1. INTRODUCTION
DNA modification is one of the most widely studied epigenetic modifications in high eukaryotic systems. Modified DNA bases impact gene regulation beyond the primary DNA sequence and affect a broad range of different biological pathways. While these epigenetic DNA modifications typically do not alter the coding of the primary sequence, the modified functional groups can encode another layer of inheritable information without affecting the base-pairing interface. The reversible installation and removal of these DNA modifications afford further dynamic regulation of gene expression.
5-Methylcytosine (5mC), the predominant DNA modification in eukaryotes, has long been accepted as the “fifth base” in mammalian genomic DNA.1 The installation of this modified base has been well-studied.2,3 Using S-adenosylmethionine (SAM) as a common electrophilic methylation source, the methyl group can be installed onto the C-5 position of the cytosine base by DNA methyltransferases (DNMTs)4 (Figure 1). The 5mC base is mainly located at the CpG dinucleotides and could be maintained by DNMT1 during DNA replication (DNMT1 converts hemimethylated CpG generated after replication to fully methylated CpG).5,6 Therefore, 5mC is a stable and inheritable epigenetic mark. In the past decades, 5mC has been shown to play crucial roles in gene regulation, genomic imprinting, X-chromosome inactivation, and many other processes.7,8 In general, the methyl modification is considered a repressive mark for chromatin status.7,8 The pattern of 5mC at specific loci affects cellular programming during development.1 From a chemical point of view, the C–C bond is chemically stable and difficult to be cleaved under mild conditions.
Figure 1.
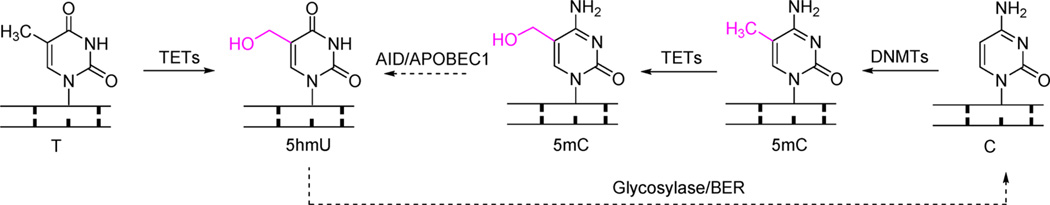
Dynamic DNA modifications in mammals mediated by TET and TDG. DNMTs can introduce a methyl group onto the C-5 position of the cytosine base. The 5mC modification can be oxidized to 5hmC/5fC/5caC in a stepwise manner by TET proteins. 5fC and 5caC can be readily excised by TDG to generate an abasic site, which can be repaired back to unmodified cytosine through the BER pathway.
However, DNA demethylation or the change of DNA methylation pattern has been observed during fertilization and early embryonic development.9–12 Two demethylation models have been established. In a passive demethylation model, the methylated cytosine is diluted during DNA replication through the downregulation of the activity of DNMT1. In a recently discovered active demethylation pathway, the methyl group is enzymatically processed and removed. While the passive pathway is a relatively straightforward mechanism, the understanding and study of the active demethylation pathway has been more complicated,10,11 particularly considering that the chemically inert C–C bond of 5mC methyl group needs to be cleaved under physiological conditions. Numerous efforts have been dedicated to the understanding of the molecular mechanism of this specific C–C bond-cleavage process.
In 2009, two breakthrough reports revealed the discovery of 5-hydroxymethylcytosine (5hmC), the oxidative product of 5mC, at surprisingly high abundance in mammals.13,14 A family of ten-eleven translocation (TET) proteins was also characterized to catalyze the oxidation reaction of the methyl group of 5mC in DNA.14 Subsequently, it was shown that TET enzymes can execute stepwise oxidation of 5hmC to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC) (Figure 1). The further oxidized 5fC and 5caC can be recognized and cleaved by thymine DNA glycosylase (TDG) and restored to normal cytosine through the base-excision repair (BER) process,15–17 which accomplishes the active demethylation of 5mC.
TET proteins were initially described as a fusion partner of the myeloid/lymphoid or mixed lineage leukemia (MLL) gene in acute myeloid leukemia (AML).18,19 In vivo studies of Tet genes have revealed their distinct expression patterns.20–23 Functional analyses of Tet-deficient mice have indicated that these proteins play regulatory roles in diverse biological processes, such as zygotic epigenetic reprogramming, germ cell development, pluripotent stem cell differentiation, and myelopoiesis.20,21,24–33
Since the discovery of 5hmC as a potential epigenetic mark in mammalian genome, quantification and sequencing methods have been developed to study the global level and genome-wide distribution of 5hmC, 5fC, and 5caC.34–51 Mapping of 5hmC, 5fC, and 5caC has revealed the association between these 5mC oxidation derivatives and gene regulatory elements, suggesting that 5hmC, 5fC, and 5caC are not only the 5mC oxidation intermediates but may also act as epigenetic marks.37,40,41,45–48 On the other hand, the development of enrichment, labeling, and sequencing methods has also facilitated functional investigations of TET proteins.13,34,52
In this review, we first summarize the oxidation activity and the mechanism of the TET proteins and TET-dependent demethylation pathways. Next, we review the interaction partners of TET proteins that may regulate TET activity and function. Finally, we discuss the potential impact of TET proteins in human cancer. Our goal is to outline the development of the TET activity study, the functional significance of TET proteins in epigenetics, and its implications for the pathogenesis of human diseases.
2. TET OXIDATION AND DNA DEMETHYLATION
2.1. TET Enzyme and Its Oxidation Activity on Cytosine Derivatives
A bioinformatics comparison of the sequences of TET genes to those of the thymine oxidases, J-binding protein 1 (JBP1) and 2 (JBP2), facilitated the discoveries of the TET enzymes as well as the 5mC oxidation activity. JBP1 and JBP2 belong to the family of iron(II)/alpha-ketoglutaric acid (α-KG)-dependent dioxygenases that sequentially catalyze the oxidation and glycosylation of thymine in DNA in the base J biosynthesis pathway.53 Both Borst and Sabatini groups predicted that JBP1 and JBP2 can oxidize thymine to 5-hydroxymethyluracil in the first step of base J synthesis.54,55 Interestingly, thymidine hydroxylase, another member of this protein family, has demonstrated the ability to oxidize the methyl group of thymidine stepwise to the hydroxymethyl group, formyl group, and carboxyl group.56,57 The reaction kinetics has been well-studied in different thymidine hydroxylases from different species.58–63 Several mammalian homologues of JBP1 and JBP2 were identified by Rao and co-workers through computational analysis.14,64 These homologues are the TET family proteins, of which the TET1 gene was previously known as a fusion partner of the MLL gene in acute myeloid leukemia (AML). Quantifications of 5hmC reveal that the new cytosine modification accumulates in most mammalian cells and tissues, as well as in even up to 40% of the total modified cytosines in postmitotic neuronal Purkinje cells.13,35,65 In vivo and in vitro experiments confirmed the oxidation activity of 5mC to 5hmC by the TET proteins;14 TET proteins significantly affect the cellular level of 5hmC.14,66 By tracking global 5hmC levels at each stage of the cell cycle in mouse embryonic stem cells (mESCs), Balasubramanian and co-workers found that 5hmC appears to be a stable DNA mark.67 These results implicate potential epigenetic roles of 5hmC as well as the epigenetic regulatory functions of TET enzymes. However, the restriction enzyme-based TET oxidation activity test assay cannot detect the oxidized modification base directly, which hampered the discovery of the further oxidation activity of TET.
In 2011, the stepwise oxidation of 5hmC by TET proteins was revealed by using synthetic nucleosides as standards (Figure 1).17,35 Using thin-layer chromatography (TLC), chemical transformation, and highly sensitive high-performance liquid chromatography–mass spectrometry (HPLC-MS) techniques, 5fC and 5caC were detected in TET-mediated 5mC oxidation reactions as well as in the genomic DNA of mESC and various mouse tissues.35,36 TLC is a classic and sensitive technique for the detection of modified bases.68 However, the resolution of TLC retention factor is relatively low. Although the resolution can be improved with 2-dimentional TLC, the technique requires an optimized buffer system and a high dose of radioactive material. The HPLC-MS technique provides multiple detection channels capable of separating and quantifying multiple base modifications simultaneously. The employment of these highly sensitive technologies largely facilitates the discovery of the new oxidation products and the stepwise oxidation activity of TET proteins. While mammalian TET proteins preferentially oxidize 5hmC all the way to 5caC in vitro,17,35,69 mushroom TET proteins can lead to the accumulation of 5fC in the same oxidation reaction,70 which may suggest functional roles of 5fC in mushrooms. The distinct chemical properties of methyl, hydroxymethyl, formyl, and carboxyl groups provide the possibility of dynamic epigenetic regulation; binding of existing and potentially new reader proteins that recognize modified cytosines in DNA could be affected by these different functional groups.
TET proteins are also known to oxidize 5mC on single-stranded DNA,71,72 although with lower activity compared to 5mC on double-stranded DNA. TET3 is known to also exist in the cytoplasm of mammalian cells. Recently, Wang and co-workers reported an interesting oxidation activity of 5mC to 5hmC on RNA,73 suggesting a potential functional role of TET on RNA.
2.2. TET Oxidation Activity on Thymine
Besides oxidation of 5mC, Carell and co-workers reported a new oxidation activity of TET enzymes in which thymine in DNA is converted to 5-hydroxymethyluracil (Figure 2).74 Using isotope-labeled nucleosides or isotope-labeled SAM, the in vivo oxidation dynamics of cytosine modifications can be monitored by highly sensitive HPLC–MS. The in vivo TET-mediated oxidation can be differentiated from the reactive oxygen species (ROS)-induced oxidation. Moreover, the isotope-labeled nucleosides can also act as internal standards that further improve quantification of these base derivatives by HPLC-MS.
Figure 2.
Proposed pathways to generate 5hmU in mammalian cells. TET proteins can oxidize thymine to 5hmU. TET proteins oxidize 5mC to 5hmC, which may be deaminated to 5hmU by AID/APOBEC1; 5hmU can be removed and repaired via BER in a proposed alternative demethylation pathway.
The change in the 5hmU level during the early differentiation phase of mESCs was shown to be similar to that of the 5mC oxidation products, further suggesting that the majority of 5hmU could be products of TET-based oxidation of thymine in DNA. Carell group reported that the level of 5hmU in the mouse cortex is relatively low compared to that in mESCs, suggesting potential different functional roles of 5hmU in mESCs and in mouse brain. Interestingly, TET proteins do not seem to further oxidize 5hmU to 5-formyluracil (5fU), which is considered as a type of ROS-induced DNA damage.
Protein pull-down analysis revealed that 5hmU can influence binding of chromatin remodeling proteins and transcription factors. Previous studies have suggested the formation of 5hmU from deamination of 5hmC in the active demethylation pathway;75,76 this thymine oxidation activity indicates that 5hmU mainly locates in the context of T:A base pairs instead of C:G base pairs. Although we have recently developed the enrichment method for 5hmU in C:G base pairs,77 the lack of a sequencing method for genome-wide 5hmU detection in T:A base pairing still hampers our understanding of the distribution and potential function of 5hmU.
