strains are very efficient in circumventing this neutrophil killing. Interestingly, only a relative small number of virulence factors have been associated with CA-MRSA, one of which are the phenol soluble modulins (PSMs). We have recently shown that the PSMs are functionally inhibited by serum lipoproteins, indicating that PSMs may exert their cytolytic function primarily in the intracellular environment. To further investigate the intracellular role of the PSMs we measured the effect of the α-type and β-type PSMs on neutrophil killing after phagocytosis. Using fluorescently labeled S. aureus, we measured bacterial survival after phagocytosis in a plate reader, which was employed next to flow cytometry and time-lapse microscopy. Phagocytosis of the CA-MRSA strain MW2 by human neutrophils resulted in rapid host cell death. Using mutant strains of MW2, we demonstrated that in the presence of serum, the intracellular expression of only the psmα operon is both necessary and sufficient for both increased neutrophil cell death and increased survival of S. aureus. Our results identify PSMα peptides as prominent contributors to killing of neutrophils after phagocytosis, a finding with major implications for our understanding of S. aureus pathogenesis and strategies for S. aureus vaccine development.
Introduction
Staphylococcus aureus (S. aureus) has the ability to cause many skin and soft tissue infections and more serious invasive diseases such as sepsis, endocarditis, osteomyelitis, toxic shock syndrome and pneumonia (Lowy, 1998). Moreover, these infections are becoming increasingly difficult to treat due to the acquisition of antibiotic resistance (Chambers et al., 2009). In recent years, there has been an emergence of community-associated (CA)-MRSA strains that cause infections outside of the healthcare setting in otherwise healthy individuals. This capacity is believed to be due to the fact that CA-MRSA strains are more virulent than hospital-associated MRSA strains (Deleo et al., 2010, David et al., 2010). This enhanced virulence potential is in part related to the ability of these strains to evade or kill human neutrophils, the most prominent cellular innate host defense against invading microorganisms (Rigby et al., 2012). Neutrophils take up pathogens by phagocytosis, which in turn triggers the production of reactive oxygen species (ROS) and the release of microbicidal granule components into the forming phagosomes. These responses are sufficient for killing most pathogens; however, S. aureus are to some extend resistant to phagocytic killing in part and cause human infections. CA-MRSA strains seem to be very efficient in circumvention of neutrophil killing (Voyich et al., 2005, Kobayashi et al., 2010).
For full virulence, S. aureus can make use of a myriad of toxins. However, only a small number of toxins have been associated with the enhanced virulence of CA-MRSA (Rigby et al., 2012). Among these are the phenol soluble modulins (PSMs). PSMs are small amphipathic α-helical peptides of approximately 20–25 (α-type) and 44 (β-type) amino acids (Wang et al., 2007). Due to their amphipatic helical structure PSMs can facilitate lysis of neutrophils, peripheral blood mononuclear cells, and erythrocytes, most likely through a receptor-independent process (Wang et al., 2007, Cheung et al., 2012). Only α-type PSMs have cytolytic activities and it is not yet understood why β-type PSMs lack such properties. In S. aureus, the α-type PSM group consists of four PSMα peptides and the δ-toxin (Wang et al., 2007). Neutrophils can recognize PSMs via their FPR2 receptor, which results in activation and chemotactic attraction (Kretschmer et al., 2010, Rautenberg et al., 2010).
Although the PSM-encoding loci are conserved in the core genome of all sequenced staphylococcal strains, the in vitro expression correlates with the more virulent phenotype between CA-MRSA and HA-MRSA (Wang et al., 2007, Li et al., 2009). The production of PSMs is strictly regulated by the accessory gene regulator (agr), the global regulatory/quorum sensing system, although independent of RNAIII (Queck et al., 2008). Multiple animal models have shown a role of PSMα peptides in experimental infection. Isogenic psmα mutant strains have significantly reduced virulence in bacteremia and skin infection models compared to their wild-type CA-MRSA strains (Li et al., 2010, Wang et al., 2007).
Recently, our group has discovered that PSMs are functionally inhibited by serum lipoproteins (Surewaard et al., 2012). These lipoproteins are abundantly present within the blood and human tissue, indicating that PSMs exert their function primarily in the intracellular environment. Thus, we hypothesized that there is an important function for PSMs as intracellular toxins and here sought to determine the role of PSMs after phagocytosis.
Results
Phagocytosis and subsequent killing of the pathogen is the most important mechanism of bacterial clearance by the host (Rigby et al., 2012). Here, we used S. aureus strain MW2 as a model CA-MRSA strain and investigated whether this strain could resist the killing mechanisms employed by human neutrophils after phagocytosis. To measure phagocytosis, freshly isolated human neutrophils were incubated in the presence of 10% human serum with a derivative of strain MW2, which constitutively expresses GFP (MW2-GFP). Complement and immunoglobulins (Ig) present in the serum will opsonize the bacterium (Rigby et al., 2012). Additionally, generated C5a and bacterial factors will prime the neutrophils, resulting in phagocytic uptake of the bacteria. To validate our bacterial killing assays, phagocytosis was blocked in two ways. First, heat-inactivation (HI) of serum at 56°C inactivated complement protein C2 and Factor B, thereby completely blocking opsonization of the bacterium by complement (Rooijakkers et al., 2005a). Additionally, formyl peptide receptor inhibitory protein-like (FLIPr-Like) protein was used to block Fcγ-receptors and prevent Ig-mediated phagocytosis (Stemerding et al, 2013). Fcγ-receptors recognize Igs and, together with complement receptors (CR), constitute the most important phagocytic receptors on human phagocytes. Secondly, cytochalasin D (CytD) was used to inhibit phagocytosis by blocking actin polymerization.
