Abstract
Background:
The drive toward the use of medicinal plants has been increasing in recent years. They have few side effects and a large variety of efficient components.
Objectives:
This study was designed to investigate the analgesic effects of hydroalcoholic Rhus coriaria leaf extract (HRCLE) in a rat model.
Materials and Methods:
A total of 42 adult male rats were divided into seven groups: a control group (the animals did not receive any drug), three HRCLE groups, (receiving 80, 100, and 300 mg/kg, intraperitoneally [ip]), a morphine group (1 mg/kg, ip) an aspirin group (1 mg/kg, ip), and a group that received 300 mg/kg of HRCLE plus naloxone (1 mg/kg, ip). The analgesic effects of HRCLE were assessed via writhing, tail flick, and formalin tests, and the data obtained were compared with the control group using one-way analysis of variance and Tukey post hoc tests.
Results:
HRCLE significantly inhibited the number of contractions induced by acetic acid in the writhing test at all doses, while anti-nociceptive activity was only shown at the 100 mg/kg dose (in the chronic phase) and at the 300 mg/kg dose (in the chronic-acute phase) in the formalin test. Interestingly, the greatest effect was observed at the 300 mg/kg HRCLE dose in the tail flick test. Simultaneous utilization of naloxone and HRCLE inhibited the anti-nociceptive effect of the extract in all tests. It is worth mentioning that aspirin and morphine revealed anti-nociceptive effects in all tests.
Conclusions:
Our findings suggest that the analgesic effect of HRCLE may be mediated via both peripheral and central mechanisms. The presence of flavonoids might be responsible for the anti-nociceptive activity of this plant.
Keywords: Pain, Formaldehyde, Medicinal Plants, Rats, Saponins, Flavonoids, Rhus coriaria
1. Background
Pain, such as touch, pressure, and proprioception, is a somatic sensation. It has always been a serious challenge in medicine, as it has an important protective role in avoiding, or treating, genuine or potential tissue damage (1, 2). Although numerous agents, such as non-steroidal anti-inflammatory or opioid drugs are primarily used to control pain (3-6), these sometimes have many adverse effects and cause gastrointestinal and renal disorders. Therefore, most people would prefer new drugs that have fewer side effects and which are cheaper and easily available (7).
There is also increasing evidence to suggest that the prescription of medicinal plants to treat pain and inflammation is prevalent in traditional medicine (8-10), although the origin and structure of many such plants remain unknown. Therefore, obtaining information regarding their pharmaceutical effects can be considered a logical research approach in order to discover new drugs (11, 12).
It is believed that plants that belong to the Anacardiaceous family have a variety of pharmaceutical effects, such as analgesic, anti-inflammatory, and antipyretic properties (13-15). Sumac, with the scientific name Rhus coriaria, is a perennial plant belonging to this family and contains over 250 individual species of flowering plants (16). Its name is derived from the word sumaga, which means “red” (17). Suma has latex stems, simple or compound leaves, small flowers, fruits, and dense clusters (18), and grows over a wide area, ranging from the Mediterranean to Iraq and Iran.
Sumac is used in traditional medicine as an anti-bacterial, anti-spasmodic, anti-viral and anti-inflammatory agent, as well as in the treatment of fever, diarrhea, gastrointestinal diseases, and dermatitis (19, 20). It also has anti-microbial and anti-oxidant effects and these properties have been proven in modern medicine (21-23). However, the analgesic effects of this plant have not been clearly shown in previous studies.
2. Objectives
As mentioned above, previous studies have not clearly shown the analgesic effects of sumac, therefore this study was designed to investigate the anti-nociceptive effect of hydroalcoholic Rhus coriaria leaf extract (HRCLE) in a rat model, using formalin, writhing, and tail flick tests.