2.3. TET-Dependent Active DNA Demethylation
During DNA replication, the level of 5mC is maintained through methylation of the newly synthesized DNA by DNMT1.5,6 Loss of this maintenance activity dilutes the methylation level after each cell cycle, which leads to passive demethylation. However, in certain biological contexts, the replication-independent removal of 5mC exists; this is known as active demethylation. During fertilization, for example, as revealed by antibody-based cell imaging, the paternal genome goes through a rapid and prevalent demethylation process while maternal and paternal genomic materials do not merge in the one-cell embryo.9 Genome-wide demethylation is thought to occur actively during this process. Immunostaining of 5mC, 5hmC, 5fC, and 5caC revealed the loss of 5mC in paternal chromosome along with the appearance of 5hmC/5fC/ 5caC.21–23,78–80 Liu and co-workers reported that deletion of the Tet3 gene abolishes the reprogramming of 5mC in mouse pronucleus, suggesting that TET-mediated oxidation is required for this active demethylation. Single-base, allele-specific DNA methylomes from mouse gametes, early embryos, and primordial germ cell (PGC) obtained recently revealed that a significant portion of 5mC and its oxidation derivatives are converted to unmodified cytosines independent of replication in both maternal and paternal genomes.81 Genes with hypomethylated promoters are enriched in embryogenesis and organ development, further confirming the association between DNA demethylation and embryonic development. Genome-wide, single-base resolution detections of 5hmC and 5fC in two-cell embryos further implicate that active demethylation can be mediated through TET proteins.81
With the emerging discoveries of TET proteins and TET-mediated 5mC oxidation, the 5mC oxidation derivatives were speculated to be involved in active demethylation. To date, the biochemically confirmed pathway involves TET-mediated 5mC oxidation followed by base-excision repair (BER), although a recent study suggested that a direct in vitro removal of the 5-position carboxyl group from 5caC in DNA oligonucleotides is chemically feasible under mild conditions.82
2.3.1. TDG-Mediated DNA Demethylation
Mammalian thymine DNA glycosylase (TDG) has been reported to interact with transcription factors, chromatin-modifying enzymes, and DNMTs.83 TDG and methyl-binding domain protein 4 (MBD4) were suggested to be involved in direct 5mC excision from double-stranded DNA in mammals.84 However, the marginal activity hardly supports this possibility.83 The knockout of TDG is embryonically lethal in mice, and this protein appears to play essential roles in epigenetic stability during embryo development.75,85
TDG has been known for a long time to act as a DNA repair protein that excises T from G:T mismatch.86 The repair activity of TDG on 5hmU in the G:5hmU context was also suggested. The 5-position substituents of cytosine can change the stability of the N-glycosidic bond by electronic effects.87 The presence of electron-withdrawing groups can increase the cleavage activity of TDG on modified cytosines. One major breakthrough was made by Xu and co-workers, who reported that TDG cleaves 5caC, a product of TET-mediated 5mC oxidation, to generate an abasic site in duplex DNA, which is further processed through BER and restored to cytosine.17 Maiti and Drohat further measured the in vitro activity of TDG on 5fC and 5caC,15 showing that TDG can more efficiently cleave 5fC than 5caC. In a computational study, Williams and Wang investigated the inherent nucleophilic cleavage chemistry of the N-glycosidic bond in both kinetics and thermodynamics, predicting the TDG selectivity toward 5fC and 5caC.88 Although TDG has a higher cleavage activity on 5fC-containing DNA oligonucleotide, it exhibits a slightly higher binding affinity to the 5caC-containing DNA oligonucleotide in the electrophoretic mobility shift assay (EMSA).16 The crystal structure of the human TDG catalytic domain in complex with 5caC-containing DNA oligonucleotide revealed a pocket in the active site of TDG that is used by the protein to selectively recognize 5caC. These biochemical and structural results confirmed the cleavage activity of TDG on 5fC/5caC-containing DNA oligonucleotides. Therefore, 5mC/5hmC sites can be converted back to unmodified cytosine once they are oxidized to 5fC/5caC by TET enzymes independent of replication83,84 (Figure 1). This is the first biochemically confirmed active demethylation pathway. Elevated 5fC/5caC levels were detected in Tdg knockdown or Tdg knockout mESC genomic DNA, supporting the involvement of TDG in 5fC/ 5caC removal.35 Sequencing methods have been developed to profile the genome-wide 5fC and 5caC sites in both wild-type mESC and Tdg-deficient mESC.45,48 The results revealed increased 5fC and 5caC peaks upon Tdg knockdown or Tdg knockout, confirming the in vivo demethylation activity mediated by TDG. Increased 5fC and 5caC sites are also associated with gene regulatory elements, suggesting the potential involvement of these base modifications in gene regulation. Whether 5fC and 5caC have direct roles on transcription regulation still requires further investigation.
2.3.2. AID/APOBEC-Mediated 5hmC Deamination
Activation-induced deaminase (AID), which is essential for demethylation of the OCT4 and NANOG promoter regions during in vitro nuclear reprogramming,89 is also required for genome-wide DNA demethylation in primordial germ cells.90 An alternative demethylation pathway was proposed, which involves TET-mediated 5mC oxidation, AID/APOBEC-based deamination of the 5hmC product, and BER.75,76,89–92
However, AID cannot work on double-stranded DNA (dsDNA) and exhibits a lower activity on 5mC compared to unmodified cytosine.93–96 Thus, in this mechanism, 5hmC generated by TET-mediated 5mC oxidation is proposed to be deaminated to 5hmU through AID/APOBEC1. The 5hmU product, a known substrate for BER, can be processed and converted to normal cytosine (Figure 2). Gadd45 was believed to be associated with AID and involved in the proposed demethylation to affect gene regulation in the nervous system. However, in vitro evidence for the deamination of 5hmC is still lacking although decreased levels of 5hmC were observed upon the overexpression of AID/APOBEC1 in the human cell lines.97,98 Kohli and co-workers further showed that AID and APOBEC1 type proteins have even lower in vitro activity on 5hmC versus 5mC due to the steric hindrance of the 5-position substitution. The levels of 5mC oxidation products barely changed upon AID/APOBEC1 overexpression. The generation of 5hmU from 5hmC through deamination and/or other pathways and its functional roles need to be further explored, perhaps with reliable sequencing methods to track 5hmU in genomic DNA.
2.4. TET Structure and Its Oxidation Mechanism
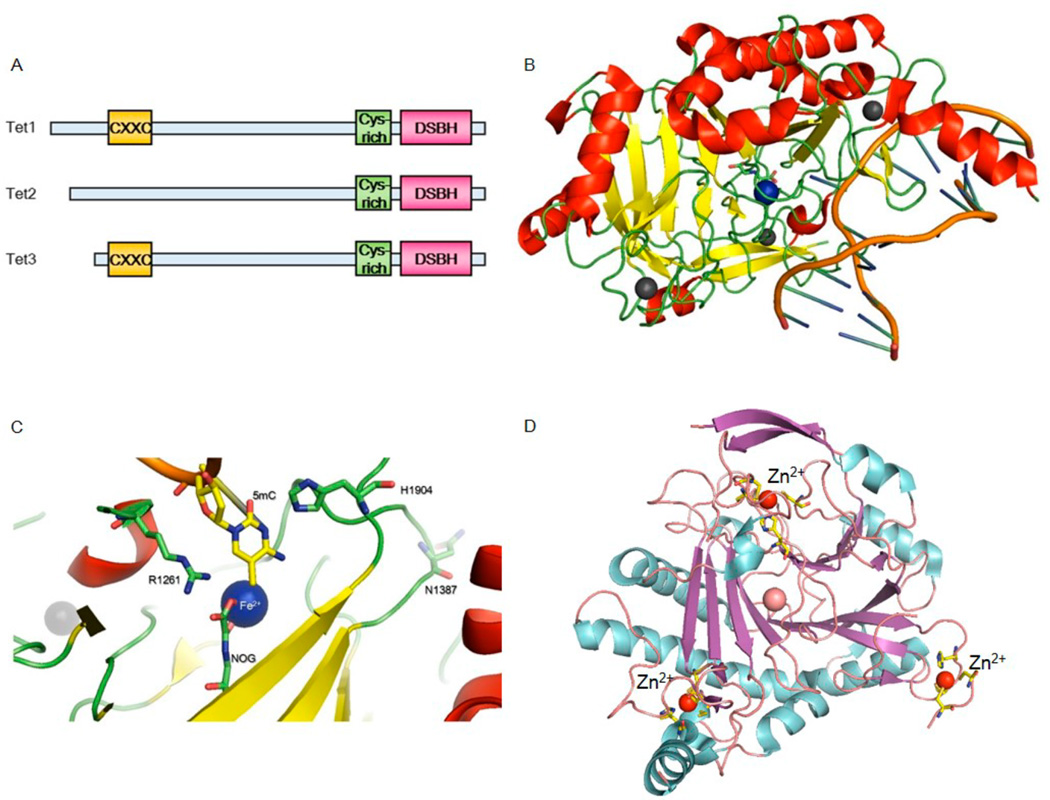
Mammalian TET proteins are evolutionarily conserved dioxygenases that contain a catalytic C-terminal domain. The catalytic domain is composed of Cys-rich and double-stranded β-helix (DSBH) regions involved in catalysis (Figure 3A). Interestingly, this domain also contains a long spacer believed to be largely unstructured with unknown functions.14 A distinct CXXC domain is shared by TET1 and TET3, which was shown to recognize the clustered unmethylated CpG.99 A crystallo-graphic study further showed that an unmodified cytosine is critical for the interaction between DNA and Xenopus TET3 CXXC domain.100
Figure 3.
Overall domain architecture of TET proteins and the published human TET2 structure. (A) TET proteins contain a CXXC DNA-binding domain and a conserved catalytic domain composed of a Cys-rich region and a DSBH fold. (B) Crystal structure of the TET2–DNA complex. The Cys-rich regions bind zinc cations and are folded together with the catalytic domain. (C) Close-up view of the active site of TET2. DNA is colored orange; 5mC, NOG, and critical residues are shown in stick representations, iron is shown in a blue sphere, and zinc is shown in a gray sphere. (D) Ribbon representations of three zinc cations in TET2. Zinc cations are shown and labeled in red spheres; corresponding coordination residues are shown in stick representations.
The Cys-rich and DSBH regions are required for the oxidation activity of TET. In the recently reported crystal structure of TET2–DNA–NOG–Fe2+, the Cys-rich domain was shown to wrap around the DSBH domain and is an integrated part of the catalytic domain (Figure 3B).101 The double-stranded DNA oligonucleotide is located above the DSBH core and interacts with two loops from the Cys-C subdomain. Besides the substrate 5mCpG dinucleotide, only DNA backbones interact directly with TET2, which explains the in vitro oxidation activity test results that TET2 shows no selectivity for DNA sequence. As expected, no specific interaction of TET2 with a methyl group was observed, which is consistent with the sequential oxidation activity of the protein that also oxidizes 5hmC and 5fC. This was not the case, however, for a Naegleria TET-like protein characterized structurally and biochemically,102 which lacks the Cys-rich domain.
The DSBH domain of human TET2 contains the characteristic HXDXnH motif that coordinates iron(II) and binds α-KG. The highly conserved histidine residues, N-oxalyl glycine (NOG) as an inactive analogue of α-KG, and a water molecule bind iron(II) to afford the octahedral coordination. The space between the target C–H bond and the iron center can accommodate the hydroxyl and formyl groups, allowing for the oxidation of these cytosine derivatives (Figure 3C). Interestingly, the overall structure of TET2 contains three zinc cations in the Cys-rich domain (Figure 3D). Two of the zinc cations are coordinated by residues from both Cys-rich and DSBH domains, suggesting that the Cys-rich domain and zinc cations play an important role in stabilizing these TET enzymes.