Phagocytosis of S. aureus MW2 induces rapid neutrophil cell-death
In the presence of human serum, neutrophils can efficiently phagocytose MW2-GFP, as demonstrated by measuring GFP fluorescence associated with neutrophils by flow cytometry (Figure 1A). At a multiplicity of infection (MOI) of 10, 96.8% of the neutrophils have phagocytosed at least one GFP-expressing bacterium and the bacterial uptake is nearly complete. Heat-inactivation of the serum in combination with FLIPr-Like treatment of neutrophils nearly completely inhibited phagocytosis, whereas CytD treatment of neutrophils used in several studies as potent inhibitor of phagocytosis only partly inhibits phagocytosis. One explanation could be that opsonized bacteria can still associate with neutrophils via CR or Fcγ-receptors, but cannot be ingested, since this process involves the polymerization of actin.
Figure 1. Impact of phagocytosis of S. aureus MW2 on neutrophil lysis and bacterial survival.
(A) Analysis of neutrophil phagocytosis of CA-MRSA MW2 by flow cytometry. Neutrophils were allowed to phagocytose strain MW2, which constitutively expresses GFP (MW2-GFP), in the presence of human serum for 30 min. Phagocytosis was blocked by either heat-inactivation (HI) of the serum combined with pre-incubation of neutrophils with Flipr-Like (FcγR-inhibitor) or by addition of cytochalasin D. One representative scatterplot out of three independent experiments is shown. (B) Time dependent lysis of human neutrophils after phagocytosis of MW2 (upper panel) and bacterial survival (lower panel). Neutrophils were mixed with pre-cultured MW2 in the presence of human serum, allowing phagocytosis, or phagocytosis was blocked, as described above. Neutrophil lysis was measured through LDH release. (C) Bacterial survival was measured by counting CFUs. Data represent means ± SEM of 4–5 independent donors. (D) Time dependent lysis of human neutrophils after phagocytosis of MW2 or MW2-GFP as described above. (E) Impact of phagocytosis on bacterial rebound measured by GFP fluorescence every 5 minutes for 10 hours. Measurement started 30 min after initiation of phagocytosis. Phagocytosis was established as described above. Data represent the mean ± 95% coincidence intervals out of 3 independent experiments.
Next, we used the wild type, non-fluorescent, MW2 strain for phagocytosis assays with human neutrophils at the same MOI of 10 and evaluated lysis of neutrophils and killing of bacteria at different time points after infection. Neutrophil lysis was determined by measuring LDH release, whereas for bacterial killing growth colony forming units (CFUs) were counted for each time point. Neutrophil phagocytosis of MW2 induced rapid neutrophil cell death (Figure 1B), which is in line with previously published data (Voyich et al., 2005, Kobayashi et al., 2010). In contrast, blocking phagocytosis by HI-serum+FLIPr-Like or CytD treatment, resulted in a considerably delayed destruction of neutrophils by MW2. Although phagocytosis resulted in killing of MW2 at early time points, there was a rebound of bacterial growth starting 4 hours after phagocytosis (and at which point there is significant lysis of neutrophils), indicating survival of bacteria after neutrophil phagocytosis (Figure 1C; black line). When phagocytosis was blocked by HI-serum+FLIPr-Like or CytD treatment, bacterial growth started 2 hours earlier, as compared to bacteria that underwent phagocytosis (Figure 1C; grey lines). Moreover, there was a clear correlation between the lysis of neutrophils and the survival of MW2 (Figure 1B).
Measuring bacterial survival using GFP
These bacterial growth results were used to validate an alternative to the labor-intensive counting of CFUs, measuring bacterial survival and outgrowth by GFP-fluorescence produced by living MW2-GFP bacteria in a fluorescence plate reader. Using fluorescence, we were able to measure bacterial outgrowth after phagocytosis, where absorbance measurements were not possible, due to the interference of neutrophils in the culture. No difference in neutrophil lysis could be observed when cells were infected with wild type MW2 or MW2-GFP S. aureus (Figure 1D), nor did these bacteria grow differently in culture medium (data not shown). To determine the effect of phagocytosis on the growth of MW2-GFP, the same experimental setup was used as before, allowing phagocytosis in the presence of serum or blocking phagocytosis by HI-serum+FLIPr-Like or CytD treatment. The only difference was the measurement of MW2-GFP growth, which was performed in a fluorescent plate reader, instead of counting CFUs. Comparable to the results shown in Figure C, blockade of phagocytosis resulted in earlier outgrowth of MW2-GFP, whereas phagocytic killing resulted in a clear delay in bacterial outgrowth, measured as GFP fluorescence (Figure 1E). Of note, adding no neutrophils or only buffer without opsonins resulted in early outgrowth, similar to blocking phagocytosis, demonstrating only minor extracellular killing of MW2-GFP by neutrophils in these assay conditions (data not shown). Overall, in accordance with previous findings, we found that phagocytosis of S. aureus strain MW2 by neutrophils results in killing of the bacteria using our assay conditions. However, some bacteria can survive in these in vitro conditions and over time escape from neutrophils and replicate.