3. Materials and Methods
3.1. Plant Material Collection
Some fresh Rhus coriaria leaves were prepared and authenticated by a botanist, after which a voucher specimen number of the plant was deposited in the herbarium of the department of biology, faculty of basic sciences, Abu Ali Sina university of Hamadan, Iran. For preparation of hydro alcoholic extract, Rhus coriaria leaves were shed-dried at room temperature in the shade, and mechanically pulverized using a grinder. A total of 100 g of powdered Rhus coriaria leaf was placed in one liter of 80% methanol for 72 hours to extract the required active ingredients. The obtained mixture was placed in a rotary device to remove the solvent, and then put in a dish and under a hood for 1 week to dehydrate the substance. The residual material at the bottom of the container, extract, was then subsequently dissolved in the appropriate amount of saline (0.9%) in order to administer different doses to the rats.
3.2. Design of Animal Experiments
A total of 42 adult male Wistar rats (weighing 200 - 250 g) were purchased from Pasteur’s institute of Iran. The animals were housed, three to four per cage, and kept at a controlled temperature of 23 ± 1°C under a light:dark cycle of 12:12 hours, with food and tap water available ad libitum. All experiments were conducted between 10:00 and 16:00 hours, and all rats were treated humanely and in accordance with the international association for the study of pain guidelines on the use of laboratory animals (24).
3.3. Drug Administration
The animals were randomly divided into seven equal groups (n = 6 rats per group): a control group (the animals did not receive any drug), three HRCLE groups, (receiving 80, 100, and 300 mg/kg, intraperitoneally [ip]), a morphine group (1 mg/kg, ip) an aspirin group (1 mg/kg, ip), and a group that received 300 mg/kg of HRCLE plus naloxone (1 mg/kg, ip). Sulfate morphine, naloxone, and aspirin were purchased from Darou Pakhsh (Iran), and acetic acid and formalin from Merck Inc. (Germany). Administration of the drug interventions and assessment of the outcomes were not blinded to group assessment.
3.4. Pain Tests
Writhing Test: The animals were sent into a standard experiment glass box to get used to the conditions 30 minutes before the experiments were conducted. The HRCLE was dissolved in sterile physiologic serum and intraperitoneally injected at doses of 80, 100, and 300 mg/kg. After 15 minutes, acetic acid with a density of 6% was intraperitoneally injected at 1 mg/kg body weight, immediately following which the number of abdominal contractions was counted for 30 minutes (with both legs of the animals stretched). It is important to mention that each animal was used only once (25). In the control group, the writhing test was carried out after intraperitoneal injection of saline.
Tail Flick Test: Acute nociception was assessed using a tail flick analgesimeter apparatus (Borj Sanat Iran Company), following the method of D’Amour and Smith (26). In brief, each rat was placed in a restrainer 30 minutes after treatment, and baseline reaction time was measured by focusing a beam of light on the distal third of the tail. The reaction time was recorded at 15-minute intervals up to 2 hours. A 15-seconds cut off time was used in order to prevent tissue damage. The percentage of maximum possible anti-nociceptive effect was calculated for each time and compared.
Formalin Test: The model suggested by Dubuisson and Dennis (1978) was used in order to evaluate chronic pain. The animals were sent into the special formalin test box 1 hour before the test, in order to get used to the experimental conditions. The box was made of Plexiglas at dimensions of 30 × 30 × 30 cm, and a mirror, positioned at 45°C and in front of the observer, was inserted below the box to allow more clear observation of the animals’ behaviors. Thirty minutes after the intraperitoneal injection of the drugs, 50 μL of 2.5% formalin solution was subcutaneously injected into the sub plantar surface of the left hind paw, after which the animal was returned to the special test box. Each animal’s motor response to pain was observed, rated, and recorded on a scale of 0, 1, 2, and 3 every 15 seconds for 60 minutes. The numbers indicated the following reactions: 0, the animal moved with complete balance and its weight was equally distributed on both feet; number 1, the animal could not tolerate its body weight on the injected foot, or take care of that foot; number 2, the animal raised the painful paw and had no contact with the box floor; and number 3, the animal licked the painful paw, chewed or moved severely. The average of grades from the first 5 minutes was considered as phase 1 (the acute phase), and the average of grades recorded during minutes 15 to 60 was considered as phase 2 (the chronic phase) (27).
