Abstract
OBJECTIVES
The long-term outcomes of patients with drug induced liver injury (DILI) are not well described. The aim of this study was to determine the frequency and severity of persistent liver biochemistry abnormalities in DILI patients followed over 2 years.
METHODS
Subjects with evidence of liver injury at 6 months after DILI onset were offered a month 12 and 24 study visit.
RESULTS
Amongst the 99 patients with definite, probable, or very likely DILI and available laboratory data at 12 months after DILI onset, 74 (75%) had persistent liver injury (persisters) defined as a serum AST or ALT > 1.5 × upper limit of normal (ULN) or an alkaline phosphatase > ULN, while 25 (25%) had resolved liver injury (resolvers). On multivariate analysis, month 12 persisters were significantly older (52.6 vs 43.7 years, p=0.01) and more likely to have a cholestatic lab profile at DILI onset (54% vs 20%, p < 0.01) than resolvers. The month 12 persisters also had significantly poorer SF-36 Physical summary scores at DILI onset and throughout follow-up compared to the resolvers (p < 0.01). Amongst the 17 subjects with a liver biopsy obtained at a median of 387 days after DILI onset, 9 had chronic cholestasis, 3 had steatohepatitis, and 3 had chronic hepatitis.
CONCLUSION
75% of subjects with liver injury at 6 months after DILI onset have laboratory evidence of persistent liver injury during prolonged follow-up. Higher serum alkaline phosphatase levels at presentation and older patient age were independent predictors of persistent liver injury. Subjects with persistent liver injury at 12 months after DILI onset should be carefully monitored and assessed for liver disease progression.
Keywords: Hepatotoxicity, cholestasis, liver histology, ductular reaction
Introduction
Idiosyncratic drug induced liver injury (DILI) is a leading cause of acute liver failure (ALF) in western countries (1,2). We recently dmonstrated that approximately 60% of patients with bonafide DILI in the US required hospitalization and that 10% of patients died or underwent liver transplantation within 6 months of DILI onset (2,3). Furthermore, 19% had evidence of liver injury 6 months after DILI onset defined by an elevated liver biochemistry level, abnormal liver imaging or histopathology (2). Whether these patients will develop clinically significant liver disease or simply experience slowly resolving liver injury during prolonged follow-up is unknown. Prior studies have suggested that continued administration of the suspect drug and presentation with an acute cholestatic liver injury profile may result in a slower rate of laboratory improvement and an increased risk of developing chronic liver disease (4–6). However, the number of patients reported in these studies was limited and varying criteria were used to define chronic liver injury (4–8).
The aim of the current study is to describe the 2 year outcomes of consecutive adult DILI patients enrolled in the ongoing Drug Induced Liver Injury Network (DILIN) prospective study with evidence of ongoing liver injury 6 months after DILI onset. DILIN is a multicenter consortium sponsored by the National Institutes of Health that is conducting prospective studies focused on the etiology, risk factors, and natural history of DILI (8). For the current study, patients with persistent liver injury (persisters) were defined by a serum AST or ALT > 1.5 × upper limit of normal (ULN) or an alkaline phosphatase > ULN at 12 months after DILI onset. Those not meeting these criteria were considered to have minimal to resolved liver injury (resolvers). Baseline and longitudinal parameters associated with persistent versus resolving liver injury at 12 months after DILI onset were identified. In addition to describing the evolution of laboratory parameters and liver histology, health related quality of life parameters were compared in the month 12 persisters and resolvers through 2 years of follow-up.
METHODS
Patient population
991 patients were enrolled in the DILIN prospective registry study from September 2004 through July 31, 2011. The research protocol was approved by the local institutional review boards and all participants provided written informed consent. The causal relationship between the liver injury episode and the implicated agent(s) were evaluated in a standardized fashion by the DILIN causality committee (8, 9). An overall DILIN causality score varying from 1 (Definite > 95% likelihood), 2 (Very Likely 75%–95% likelihood), 3 (Probable 50%–74% likelihood), 4 (Possible 25%–49% likelihood) to 5 (unlikely < 25% likelihood) was assigned by consensus agreement of committee members (9). In cases with 2 or more implicated drugs, an overall DILIN causality score was assigned and then an individual causality score was given for each suspect drug or herbal and dietary supplement (HDS). Of the 991 enrolled patients, 801 were adjudicated as definite, highly likely, or probable DILI. Of these 801 DILI patients, 143 were excluded from the analysis of early outcomes due to age < 18 (n=38), pre-existing chronic hepatitis B or C infection (n=28) or dropping out of the study before 6 month follow-up (n=77). (Figure 1) Of the remaining 660 patients, there were 62 patients who either died or underwent liver transplantation within 6 months of DILI onset. Thus, 598 adult DILIN patients had data available at baseline and 6 months after DILI onset for analysis of chronic DILI risk factors.
Figure 1. Overview of Study population.
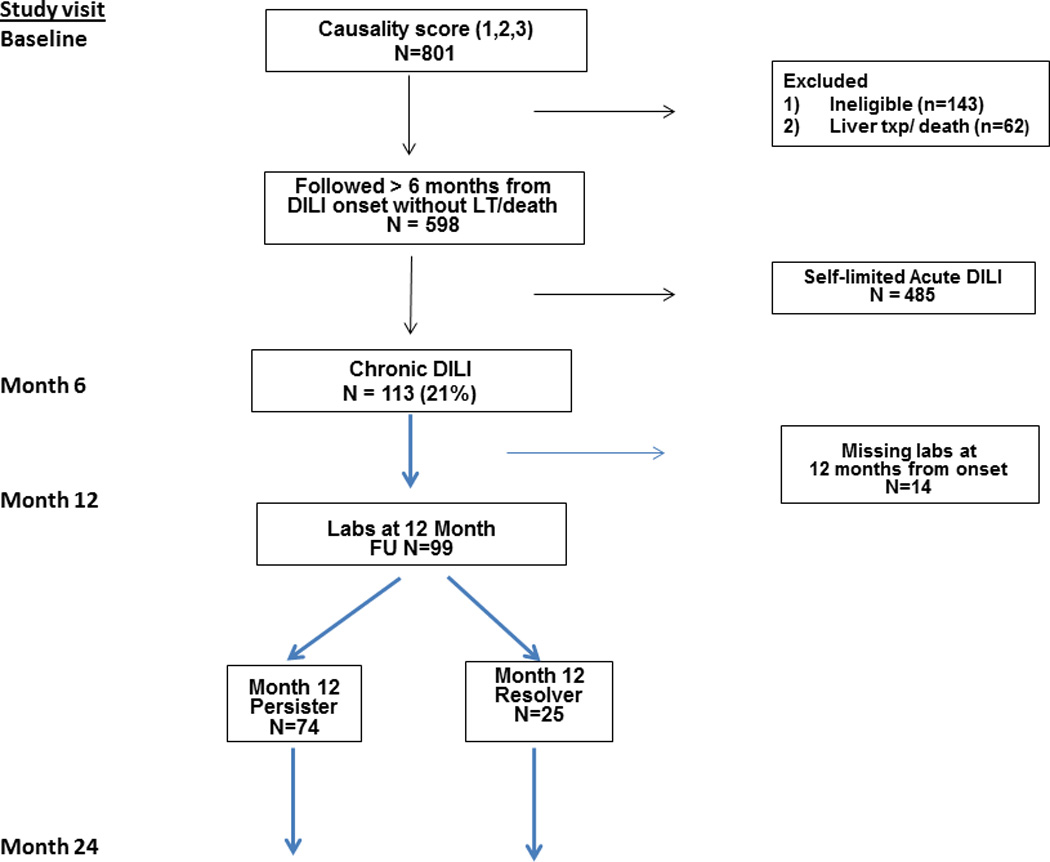
Amongst the 598 patients enrolled in DILIN between 9/04 and 7/11, 113 patients met protocol defined criteria for ongoing liver injury at 6 months after DILI onset. Amongst those patients, the 99 subjects with laboratory data available at month 12 after DILI onset were categorized as Persisters (n=75) if they had abnormal liver biochemistries or resolvers (n=24).