Time lapse fluorescent microscopy was used to visualize neutrophil lysis and bacterial growth after MW2-GFP phagocytosis on a single cell basis. MW2-GFP was incubated with neutrophils as described above to allow for phagocytosis. Both the growth of the bacteria (GFP) and the disruption of the neutrophil membranes (PI, red staining) were monitored over time. The results obtained using this setup were similar compared to those obtained in the experiments described above. When no phagocytosis takes place, the bacteria are able to rapidly replicate in the growth medium, and eventually most neutrophils become PI positive. However, when MW2-GFP is phagocytosed, bacterial growth is clearly delayed, but neutrophil lysis is advanced (Figure 2 and Supplementary movies 1–4), illustrating that neutrophil phagocytosis of staphylococci promotes lysis of human neutrophils. Moreover, a certain percentage of S. aureus MW2-GFP escapes after phagocytosis by neutrophils and replicate in the extracellular milieu.
Figure 2. Time lapse analysis of neutrophil lysis and bacterial outgrowth.
(A) Analysis of neutrophil phagocytosis of CA-MRSA strain MW2 by time lapse fluorescence microscopy. Neutrophils were incubated with strain MW2, which constitutively expresses GFP (MW2-GFP) for 30 min, in the presence of human serum (phagocytosis), or absence of opsonin (Buffer) (No phagocytosis), or serum with addition of cytochalasin D (CytD), or heat-inactivated serum together with pre-incubation of neutrophils with FLIPR-Like (FcγR-inhibitor) (HI-HPS+Flipr-L). A representative frame series out of three independent experiments is shown. Brightfield (grey), GFP (green) and PI (red) channels are combined.
PSMα peptides produced inside neutrophils promote lysis of neutrophils and survival of S. aureus
Many studies have suggested a prominent role for PSMs in staphylococcal virulence, based on their capacity to lyse and activate neutrophils (Wang et al., 2007). However, as we described before, in the presence of serum lipoproteins PSMs may be functionally neutralized (Surewaard et al., 2012). We reasoned that PSMs could function after phagocytosis and promote neutrophil lysis from inside the phagosome. To address this possibility, neutrophils were infected with MW2 and MW2 derivatives, in which single or all psm operons, or the agr system were deleted (Δagr, Δα, Δβ,Δhld,Δαβhld). In addition, we included a PVL-negative mutant of strain MW2 (MW2ΔlukSF) in our experiments to compare the effects of PSMs on intracellular lysis to PVL. Furthermore, a PSMα-complemented Δα pTXα strain was tested. First, we investigated whether the phagocytic uptake is similar for all strains. Therefore, we fluorescently labeled these strains and measured phagocytosis 30 min after infection.
No difference in the rate of phagocytosis was observed, demonstrating that all strains were equally well ingested by human neutrophils (Figure 3A). Three hours after infection, lysis of neutrophils was monitored by LDH release. The production of many virulence toxins are under control of the agr system, including the PSMs. Significantly less neutrophils were lysed when cultured with the Δagr strain (Figure 3B), although not to the extent of those lysed by the wild-type strain, indicating mechanisms of agr-independent neutrophil lysis exist. Interestingly, the psmα operon is fully responsible for this effect, as the neutrophil lysis caused by Δα and Δαβhld strains was comparable to that caused by the Δagr strain. In addition, with the psmα-complemented Δα pTXα strain recovery of the wild type MW2 phenotype was accomplished. Other PSM loci and PVL are not important for neutrophil lysis after phagocytosis, as they did not influence the neutrophil lysis compared to wild type MW2. Taken together, these date indicate that PSMα peptides produced by intracellular S. aureus promote lysis of human neutrophils.
Figure 3. Impact of PSM deletions on neutrophil lysis after phagocytosis.
(A) Analysis of neutrophil phagocytosis of FITC-labeled MW2 and isogenic agr, PSM, and PVL mutant strains. Neutrophils were allowed to phagocytose MW2 strains in the presence of human serum. After 30 min phagocytosis was measured by flow cytometry. (B) Neutrophil lysis 3hrs after phagocytosis (MOI 10) of MW2 strains, as determined by LDH release. Data represent the mean ± SEM of 4–5 independent experiments with neutrophils isolated from different donors.
Next, we investigated whether the observed decrease in neutrophil lysis when using the MW2Δα and Δαβhld strains impacts bacterial survival and subsequent outgrowth. For this purpose, neutrophils were fed MW2, Δα, Δβ,Δhld, and Δαβhld strains, which constitutively express GFP on a plasmid. Subsequently, GFP fluorescence was monitored in a fluorescence plate reader for 10 hours to investigate bacterial growth. When phagocytosis was blocked, no difference in bacterial growth was observed between the different mutants (Figure 4). When phagocytosis occurred, there was a clear delay in outgrowth of the Δα and Δαβhld strains, whereas the growth curves of the other psm mutant strains were indistinguishable from that of the wild type MW2 strain. Previously we and others have shown that PSM expression is upregulated after phagocytosis of staphylococci (Surewaard et al., 2012, Geiger et al., 2012). These results further confirm that PSMα peptides produced in the intracellular space promote the escape of S. aureus from the neutrophil.