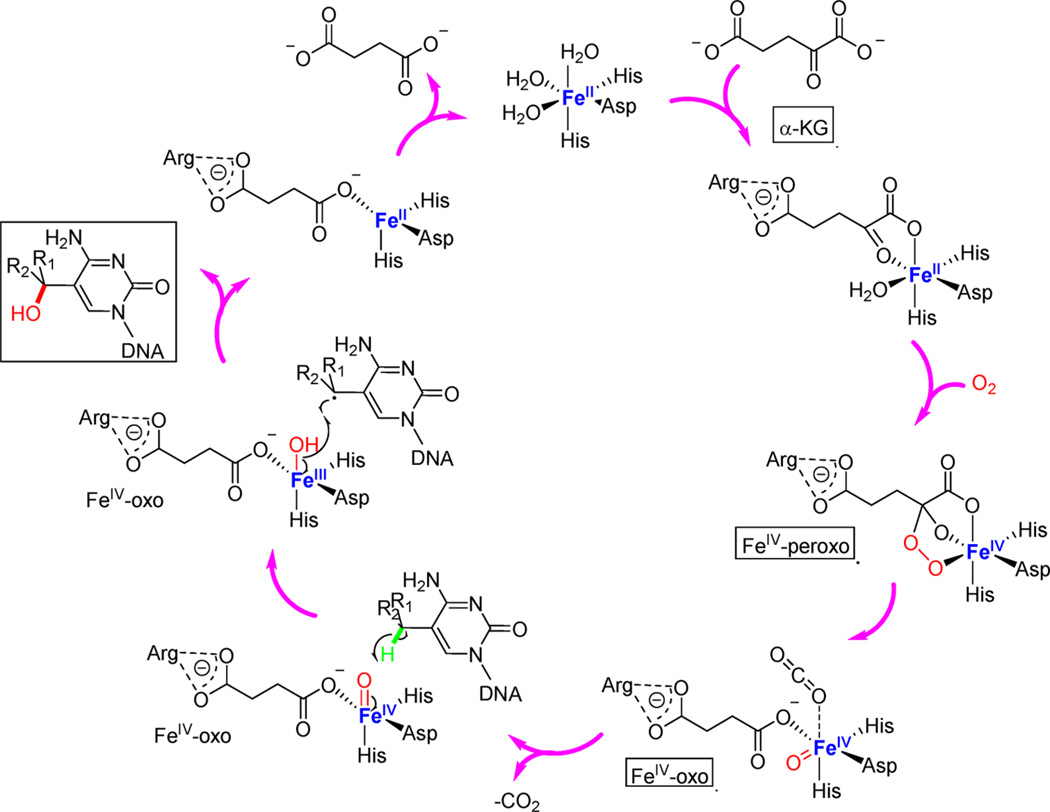
Although the detailed molecular mechanism of the TET-mediated oxidation is yet to be fully elucidated, the oxidation mechanism of AlkB, which has a similar active site, has been well-studied and reviewed recently.103 In brief, the mono-nuclear iron(II) center donates two electrons for the reduction of dioxygen, while α-KG serves as a cosubstrate to provide the other two electrons, completing the four-electron reduction.104–109 With the four electrons available, the active site iron(II) binds and activates a dioxygen molecule to form a proposed iron(IV)–oxo species with α-KG converting to succinate and releasing one molecule of CO2 during the process.107,110–114 The formed iron(IV)–oxo is oriented to target the methyl C–H group115,116 and activate the C–H bond in either a radical rebound mechanism (Figure 4) or potentially a more concerted mechanism as previously studied computationally for the AlkB family proteins.117,118 As TET proteins mediate oxidation of three distinct substrates, the actual mechanism may be more complex, which needs to be investigated in detail both experimentally and computationally.
Figure 4.
Proposed mechanism of TET-mediated oxidation of 5mC and its derivatives. The oxidation substrates can be 5mC, 5hmC, or 5fC. The coordination residues of iron(II) and α-KG are drawn based on the human TET2 structure.[san]98 A radical rebound mechanism is shown, but the C–H activation could also go through a more concerted process.
2.5. Genome-wide Distribution and Regulatory Function of TET Oxidation Products
The development of next-generation sequencing technologies for cytosine modifications has notably facilitated functional characterizations of TET proteins. Since the discovery of TET oxidation activity, various sequencing methods have been invented to unveil the genome-wide distribution of the oxidation products of TET. These methods have been reviewed by us and others, including an article in this issue.51 In general, 5hmC has been shown to be enriched at transcription start sites, promoters, gene bodies (exons), CCCTC-binding factor (CTCF)-binding sites, and enhancers in ESCs using profiling approaches.37,40,42,46 Recently, we and others reported the genome-wide distribution of 5fC and 5caC in mESC.41,45,48 Using a chemical selective labeling strategy (5fC-Seal), we showed that 5fC is preferentially enriched at poised enhancers and correlates with p300 binding.45 By applying 5fC- and 5caC-specific antibodies, Zhang and co-workers revealed that 5fC and 5caC are enriched at major satellite repeats but not at nonrepetitive loci in wild-type mESCs, while 5fC and 5caC are enriched at a large number of proximal and distal gene regulatory elements upon Tdg depletion.48
Single-base resolution methods were developed to provide high-resolution maps of 5hmC with absolute abundance at modification sites. Different strategies have been used in realizing single-base resolution detection of 5hmC.38,39,43,47 We and Balasubramanian group each independently reported Tet-assisted bisulfite sequencing (TAB-seq) and oxidative bisulfite sequencing (oxBS-seq) to realize quantitative single-nucleotide-resolution mapping of 5hmC and 5mC in mammalian DNA using different properties of modified cytosines in bisulfite sequencing.38,47 Single-base resolution methods of 5fC and 5caC have also been reported recently using the similar bisulfite sequencing principles. We reported the chemical-assisted bisulfite (CAB-seq) for both 5fC and 5caC. EtONH2 protection was applied for the selective protection of 5fC, while the reduction of 5fC to 5hmC by NaBH4 was also reported as an alternative strategy.45 1-Ethyl-3-(3-(dimethylamino)propyl)-carbodiimide (EDC)-based amide bond formation was applied for 5caC.50 Xu and co-workers reported another 5fC detection strategy by protecting all normal cytosines as 5mC with methyltransferase, therefore reading out 5fC and 5caC in bisulfite sequencing.50,119
Recently, the Balasubramanian group optimized the NaBH4 reduction conditions and significantly improved the efficiency of 5fC reduction to 5hmC, which allowed genome-wide mapping of 5fC in a reduced representative manner with reduced bisulfite sequencing (redBS-seq).49 Zhang and co-workers independently developed M.SssI methylase-assisted bisulfite sequencing (MAB-seq), a method that detects both 5fC and 5caC at single-base resolution when coupled with selective reduction of 5fC to 5hmC.120 All these results revealed genome-wide active demethylation in mouse ESCs and the association of 5hmC, 5fC, and 5caC with functional elements in the genome.
Despite the rapid method development, most single-base resolution methods still demand high sequencing costs due to the low abundance of these modified bases. In particular, in genome-wide single-base detection of 5fC/5caC, the limitation of the sequencing depth hampers the detection of low-abundance 5fC/5caC sites. Current sequencing methods also require microgram scales of input DNA, which limits the application scope in processes where only small amounts of genomic DNA can be isolated.81
3. TET REGULATION
3.1. TET Regulation by Its Interacting Molecules
3.1.1. Tet Regulation via Small-Molecule Metabolites
3.1.1.1. Vitamin C Facilitates Tet-Mediated Oxidation Both In Vitro And In Vivo
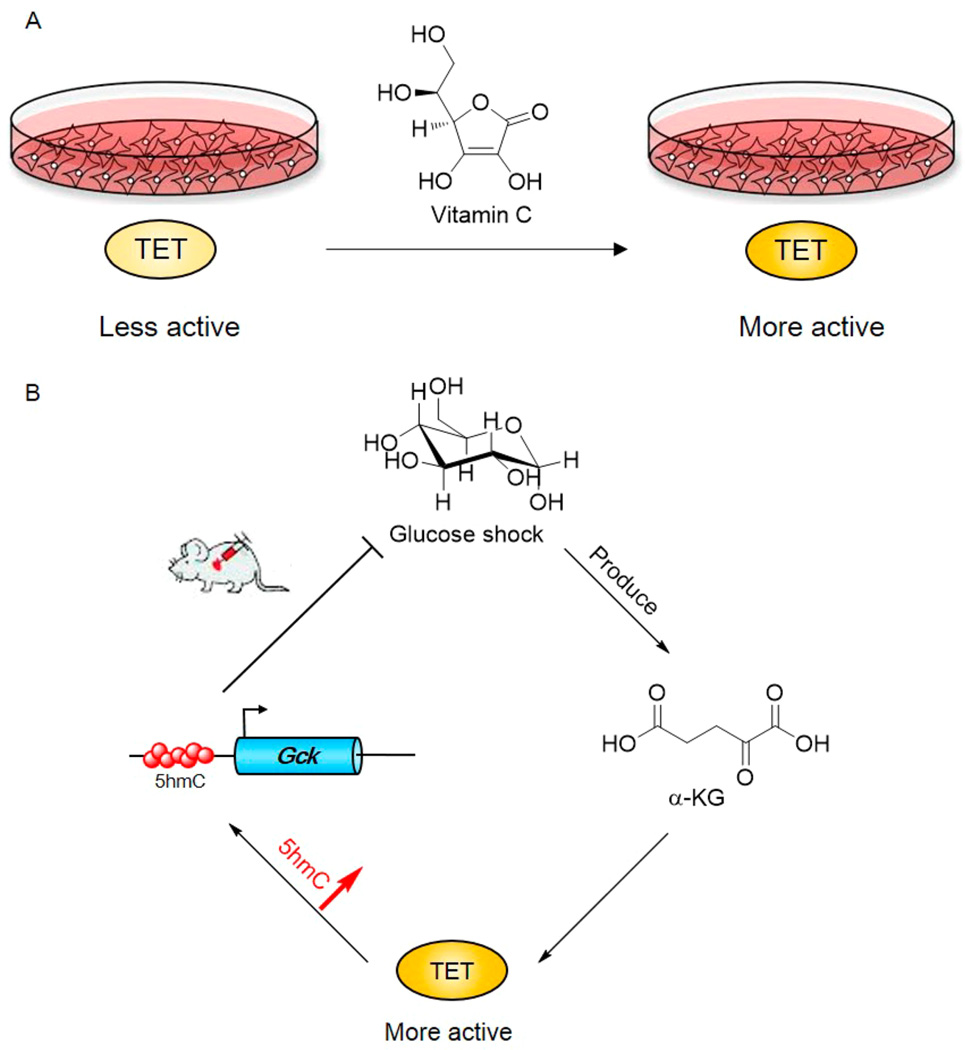
Ascorbic acid, or vitamin C, is a known reducing agent used to maintain the active iron(II) status in certain nonheme iron dioxygenases.121 It is also used as the iron reducing agent in in vitro TET oxidation assays.14,17,47 However, vitamin C is not required for in vitro TET activity.14 Two groups observed elevated DNA demethylation in the presence of supplemented vitamin C.122,123 Measurements of in vitro activity revealed that vitamin C can stimulate the oxidation activity of TET enzymes122 (Figure 5A). Cell-based experiments further showed that vitamin C can significantly promote 5mC oxidation and induce the demethylation of certain promoters. Other reducing agents, such as DTT or glutathione, did not produce such effects. Biochemical investigations suggested that vitamin C selectively interacts with the C-terminal catalytic domain of mouse TET2, which may allosterically tune the activity of TET1. Although cocrystallization of vitamin C and TET proteins has not been reported, this biochemical observation provides a possible mechanism for the functional role of vitamin C in ESC self-renewal, normal myelopoiesis, and myeloid leukemia.124–127
Figure 5.
Small molecule-induced 5mC oxidation. (A) Vitamin C can promote the oxidation activity of TET proteins both in vitro and in vivo. (B) Elevated cellular glucose can increase the cellular levels of α-KG and 5hmC and promote oxidation by the TET proteins. The Gck gene is rapidly activated to consume the excess glucose through loci-specific demethylation and hydroxymethylation.
3.1.1.2. TET Regulation by α-KG under Metabolism Pressure
Ye and co-workers reported that an injection of glucose source could promote levels of α-KG in mice, accompanied by increased levels of 5hmC and 5fC without a noticeable change in the global level of 5mC128 (Figure 5B). α-KG can be produced and consumed in several metabolic pathways. As described above, α-KG is also the cofactor for TET oxidation, in which one equivalent of α-KG is required per oxidation reaction. Interestingly, cellular 5hmC and 5fC could recover to original levels with the reduction of both the blood glucose concentration and the α-KG level. The Gck gene, which encodes glucokinase that catalyzes the phosphorylation of glucose to glucose-6-phosphate, was activated rapidly under glucose shock, accompanied by reduced 5mC and increased 5hmC at the CpG-rich region of Gck. Thus, the Gck gene can efficiently counteract changes in environmental metabolism pressure via TET-mediated demethylation. Future studies of connections between metabolism and TET proteins will provide insights into the understanding of epigenetic regulation under environmental stress or nutrient fluctuations.