Of these 598 patients, 113 had chronic DILI at least 6 months after DILI onset defined by one or more of the following criteria: elevated serum AST, ALT, alkaline phosphatase or total bilirubin level, histological evidence of liver injury, or radiological evidence of liver disease (i.e. ascites on imaging) (3,8). These 113 are the focus of this study, and per protocol, they were offered follow up through month 24 after DILI onset. Patients were categorized as either persisters or resolvers, based on the liver enzyme values at 12 months from DILI onset. Persisters were defined as serum AST or ALT > 1.5×upper limit of normal (ULN) or an alkaline phosphatase > ULN at 12 months from DILI onset. Resolvers did not meet these criteria and were considered to have minimal or no ongoing liver injury at 12 months after DILI onset.
Clinical Data
A detailed medical and medication history was obtained at the baseline study visit and additional laboratory and radiological testing were performed to more fully characterize the DILI event and exclude competing etiologies of liver injury (2,8). Alcohol use was determined using the modified Skinner instrument. Heavy alcohol use in the 5 years prior to DILI onset was defined as a mean consumption of > 21 alcoholic beverages per week for men and >14 drinks per week for women. All enrolled patients were seen for a follow-up study visit at 6 months after initial enrollment. The subjects with ongoing liver injury at 6 months after DILI onset returned for additional follow-up study visits at 12 and 24 months after DILI onset. Severity of DILI injury was assessed on a 5 point scale used in the DILIN protocol with 1= asymptomatic to 5= Death/ liver transplant (8).
Assessment of symptoms
A 12 question likert scale was completed by subjects at baseline and month 6, 12, and 24 study visits to assess for symptoms frequently reported in patients with liver disease including nausea, abdominal pain, fatigue, pruritus, pain over liver area, poor appetite, muscle aches, weakness, fevers and chills, rash, sadness, and overall feeling. The 10 point likert scale varied from 1 = Not at all to 10= worse ever.
Health related Quality of life (QOL)
The SF-36 (version 1.0) was completed at the baseline and months 6, 12, and 24 study visits (10). The questionnaire is comprised of 8 scales: physical functioning, social functioning, role limitations due to physical health, role limitations due to emotional problems, mental health, vitality, bodily pain, and general health perceptions. Responses to questions in each scale are combined to create a score ranging between 0 (low) to 100 (high). In addition, these individual scale scores can be combined to create separate physical and mental health summary scores. Population norms were obtained from the SF-36 scoring manual that consists of 2,474 individuals with 47% between the ages of 18–44 and 57% female. Population norms were available only for the physical and mental health composite scores.
Liver histopathology
Available liver biopsies were reviewed by a single expert liver histopathologist (DEK). All samples were scored for multiple histological features as well as an overall pattern of liver injury (11).
Statistics
The 113 patients with chronic DILI at 6 months after DILI onset were analyzed in this paper with available data through March 10th 2014 (2). The two time scales of time from DILI onset and time from baseline visit were used for different analyses due to the nature of data collection. For variables related to liver injury pattern over time (e.g aminotransferase values), the time from DILI onset was used. For variables related to data collected at the specific study visits (e.g. symptom likert scale and quality of life), the time scale from baseline visit was used. The classification of patients as persisters versus resolvers was computed based on available laboratory values within 12 months of the DILI onset.
Non-parametric Wilcoxon test was used for comparison of the two groups for continuous variables, and chi-square test for categorical variables. Stepwise multivariate logistic regression analyses were used to identify independent factors at DILI onset or baseline study visit selected by clinical judgment for predicting month 12 persistent versus resolved liver injury status. All model selection used an initial univariate analysis to screen potential candidates for multivariate modeling and only variables with < 50% missing data were used. Variables with a univariate p-value of 0.1 or less were considered including demographic variables (age, gender, race, weight, BMI) at baseline visit, signs and symptoms at DILI onset (except jaundice), medical histories, and various lab parameters at DILI onset. The model results are reported as odds ratio (OR) with 95% confidence interval (CI). A c-statistic was used to describe the fit of the final models.
SF-36 quality of life scores were compared between the groups by linear regression models with adjustment for age and gender. All p values reported are two-sided and a level of 0.05 or less is considered statistically significant. All data were analyzed by SAS 9.2.
RESULTS
Patient population
Between September 2004 and August 2013, 113 of the 598 (18.8%) DILI patients had evidence of liver injury at 6 months after DILI onset. The manifestations of liver injury included serum AST, ALT, or alkaline phosphatase levels > baseline in 97 patients, liver function test 1.25 times of baseline on 2 occasions in 6, portal hypertension after 6 month from DILI onset in 8, radiological evidence of chronic liver injury in 23, histological evidence of chronic DILI in 6 and clinical evidence of chronic DILI in 36. Forty-three (38.1%) of the subjects met more than one criteria for chronic DILI. All of these patients were offered further study visits at 12 and 24 months after their baseline study visit wherein standardized laboratory, radiological and clinical assessments were undertaken. The 99 patients with laboratory data obtained at 12 months from DILI onset form the basis of this report (Figure 1). Of note the age, gender, DILIN severity score, and DILIN causality score were not significantly different in the 14 patients with missing data at 12 months from DILI onset compared to the 99 included in this analysis (data not shown).
Persiters and Resolvers at 12 months after DILI onset
74 subjects had persistent laboratory evidence of liver injury at 12 months after DILI onset while 25 subjects were resolvers. Subjects with persistent liver injury had a similar BMI, gender, and racial distribution to those without persistent liver injury (Table 1). However, the month 12 persisters were significantly older (52.6 vs 43.7 years, p=0.01) and were more likely to have a history of heart disease (35.1% vs 4%, p < 0.01). Interestingly, those with persistent liver injury had a significantly higher initial and peak serum alkaline phosphatase level and a lower initial and peak serum ALT level. Not surprisingly, the proportion of subjects with cholestatic liver injury at presentation was higher in the persisters versus resolvers (54.1% vs 20%, p < 0.01). However, the initial and peak total bilirubin, albumin, and INR levels in the two groups were similar. The frequency of anti-nuclear antibodies (ANA) and smooth muscle antibodies were similar in the two groups. On multivariate analysis, subject age and higher serum alkaline phosphatase levels at DILI onset were independent predictors of persistent liver injury at 12 months after DILI onset (C-statistic = 0.74 95% CI: 0.63: 0.85, See Supplemental Table 1).
Table 1.