Figure 4. Staphylococcal outgrowth after phagocytosis.
Neutrophils were allowed to phagocytose MW2, Δα, Δβ,ΔHld,ΔαβHld strains, which are transformed to constitutively express GFP (MOI 10). Alternatively, phagocytosis was blocked by HI-serum and FcγR-inhibitor FLIPr-Like or by 20 μg/mL Cytochalasin D. Bacterial growth represented by GFP Fluorescence was monitored for 10 hours after infection. Results represent the means out of 4 replicates.
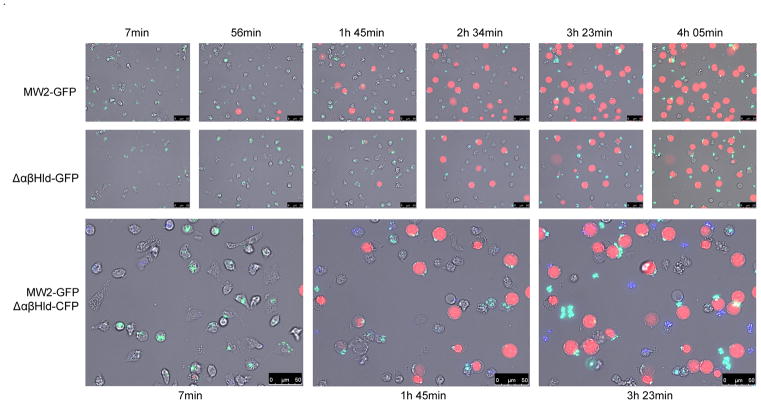
Time lapse video microscopy was used to monitor the effect of PSMs produced in the intracellular space on the growth of the bacteria (GFP) and on the disruption of the neutrophil membranes (PI staining) over time. Again, the results indicated that PSMs contribute to the lysis of neutrophils after phagocytosis of S. aureus, as there was a clear delay in the timing of neutrophil lysis when neutrophils were infected with the psm triple mutant compared to the wild-type strain (Figure 5; MW2-GFP versus Δαβhld-GFP). Additionally, we tested the difference between neutrophil death and bacterial growth when neutrophils, after separate phagocytosis of wild type MW2 or Δαβhld, were incubated in the same well. To enable separate visualization of both strains, a MW2Δαβhld strain was used that constitutively expresses CFP (Δαβhld-CFP; blue staining). When neutrophils were infected with MW2-GFP or Δαβhld-CFP, killing of neutrophils infected with MW2-GFP was considerably advanced (Figure 5; MW2-GFP/Δαβhld-CFP), as neutrophils containing green (MW2-GFP) bacteria stained PI-positive earlier then Δαβhld-CFP-positive neutrophils. These data further confirm that PSMs contribute to lysis of the neutrophils after phagocytosis of S. aureus, and thereby promote staphylococcal escape from the neutrophil, as graphically depicted in Figure 6.
Figure 5. Time lapse analysis of neutrophil lysis and bacterial outgrowth of MW2 and the isogenic ΔαβHld mutant.
Neutrophils were incubated with strain MW2-GFP, ΔαβHld-GFP or ΔαβHld-CFP, for 30 min, in the presence of 10% human serum to allow proper phagocytosis. After phagocytosis neutrophil killing was analyzed separately (upper two lanes), or together (bottom lane). A representative frame series out of three independent experiments is shown. Bright field (grey), GFP (green) CFP (blue) and PI (red) channels are combined.
Figure 6. Staphylococcal PSMs contribute to phagosomal escape and intracellular lysis in the presence of serum.
Graphic illustration depicting the role of staphylococcal PSMs during infection. Neutrophils recognize PSMs via their Formylated Peptide Receptor 2, are subsequently activated, attracted and will phagocytose the bacteria. Extracellular PSMs will be neutralized by serum lipoproteins. After phagocytosis, the pathogen can either be destroyed by the neutrophil or PSMα can contribute in the escape from the phagosome by lysing the neutrophil from the inside, which ultimately results in escape of S. aureus from the neutrophil.