3.1.2. TET Regulation via Protein Partners
3.1.2.1. Calpain-Mediated TET Degradation
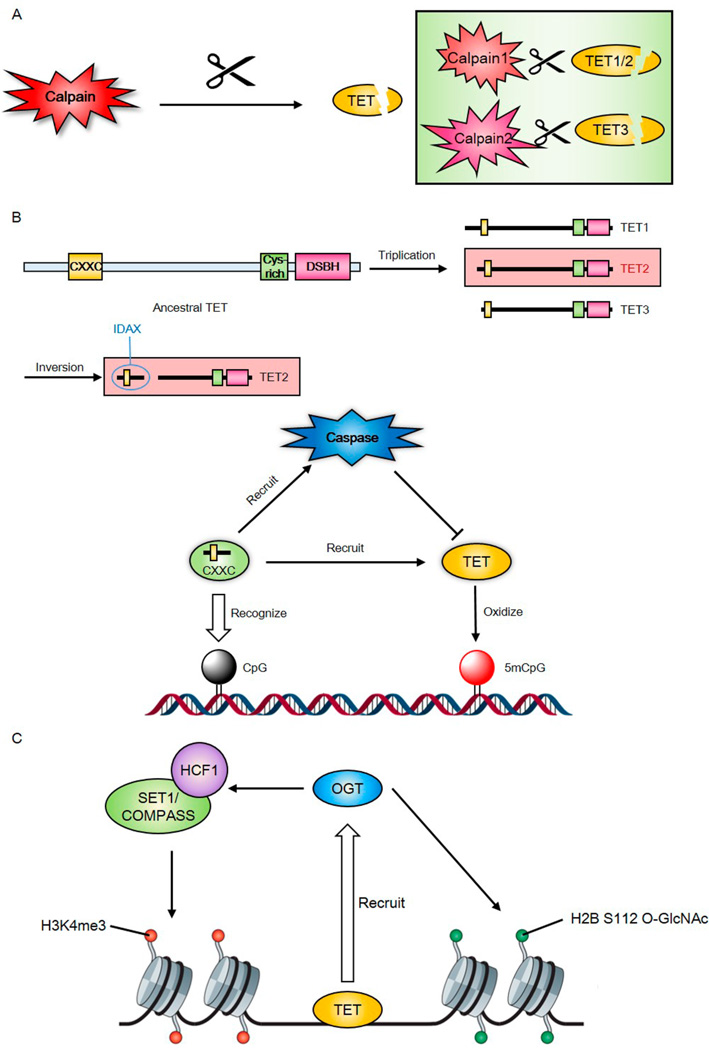
Calpains are a family of calcium-dependent cysteine proteases, which is one of the major regulators in protein degradation.129 A half-life study of TET in cycloheximide-treated cells reported by Zhang group revealed that calpains can regulate the turnover of TET proteins130 (Figure 6A). The coexpression of calpain1 or calpain2 can significantly decrease TET levels. In vitro assays confirmed the degradation of TET proteins by calpain1 and calpain2. Interestingly, the in vivo knockout experiment in mESC suggested that calpain1 may regulate TET1 and TET2, and calpain2 may regulate TET3.
Figure 6.
TET interacting proteins. (A) Calpains can regulate the half-life of TET enzymes. Calpain1 may regulate the degradation of TET1 and TET2, and calpain2 may regulate TET3. (B) IDAX can affect TET2 expression via caspase activation and the degradation of TET2. IDAX is separated from the catalytic domain of TET in a chromosomal gene inversion of an ancestral TET2 gene during evolution. IDAX CXXC domain binds unmethylated CpG dinucleotides and recruits TET2 to DNA. (C) TET enzymes recruit OGT to the chromatin and promote O-GlcNAcylation of histone 2B at Ser112, increasing the transcription of the corresponding genes. TET2/3 can also promote the O-GlcNAcylation activity of OCT on the host cell factor 1 (HCF1), stabilizing the integrity of H3K4 methyltransferase Set1/COMPASS.
Although calpain1 knockout has no effect on mESC pluripotency maintenance, calpain1 knockout does alter the level of 5hmC and lineage-specific gene expression in a way opposite to TET1 gene knockdown, which is consistent with the proposed calpain1-dependent TET1 and TET2 degradation. Calpain2-mediated degradation of TET3 is suggested to modulate the neuronal gene expression program and the in vitro efficiency of neural differentiation.
3.1.2.2. Regulation of TET2 Expression and Stability by the CXXC Domain
IDAX, also known as CXXC4, has previously been reported as an inhibitor of Wnt signaling.131–133 Rao and co-workers revealed that IDAX can affect TET2 protein expression via caspase activation and degradation of TET2.134 IDAX is believed to have been separated from the TET catalytic domain in a chromosomal gene inversion of an ancestral TET2 gene during evolution. Similar to the TET1 CXXC domain, the IDAX CXXC domain can also bind unmethylated CpG dinucleotides in DNA. This domain was shown to be localized to promoters and CpG islands in genomic DNA and helped recruit TET2 to DNA through protein–protein interaction (Figure 6B), without directly affecting the enzymatic activity of TET2. Interestingly, the study of TET3 and its CXXC domain showed that both intrinsic and extrinsic CXXC domains can regulate the expression of TET3. The cellular and subcellular distribution of IDAX may contribute to the levels of TET2 and 5hmC in different cells or tissues. Altered IDAX and TET2 expression in tumor cells has been observed. This study records the potential effects of IDAX and TET2 regulation in tumor cells for future work.
3.1.2.3. TET Proteins Bind OGT To Facilitate Chromatin O-GlcNAcylation
The mammalian O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT) is responsible for transferring O-GlcNAc from UDP-GlcNAc to the hydroxyl group of serine or threonine residues of various cellular proteins.135–138 The reverse reaction is realized by O-GlcNAcase that catalyzes the removal of O-GlcNAc.139 This OGT-mediated protein post-translational modification has been shown to occur with gene expression in multiple ways.140
Recent studies have confirmed the interaction of OGT with all three TET proteins.141–143 OGT interacts with the catalytic DSBH domain of TET2. Knockdown of TET2 in embryonic stem cells prevented the recruitment of OGT to chromatin, while knockdown of OGT had little effect on the TET activity at these loci. Overexpression of TET2 in human cells increased the OGT level on chromatin. An OGT mutant that has no binding affinity to TET failed to bind chromatin. Chromatin immunoprecipitation sequencing (ChIP-seq) analysis of OGT and TET2/3 indicated genome-wide colocalization of both proteins, mainly at CpG islands and transcription start sites.
The recruitment of OGT by TET2/3 proteins also increased the O-GlcNAcylation activity of OGT. Knockdown of TET proteins led to decreased O-GlcNAcylation without affecting the global level of OGT. Through monitoring of the 5hmC level in genomic DNA, it was shown that OGT has negligible influence on the oxidation activity of TET2/3.
TET2 can promote O-GlcNAcylation of histone 2B at Ser112 and increase the corresponding gene transcription (Figure 6C). TET2/3 also promotes the OGT O-GlcNAcylation activity of host cell factor 1 (HCF1), which stabilizes the integrity of Set1/COMPASS, the main complex of the H3K4 methyltransferase.142 ChIP-S analysis demonstrated that the majority loci targeted by TET2/3 and OGT overlap with the H3K4me3 mark. The study of Tet2 knockout mice also showed the decreased global level of O-GlcNAcylation and H3K4me3, especially at certain key regulators of hematopoiesis.
3.2. TET and 5hmC in Human Cancers
3.2.1. Decreased Levels of 5hmC and TET Expression in Human Cancers
5hmC quantification by dot blot and cell imaging revealed that global 5hmC levels are lower in a variety of human cancers including breast, liver, lung, pancreatic, and prostate cancers than in normal tissue samples. Corresponding TET gene expression in these tumors is also decreased, suggesting potential TET-dependent epigenetic patterns in cancers. Decreased levels of 5hmC and downregulation of TET proteins have also been observed during tumor progression, including hepatocellular carcinoma and melanoma.144,145 In these cases, levels of 5hmC are associated with different stages of tumor progression and may be associated with the overall survival rate of patients. Genome-wide profiling of 5hmC in nevi and melanomas revealed the loss of 5hmC during melanoma progression in genome-wide events while the global distribution of 5mC is rarely changed.145 Reintroducing TET2 in human melanoma may reestablish the 5hmC landscape and potentially suppress tumor invasion and growth.
3.2.2. TET Mutants in Hematopoietic Malignancies
After identification of the oxidation function of TET proteins, the involvement of TET family proteins during hematopoietic transformation has come into the spotlight with the discovery of somatic mutants of TET genes in myeloproliferative neoplasm (MPN) and myelodysplastic syndrome (MDS) patients.146 Somatic TET2 mutants in MPN and MDS patients include deletions and missense, nonsense, and frameshift mutations.147,148 These TET2 gene mutants were soon discovered to be TET2 inactive mutants, which lead to significant reductions in global 5hmC level.149 MPN patients with TET2 mutations exhibited advanced engraftment, which is consistent with the improved self-renewal ability of the Tet2 deletion mouse hematopoietic stem and progenitor cells (HSPCs).30–33
All three TET genes in multiple patients with different myeloid malignancies have been sequenced.150 While SNPs were identified in TET1 and TET3, the frequent somatic mutants are mainly located in the TET2 gene. Notably, TET mutants in both alleles are identified in a significant percentage of the patient samples, suggesting a similar dose-dependent effect of TET activity loss as in Tet2 deletion mice.
TET2 genetic mutants are the major abnormality identified in MDS.151–154 Although the relationship between TET2 mutants and a clinical phenotype or correlations with an overall survival rate is still unclear, TET2 mutants can be used to predict responses to azacytidine in higher-risk MDS and AML.154 Azacytidine is a chemical analogue of cytidine that inhibits DNA methyltransferase and causes DNA hypomethy-lation. The methylation pattern shaped by TET2 mutants may correspond to the sensitivity of response to the azacytidine treatment and has the potential to be a biomarker for clinical purposes. As indicated by the CMML-like disease in Tet2 deletion mice, CMML is also host to the high rate of TET2 genetic mutants. However, the impact of TET2 mutants in CMML is still unclear despite the analysis of a large number of cases.
TET2-acquired somatic mutants are also identified in classical MPNs.151,155 Although mutants occur at the early stage of the MPN and have no clear prognostic impact on the leukemic transformation risk, the appearance of some TET2 mutations is associated with the disease progression to the acute phase.156,157
TET2 mutants have also been reported in AML patients, especially in secondary AMLs.158–162 Detailed analysis has revealed that TET2 mutations have a poor prognostic impact on the overall survival rate of AML patients, thus promoting the utilization of risk-adapted therapeutic strategies for these AML patients.163 Interestingly, the global 5hmC level in TET2 mutated AML patients is significantly decreased compared to patients without TET2 mutants. Due to the complexity of the TET2 mutants, the level or distribution pattern of 5hmC may serve as a prognostic marker for AML.
3.2.3 IDH Mutants Inhibit TET Function
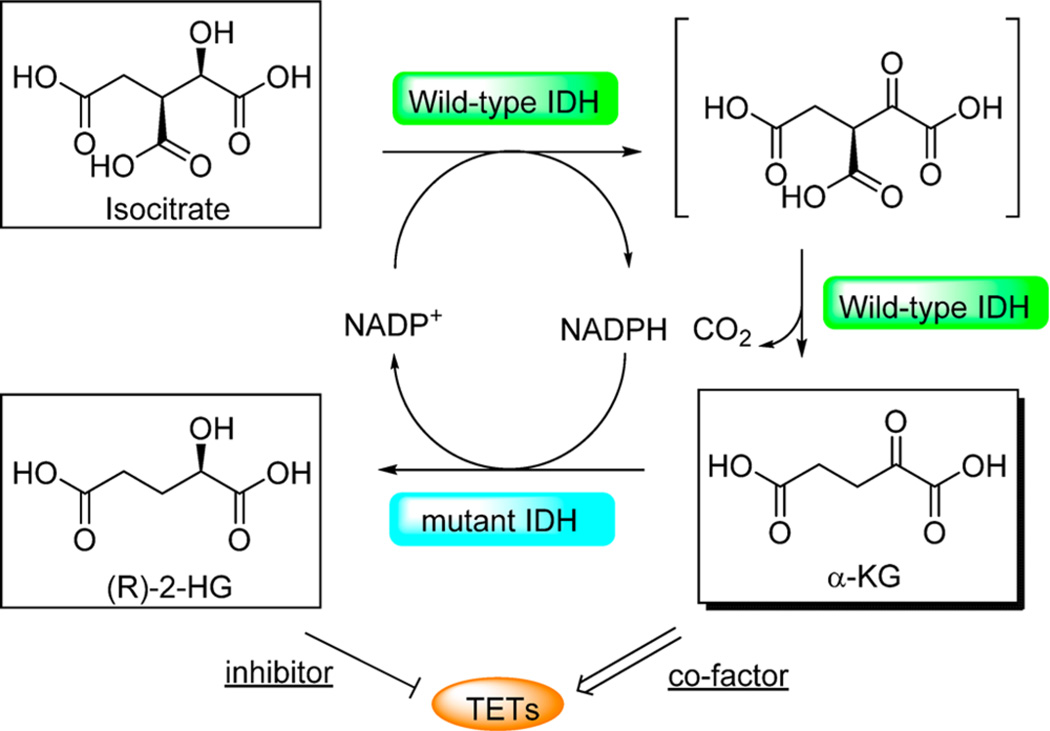
Isocitrate dehydrogenase 1 (IDH1) and IDH2 catalyze the conversion of isocitrate to α-KG, the key cofactor for TET enzymes, via a sequential NADP-based oxidation and decarboxylation. Mutations of IDH1 and IDH2 are commonly discovered in a diverse range of cancers or solid tumors, including gliomas, AML, MDS, MPN, and CMM.164–177 In myeloid malignancies, IDH mutants are exclusive to TET2 mutants and exhibit similar phenotypes as TET mutants, such as decreased levels of global 5hmC.145 Thus, IDH mutations and TET2 inactive mutants are believed to share the mechanism of hematopoietic transformation through the regulation of global DNA methylation and hydroxymethylation, suggesting the inhibition of TET activity in IDH mutants.