3.5. Determination of Acute Lethal Dose
The acute toxicity was determined in a previous laboratory model (28). Various doses of HRCLE were separately and intraperitoneally administered to the male rats. The mortality rate of the animals was calculated within the following 72 hours, and the LD50 of the plant extract was determined.
3.6. Data Analysis
All data were expressed as mean ± SEM. A one-way analysis of variance, followed by Tukey’s post hoc test was used for analysis of data, and P < 0.05 was considered statistically significant.
4. Results
4.1. Writhing Test
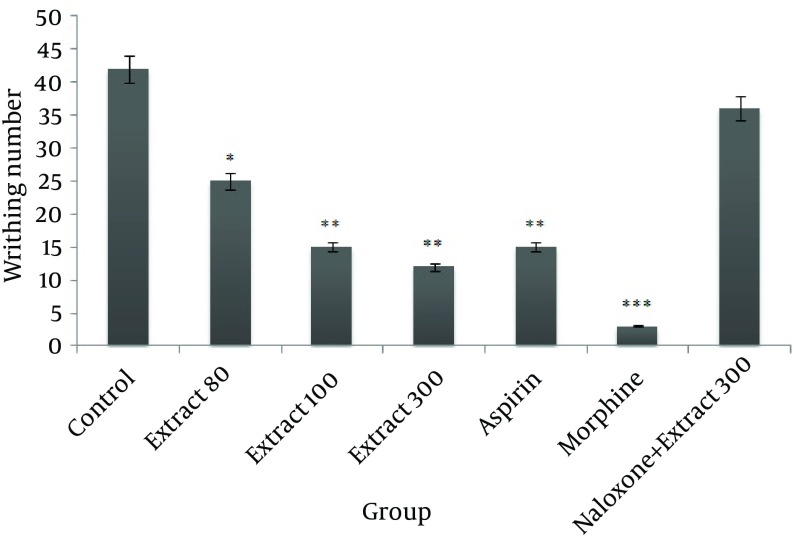
Statistical analysis revealed that doses of 80, 100, and 300 mg/kg of HRCLE led to a significant reduction in writhing, compared to the control group (P < 0.05, P < 0.01, and P < 0.01, respectively). There was a highly significant reduction (P < 0.001 and P < 0.01, respectively) in writhing in the morphine and aspirin groups compared to the control group, as shown in Figure 1.
Figure 1. The Effect of Different Doses of Hydroalcoholic Rhus coriaria Leaf Extract on Acetic Acid-Induced Writhing in Rats.
* P < 0.05, ** P < 0.01, and *** P < 0.001, compared to control (n = 6, mean ± SEM).
4.2. Tail Flick Test
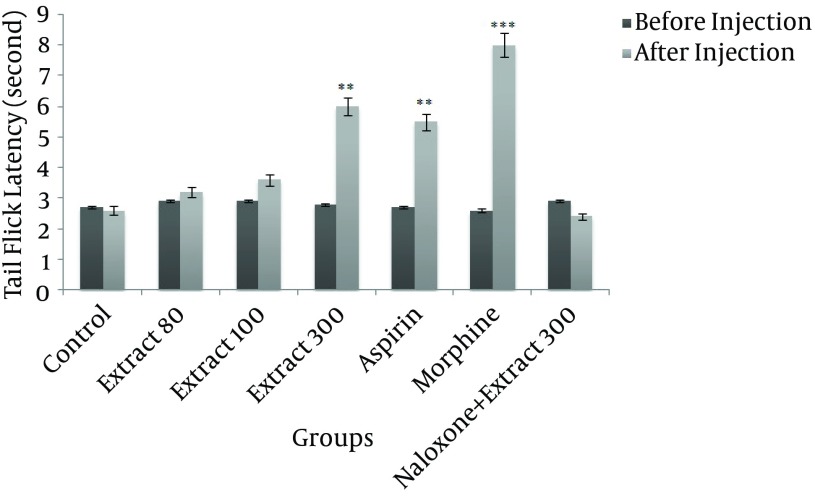
A The HRCLE 300 mg/kg group showed a very significant increase in tail flick latency when compared to the control group (P < 0.01), as shown in Figure 2. Injection of morphine and aspirin also significantly increased tail flick latency (P < 0.001 and P < 0.01, respectively).