Discussion
Neutrophils play a crucial role in clearing staphylococcal infections. Nevertheless, a fraction of S. aureus is capable of evading this innate immune defense, as it has evolved multiple and often redundant ways to evade neutrophils and remain undetected (Rooijakkers et al., 2005, Serruto et al., 2010). In addition, S. aureus makes use of a whole arsenal of cytolytic toxins. Leukocidins such as PVL (Panton et al., 1932), γ-hemolysins (HlgAB and HlgCB) (Cooney et al., 1988), and the recently described leukocidin GH (LukGH) (Ventura et al., 2010), also known as LukAB (Dumont et al., 2011), are bi-component toxins that can specifically lyse neutrophils, most probably by formation of an octameric pore (Yamashita et al., 2011). Of these bi-component toxins, PVL has recently received most attention, as this toxin is associated with CA-MRSA strains (Labandeira-Rey et al., 2007, Vandenesch et al., 2003). However, there has been controversy about the extent to which this toxin contributes to S. aureus pathogenesis (Bubeck Wardenburg et al., 2008, Kobayashi et al., 2011). Many of the controversial findings that were obtained may be explained by species specificity, since murine leukocytes are highly resistant to cytolysis in vitro, whereas human and rabbit cells are highly susceptible to the toxin (Loffler et al., 2010). The species specificity of these bi-component toxins most likely is determined by the interaction with host G protein-coupled receptors, as was demonstrated recently for leukocidin ED (LukED) (Francis et al., 2012). In contrast, S. aureus virulence attributed to PSMs is not species specific. Multiple cell types can be lysed, liposomes can be perforated (Cogen et al., 2010) and genetic deletion of the PSMs impacts virulence in mice and rodents (Li et al., 2010, Wang et al., 2007). Together with the notion that PSMs are efficiently inhibited by the abundantly present lipoproteins, we hypothesized that PSMs could elicit their toxicity not as extracellular toxins but rather function from within the neutrophil after phagocytosis. Here, we show that PSMs are involved in neutrophil lysis after phagocytosis and that the PSMα operon is necessary and sufficient for this effect, inasmuch as psmα deletion strains have a significantly decreased capacity to lyse neutrophils after phagocytosis. Moreover, psmα deletion results in decreased bacterial survival and subsequent outgrowth, as compared to the wild type strain. In figure 6 we present a model of what happens to the S. aureus cell after it is phagocytosed by a neutrophil. By default phagocytosis will lead to destruction of the pathogen by the neutrophil. However, if S. aureus manages to produce enough PSMα in the phagosome, this will lead to lysis of the neutrophil from the inside, resulting in survival and escape of the bacterium.
Geiger et al. recently showed that plasmid-based expression of the psmα or psmβ operon results in increased escape from neutrophils after phagocytosis (Geiger et al., 2012). They also observed diminished lysis from the intracellular space using a psmα/psmβ double mutant versus wild-type S. aureus Newman. These findings contradict ours, inasmuch as we did not detect a role of PSMβ peptides in that phenotype, which is in accordance with previous findings showing no cytolytic activity of PSMβ peptides (Wang et al., 2007). Notably, our experiments were performed with single isogenic psm deletion strains in a CA-MRSA strain, pinpointing lysis after phagocytosis to the PSMα peptides and allowing a conclusion on the contribution of the investigated toxins to their biological role in the background of an important clinical strain.
Multiple lines of evidence have accumulated in recent years demonstrating that S. aureus can survive within cells, or even use host cells as transport to disseminate from the site of infection (Thwaites et al., 2011, Gresham et al., 2000, Rogers, 1956). One strategy involves the formation of small colony variants (SCVs), which is crucial for establishing chronic infections such as osteomyelitis (Tuchscherr et al., 2011). Using this strategy, staphylococcal cells within epithelial cells are less susceptible to antibiotics and remain undetected by lowering their metabolism and growth and expression of virulence factors and regulators. However, to escape from the phagosome, staphylococci need to adopt a more virulent phenotype (Rigotti et al., 1997). This switch is dependent on the intracellular activation of the agr system (Ng et al., 1997). Inside host cells the concentration of the quorum sensing pheromone AIP can reach the critical concentration necessary for agr activation (Pang et al., 2010, Carnes et al., 2010). In line with these studies, we have shown with Ppsm-GFP reporter constructs that the psmα operon is activated after phagocytosis of S. aureus by neutrophils (Surewaard et al., 2012). Very recently, it was demonstrated that the stringent response, characterized by the rapid synthesis of (p)ppGpp as messenger of environmental stress conditions, is preceding the quorum sensing mechanism. Both systems appear to be crucial for up-regulating PSMs in the neutrophil phagosome and contribute to the lysis of neutrophils after phagocytosis and subsequent escape of the staphylococci (Geiger et al., 2012).
The escape of S. aureus from endosomes in epithelial cells was reported to be dependent on β-toxin, β-PSM and Hld (Giese et al., 2011). However, the corresponding studies were also only performed using plasmid-based artificial overexpression. Whether especially the PSMβ peptides contribute to that phenotype in a natural S. aureus background remains to be determined. Interestingly, while α-haemolysin (α-toxin, Hla) does not cause lysis of human neutrophils when added exogenously, a role for that toxin in phagosomal escape from human neutrophils and intracellular vacuoles of airway epithelial cells and was described previously (Pang et al., 2010). In strain USA300 it was demonstrated that also LukGH plays a role in neutrophil lysis after phagocytosis (Ventura et al., 2010). Therefore, among different S. aureus strains multiple mechanisms, instead of a single virulence factor, may play a role in staphylococcal escape after phagocytosis; and different toxins may cause escape in different cell types.