Characteristics of patients with persistent vs resolved liver injury at 12 months after DILI onset.
| Characteristic | Month 12 persisters N=74 |
Month 12 resolvers N=25 |
P-value ^ |
|---|---|---|---|
| Age (yrs) | 52.6 ± 14.0 | 43.7 ± 17.3 | 0.01 |
| Female (%) | 69% | 68% | 1.00 |
| Race (%) | 1.00 | ||
| Caucasian | 74% | 76% | |
| Black | 18% | 20% | |
| Asian | 5% | 4% | |
| Others | 3% | 0% | |
| Hispanic (%) | 5% | 8% | 0.64 |
| BMI (kg/m2) | 27.5 ± 6.4 | 28.1 ± 9.2 | 0.77 |
| Self-reported drug allergies (%) | 49% | 28% | 0.10 |
| Medical History | |||
| Diabetes mellitus (%) | 34% | 24% | 0.46 |
| Heart disease (%) | 35% | 4% | < 0.01 |
| Any alcohol prior to DILI onset (%) a | 40% | 68% | 0.02 |
| Amongst drinkers b | |||
| Heavy alcohol use (%) | 17% | 23% | 0.70 |
| Implicated agents | |||
| Duration of agent use (days) | 54 (1, 2343) | 32 (1, 2120) | 0.70 |
| Single drug (%) | 50% | 56% | 0.23 |
| Single HDS (%) | 4% | 12% | |
| Multiple drug(s) /or HDS (%) | 46% | 32% | |
| Primary implicated agent class | 0.88 | ||
| Antimicrobial (%) | 38% | 36% | 1.00 |
| Antineoplastic (%) | 13% | 12% | 1.00 |
| Cardiovascular (%) | 15% | 8% | 0.51 |
| HDS (%) | 8% | 24% | 0.07 |
| Endocrine (%) | 7% | 0% | 0.33 |
| 7% | 0% | 0.33 | |
| Gastrointestinal (%) | 3% | 8% | 0.26 |
| Analgesic (%) | 3% | 8% | 0.26 |
| CNS (%) | 3% | 0% | 1.00 |
| Immunolomodulatory (%) | 4% | 4% | 1.00 |
| Liver biochemistries at DILI onset | |||
| Serum AST (U/L) | 266 (29, 2125) | 367 (45, 2077) | 0.07 |
| Serum ALT (U/L) | 275 (14, 2733) | 553 (121, 2412) | 0.01 |
| Alk P (U/L) | 394 (41, 1730) | 219 (74, 783) | <.01 |
| Total bilirubin (mg/dl) | 5.2 (0.2, 29.7) | 7.2 (0.2, 36.2) | 0.24 |
| 1.2 (0.9, 3.5) | 1.1 (0.9, 3.2) | 0.48 | |
| INR | |||
| Albumin (g/dL) | 3.5 (1.7, 4.6) | 3.7 (2.3, 4.4) | 0.29 |
| MELD score | 16.5 (6.0, 40.0) | 18.0 (6.0, 40.0) | 0.65 |
| R-value | 1.8 (0.1, 70.2) | 8.0 (1.0, 27.8) | <.01 |
| Pattern of Injury | |||
| Cholestatic (%) | 54% | 20% | <.01 |
| Mixed (%) | 18% | 20% | |
| Hepatocellular (%) | 28% | 60% | |
| Causality score (%) | 0.49 | ||
| Definite | 15% | 16% | |
| Very likely | 53% | 64% | |
| Probable | 32% | 20% | |
| Corticosteroid use (%) | 47% | 44% | 1.00 |
| Ursodeoxycholic acid use (%) | 26% | 12% | 0.18 |
| Peak liver biochemistries within 6 months of DILI onset | |||
| Serum AST (U/L) | 354 (57, 3229) | 462 (116, 2343) | 0.33 |
| Serum ALT (U/L) | 428 (62, 3742) | 630 (133, 2412) | 0.05 |
| Alk P (U/L) | 599 (65, 2865) | 246 (90, 1016) | <.01 |
| Total bilirubin (mg/dl) | 13.8 (0.5, 54.8) | 10.8 (0.4, 38.2) | 0.66 |
| INR | 1.2 (0.9, 13.1) | 1.2 (0.9, 3.2) | 0.22 |
| Lowest Albumin (g/dL | 2.7 (0.6, 4.9) | 3.3 (1.3, 4.2) | 0.04 |
Data reported as mean ± standard deviation or median (range)
Month 12 persisters vs resolvers
% with any alcohol use prior to DILI onset
Determined from Skinner Survey in 5 years prior to DILI onset.
Heavy alcohol use defined as > 21 drinks/ week in men and > 14 drinks/week in women
The suspect drugs implicated in the month 12 persisters and resolvers were similar with antimicrobial antibiotics being the most commonly implicated agents in both groups (Supplemental Table 2). The mean causality scores were also similar in the two groups as were the DILIN severity scores (Table 1). In addition, the frequency of prednisone and ursodeoxycholic acid use during follow-up was similar in the two patient groups.
Evolution of Liver biochemistries over time
The month 12 persisters had significantly higher serum alkaline phosphatase levels at DILI onset compared to those who resolved their liver injury at month 12. Although the serum alkaline phosphatase, total bilirubin and aminotransferase levels tended to improve in both groups over time, the persisters had significantly higher alkaline phosphatase levels at months 6, 12, and 24 compared to the resolvers (p < 0.01). In addition, although the serum AST and ALT levels were significantly lower at DILI onset in the persisters, they were subsequently higher than the resolvers at the month 6, 12, and 24 study visits (Table 2). The proportion of month 12 persisters and resolvers with serum ALT > 3 × ULN at DILI onset, month, 6, 12, and 24 were 73%, 27%, 27%, and 21% and 88%, 8%, 0%, and 5%, respectively.
Table 2.
Evolution of liver biochemistries in the Month 12 persisters vs resolvers over time
| Liver biochemistries | Month 12 Persister |
Month 12 Resolver |
p-value |
|---|---|---|---|
| At DILI Onset (n) | (74) | (25) | |
| Serum AST (U/L) | 266 (29, 2125) | 367 (45, 2077) | 0.07 |
| Serum ALT (U/L) | 275 (14, 2733) | 553 (121, 2412) | 0.01 |
| Alk P (U/L) | 394 (41, 1730) | 219 (74, 783) | <.01 |
| Total bilirubin (mg/dl) | 5.2 (0.2, 29.7) | 7.2 (0.2, 36.2) | 0.24 |
| T bilirubin > 2.5 mg/dl (%) | 71% | 79% | 0.6 |
| At Month 6 (n) | (70) | (24) | |
| Serum AST (U/L) | 73 (27, 1258) | 47 (18, 140) | <.01 |
| Serum ALT (U/L) | 78 (13, 1627) | 66 (13, 216) | 0.09 |
| Alk P (U/L) | 275 (53, 1487) | 116 (32, 277) | <.01 |
| Total bilirubin (mg/dl) | 0.9 (0.1, 16.3) | 0.6 (0.4, 2.4) | 0.01 |
| T Bilirubin > 2.5 mg/dl (%) | 13% | 0% | 0.11 |
| At Month 12 (n) | (74) | (25) | |
| Serum AST (U/L) | 67 (29, 2335) | 29 (15, 50) | <.01 |
| Serum ALT (U/L) | 79 (16, 1223) | 40 (13, 73) | <.01 |
| Alk P (U/L) | 245 (58, 1333) | 92 (35, 151) | <.01 |
| Total bilirubin (mg/dl) | 0.9 (0.3, 33.4) | 0.6 (0.1, 2.4) | 0.03 |
| T bilirubin > 2.5 mg/dl (%) | 7% | 0% | 0.33 |
| At Month 24 (n) | (66) | (19)* | |
| Serum AST (U/L) | 50 (17, 410) | 30 (16, 558) | <.01 |
| Serum ALT (U/L) | 50 (19, 407) | 32 (9, 1211) | <.01 |
| Alk phos | 157 (54, 1389) | 82 (47, 228) | <.01 |
| Total bilirubin (mg/dl) | 0.8 (0.3, 3.8) | 0.6 (0.2, 3.7) | 0.28 |
| T Bilirubin > 2.5 mg/dl (%) | 3% | 5% | 0.54 |
Data reported as median (range)
A single patient with normal liver biochemistries at 12 months after DILI onset subsequently developed acute cholestatic liver injury at month 24 attributed to symptomatic gallstones.