The tumor-associated IDH mutations have a gain of function to generate (R)-2-hydroxyglutarate ((R)-2-HG).178,179 The active site residues, such as Arg100 and Arg132 in IDH1 and Arg140 and Arg172 in IDH2, form hydrogen bonds to the isocitrate substrate.180 Mutations at these sites not only decrease the binding affinity of isocitrate, which abolishes the decarboxylation activity, but also increase the binding affinity for NADPH, which facilitates a reverse reduction reaction,179 thereby leading to the conversion of α-KG to (R)-2HG, which is an analogue inhibitor for α-KG-dependent proteins (Figure 7). The inhibition of TET proteins explains the globally reduced 5hmC in IDH mutants. However, IDH mutants are more pervasive in solid tumors than TET2 mutants. Thus, the inhibition effect of IDH mutants may have a broader impact on a range of α-KG-dependent dioxygenases, such as histone demethylases and RNA demethylases, or other proteins. These mutant IDH proteins serve as novel anticancer targets for chemists to explore.
Figure 7.
Catalytic activity of the wild-type and mutant IDH1 and IDH2. Wild-type IDH1 and IDH2 catalyze the oxidation of isocitrate to the intermediate oxalosuccinate, in which NADP+ is reduced to NADPH, serving as the hydrogen acceptor. In the second decarboxylation reaction, wild-type IDH1 and IDH2 catalyze the formation of α-KG, which is the key cofactor of TET and many other proteins. Mutant IDH1 and IDH2 catalyze the reduction of α-KG to 2-HG, in which NADPH is oxidized to NADP+. 2-HG can act as the inhibitor of TET proteins due to its structural similarity to α-KG.
3.2.4. TET Regulation in Human Cancer
MicroRNA miR-22 is reported to trigger epithelial–mesenchymal transition (EMT), and it enhances invasiveness and promotes metastasis in mouse xenografts.181 This function is realized by silencing antimetastatic miR-200 through direct targeting of the TET family protein (Figure 7). Overexpression of miR-22 led to reduced TET1 expression in cultured cell lines and significantly reduced 5hmC levels both globally and specifically on mir-200 promoter CpG islands, thus silencing the expression of miR-200. Knockdown of TET1 introduced a similar phenotype known to be induced by miR-22, and the ectopic expression of TET1 rescues this phenotype, restoring both global 5hmC level and miR-200 expression.
The analysis of the TET1 expression level in breast cancer patient samples compared to normal samples also suggests that miR-22 is associated with TET1 in an anticorrelated way. In this model, miR-22 suppresses the expression of another miRNA via direct targeting of TET family proteins. In other words, TET proteins can regulate the downstream miRNA expression through the demethylation of the promoter region upon upstream stimulation. Although no global demethylation has been observed, the loci-specific epigenetic dynamics regulated by TET enzymes may have a profound impact on gene expression regulation and cell fate.
A recent study of breast cancer also suggests that TET1 can simulate the expression of homeobox A (HOXA) genes by demethylating its promoter and suppress breast tumor growth and metastasis in mouse xenografts.182 The architectural transcription factor high mobility group AT-hook (HMGA2) was shown to induce TET1, implicating the HMGA2-TET1-HOX signaling pathway.
In contrast to the downregulation of TET proteins, TET1 was also shown to be significantly upregulated in MLL-rearranged leukemia, leading to a global increase in cellular 5hmC levels.183 The in vitro and in vivo functional studies revealed that TET1 coordinates with MLL-fusion protein, regulating cotargets like homeobox A9 (Hoxa9)/myeloid ecotropic viral integration 1 (Meis1)/pre-B-cell leukemia homeobox 3 (Pbx3) genes.
4. CONCLUSIONS AND PERSPECTIVE
In this review, we have summarized the activity of TET proteins and the corresponding demethylation pathway. The discovery of 5mC oxidation by TET proteins opens up new avenues in our understanding of reversible methylation in modulating gene expression. Sensitive and efficient quantification and sequencing methods have been established to illustrate the cellular abundance and distribution of the DNA cytosine modifications, which may encode a complex layer of transcription regulation. However, the functions of 5hmC/5fC/ 5caC still require future research. The distribution and roles of 5hmU need to be elucidated. The versatile interaction among TET with its regulatory factors, including small molecules, proteins, and miRNA, provides a glimpse of the complexity of epigenetic machinery acting on the modified bases. The functions and regulation mechanism of TET-mediated epigenetic dynamics will continue to be unveiled. The emerging roles of TET2 mutants in human cancers and their oxidation activity will stimulate investigations of epigenetic modifications as prognostic markers in cancer diagnostics.
Acknowledgments
We apologize for any references that we might have omitted due to space limitations. This study was supported by the National Institutes of Health HG006827.
ABBREVIATIONS
- 2-HG
2-Hydroxyglutarate
- 5caC
5-Carboxylcytosine
- 5fC
5-Formylcytosine
- 5fU
5-Formyluracil
- 5hmC
5-Hydroxymethylcytosine
- 5hmU
5-Hydroxymethyluracil
- 5mC
5-Methylcytosine
- AID
Activation-induced cytidine daminase
- AML
Acute myeloid leukemia
- APOBEC
Apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like
- BER
Base-excision repair
- CAB-seq
Chemical-assisted bisulfite sequencing
- ChIP-seq
Chromatin immunoprecipitation sequencing
- CMML
Chronic myelomonocytic leukemia
- CTCF
CCCTC-binding factor
- DNMT
Methyl transferase enzymes
- DSBH
Double-stranded β-helix
- DTT
Dithiothreitol
- EDC
1-Ethyl-3-(3-(dimethylamino)propyl)carbodiimide
- EMSA
Electrophoretic mobility shift assay
- EMT
Epithelial–mesenchymal transition
- Gadd45
Growth arrest and DNA damage
- HCF1
Host cell factor 1
- HMGA
High mobility group AT-hook
- HOXA
Homeobox A
- HPLC-MS
High-performance liquid chromatography–mass spectrometry
- IDH
Isocitrate dehydrogenase
- JBP
J-binding protein
- MAB-seq
M.SssI methylase-assisted bisulfite sequencing
- MDS
Myelodysplastic syndrome
- mESC
Mouse embryonic stem cell
- MPN
Myeloproliferative neoplasm
- MLL
Mixed lineage leukemia
- NADP
Nicotinamide adenine dinucleotide phosphate
- NOG
N-Oxalyl glycine
- O-GlcNAc
O-Linked N-actylglucosamine
- OGT
O-Linked N-actylglucosamine (O-GlcNac) trans-ferase
- oxBS-seq
Oxidative bisulfite sequencing
- redBS-seq
Reduced bisulfite sequencing
- PGC
Primordial germ cell
- ROS
Reactive oxygen species
- Seal
Selective chemical labeling
- TAB-seq
Tet-assisted bisulfite sequencing
- TDG
DNA thymine glycosylase
- TET
Ten-eleven translocation
- TLC
Thin-layer chromatography
- SAM
S-Sdenosylmethionine
- α-KG
α-Ketoglutarate
Biographies

Xingyu Lu was born in P. R. China in 1988 and received his B.S. (2010) in Chemistry from Peking University. He then moved to the United States and began to work on the sequencing method of DNA modifications in Dr. Chuan He’s laboratory as a Ph.D candidate at the University of Chicago. Since then, he has focused on the selective chemical labeling of cytosine modifications, such as 5hmC, 5fC, and 5caC.

Boxuan Simen Zhao received his B.S. degree in Chemistry from Peking University in 2012. He then received his M.S. degree in 2013 and is pursuing his Ph.D degree under Dr. Chuan He’s instruction, both at the University of Chicago. His research interests include DNA and RNA epigenetics, specifically the investigation of distribution and biological functions of modified bases on DNA (5hmC and 5fC) and RNA (m6A).