Surprisingly, even in the total absence of PSMs (e.g. in agr or psmαβhld mutants) strain MW2 can induce lysis and escape from neutrophil-mediated killing, although at lower levels. This is in accordance with previously published data indicating that staphylococci escape from neutrophils independently of toxins when assayed six hours post infection (Kobayashi et al., 2010). Importantly, next to its array of toxins, S. aureus appears to make use of the golden pigment staphyloxanthin and several catalases to survive the hostile environment of the neutrophil phagosome. Both these factors protect against reactive oxygen species by functioning as antioxidants (Liu et al., 2005). Furthermore, modification of the cell wall by O-acetylation of peptidoglycan leads to resistance against lysozyme; and surface-located teichoic acids protect against antimicrobial peptides (Weidenmaier et al., 2004). In in vitro model systems, S. aureus may use these factors to prolong survival until the neutrophil is weakened enough for the bacteria to escape. During infection of the host, the ratio of neutrophils to S. aureus is likely much more in favor of the neutrophil, making it necessary for S. aureus to employ aggressive methods to survive the concerted attack of the innate immune system.
In conclusion, we show in this study that the PSMα peptides play an important role in the escape from neutrophils by killing the neutrophils from within. These data not only contribute to our understanding of the mechanisms virulent S. aureus strains use to circumvent killing by neutrophils, they also have major implications for the development of S. aureus vaccine strategies. First, strategies to specifically target PSM peptides will have to consider that these virulence factors exert a major function in pathogenesis within the neutrophil, which might make strategies employing anti-PSM antibodies difficult. Second, our findings may explain why traditional active vaccination strategies against S. aureus have so far failed, inasmuch as efficient killing of the neutrophil after phagocytosis undermines the increase of opsonophagocytosis by neutrophils, by which those vaccines mainly are supposed to function (DeLeo et al., 2008).
Experimental procedures
Ethics statement
Informed written consent was obtained from all donors and was provided in accordance with the Declaration of Helsinki. Approval was obtained from the medical ethics committee of the University Medical Center Utrecht (Utrecht, The Netherlands).
Bacterial strains and culture conditions
The CA-MRSA strain MW2 (1999) and isogenic lukSF, psm and agr deletion mutants (Wang et al., 2007, Periasamy et al., 2012) were used for all experiments. In the Δhld strain, translation of hld is interrupted by a single base mutation of the start codon, which allows for maintained function of RNAIII. The psmα gene locus was complemented in Δα strain as described (Wang et al., 2007). The MW2 strain and all isogenic psm mutant strains were transformed with pCM29 (Pang et al., 2010) or pTH3 (Nijland et al, in prep.) that from the sarAP1 promoter constitutively and robustly express the superfolder green fluorescent protein (sGFP) or the cyan fluorescent protein Cerulean (CFP) respectively. Additionally, the Δαβhld strain was transformed with pCM29 expressing cyan fluorescent protein (CFP). Bacteria were grown in Müller-Hinton broth (MHb) at 37°C while shaking. When appropriate, tetracycline (12.5 μg/mL) or chloramphenicol (10 μg/mL) was added for o/n maintenance of the plasmids.
Ex vivo phagocytosis assay
Neutrophils were isolated as previously described (Surewaard et al., 2012). To investigate the S. aureus-mediated killing of human neutrophils after phagocytosis, MW2, the isogenic psm, lukSF and agr mutants or similar strains constitutively expressing GFP were used. Bacterial strains were grown o/n in MHb supplemented with 10 μg/mL chloramphenicol. On the day of the experiment, the strains were diluted 20x in MHb without antibiotics and cultured until late log phase (OD660nm 1.0). Bacteria were washed and resuspended in RPMI-1640 containing 25 mM HEPES, L-glutamine (Biowhittaker), and 0,05% human serum albumin (HSA; Sanquin) (RPMI-HSA). Freshly isolated neutrophils were pre-treated with or without 10 μg/mL FLIPr-Like (FcγR-inhibitor) or with and without cytochalasin D (Sigma) to investigate the influence of phagocytosis. Subsequently, 5×105 neutrophils were mixed with 5×106 bacteria (MOI=10) in the presence of 10% normal human pooled serum (15 donors) or heat-inactivated serum (from same serum pool) in a final volume of 100 μL RPMI-HSA. Phagocytosis was initiated at 37°C with shaking for 15 min. For some experiments phagocytosis was measured by flow cytometry. Neutrophils were gated based on their forward and side scatter profiles and the percentage of neutrophil-associated GFP-positive bacteria was used as measurement for phagocytosis. In these conditions both ingested bacteria as well as bacteria bound to the neutrophil surface are measured. We will refer to both these phenomenons as phagocytosis. For other experiments, phagocytosis was followed by various incubation times at 37°C while gently shaking. Cells were pelleted by centrifugation at different time points after infection and neutrophil lysis was determined in the supernatant by detection of lactate dehydrogenase (LDH) using the CytoTox 96 Non-Radioactive Cytotoxicity kit (Promega), according to the manufacturer’s protocol.