SF-36 scores at baseline and follow-up study visits
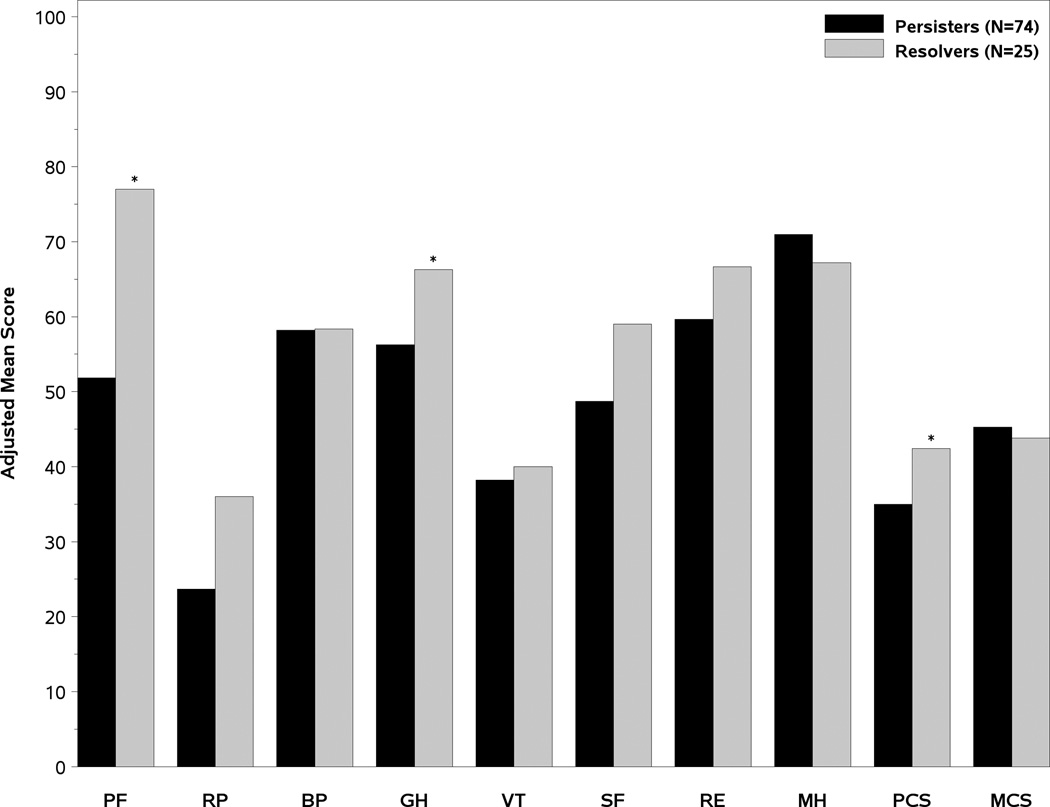
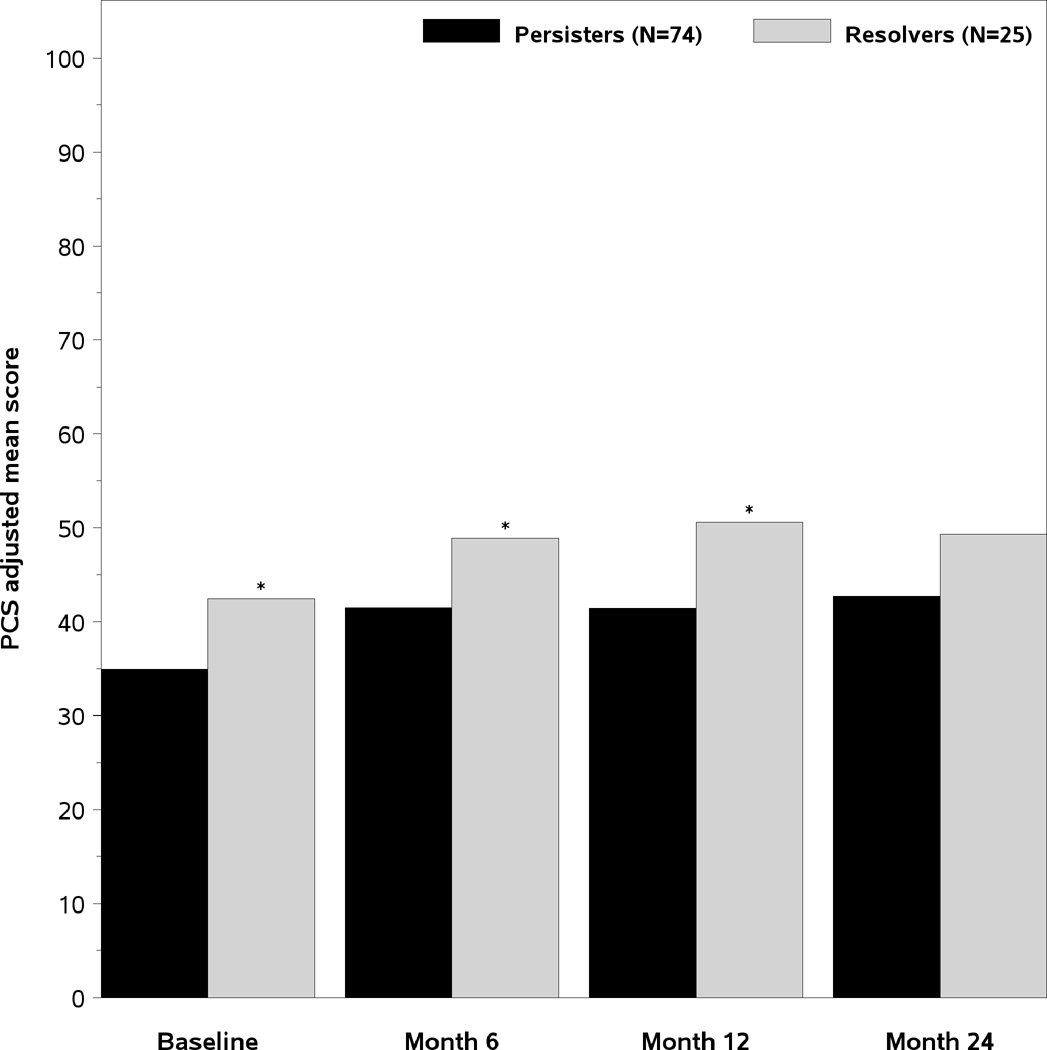
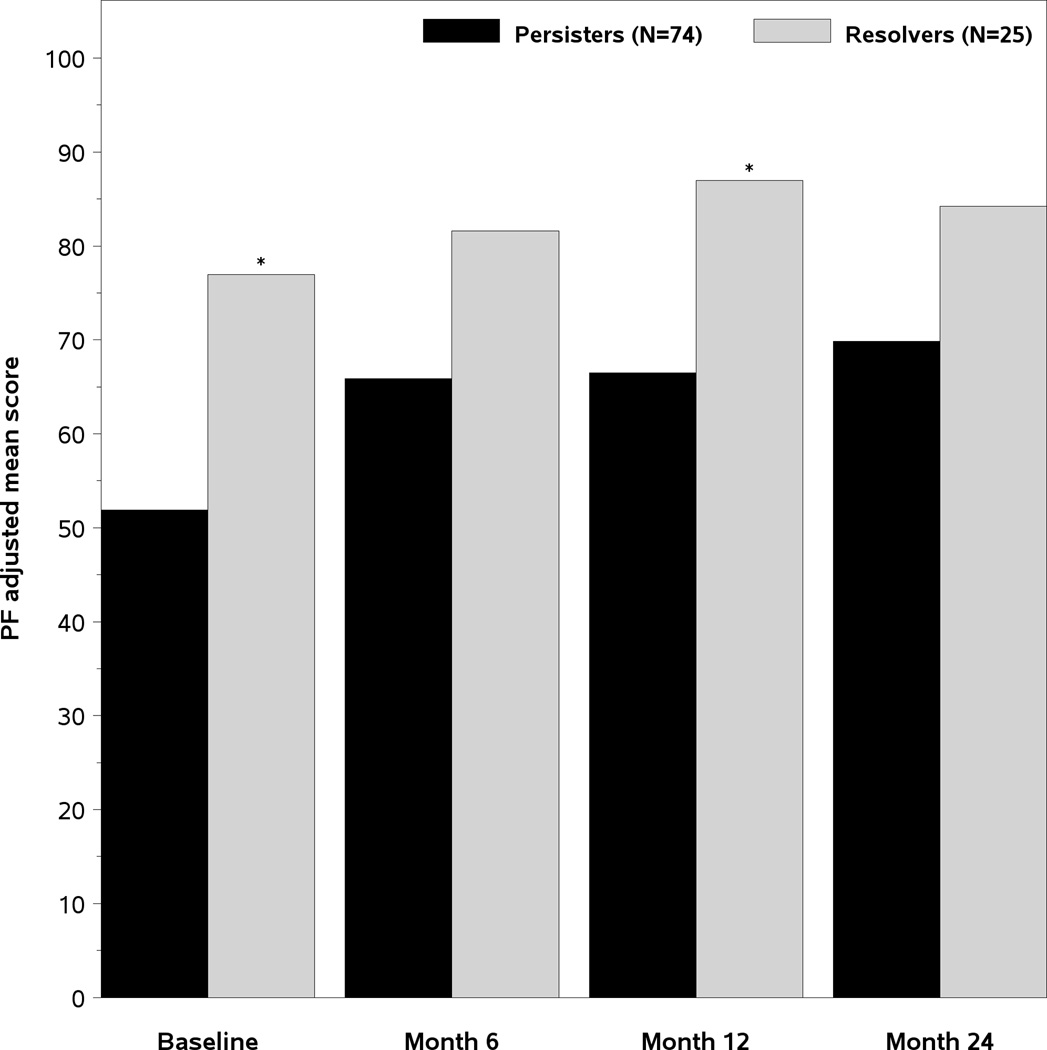
The age and gender adjusted SF-36 scores of the two groups of patients are presented in Figure 2. At the baseline DILIN study visit, the persisters had significantly lower Physical Component Summary (PCS) scores (35 vs 42.4, p=0.003), Physical Function (PF) scores (51.9 vs 77 p=0.001), and General Health (GH) scores (56.2 vs 66.3, p=0.044) (Figure 2A). However, the Mental Health summary (MCS) scores and other subscale scores were similar. The PCS remained significantly lower in the month 12 persisters vs resolvers at month 6 and 12 and were also lower at the month 24 study visit (Figure 2B). Furthermore, the Physical Function scores in the month 12 persisters were consistently lower compared to resolvers during follow-up as well (Figure 2C). These data indicate that the subjects with laboratory evidence of persistent liver injury 12 months after DILI onset have significantly poorer quality of life at initial enrollment that persists for 2 years compared to those with resolving liver injury.
Figure 2. Quality of life scores.
A- Mean adjusted SF-36 summary and subscale scores at the Baseline study visit. The mean PCS, PF, and General health (GH) scores were significantly lower in the month 12 persisters versus resolvers. 2B). The PCS scores improved in both groups of patients but significant differences remained in the month 6, month 12, and month 24 PCS scores between the subjects with persistent and resolved liver injury 2C). The Physical function (PF) scores of the month 12 persisters remained significantly lower than the resolvers at the baseline, month 6, month 12, and month 24 study visits. PF= Physical function BP= Bodily pain RP= role physical GH= general health VT= vitality SF= social functioning RE= Role emotional MH= mental health PCS= Physical summary score, MCS= Mental health summary score
Hepatitis symptoms at baseline and follow-up visits