Chuan He received his B.S. degree in Chemistry from the University of Science and Technology of China (USTC) in 1994. He obtained his Ph.D. degree at Massachusetts Institute of Technology with Professor Stephen J. Lippard in 2000 and received postdoctoral training with Professor Gregory L. Verdine at Harvard University. He is a currently a professor at the University of Chicago and an investigator of Howard Hughes Medical Institute. His research interests cover nucleic acid modifications, epigenetics, chemical biology, RNA metabolisms, and bioinorganic chemistry.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Klose RJ, Bird AP. Trends Biochem. Sci. 2006;31:89. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Okano M, Bell DW, Haber DA, Li E. Cell. 1999;99:247. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 3.Okano M, Xie S, Li E. Nat. Genet. 1998;19:219. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 4.Goll MG, Bestor TH. Annu. Rev. BioChem. 2005;74:481. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 5.Bestor T, Laudano A, Mattaliano R, Ingram V. J. Mol. Biol. 1988;203:971. doi: 10.1016/0022-2836(88)90122-2. [DOI] [PubMed] [Google Scholar]
- 6.Hermann A, Goyal R, Jeltsch A. J. Biol. Chem. 2004;279:48350. doi: 10.1074/jbc.M403427200. [DOI] [PubMed] [Google Scholar]
- 7.Bestor TH. Bourc’his, D. Cold Spring Harbor Symp. Quant. Biol. 2004;69:381. doi: 10.1101/sqb.2004.69.381. [DOI] [PubMed] [Google Scholar]
- 8.Jaenisch R, Bird A. Nat. Genet. 2003;33(Suppl):245. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 9.Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Nature. 2000;403:501. doi: 10.1038/35000656. [DOI] [PubMed] [Google Scholar]
- 10.Wu SC, Zhang Y. Nat. Rev. Mol. Cell Biol. 2010;11:607. doi: 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ooi SK, Bestor TH. Cell. 2008;133:1145. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Surani MA, Hajkova P. Cold Spring Harbor Symp. Quant. Biol. 2010;75:211. doi: 10.1101/sqb.2010.75.010. [DOI] [PubMed] [Google Scholar]
- 13.Kriaucionis S, Heintz N. Science. 2009;324:929. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Science. 2009;324:930. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maiti A, Drohat AC. J. Biol. Chem. 2011;286:35334. doi: 10.1074/jbc.C111.284620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Lu X, Lu J, Liang H, Dai Q, Xu GL, Luo C, Jiang H, He C. Nat. Chem. Biol. 2012;8:328. doi: 10.1038/nchembio.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, Sun Y, Li X, Dai Q, Song CX, Zhang K, He C, Xu GL. Science. 2011;333:1303. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono R, Taki T, Taketani T, Taniwaki M, Kobayashi H, Hayashi Y. Cancer Res. 2002;62:4075. [PubMed] [Google Scholar]
- 19.Lorsbach RB, Moore J, Mathew S, Raimondi SC, Mukatira ST, Downing JR. Leukemia. 2003;17:637. doi: 10.1038/sj.leu.2402834. [DOI] [PubMed] [Google Scholar]
- 20.Wu H, Zhang Y. Genes Dev. 2011;25:2436. doi: 10.1101/gad.179184.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, Iqbal K, Shi YG, Deng Z, Szabo PE, Pfeifer GP, Li J, Xu GL. Nature. 2011;477:606. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal K, Jin SG, Pfeifer GP, Szabo PE. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3642. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Arand J, Nakano T, Reik W, Walter J. Nat. Commun. 2011;2:241. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- 24.Cimmino L, Abdel-Wahab O, Levine RL, Aifantis I. Cell Stem Cell. 2011;9:193. doi: 10.1016/j.stem.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawlaty MM, Breiling A, Le T, Raddatz G, Barrasa MI, Cheng AW, Gao Q, Powell BE, Li Z, Xu M, Faull KF, Lyko F, Jaenisch R. Dev. Cell. 2013;24:310. doi: 10.1016/j.devcel.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawlaty MM, Ganz K, Powell BE, Hu YC, Markoulaki S, Cheng AW, Gao Q, Kim J, Choi SW, Page DC, Jaenisch R. Cell Stem Cell. 2011;9:166. doi: 10.1016/j.stem.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, Laiho A, Tahiliani M, Sommer CA, Mostoslavsky G, Lahesmaa R, Orkin SH, Rodig SJ, Daley GQ, Rao A. Cell Stem Cell. 2011;8:200. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcucci G, Maharry K, Wu YZ, Radmacher MD, Mrozek K, Margeson D, Holland KB, Whitman SP, Becker H, Schwind S, Metzeler KH, Powell BL, Carter TH, Kolitz JE, Wetzler M, Carroll AJ, Baer MR, Caligiuri MA, Larson RA, Bloomfield CD. J. Clin. Oncol. 2010;28:2348. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaguchi S, Hong K, Liu R, Shen L, Inoue A, Diep D, Zhang K, Zhang Y. Nature. 2012;492:443. doi: 10.1038/nature11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, Figueroa ME, Vasanthakumar A, Patel J, Zhao X, Perna F, Pandey S, Madzo J, Song C, Dai Q, He C, Ibrahim S, Beran M, Zavadil J, Nimer SD, Melnick A, Godley LA, Aifantis I, Levine RL. Cancer Cell. 2011;20:11. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko M, Bandukwala HS, An J, Lamperti ED, Thompson EC, Hastie R, Tsangaratou A, Rajewsky K, Koralov SB, Rao A. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14566. doi: 10.1073/pnas.1112317108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Cai X, Cai CL, Wang J, Zhang W, Petersen BE, Yang FC, Xu M. Blood. 2011;118:4509. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quivoron C, Couronne L, Della Valle V, Lopez CK, Plo I, Wagner-Ballon O, Do Cruzeiro M, Delhommeau F, Arnulf B, Stern MH, Godley L, Opolon P, Tilly H, Solary E, Duffourd Y, Dessen P, Merle-Beral H, Nguyen-Khac F, Fontenay M, Vainchenker W, Bastard C, Mercher T, Bernard OA. Cancer Cell. 2011;20:25. doi: 10.1016/j.ccr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Globisch D, Munzel M, Muller M, Michalakis S, Wagner M, Koch S, Bruckl T, Biel M, Carell T. PLoS One. 2010;5:e15367. doi: 10.1371/journal.pone.0015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Science. 2011;333:1300. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfaffeneder T, Hackner B, Truss M, Munzel M, Muller M, Deiml CA, Hagemeier C, Carell T. Angew. Chem. Int. Ed. Engl. 2011;50:7008. doi: 10.1002/anie.201103899. [DOI] [PubMed] [Google Scholar]
- 37.Song CX, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen CH, Zhang W, Jian X, Wang J, Zhang L, Looney TJ, Zhang B, Godley LA, Hicks LM, Lahn BT, Jin P, He C. Nat. Biotechnol. 2011;29:68. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Booth MJ, Branco MR, Ficz G, Oxley D, Krueger F, Reik W, Balasubramanian S. Science. 2012;336:934. doi: 10.1126/science.1220671. [DOI] [PubMed] [Google Scholar]
- 39.Li WW, Gong L, Bayley H. Angew. Chem. Int. Ed. 2013;52:4350. doi: 10.1002/anie.201300413. [DOI] [PubMed] [Google Scholar]
- 40.Pastor WA, Pape UJ, Huang Y, Henderson HR, Lister R, Ko M, McLoughlin EM, Brudno Y, Mahapatra S, Kapranov P, Tahiliani M, Daley GQ, Liu XS, Ecker JR, Milos PM, Agarwal S, Rao A. Nature. 2011;473:394. doi: 10.1038/nature10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raiber EA, Beraldi D, Ficz G, Burgess H, Branco MR, Murat P, Oxley D, Booth MJ, Reik W, Balasubramanian S. Genome Biol. 2012;13:R69. doi: 10.1186/gb-2012-13-8-r69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robertson AB, Dahl JA, Vagbo CB, Tripathi P, Krokan HE, Klungland A. Nucleic Acids Res. 2011;39:e55. doi: 10.1093/nar/gkr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song CX, Clark TA, Lu XY, Kislyuk A, Dai Q, Turner SW, He C, Korlach j. Nat. Methods. 2012;9:75. doi: 10.1038/nmeth.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song CX, Sun Y, Dai Q, Lu XY, Yu M, Yang CG, He C. ChemBioChem. 2011;12:1682. doi: 10.1002/cbic.201100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song CX, Szulwach KE, Dai Q, Fu Y, Mao SQ, Lin L, Street C, Li Y, Poidevin M, Wu H, Gao J, Liu P, Li L, Xu GL, Jin P, He C. Cell. 2013;153:678. doi: 10.1016/j.cell.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stadler MB, Murr R, Burger L, Ivanek R, Lienert F, Scholer A, van Nimwegen E, Wirbelauer C, Oakeley EJ, Gaidatzis D, Tiwari VK, Schubeler D. Nature. 2011;480:490. doi: 10.1038/nature10716. [DOI] [PubMed] [Google Scholar]
- 47.Yu M, Hon GC, Szulwach KE, Song CX, Zhang L, Kim A, Li X, Dai Q, Shen Y, Park B, Min JH, Jin P, Ren B, He C. Cell. 2012;149:1368. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen L, Wu H, Diep D, Yamaguchi S, D’Alessio AC, Fung HL, Zhang K, Zhang Y. Cell. 2013;153:692. doi: 10.1016/j.cell.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Booth MJ, Marsico G, Bachman M, Beraldi D, Balasubramanian S. Nat. Chem. 2014;6:435. doi: 10.1038/nchem.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu X, Song CX, Szulwach K, Wang Z, Weidenbacher P, Jin P, He C. J. Am. Chem. Soc. 2013;135:9315. doi: 10.1021/ja4044856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song CX, Yi C, He C. Nat. Biotechnol. 2012;30:1107. doi: 10.1038/nbt.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munzel M, Globisch D, Bruckl T, Wagner M, Welzmiller V, Michalakis S, Muller M, Biel M, Carell T. Angew. Chem. Int. Ed. 2010;49:5375. doi: 10.1002/anie.201002033. [DOI] [PubMed] [Google Scholar]
- 53.Borst P, Sabatini R. Annu. Rev. Microbiol. 2008;62:235. doi: 10.1146/annurev.micro.62.081307.162750. [DOI] [PubMed] [Google Scholar]
- 54.Yu Z, Genest PA, ter Riet B, Sweeney K, DiPaolo C, Kieft R, Christodoulou E, Perrakis A, Simmons JM, Hausinger RP, van Luenen HG, Rigden DJ, Sabatini R, Borst P. Nucleic Acids Res. 2007;35:2107. doi: 10.1093/nar/gkm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cliffe LJ, Kieft R, Southern T, Birkeland SR, Marshall M, Sweeney K, Sabatini R. Nucleic Acids Res. 2009;37:1452. doi: 10.1093/nar/gkn1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thornburg LD, Lai MT, Wishnok JS, Stubbe J. Biochemistry. 1993;32:14023. doi: 10.1021/bi00213a036. [DOI] [PubMed] [Google Scholar]
- 57.Liu CK, Hsu CA, Abbott MT. Arch. Biochem. Biophys. 1973;159:180. doi: 10.1016/0003-9861(73)90443-8. [DOI] [PubMed] [Google Scholar]
- 58.Wondrack LM, Hsu CA, Abbott MT. J. Biol. Chem. 1978;253:6511. [PubMed] [Google Scholar]
- 59.Holme E. Biochemistry. 1975;14:4999. doi: 10.1021/bi00693a033. [DOI] [PubMed] [Google Scholar]
- 60.Smiley JA, Kundracik M, Landfried DA, Barnes VR, Axhemi AA. Biochim. Biophys. Acta. 2005;1723:256. doi: 10.1016/j.bbagen.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 61.Bankel L, Lindstedt G, Lindstedt S. Biochim. Biophys. Acta. 1977;481:431. doi: 10.1016/0005-2744(77)90276-5. [DOI] [PubMed] [Google Scholar]
- 62.Simmons JM, Koslowsky DJ, Hausinger RP. Exp. Parasitol. 2010;124:453. doi: 10.1016/j.exppara.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao C, Huang T, Chen W, Deng Z. Appl. Environ. Microbiol. 2010;76:7343. doi: 10.1128/AEM.01257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iyer LM, Tahiliani M, Rao A, Aravind L. Cell Cycle. 2009;8:1698. doi: 10.4161/cc.8.11.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu S, Wang J, Su Y, Guerrero C, Zeng Y, Mitra D, Brooks PJ, Fisher DE, Song H, Wang Y. Nucleic Acids Res. 2013;41:6421. doi: 10.1093/nar/gkt360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Nature. 2010;466:1129. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bachman M, Uribe-Lewis S, Yang X, Williams M, Murrell A, Balasubramanian S. Nat. Chem. 2014;6:1049. doi: 10.1038/nchem.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grippo P, Iaccarino M, Rossi M, Scarano E. Biochim. Biophys. Acta. 1965;95:1. doi: 10.1016/0005-2787(65)90204-2. [DOI] [PubMed] [Google Scholar]