Staphylococcal outgrowth after phagocytosis
Neutrophil-mediated killing of S. aureus and staphylococcal outgrowth after phagocytosis was monitored by culturing and phagocytosis of the staphylococcal strains, as described above, with the exception that neutrophils were seeded at 2.5×105 cells per well and infected with 2.5×106 bacteria (MOI=10). At different time points post phagocytosis the neutrophils were lysed by 10 times dilution in 0.1% Saponin H20 (pH 11.0) for 15 min on ice. Bacteria were than serially diluted in 10 fold dilutions in PBS and plated on sheep blood agar plates (Oxoid). Recovered bacteria were determined by counting CFUs and normalized at each time point to the input CFU. Alternatively, bacterial outgrowth was measured as GFP fluorescence. After initiation of phagocytosis, neutrophils were transferred to a clear 96 well flat bottom polystyrene tissue culture plate (Greiner) and 50 μl RPMI-HSA was added, resulting in 150 μL culture/well. The plate was grown in a Fluostar Omega plate reader (BMG labtech) at 37°C with constant double orbital shaking (400 rpm) in between measurements. Both the absorbance at 660 nm and GFP fluorescence (excitation 485 nm/emission 520 nm) were measured every 10 minutes for each well. The signal from 4 identical wells was averaged and corrected for blank wells containing only medium.
Time-lapse microscopy
Each well of a 8 well Lab-Tek II chambered coverglass (Thermo Scientific) was loaded with 200 μl of RPMI-HSA. 1×105 neutrophils that had phagocytosed GFP-labeled MW2 strains as described above were added and were allowed to settle at the bottom of the well. Propidium iodine (Invitrogen) was added at a final concentration of 4 μM to visualize disruption of neutrophil membranes in time. Microscopic image acquisition of neutrophils and bacteria was performed using a Leica TSC SP5 inverted microscope equipped with a HCX PL APO 40 × 0.85 objective (Leica Microsystems, The Netherlands). The microscope was encased in a dark environment chamber that was maintained at 37°C. The cells and bacteria were monitored for CFP (CFP ET filter cube) and/or GFP (GFP ET filter cube), PI (N21 filter cube), and brightfield every 7 minutes at four positions in each well. To create a time lapse movie of the interaction between the neutrophils and the MW2 strains, the separate channels were combined and rendered as a time-lapse movie using Leica LAS AF software.
Statistics
Statistical analysis was performed using Graph Pad Prism Version 5 with two-tailed unpaired T-tests (two groups) or one-way ANOVA with Bonferoni posttest (multiple groups)
Supplementary Material
Acknowledgments
The authors thank Alexander Horswill for providing the GFP expression plasmid pCM29. RN was partially funded by a Marie Curie European Re-integration Grants (ERG) from the European Community’s Seventh Framework Programme, project number 268324. MO, KR and FD are supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health.
References
- 1.From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus--Minnesota and North Dakota 1997–1999. JAMA. 282:1123–1125. [PubMed] [Google Scholar]
- 2.Bubeck Wardenburg J, Palazzolo-Ballance AM, Otto M, Schneewind O, DeLeo FR. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J Infect Dis. 2008;198:1166–1170. doi: 10.1086/592053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnes EC, Lopez DM, Donegan NP, Cheung A, Gresham H, Timmins GS, Brinker CJ. Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nature Chemical Biology. 2010;6:41–45. doi: 10.1038/nchembio.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers HF, DeLeo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nature Reviews Microbiology. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung GY, Duong AC, Otto M. Direct and synergistic hemolysis caused by Staphylococcus phenol-soluble modulins: implications for diagnosis and pathogenesis. Microbes Infect. 2012;14:380–386. doi: 10.1016/j.micinf.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cogen AL, Yamasaki K, Sanchez KM, Dorschner RA, Lai Y, MacLeod DT, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooney J, Mulvey M, Arbuthnott JP, Foster TJ. Molecular cloning and genetic analysis of the determinant for gamma-lysin, a two-component toxin of Staphylococcus aureus. J Gen Microbiol. 1988;134:2179–2188. doi: 10.1099/00221287-134-8-2179. [DOI] [PubMed] [Google Scholar]
- 8.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: Epidemiology and clinical consequences of an emerging epidemic. Clinical Microbiology Reviews. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLeo FR, Otto M. An antidote for Staphylococcus aureus pneumonia? Journal of Experimental Medicine. 2008;205:271–274. doi: 10.1084/jem.20080167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deleo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, et al. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol. 2011;79:814–825. doi: 10.1111/j.1365-2958.2010.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis A, III, Lina K, Stephen AR, Tamara R-R, Ashley LD, David GM, et al. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature. 2012 doi: 10.1038/nature11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, et al. The Stringent Response of Staphylococcus aureus and Its Impact on Survival after Phagocytosis through the Induction of Intracellular PSMs Expression. PLoS Pathog. 2012;8:e1003016. doi: 10.1371/journal.ppat.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giese B, Glowinski F, Paprotka K, Dittmann S, Steiner T, Sinha B, Fraunholz MJ. Expression of δ-toxin by Staphylococcus aureus mediates escape from phago-endosomes of human epithelial and endothelial cells in the presence of β-toxin. Cellular Microbiology. 2011;13:316–329. doi: 10.1111/j.1462-5822.2010.01538.x. [DOI] [PubMed] [Google Scholar]