11 clinical symptoms associated with acute hepatitis were recorded from all patients at the baseline, month 6, month 12, and 24 study visits (Supplemental Table 3). Subjects with laboratory evidence of persistent liver injury at 12 months had similar clinical symptoms at presentation to those who eventually resolved both at DILI onset and throughout follow-up.
Histopathologic evolution of DILI over time
A liver biopsy was not required for enrollment into the DILIN study and protocolized biopsies were not obtained during follow-up. Nonetheless, liver biopsy slides obtained by local physicians for clinical management purposes were available for review in 17 patients of whom 12 also had one or two biopsies during their acute DILI episode. For the 3 patients with 2 acute DILI biopsies, data from the second biopsy was utilized in Table 3. The mean age of these 17 subjects was 48.3 years and 70% were female. The median time from DILI onset to their initial liver biopsy was 22 days and 446 days to their additional biopsy done during follow-up. There were 14 individual agents implicated in these cases including 3 patients with nitrofurantoin and 2 with azithromycin induced hepatotoxicity. The most common pattern of liver injury observed on follow-up biopsy was chronic cholestasis in 9 patients (53%). Three patients each had either chronic hepatitis or steatohepatitis patterns. Of note, seven patients had evidence of bile duct loss, including the only patient classified in blinded review as acute cholestasis (based on prominent zone 3 cholestasis and absence of inflammation or significant fibrosis). In the 12 patients with more than one biopsy, 8 demonstrated fibrosis progression of at least one Ishak stage over a mean time interval of 397 days. The only patient to show regression of fibrosis had DILI due to tamoxifen. This patient had three biopsies, demonstrating progression of steatohepatitis features and fibrosis between the first and second biopsies while she was receiving tamoxifen. However, following the discontinuation of tamoxifen, the third biopsy demonstrated regression of fibrosis as well as reduction in ballooning injury and steatosis. The 8 patients with fibrosis progression all had cholestatic liver injury and included all six of the patients with paired biopsies who showed bile duct loss.
Table 3.