- 69.Yu M, Hon GC, Szulwach KE, Song CX, Jin P, Ren B, He C. Nat. Protoc. 2012;7:2159. doi: 10.1038/nprot.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L, Chen W, Iyer LM, Hu J, Wang G, Fu Y, Yu M, Dai Q, Aravind L, He C. J. Am. Chem. Soc. 2014;136:4801. doi: 10.1021/ja500979k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L, Yu M, He C. Acta Chim. Sin. 2012;70:2123. [Google Scholar]
- 72.Kizaki S, Sugiyama H. Org. Biomol. Chem. 2014;12:104. doi: 10.1039/c3ob41823e. [DOI] [PubMed] [Google Scholar]
- 73.Fu L, Guerrero CR, Zhong N, Amato NJ, Liu Y, Liu S, Cai Q, Ji D, Jin SG, Niedernhofer LJ, Pfeifer GP, Xu GL, Wang Y. J. Am. Chem. Soc. 2014;136:11582. doi: 10.1021/ja505305z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pfaffeneder T, Spada F, Wagner M, Brandmayr C, Laube SK, Eisen D, Truss M, Steinbacher J, Hackner B, Kotljarova O, Schuermann D, Michalakis S, Kosmatchev O, Schiesser S, Steigenberger B, Raddaoui N, Kashiwazaki G, Muller U, Spruijt CG, Vermeulen M, Leonhardt H, Schar P, Muller M, Carell T. Nat. Chem. Biol. 2014;10:574. doi: 10.1038/nchembio.1532. [DOI] [PubMed] [Google Scholar]
- 75.Cortellino S, Xu J, Sannai M, Moore R, Caretti E, Cigliano A, Le Coz M, Devarajan K, Wessels A, Soprano D, Abramowitz LK, Bartolomei MS, Rambow F, Bassi MR, Bruno T, Fanciulli M, Renner C, Klein-Szanto AJ, Matsumoto Y, Kobi D, Davidson I, Alberti C, Larue L, Bellacosa A. Cell. 2011;146:67. doi: 10.1016/j.cell.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo JU, Su Y, Zhong C, Ming GL, Song H. Cell. 2011;145:423. doi: 10.1016/j.cell.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu M, Song C-X, He C. Methods. 2011;15:16. [Google Scholar]
- 78.Inoue A, Zhang Y. Science. 2011;334:194. doi: 10.1126/science.1212483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Inoue A, Shen L, Dai Q, He C, Zhang Y. Cell Res. 2011;21:1670. doi: 10.1038/cr.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, Meissner A. Nature. 2012;484:339. doi: 10.1038/nature10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang L, Zhang J, Duan J, Gao X, Zhu W, Lu X, Yang L, Li G, Ci W, Li W, Zhou Q, Aluru N, Tang F, He C, Huang X, Liu J. Cell. 2014;157:979. doi: 10.1016/j.cell.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schiesser S, Hackner B, Pfaffeneder T, Muller M, Hagemeier C, Truss M, Carell T. Angew. Chem. Int. Ed. 2012;51:6516. doi: 10.1002/anie.201202583. [DOI] [PubMed] [Google Scholar]
- 83.Dalton SR, Bellacosa A. Epigenomics. 2012;4:459. doi: 10.2217/epi.12.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu JK. Annu. Rev. Genet. 2009;43:143. doi: 10.1146/annurev-genet-102108-134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cortazar D, Kunz C, Selfridge J, Lettieri T, Saito Y, MacDougall E, Wirz A, Schuermann D, Jacobs AL, Siegrist F, Steinacher R, Jiricny J, Bird A, Schar P. Nature. 2011;470:419. doi: 10.1038/nature09672. [DOI] [PubMed] [Google Scholar]
- 86.Cortazar D, Kunz C, Saito Y, Steinacher R, Schar P. DNA Repair. 2007;6:489. doi: 10.1016/j.dnarep.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Bennett MT, Rodgers MT, Hebert AS, Ruslander LE, Eisele L, Drohat AC. J. Am. Chem. Soc. 2006;128:12510. doi: 10.1021/ja0634829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williams RT, Wang Y. Biochemistry. 2012;51:6458. doi: 10.1021/bi300797q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Nature. 2010;463:1042. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, Jacobsen SE, Reik W. Nature. 2010;463:1101. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar R, DiMenna L, Schrode N, Liu TC, Franck P, Munoz-Descalzo S, Hadjantonakis AK, Zarrin AA, Chaudhuri J, Elemento O, Evans T. Nature. 2013;500:89. doi: 10.1038/nature12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rai K, Huggins IJ, James SR, Karpf AR, Jones DA, Cairns BR. Cell. 2008;135:1201. doi: 10.1016/j.cell.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bransteitter R, Pham P, Scharff MD, Goodman MF. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4102. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Conticello SG, Langlois MA, Yang Z, Neuberger MS. Adv. Immunol. 2007;94:37. doi: 10.1016/S0065-2776(06)94002-4. [DOI] [PubMed] [Google Scholar]
- 95.Di Noia JM, Neuberger MS. Annu. Rev. BioChem. 2007;76:1. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 96.Larijani M, Frieder D, Sonbuchner TM, Bransteitter R, Goodman MF, Bouhassira EE, Scharff MD, Martin A. Mol. Immunol. 2005;42:599. doi: 10.1016/j.molimm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Nabel CS, Jia H, Ye Y, Shen L, Goldschmidt HL, Stivers JT, Zhang Y, Kohli RM. Nat. Chem. Biol. 2012;8:751. doi: 10.1038/nchembio.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rangam G, Schmitz KM, Cobb AJ, Petersen-Mahrt SK. PLoS One. 2012;7:e43279. doi: 10.1371/journal.pone.0043279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu C, Bian C, Lam R, Dong A, Min J. Nat. Commun. 2011;2:227. doi: 10.1038/ncomms1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu Y, Xu C, Kato A, Tempel W, Abreu JG, Bian C, Hu Y, Hu D, Zhao B, Cerovina T, Diao J, Wu F, He HH, Cui Q, Clark E, Ma C, Barbara A, Veenstra GJ, Xu G, Kaiser UB, Liu XS, Sugrue SP, He X, Min J, Kato Y, Shi YG. Cell. 2012;151:1200. doi: 10.1016/j.cell.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu L, Li Z, Cheng J, Rao Q, Gong W, Liu M, Shi YG, Zhu J, Wang P, Xu Y. Cell. 2013;155:1545. doi: 10.1016/j.cell.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 102.Hashimoto H, Pais JE, Zhang X, Saleh L, Fu ZQ, Dai N, Correa IR, Zheng Y, Cheng XD. Nature. 2014;506:391. doi: 10.1038/nature12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zheng G, Fu Y, He C. Chem. Rev. 2014;114:4602. doi: 10.1021/cr400432d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mishina Y, He CJ. Inorg. BioChem. 2006;100:670. doi: 10.1016/j.jinorgbio.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mishina Y, Chen LX, He C. J. Am. Chem. Soc. 2004;126:16930. doi: 10.1021/ja045066z. [DOI] [PubMed] [Google Scholar]
- 106.Henshaw TF, Feig M, Hausinger RP. J. Inorg Bio. Chem. 2004;98:856. doi: 10.1016/j.jinorgbio.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 107.Liu H, Llano J, Gauld JW. J. Phys. Chem. B. 2009;113:4887. doi: 10.1021/jp810715t. [DOI] [PubMed] [Google Scholar]
- 108.Cisneros GA. Interdiscip. Sci. 2010;2:70. doi: 10.1007/s12539-010-0092-z. [DOI] [PubMed] [Google Scholar]
- 109.Bleijlevens B, Shivarattan T, van den Boom KS, de Haan A, van der Zwan G, Simpson PJ, Matthews SJ. Biochemistry. 2012;51:3334. doi: 10.1021/bi201699e. [DOI] [PubMed] [Google Scholar]
- 110.Que L., Jr Acc. Chem. Res. 2007;40:493. doi: 10.1021/ar700024g. [DOI] [PubMed] [Google Scholar]
- 111.Bleijlevens B, Shivarattan T, Sedgwick B, Rigby SE, Matthews SJ. J. Inorg. Bio. Chem. 2007;101:1043. doi: 10.1016/j.jinorgbio.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 112.Bleijlevens B, Shivarattan T, Flashman E, Yang Y, Simpson PJ, Koivisto P, Sedgwick B, Schofield CJ, Matthews S. J. EMBO Rep. 2008;9:872. doi: 10.1038/embor.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shivarattan T, Chen HA, Simpson P, Sedgwick B, Matthews S. J. Biomol. NMR. 2005;33:138. doi: 10.1007/s10858-005-3040-1. [DOI] [PubMed] [Google Scholar]
- 114.Yu B, Edstrom WC, Benach J, Hamuro Y, Weber PC, Gibney BR, Hunt JF. Nature. 2006;439:879. doi: 10.1038/nature04561. [DOI] [PubMed] [Google Scholar]
- 115.Chen H, Costa M. Biometals. 2009;22:191. doi: 10.1007/s10534-008-9190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chervona Y, Arita A, Costa M. Metallomics. 2012;4:619. doi: 10.1039/c2mt20033c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu H, Llano J, Gauld JW. J. Phys. Chem. B. 2009;113:4887. doi: 10.1021/jp810715t. [DOI] [PubMed] [Google Scholar]
- 118.Fang D, Cisneros GA. J.Chem. Theory Comput. 2014;10:5136. doi: 10.1021/ct500572t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Guo F, Li X, Liang D, Li T, Zhu P, Guo H, Wu X, Wen L, Gu TP, Hu B, Walsh CP, Li J, Tang F, Xu GL. Cell Stem Cell. 2014;15:447. doi: 10.1016/j.stem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 120.Wu H, Wu X, Shen L, Zhang Y. Nat. Biotechnol. 2014;32:1231. doi: 10.1038/nbt.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gorres KL, Raines RT. Crit.Rev.Bio.chem.Mol. Biol. 2010;45:106. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yin R, Mao SQ, Zhao B, Chong Z, Yang Y, Zhao C, Zhang D, Huang H, Gao J, Li Z, Jiao Y, Li C, Liu S, Wu D, Gu W, Yang YG, Xu GL, Wang H. J. Am. Chem. Soc. 2013;135:10396. doi: 10.1021/ja4028346. [DOI] [PubMed] [Google Scholar]
- 123.Blaschke K, Ebata KT, Karimi MM, Zepeda-Martinez JA, Goyal P, Mahapatra S, Tam A, Laird DJ, Hirst M, Rao A, Lorincz MC, Ramalho-Santos M. Nature. 2013;500:222. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JMC, Thomson JA. Nat. Methods. 2011;8:424. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J, Li W, Weng Z, Chen J, Ni S, Chen K, Li Y, Liu X, Xu J, Zhang S, Li F, He W, Labuda K, Song Y, Peterbauer A, Wolbank S, Redl H, Zhong M, Cai D, Zeng L, Pei D. Cell Stem Cell. 2010;6:71. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 126.Wang Y, Mackenzie B, Tsukaguchi H, Weremowicz S, Morton CC, Hediger MA. Biochem. Biophys. Res. Commun. 2000;267:488. doi: 10.1006/bbrc.1999.1929. [DOI] [PubMed] [Google Scholar]
- 127.Agus DB, Vera JC, Golde DW. Cancer Res. 1999;59:4555. [PubMed] [Google Scholar]
- 128.Yang H, Lin H, Xu H, Zhang L, Cheng L, Wen B, Shou J, Guan K, Xiong Y, Ye D. Cell Res. 2014;24:1017. doi: 10.1038/cr.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cohen GM. BioChem. J. 1997;326(Pt1):1. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Zhang Y. Cell Rep. 2014;6:278. doi: 10.1016/j.celrep.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kojima T, Shimazui T, Hinotsu S, Joraku A, Oikawa T, Kawai K, Horie R, Suzuki H, Nagashima R, Yoshikawa K, Michiue T, Asashima M, Akaza H, Uchida K. Oncogene. 2009;28:297. doi: 10.1038/onc.2008.391. [DOI] [PubMed] [Google Scholar]
- 132.Hino S, Kishida S, Michiue T, Fukui A, Sakamoto I, Takada S, Asashima M, Kikuchi A. Mol.Cell. Biol. 2001;21:330. doi: 10.1128/MCB.21.1.330-342.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nguyen AV, Albers CG, Holcombe RF. Int.J. Mol. Med. 2010;26:121. doi: 10.3892/ijmm_00000443. [DOI] [PubMed] [Google Scholar]
- 134.Ko M, An J, Bandukwala HS, Chavez L, Aijo T, Pastor WA, Segal MF, Li H, Koh KP, Lahdesmaki H, Hogan PG, Aravind L, Rao A. Nature. 2013;497:122. doi: 10.1038/nature12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Holt GD, Hart GW. J. Biol. Chem. 1986;261:8049. [PubMed] [Google Scholar]
- 136.Haltiwanger RS, Blomberg MA, Hart GW. J. Biol. Chem. 1992;267:9005. [PubMed] [Google Scholar]