- 15.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi SD, Braughton KR, Palazzolo-Ballance AM, Kennedy AD, Sampaio E, Kristosturyan E, et al. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J Innate Immun. 2010;2:560–575. doi: 10.1159/000317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, et al. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. Journal of Infectious Diseases. 2011;204:937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kretschmer D, Gleske AK, Rautenberg M, Wang R, Koberle M, Bohn E, et al. Human formyl peptide receptor 2 senses highly pathogenic Staphylococcus aureus. Cell Host Microbe. 2010;7:463–473. doi: 10.1016/j.chom.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labandeira-Rey M, Couzon F, Boisset S, Brown EL, Bes M, Benito Y, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Cheung GY, Hu J, Wang D, Joo HS, Deleo FR, Otto M. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J Infect Dis. 2010;202:1866–1876. doi: 10.1086/657419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Diep BA, Villaruz AE, Braughton KR, Jiang X, DeLeo FR, et al. Evolution of virulence in epidemic community-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2009;106:5883–5888. doi: 10.1073/pnas.0900743106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, et al. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. Journal of Experimental Medicine. 2005;202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loffler B, Hussain M, Grundmeier M, Bruck M, Holzinger D, Varga G, et al. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 25.Ng DS, Francone OL, Forte TM, Zhang J, Haghpassand M, Rubin EM. Disruption of the murine lecithin:cholesterol acyltransferase gene causes impairment of adrenal lipid delivery and up-regulation of scavenger receptor class B type I. J Biol Chem. 1997;272:15777–15781. doi: 10.1074/jbc.272.25.15777. [DOI] [PubMed] [Google Scholar]
- 26.Pang YY, Schwartz J, Thoendel M, Ackermann LW, Horswill AR, Nauseef WM. agr-Dependent Interactions of Staphylococcus aureus USA300 with Human Polymorphonuclear Neutrophils. Journal of Innate Immunity. 2010;2:546–559. doi: 10.1159/000319855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panton PN, Valentine FCO. Staphylococcal Toxin. The Lancet. 1932:506–508. [Google Scholar]
- 28.Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, et al. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A. 2012;109:1281–1286. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, et al. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell. 2008;32:150–158. doi: 10.1016/j.molcel.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rautenberg M, Joo HS, Otto M, Peschel A. Neutrophil responses to staphylococcal pathogens and commensals via the formyl peptide receptor 2 relates to phenol-soluble modulin release and virulence. FASEB J. 2010 doi: 10.1096/fj.10-175208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997;94:12610–12615. doi: 10.1073/pnas.94.23.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers DE. Studies on bacteriemia. I. Mechanisms relating to the persistence of bacteriemia in rabbits following the intravenous injection of staphylococci. J Exp Med. 1956;103:713–742. doi: 10.1084/jem.103.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, et al. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005a;6:920–927. doi: 10.1038/ni1235. [DOI] [PubMed] [Google Scholar]
- 35.Rooijakkers SH, van Kessel KP, van Strijp JA. Staphylococcal innate immune evasion. Trends Microbiol. 2005b;13:596–601. doi: 10.1016/j.tim.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Serruto D, Rappuoli R, Scarselli M, Gros P, van Strijp JA. Molecular mechanisms of complement evasion: learning from staphylococci and meningococci. Nat Rev Microbiol. 2010;8:393–399. doi: 10.1038/nrmicro2366. [DOI] [PubMed] [Google Scholar]
- 37.Stemerding A, KJ, Pandey M, Kuipers A, Leusen J, Boross P, Nederend M, Vidarsson G, Weersink A, Winkel J, van Kessel K, van Strijp J. Staphylococcus aureus Formyl Peptide Receptor-Like 1 Inhibitor (FLIPr) and its Homologue FLIPr-like are Potent Fcγ Receptor Antagonists that Inhibit IgG-Mediated Effector Functions. 2013 doi: 10.4049/jimmunol.1203243. [DOI] [PubMed] [Google Scholar]
- 38.Surewaard BG, Nijland R, Spaan AN, Kruijtzer JA, de Haas CJ, van Strijp JA. Inactivation of staphylococcal phenol soluble modulins by serum lipoprotein particles. PLoS Pathog. 2012;8:e1002606. doi: 10.1371/journal.ppat.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thwaites GE, Gant V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nat Rev Microbiol. 2011;9:215–222. doi: 10.1038/nrmicro2508. [DOI] [PubMed] [Google Scholar]
- 40.Tuchscherr L, Medina E, Hussain M, Volker W, Heitmann V, Niemann S, et al. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med. 2011;3:129–141. doi: 10.1002/emmm.201000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al. Community-acquired methicillin-resistant staphylococcus aureus carrying panton-valentine leukocidin genes: Worldwide emergence. Emerging Infectious Diseases. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ventura CL, Malachowa N, Hammer CH, Nardone GA, Robinson MA, Kobayashi SD, DeLeo FR. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One. 2010;5:e11634. doi: 10.1371/journal.pone.0011634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Saïd-Salim B, Porcella SF, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. Journal of Immunology. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 44.Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 45.Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, et al. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- 46.Yamashita K, Kawai Y, Tanaka Y, Hirano N, Kaneko J, Tomita N, et al. Crystal structure of the octameric pore of staphylococcal gamma-hemolysin reveals the beta-barrel pore formation mechanism by two components. Proc Natl Acad Sci U S A. 2011;108:17314–17319. doi: 10.1073/pnas.1110402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.