Histological and clinical features of DILI patients with follow-up liver biopsies
| Age/ sex |
Primary Implicated (days of therapy) |
R value at DILI onset |
Time between biopsies (days) |
*Stage at 1st |
Stage at 2nd |
R value closest to 2nd biopsy |
Histological Diagnosis on f/u biopsy |
Duct Loss/Ductular Reaction on F/U |
|---|---|---|---|---|---|---|---|---|
| 53 F | Trimethoprim-sulfamethoxasole | 1.9 | 490 | 0 | 0 | 1.0 | Chronic. Cholestasis | No/No |
| 38 F | Celecoxib | 8.4 | 382 | 0 | 0 | 0.7 | Chronic Cholestasis | No/No |
| 20 F | Azithromycin | 7.3 | 283 | 0 | 2 | 4.5 | Chronic Cholestasis. | Yes/No |
| 45 M | Azithromycin | 62.6 | 392 | 1 | 1 | 18.6 | Chronic Hepatitis | No/No |
| 58 F | Ultravist vitamin preparation | 0.42 | 246 | 4 | 6 | 27.3 | Steatohepatitis + Cholestasis | No/Yes |
| 37 F | Tamoxifen | 17.0 | 675 | 3 | 0 | 1.3 | Steatohepatitis | No/No |
| 20 F | Olanzapine | 1.6 | 633 | 0 | 4 | 1.8 | Chronic Cholestasis | Yes/Yes |
| 52 M | Metoclopramide | 1.8 | 404 | 1 | 2 | 0.7 | Chronic Cholestasis | Yes/Yes |
| 54 F | Omeprazole | 2.5 | 331 | 0 | 2 | 1.0 | Chronic Cholestasis. | Yes/No |
| 64 M | Amoxicillin-clavulanate | 6.8 | 183 | 0 | 2 | 0.8 | Chronic Cholestasis | No/Yes |
| 56 M | Gluco-Ease Plus | 1.1 | 522 | 0 | 2 | 0.2 | Chronic Cholestasis. | Yes/No |
| 70 F | Lansoprazole | 2.4 | 217 | 0 | 1 | 3.7 | Acute Cholestasis | Yes/No |
Ishak fibrosis score with scale of 0 (none) to 5/6 (cirrhosis)
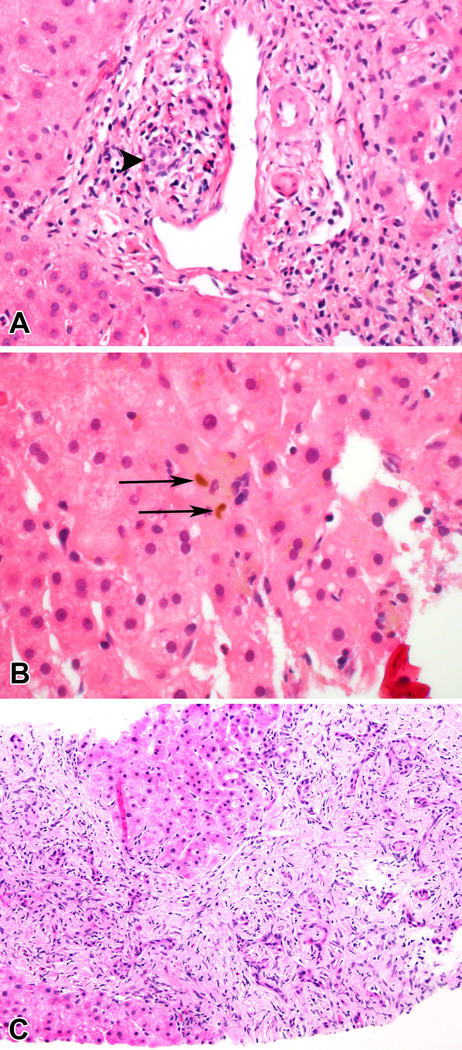
An informative case of the histopathological evolution of persistent DILI is seen in Figure 3. A 51 year old Caucasian male presented with jaundice, pruritus and acholic stools after taking metoclopramide for 1 month for refractory gastroesophageal reflux disease. His initial ALT was 779 IU/l, alkaline phosphatase 859 IU/L, and total bilirubin 6.9 mg/dl and his bilirubin peaked 3 weeks later at 20.4 mg/dl. A liver biopsy obtained 89 days after DILI onset when his alkaline phosphatase was 1150 IU/l demonstrated mild portal infiltrates with periductal inflammation but no fibrosis. With persistent fatigue at approximately 16 months after DILI onset and an alkaline phosphatase of 659 IU/L and total bilirubin of 0.7 mg/dl, he underwent a 2nd liver biopsy that demonstrated marked chronic cholestatic changes with ductular reaction and ductopenia. The patient continued to have ongoing liver injury at his month 24 study visit with symptoms of fatigue and a serum ALT of 240 IU/L, alkaline phosphatase of 497 IU/L, and total bilirubin of 0.5 mg/dl.
Figure 3. Chronic liver injury due to metaclopramide.
A. In the initial biopsy, taken 89 days after DILI onset, there is a mild portal infiltrate with periductal inflammation and duct injury (arrowhead). No fibrosis was present.(Magnification 400 ×) B. Visible bile (arrows) was present in the parenchyma. (Magnification 600 ×) C. Enzyme abnormalities persisted and a second biopsy, taken 492 days after DILI onset, showed chronic cholestatic changes with marked ductular reaction and ductopenia. The portal areas were greatly expanded by fibrosis. (Magnification 200 ×).
Discussion
Prior studies have demonstrated that 5 to 10% of patients with idiosyncratic DILI will die or undergo liver transplantation within 6 months of DILI onset (1–3). In addition to characterizing early adverse outcomes, a primary aim of the DILIN prospective study is to better determine the long-term clinical outcomes in DILI patients with ongoing liver injury at 6 months or more after DILI onset (2,3). As a result, all eligible subjects had a standardized battery of liver biochemistries drawn and also had symptoms, medications, and interval medical history recorded at each follow-up study visit through 2 years. Early on, we noted that some of the patients completely normalized their labs during follow-up while others continued to have persistent laboratory abnormalities. Persistent liver injury was defined in this study as a patient with a serum AST or ALT > 1.5 × ULN or an elevated serum alkaline phosphatase level at 12 months after DILI onset (12,13). We feel that the objective, laboratory criteria we selected to define persistent liver injury at 12 months after DILI onset are reasonable since all of the patients had an episode of well-documented acute liver injury at enrollment (Table 1). Furthermore, serum AST and ALT levels were normal in 88 of the 99 patients included in this analysis who had labs available prior to starting the suspect medication. Using this definition, nearly 75% of the current cohort had laboratory evidence of ongoing liver injury at 12 months after DILI onset while the remaining 25% had resolved their liver injury as evidenced by normalization of laboratory results. Of note, the majority of subjects were not jaundiced at month 6 and this proportion remained low during follow-up. However, the mean serum AST, ALT, and alk phos levels remained significantly higher in the month 12 persisters at month 24 compared to the resolvers (Table 2).
To determine if the month 12 persisters had more severe symptoms or functional impairment from their liver disease, we compared the self-reported symptom scores and SF-36 scores of the two patient groups at multiple time points. The clinical symptoms reported by both groups of patients were generally mild at their baseline study visit which took place a mean of 93 (range: 12 to 342) days after DILI onset in the persisters and 45 (range: 5 to 287) days after DILI onset in the resolvers. Furthermore, no significant differences in clinical symptoms were noted in the two groups during follow-up (Supplemental Table 3). However, clinically significant differences were noted in multiple SF-36 subscale and summary scores at the baseline study visit in the two groups of patients (Figure 2A). In particular, the physical function and role physical scores were markedly lower as was the PCS. The persistence of these differences during 2 years of follow-up suggest that subjects destined to have persistent DILI truly have a poorer quality of life and do not feel as well from DILI onset through 2 years of follow-up compared to those who will resolve their liver injury. It also suggests that our definition of persistent liver injury may have discerning value of clinical importance. Whether persisters go on to develop cirrhosis, portal hypertension, or symptoms of subclinical hepatic encephalopathy with more prolonged follow-up is an intriguing but unknown possibility worthy of further study (6).