Figure 2. The Effect of Different Doses of Hydroalcoholic Rhus coriaria Leaf Extract on Reaction Time in Rats.
** P < 0.01 and *** P < 0.001, compared to control (n = 6, mean ± SEM).
4.3. Formalin Test
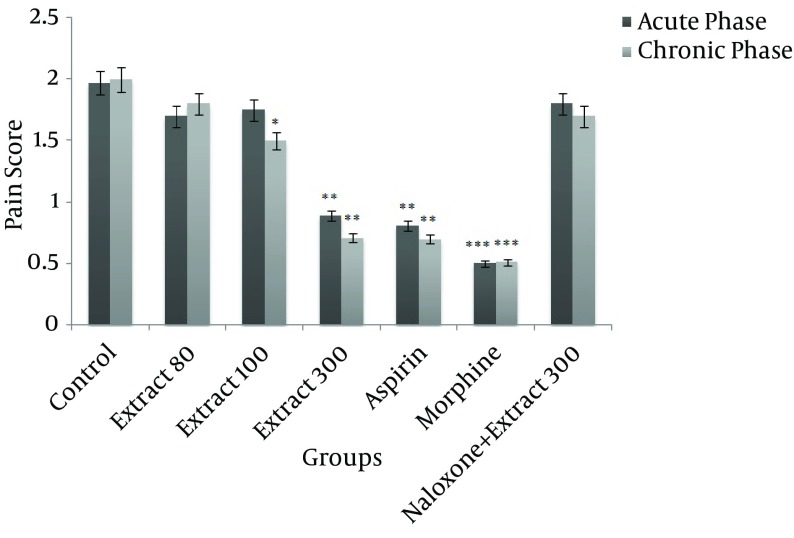
Administration of 300 mg/kg of HRCLE significantly decreased pain score in both the acute and chronic phases, compared to the control group (P < 0.01), while 100 mg/kg of HRCLE led to a significant decrease in the pain score in the chronic phase (P < 0.05). However, no significant change in pain score was observed with the 80 mg/kg HRCLE dose. The aspirin and morphine groups also showed significantly decreased pain scores when compared to the control group (P < 0.001; Figure 3).
Figure 3. The Effect of Different Doses of Hydroalcoholic Rhus coriaria leaf extract on Formalin-Induced Nociception in Rats.
P < 0.05, ** P < 0.01 and *** P < 0.001, compared to control (n = 6, mean± SEM).
Naloxane plus HRCLE (300 mg/kg) inhibited the anti-nociceptive effect of HRCLE in all of the pain tests. The intraperitoneal lethal dose of the plant was 5100 mg/kg. There was no significant change in the body weight of the animals in each group.
5. Discussion
The correct management of pain has been identified as a primary indicator of quality assurance. Pain and substance abuse frequently co-occur, and each can make the other more difficult to treat (29). Non-steroidal anti-inflammatory drugs, such as aspirin, are widely used in the treatment of pain, but they often cause gastrointestinal damage (30).
In the present study, the standard writhing, tail flick, and formalin tests were used in order to investigate the anti-nociceptive effects of HRCLE. One of the most important tests is the writhing test, which is usually used to screen possible anti-nociceptive mixtures. In this test, acetic acid is extensively used to evaluate peripheral anti-nociceptive activity (31). The HRCLE prevented abdominal constriction caused by acetic acid; therefore, it is suggested that its alleviative effects are supported by environmental mechanisms. Intraperitoneal injection of acetic acid can cause acute inflammation of the peritoneum (32). It appears that mediators such as bradykinin, serotonin, histamine, substance P, and prostaglandin, play a role in this model (8, 10, 14, 15). It is justifiable that all of these mediators are associated with the stimulation of peripheral nociceptive neurons (31).