- 137.Vosseller K, Sakabe K, Wells L. Hart, G. W. Curr. Opin. Chem. Biol. 2002;6:851. doi: 10.1016/s1367-5931(02)00384-8. [DOI] [PubMed] [Google Scholar]
- 138.Kreppel LK, Blomberg MA, Hart GW. J. Biol. Chem. 1997;272:9308. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 139.Dong DL, Hart GW. J. Biol. Chem. 1994;269:19321. [PubMed] [Google Scholar]
- 140.Hanover JA, Krause MW, Love DC. Nat. Rev. Mol. Cell Biol. 2012;13:312. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- 141.Vella P, Scelfo A, Jammula S, Chiacchiera F, Williams K, Cuomo A, Roberto A, Christensen J, Bonaldi T, Helin K, Pasini D. Mol. Cell. 2013;49:645. doi: 10.1016/j.molcel.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 142.Deplus R, Delatte B, Schwinn MK, Defrance M, Mendez J, Murphy N, Dawson MA, Volkmar M, Putmans P, Calonne E, Shih AH, Levine RL, Bernard O, Mercher T, Solary E, Urh M, Daniels DL, Fuks F. EMBO J. 2013;32:645. doi: 10.1038/emboj.2012.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen Q, Chen Y, Bian C, Fujiki R, Yu X. Nature. 2013;493:561. doi: 10.1038/nature11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu C, Liu L, Chen X, Shen J, Shan J, Xu Y, Yang Z, Wu L, Xia F, Bie P, Cui Y, Bian XW, Qian C. PLoS One. 2013;8:e62828. doi: 10.1371/journal.pone.0062828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lian CG, Xu Y, Ceol C, Wu F, Larson A, Dresser K, Xu W, Tan L, Hu Y, Zhan Q, Lee CW, Hu D, Lian BQ, Kleffel S, Yang Y, Neiswender J, Khorasani AJ, Fang R, Lezcano C, Duncan LM, Scolyer RA, Thompson JF, Kakavand H, Houvras Y, Zon LI, Mihm MC, Kaiser UB, Schatton T, Woda BA, Murphy GF, Shi YG., Jr Cell. 2012;150:1135. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.James C, Mazurier F, Dupont S, Chaligne R, Lamrissi-Garcia I, Tulliez M, Lippert E, Mahon FX, Pasquet JM, Etienne G, Delhommeau F, Giraudier S, Vainchenker W, de Verneuil H. Blood. 2008;112:2429. doi: 10.1182/blood-2008-02-137877. [DOI] [PubMed] [Google Scholar]
- 147.Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, Kosmider O, Le Couedic JP, Robert F, Alberdi A, Lecluse Y, Plo I, Dreyfus FJ, Marzac C, Casadevall N, Lacombe C, Romana SP, Dessen P, Soulier J, Viguie F, Fontenay M, Vainchenker W, Bernard OAN. Engl. J. Med. 2009;360:2289. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 148.Langemeijer SM, Kuiper RP, Berends M, Knops R, Aslanyan MG, Massop M, Stevens-Linders E, van Hoogen P, van Kessel AG, Raymakers RA, Kamping EJ, Verhoef GE, Verburgh E, Hagemeijer A, Vandenberghe P, de Witte T, van der Reijden BA, Jansen JH. Nat. Genet. 2009;41:838. doi: 10.1038/ng.391. [DOI] [PubMed] [Google Scholar]
- 149.Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS, An J, Lamperti ED, Koh KP, Ganetzky R, Liu XS, Aravind L, Agarwal S, Maciejewski JP, Rao A. Nature. 2010;468:839. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abdel-Wahab O, Mullally A, Hedvat C, Garcia-Manero G, Patel J, Wadleigh M, Malinge S, Yao J, Kilpivaara O, Bhat R, Huberman K, Thomas S, Dolgalev I, Heguy A, Paietta E, Le Beau MM, Beran M, Tallman MS, Ebert BL, Kantarjian HM, Stone RM, Gilliland DG, Crispino JD, Levine RL. Blood. 2009;114:144. doi: 10.1182/blood-2009-03-210039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, Kantarjian H, Raza A, Levine RL, Neuberg D, Ebert BLN. Engl.J. Med. 2011;364:2496. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tefferi A, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, Patnaik MM, Hanson CA, Pardanani A, Gilliland DG, Levine RL. Leukemia. 2009;23:1343. doi: 10.1038/leu.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kosmider O, Gelsi-Boyer V, Cheok M, Grabar S, Della-Valle V, Picard F, Viguie F, Quesnel B, Beyne-Rauzy O, Solary E, Vey N, Hunault-Berger M, Fenaux P, Mansat-De Mas V, Delabesse E, Guardiola P, Lacombe C, Vainchenker W, Preudhomme C, Dreyfus F, Bernard OA, Birnbaum D, Fontenay M. Blood. 2009;114:3285. doi: 10.1182/blood-2009-04-215814. [DOI] [PubMed] [Google Scholar]
- 154.Itzykson R, Kosmider O, Cluzeau T, Mansat-De Mas V, Dreyfus F, Beyne-Rauzy O, Quesnel B, Vey N, Gelsi-Boyer V, Raynaud S, Preudhomme C, Ades L, Fenaux P, Fontenay M. Leukemia. 2011;25:1147. doi: 10.1038/leu.2011.71. [DOI] [PubMed] [Google Scholar]
- 155.Tefferi A, Pardanani A, Lim KH, Abdel-Wahab O, Lasho TL, Patel J, Gangat N, Finke CM, Schwager S, Mullally A, Li CY, Hanson CA, Mesa R, Bernard O, Delhommeau F, Vainchenker W, Gilliland DG, Levine RL. Leukemia. 2009;23:905. doi: 10.1038/leu.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Beer PA, Delhommeau F, LeCouedic JP, Dawson MA, Chen E, Bareford D, Kusec R, McMullin MF, Harrison CN, Vannucchi AM, Vainchenker W, Green AR. Blood. 2010;115:2891. doi: 10.1182/blood-2009-08-236596. [DOI] [PubMed] [Google Scholar]
- 157.Schaub FX, Looser R, Li S, Hao-Shen H, Lehmann T, Tichelli A, Skoda RC. Blood. 2010;115:2003. doi: 10.1182/blood-2009-09-245381. [DOI] [PubMed] [Google Scholar]
- 158.Nibourel O, Kosmider O, Cheok M, Boissel N, Renneville A, Philippe N, Dombret H, Dreyfus F, Quesnel B, Geffroy S, Quentin S, Roche-Lestienne C, Cayuela JM, Roumier C, Fenaux P, Vainchenker W, Bernard OA, Soulier J, Fontenay M, Preudhomme C. Blood. 2010;116:1132. doi: 10.1182/blood-2009-07-234484. [DOI] [PubMed] [Google Scholar]
- 159.Weissmann S, Alpermann T, Grossmann V, Kowarsch A, Nadarajah N, Eder C, Dicker F, Fasan A, Haferlach C, Haferlach T, Kern W, Schnittger S, Kohlmann A. Leukemia. 2012;26:934. doi: 10.1038/leu.2011.326. [DOI] [PubMed] [Google Scholar]
- 160.Konstandin N, Bultmann S, Szwagierczak A, Dufour A, Ksienzyk B, Schneider F, Herold T, Mulaw M, Kakadia PM, Schneider S, Spiekermann K, Leonhardt H, Bohlander SK. Leukemia. 2011;25:1649. doi: 10.1038/leu.2011.134. [DOI] [PubMed] [Google Scholar]
- 161.Kosmider O, Delabesse E, de Mas VM, Cornillet-Lefebvre P, Blanchet O, Delmer A, Recher C, Raynaud S, Bouscary D, Viguie F, Lacombe C, Bernard OA, Ifrah N, Dreyfus F, Fontenay M. Haematologica. 2011;96:1059. doi: 10.3324/haematol.2011.040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chou WC, Chou SC, Liu CY, Chen CY, Hou HA, Kuo YY, Lee MC, Ko BS, Tang JL, Yao M, Tsay W, Wu SJ, Huang SY, Hsu SC, Chen YC, Chang YC, Kuo KT, Lee FY, Liu MC, Liu CW, Tseng MH, Huang CF, Tien HF. Blood. 2011;118:3803. doi: 10.1182/blood-2011-02-339747. [DOI] [PubMed] [Google Scholar]
- 163.Kroeze LI, Aslanyan MG, van Rooij A, Koorenhof-Scheele TN, Massop M, Carell T, Boezeman JB, Marie JP, Halkes CJ, de Witte T, Huls G, Suciu S, Wevers RA, van der Reijden BA, Jansen JH. Blood. 2014;124:1110. doi: 10.1182/blood-2013-08-518514. [DOI] [PubMed] [Google Scholar]
- 164.Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Acta. Neuropathol. 2008;116:597. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 165.Bleeker FE, Lamba S, Leenstra S, Troost D, Hulsebos T, Vandertop WP, Frattini M, Molinari F, Knowles M, Cerrato A, Rodolfo M, Scarpa A, Felicioni L, Buttitta F, Malatesta S, Marchetti A, Bardelli A. Hum. Mutat. 2009;30:7. doi: 10.1002/humu.20937. [DOI] [PubMed] [Google Scholar]
- 166.Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, Weller M, Herold-Mende C, Unterberg A, Jeuken JW, Wesseling P, Reifenberger G, von Deimling A. Acta. Neuropathol. 2009;118:469. doi: 10.1007/s00401-009-0561-9. [DOI] [PubMed] [Google Scholar]
- 167.Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DDN. Engl. J. Med. 2009;360:765. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Capper D, Weissert S, Balss J, Habel A, Meyer J, Jager D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H, Hartmann C, von Deimling A. Brain Pathol. 2010;20:245. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Byeon SJ, Myung JK, Kim SH, Kim SK, Phi JH, Park SH. Childs Nerv. Syst. 2012;28:1025. doi: 10.1007/s00381-012-1773-1. [DOI] [PubMed] [Google Scholar]
- 170.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, Fulton LA, Locke DP, Magrini VJ, Abbott RM, Vickery TL, Reed JS, Robinson JS, Wylie T, Smith SM, Carmichael L, Eldred JM, Harris CC, Walker J, Peck JB, Du F, Dukes AF, Sanderson GE, Brummett AM, Clark E, McMichael JF, Meyer RJ, Schindler JK, Pohl CS, Wallis JW, Shi X, Lin L, Schmidt H, Tang Y, Haipek C, Wiechert ME, Ivy JV, Kalicki J, Elliott G, Ries RE, Payton JE, Westervelt P, Tomasson MH, Watson MA, Baty J, Heath S, Shannon WD, Nagarajan R, Link DC, Walter MJ, Graubert TA, DiPersio JF, Wilson RK, Ley TJN. Engl. J. Med. 2009;361:1058. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kosmider O, Gelsi-Boyer V, Slama L, Dreyfus F, Beyne-Rauzy O, Quesnel B, Hunault-Berger M, Slama B, Vey N, Lacombe C, Solary E, Birnbaum D, Bernard OA, Fontenay M. Leukemia. 2010;24:1094. doi: 10.1038/leu.2010.52. [DOI] [PubMed] [Google Scholar]
- 172.Pardanani A, Lasho TL, Finke CM, Mai M, McClure RF, Tefferi A. Leukemia. 2010;24:1146. doi: 10.1038/leu.2010.77. [DOI] [PubMed] [Google Scholar]
- 173.Tefferi A, Lasho TL, Abdel-Wahab O, Guglielmelli P, Patel J, Caramazza D, Pieri L, Finke CM, Kilpivaara O, Wadleigh M, Mai M, McClure RF, Gilliland DG, Levine RL, Pardanani A, Vannucchi AM. Leukemia. 2010;24:1302. doi: 10.1038/leu.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Patnaik MM, Hanson CA, Hodnefield JM, Lasho TL, Finke CM, Knudson RA, Ketterling RP, Pardanani A, Tefferi A. Leukemia. 2012;26:101. doi: 10.1038/leu.2011.298. [DOI] [PubMed] [Google Scholar]
- 175.Rakheja D, Konoplev S, Medeiros LJ, Chen W. Hum. Pathol. 2012;43:1541. doi: 10.1016/j.humpath.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 176.Zhou KG, Jiang LJ, Shang Z, Wang J, Huang L, Zhou JF. Leuk. Lymphoma. 2012;53:2423. doi: 10.3109/10428194.2012.695359. [DOI] [PubMed] [Google Scholar]
- 177.Abbas S, Lugthart S, Kavelaars FG, Schelen A, Koenders JE, Zeilemaker A, van Putten WJ, Rijneveld AW, Lowenberg B, Valk P. J.Blood. 2010;116:2122. doi: 10.1182/blood-2009-11-250878. [DOI] [PubMed] [Google Scholar]
- 178.Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, Cross JR, Fantin VR, Hedvat CV, Perl AE, Rabinowitz JD, Carroll M, Su SM, Sharp KA, Levine RL, Thompson CB. Cancer Cell. 2010;17:225. doi: 10.1016/j.ccr.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM. Nature. 2009;462:739. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Xu X, Zhao J, Xu Z, Peng B, Huang Q, Arnold E, Ding J. J. Biol. Chem. 2004;279:33946. doi: 10.1074/jbc.M404298200. [DOI] [PubMed] [Google Scholar]
- 181.Song SJ, Poliseno L, Song MS, Ala U, Webster K, Ng C, Beringer G, Brikbak NJ, Yuan X, Cantley LC, Richardson AL, Pandolfi PP. Cell. 2013;154:311. doi: 10.1016/j.cell.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sun M, Song CX, Huang H, Frankenberger CA, Sankarasharma D, Gomes S, Chen P, Chen J, Chada KK, He C, Rosner MR. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9920. doi: 10.1073/pnas.1305172110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Huang H, Jiang X, Li Z, Li Y, Song CX, He C, Sun M, Chen P, Gurbuxani S, Wang J, Hong GM, Elkahloun AG, Arnovitz S, Szulwach K, Lin L, Street C, Wunderlich M, Dawlaty M, Neilly MB, Jaenisch R, Yang FC, Mulloy JC, Jin P, Liu PP, Rowley JD, Xu M, Chen J. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11994. doi: 10.1073/pnas.1310656110. [DOI] [PMC free article] [PubMed] [Google Scholar]