Another aim of the current study was to identify baseline features associated with persistent liver injury that may be of use to clinicians in counseling future patients. Of note, there was no association between the type of suspect medication or duration of use with persistent DILI. However, the mean duration of suspect medication use in the 113 patients who formed the basis of this report was significantly longer than the 485 patients with self-limited DILI (2). Furthermore, gender, BMI, immunoallergic features (i.e. eosinophilia, rash, fever) and autoantibodies at DILI onset were also not associated with persistent liver injury. However, older patient age and higher serum alkaline phosphatase levels at presentation were both significant and independent risk factors for persistent DILI on multivariate analysis (Supplemental Table 1). The observed association of persistent liver injury with increasing patient age is not surprising. Studies of ALF patients have demonstrated that older subjects are more likely to die or experience greater morbidity presumably due to impaired hepatic regeneration (14). Older age is a strong determinant of developing chronic hepatitis C virus infection after initial exposure and older patients demonstrate greater evidence of hepatic inflammation and fibrosis progression during follow-up (15, 16). Older donor livers also do not fare as well in living and deceased donor liver transplantation and aging promotes the development of hepatic inflammation and liver cell injury in animal models of hepatic steatosis (17–19). These data cumulatively suggest that older individuals are at greater risk of developing severe and potentially progressive liver injury following an acute DILI episode compared to younger individuals.
The observation that subjects with cholestatic liver injury at DILI onset are at greater risk of having persistent liver injury during prolonged follow-up is perhaps one of the most important findings from our study. Some drugs associated with cholestatic liver injury early on can lead to severe prolonged cholestasis and even ductopenia during follow-up in a minority of afflicted patients (20–22). In particular, some patients with severe acute cholestatic drug reactions may go on to develop protracted liver disease with repeated hospitalizations and even development of cirrhosis (6). Furthermore, subjects with a cholestatic DILI episode in one study were more likely to develop chronic liver injury and a small proportion of these patients also developed progressive liver fibrosis (5). However, the mechanism(s) behind this association remains unclear. In primary biliary cirrhosis, there is evidence that the Canals of Hering along with the small bile ducts, are targets of an immune-mediated attack (23, 24). This disrupts the normal relationship of hepatocytes to bile ducts, stimulating a ductular reaction that can lead to subsequent hepatic fibrosis (25). Among our eight cases with fibrosis progression, six showed duct loss and four showed ductular reaction, while none of the four cases without fibrosis progression had either duct loss or ductular reaction (Table 3). Therefore, we speculate that the mechanism(s) of fibrosis involved in our chronic cholestatic DILI cases may be similar to those involved in other immune-mediated chronic cholestatic liver diseases but additional studies of a larger group of consecutive DILI patients are needed. The patients with clinically driven follow-up liver biopsies available for review in our cohort provided us a unique opportunity to explore the pathological evolution of chronic liver injury from DILI. Although only a few patients had follow-up biopsies, in those that did, the biopsies mainly showed chronic injury patterns with varying severity of hepatic fibrosis and just over half showed chronic cholestasis. This histological pattern of injury is in keeping with the persistent cholestatic laboratory injury profile and is unlikely to be the result of pre-existing liver disease. In patients with a baseline and a follow-up biopsy, some fibrosis progression was seen in two-thirds of cases, even though the time between biopsies was relatively short. All six patients who showed duct loss on follow-up had fibrosis progression and may be at risk for the development of a biliary cirrhosis. The only patient that showed clear regression of fibrosis had steatohepatitis due to tamoxifen.
There are several limitations of our study. Firstly, only a minority of patients had liver biopsy performed during follow-up and these were for clinical indications which may have led to selection bias. In addition, the biopsies were not obtained at a uniform time after DILI onset. Furthermore, we cannot completely exclude the possibility that some of the persisters may have had hepatic steatosis prior to taking the suspect drug. However, only 16% of the patients with labs available prior to starting the suspect medication had abnormal liver biochemistries. In addition, the mean BMI and frequency of diabetes mellitus and other components of the metabolic syndrome were not more prevalent in the persisters (Table 1). Furthermore, the predominance of cholestatic enzyme injury at DILI onset and at 12 months in the persisters suggests more than just mild fatty liver was detected by our enzyme criteria. Lastly, a multitude of suspect drugs were implicated in these 99 patients precluding us from linking the likelihood of persistent liver injury developing to a specific drug or class of drugs. Nevertheless, these data represent the largest group of patients with bona fide DILI (i.e. high causality assessment scores) who have been prospectively monitored for long-term clinical outcomes. In addition, clinically and statistically significant impairments in health related quality of life were noted in month 12 persisters compared to the resolvers throughout the study providing clinical relevance to our definition of persistent liver injury at 12 months after DILI onset.
In conclusion, 75% of subjects with liver injury at 6 months after DILI onset have persistent laboratory evidence of liver injury during further follow-up. Older patients and those that present with a higher serum alkaline phosphatase level are more likely to have persistent laboratory abnormalities during follow-up and also report a significantly poorer quality of life through month 24. Therefore, patients with persistent laboratory abnormalities at 12 months after DILI onset should be carefully monitored for clinical and histological progression of liver disease. Our natural history data also suggest that selected patients may benefit from a medical therapy aimed at reducing ductular damage and fibrosis progression but additional studies are needed.
Supplementary Material
Study Highlights.
What is current knowledge
-
-
Idiosyncratic drug induced liver injury (DILI) is an important cause of clinically significant liver injury that is associated with a 10% 6-month mortality.
-
-
Little is known about the long-term outcomes of DILI patients including the evolution of laboratory abnormalities, liver histology, and HRQOL.
-
-
The NIH Drug Induced Liver Injury Network (DILIN) is conducting an ongoing prospective study of 2 year outcomes in patients who have evidence of liver injury 6 months after DILI onset.
What is new here
-
-
Amongst 99 patients with probable DILI, 75% had laboratory evidence of persistent liver injury at 12 months after DILI onset with clinically significant reductions in various HRQOL domains throughout follow-up compared to resolvers.
-
-
Older patient age and a cholestatic lab profile at DILI onset were associated with a greater likelihood of persistent liver injury during follow-up.
-
-
Analysis of available serial liver biopsy specimens demonstrated evidence of progressive liver injury with chronic cholestasis and progressive hepatic fibrosis in over 2/3rd of the patients.
-
-
Persistent liver biochemical abnormalities are common in DILI patients with injury at 6 months after DILI onset; these patients require careful monitoring for liver disease progression
Acknowledgments
Financial support: The DILIN Network is structured as a U01 cooperative agreement with funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under grants: 2U01-DK065176-06 (Duke), 2U01-DK065201-06 (UNC), 2U01-DK065184-06 (Michigan), 2U01-DK065211-06 (Indiana), 5U01DK065193-04 (UConn), 5U01-DK065238-08 (UCSF/CPMC), 1U01-DK083023-01 (UTSW), 1U01-DK083027-01 (TJH/UPenn), 1U01-DK082992-01 (Mayo), 1U01-DK083020-01 (USC). Additional funding is provided by CTSA grants: UL1 RR025761 (Indiana), UL1TR000083 (UNC), UL1 RR024134 (UPenn), UL1 RR024986 (Michigan), UL1 RR024982 (UTSW), UL1 RR024150 (Mayo) and in part by the Intramural Research Program of the NIH, National Cancer Institute.
Dr. Fontana has received research support from Vertex Pharmaceuticals, BMS, Janssen, and Gilead; he has also served as a consultant to Tibotec in the past year.
Dr. Reddy has received research support from Merck, Gilead, Abbvie, Bristol-MyersSquibb, Janssen, Ikaria, and Genfit and served as an advisor to Merck, Genentech-Roche, Gilead, BMS, Vertex, Janssen, Idenix, and Abbvie.
Dr. Lee receives research support from BI, BMS, Anadys, Gilead, Vertex, Merck, Roche. Consulting: Lilly, Novartis, GSK
Dr. Chalasani has served as a consultant to Merck, Aegerion, BMS, Abbvie, Lilly and Salix over the past 12 months and received financial compensation from these entities. He has received research support from Intercept, Cumberland, Gilead, Enterome and Takeda.
Abbreviations
- ALF
Acute liver failure
- ALT
Alanine aminotransferase
- ANA
Anti-nuclear antibody
- AST
Aspartate aminotransferase
- DILI
Drug induced liver injury
- DILIN
Drug induced Liver Injury Network
- GH
General Health
- HDS
Herbal and Dietary Supplement
- MCS
Mental component summary
- PCS
Physical Component summary
- PF
Physical function
- QOL
Quality of life
- SF-36
Short-Form 36
- ULN
Upper limit of normal
Footnotes
Specific Author contributions: All of the authors were involved in study concept and design, acquisition of data, analysis and interpretation of data, and critical review of the final draft of the manuscript. Drafting of the manuscript (Fontana, Hayashi, Reddy, Barnhart, Kleiner), statistical analyses (Barnhart, Phillips), review and finalization (Fontana, Hayashi, Barnhart, Kleiner, Reddy, Chalasani, Lee, Stolz, Serrano, Watkins)
Potential Conflicts of interest
Dr’s Barnhart, Hayashi, Stolz, and Serrano have no conflicts of interest
Contributor Information
Paul H. Hayashi, Email: paul_hayashi@med.unc.edu.
Huiman Barnhart, Email: huiman.Barnhart@duke.edu.
K. Rajender Reddy, Email: rajender.reddy@uphs.upenn.edu.
Naga Chalasani, Email: nchalasa@iupui.edu.
William M Lee, Email: lee03@utsw.swmed.edu.
Andrew Stolz, Email: astolz@usc.edu.
Thomas Phillips, Email: thomas.a.phillips@dm.duke.edu.
Jose Serrano, Email: js362q@NIH.GOV.
Paul B. Watkins, Email: pbwatkins@med.unc.edu.
References
- 1.Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Fontana RJ, Hayashi PH, Gu J, et al. Idiosyncratic drug induced liver injury is associated with substantial morbidity and mortality within 6 months from onset. Gastroenterology. 2014;147:96–108. doi: 10.1053/j.gastro.2014.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aithal PG, Day CP. The natural history of histologically proved drug-induced liver disease. Gut. 1999;44:731–735. doi: 10.1136/gut.44.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade RJ, Lucena MI, Kaplowitz N, et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–1588. doi: 10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- 6.Bjornsson E, Davidsdottir L. The long-term follow-up after idiosyncratic drug-induced liver injury with jaundice. J Hepatology. 2009;50:511–517. doi: 10.1016/j.jhep.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Bjornosson E, Kalaitzakis E, Klintbert AV, et al. Long-term follow-up of patients with mild to moderate drug-induced liver injury. Aliment Pharmacol Ther. 2007;26:79–85. doi: 10.1111/j.1365-2036.2007.03355.x. [DOI] [PubMed] [Google Scholar]
- 8.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rockey DC, Seeff LB, Rochon J, et al. for the US DILIN. Causality assessment in drug induced Liver Injury using a structured Expert Opinion Process: Comparison to RUCAM. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware JE, Bayliss MS, Mannocchia M. Health-related quality of life in chronic hepatitis C: impact of disease and treatment response. Hepatology. 1999;30:264–270. doi: 10.1002/hep.510300203. [DOI] [PubMed] [Google Scholar]
- 11.Kleiner DE, Chalasani NP, Lee WM, et al. Hepatic histological findings in suspected drug-Induced liver Injury: Systematic evaluation and clinical associations. Hepatology. 2014;59:661–670. doi: 10.1002/hep.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams CD, Stenger J, Asike MI, et al. Prevalence of non-alcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 13.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 14.Fontana RJ, Ellerbe C, Durkalski V, et al. 2-year outcomes in initial survivors with acute liver failure: Results from a prospective, multicenter study. Liver International. 2014 doi: 10.1111/liv.12632. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiese M, Berr F, Lafrenz M, et al. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single source outbreak in Germany: a 20 year multicenter study. Hepatology. 2000;32:91–96. doi: 10.1053/jhep.2000.8169. [DOI] [PubMed] [Google Scholar]
- 16.Seeff LB, Hollinger FB, Alter HJ, et al. Long-term morbidity and mortality of transfusion-associated non-A, non-B, and type C hepatitis: A National Heart, Lung, and Blood institute Collaborative Study. Hepatology. 2001;33:455–463. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 17.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 18.Yamasahiki N, Sugawara Y, Tamura S, et al. Outcomes after living donor liver transplantation for acute liver failure in Japan: Results of a nationwide survey. Liver Transpl. 2012;18:1069–1077. doi: 10.1002/lt.23469. [DOI] [PubMed] [Google Scholar]
- 19.Fontana L, Zhao E, Amir M, et al. Aging promotes the development of diet induced murine steatohepatitis but not steatosis. Hepatology. 2013;57:995–1004. doi: 10.1002/hep.26099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy C, Lindor KD. Drug-induced cholestasis. Clin Liv Dis. 2003;7:311–330. doi: 10.1016/s1089-3261(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 21.Mandapoor D, Altorfer J, Flury R, et al. Chlorpromazine-induced vanishing bile duct syndrome leading to biliary cirrhosis. Hepatology. 1994;20:1437–1441. doi: 10.1002/hep.1840200610. [DOI] [PubMed] [Google Scholar]
- 22.Davies MH, Harrison RF, Elias E, et al. Antibiotic-associated acute vanishing bile duct syndrome: A pattern associated with severe, prolonged, intrahepatic cholestasis. J Hepatology. 1994;20:112–116. doi: 10.1016/s0168-8278(05)80476-3. [DOI] [PubMed] [Google Scholar]
- 23.Saxena RP, Hytiroglou P, Thung SN, et al. Destruction of Canals of hering in primary biliary cirrhosis. Hum Pathol. 2002;33(10):983–988. doi: 10.1053/hupa.2002.128060. [DOI] [PubMed] [Google Scholar]
- 24.Khan FM, Komarla AR, Mendoza PG, et al. Keratin 19 demonstration of canal of Hering loss in primary biliary cirrhosis:"minimal change PBC"? Hepatology. 2013;57(2):700–707. doi: 10.1002/hep.26020. [DOI] [PubMed] [Google Scholar]
- 25.Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54(5):1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.