The tail flick test, in which thermal stimuli are used, is among the most significant parameters in the evaluation of anti-nociceptive activities (26). In the current study, injection of moderate or high doses of HRCLE decreased the pain resulting from thermal stimulation in the tail flick test. Since this test is used to evaluate spinal reflexes and central anti-nociceptive pathways (33), it can therefore be suggested that the anti-nociceptive effect of HRCLE involves a central nervous component that may be elicited from several defined areas in the central nervous system.
Of the various models of persistent nociception, the formalin test has been well established as one that is valid for the screening of anti-inflammatory and anti-nociceptive agents that act through the central pain route from peripheral pain (34). Intraplantar injection of formalin evokes signs of nociception (flinching and licking of the injected paw) with early phase 1, followed by a quiescent period characterized by fewer pain behaviors, and late hyperalgesic (phase 2) components that last for approximately 1 hour. The early phase, or neurogenic nociception, results in direct activation of peripheral nociceptors, whereas the late phase is due to inflammatory nociception, which reflects the induction of a spinal state of facilitation, central sensitization, development of inflammation, and enlargement of receptive fields, as well as the concurrent presence of low level input from both large and small afferents (35). The results showed that HRCLE had an inhibitory effect on the pain and showed anti-nociceptive activity in both phases of formaldehyde-induced pain in the rat. It was found that its decreasing effect was more potent in the chronic phase than in the acute phase. HRCLE-facilitated inhibition of the chronic phase of the formalin test can be a result of inflammation, so that aspect of the anti-nociceptive effect appears to be mediated by the release of compounds such as prostaglandins F2α and E2, which are to a degree sensitized by central nociceptive neurons (36).
To evaluate opioid system interference with the anti-nociceptive effect of HRCLE, we used an opioid antagonist, naloxane, which prevents the activation of opioid receptors (37). The results indicate that naloxone attenuates the anti-nociceptive effect of the extract. Therefore, it seems that the effect exerted by HRCLE in pain relief is due to opioid receptors, although we are unable ignore the role of other substances, such as endorphins.
The biologic or therapeutic activity of herbs has a close relationship with their chemical composition (38). It is known that Rhus coriaria contains several flavonoids, quercetin, myricitrin, myricetin, tannic acid, and tannins (19), which possess antioxidant, anti-inflammatory and anti-nociceptive properties (39). Previous studies have shown that inhibition of the N-methyl-D-aspartate receptor decreases intracellular calcium. Consequently, the synthesizer enzyme of calcium-related nitric oxide and phospholipase A2 also decreases, and with the reduction of nitric oxide and prostaglandins, especially prostaglandins E2 and F2α it reveals its antinociceptive effects (40). Many flavonoids and tannins are capable of chelating free radicals, such as hydroxyl radicals, and subsequently dismutase reactive oxygen species. In this respect, some studies have shown that tannins have roles in producing anti-nociceptive and anti-inflammatory effects (41). Therefore, another aspect of the anti-nociceptive effect of HRCLE is due to tannins inside the plant.
In conclusion, the results of the present study suggest that the anti-nociceptive effect of HRCLE may be due to its flavonoid content. In the present study, a reduction in writhing, an increase in tail flick, and inhibition of both phases of the formalin test showed the anti-nociceptive effect of Rhus coriaria. Therefore, HRCLE possesses an analgesic activity that is probably due to inhibition of prostaglandin synthesis and inhibition of the central and peripheral nervous system, meaning that this extract could potentially be used to control painful disease.
Acknowledgments
We thank Masoud Mohammadi for his assistance in this study and all of those who helped us in conducting this research are greatly appreciated. This paper was extracted from the master’s thesis of Saeed Mohammadi, which was submitted at Islamic Azad University of Hamadan in Iran and financially supported by grant no. 91223 from this university.
Footnotes
Authors’ Contribution:Saeed Mohammadi designed and conducted this study. Mohammad Mahdi Zarei provided and handled all animals and drafted the manuscript and conceived and designed the study. Iraj Salehi contributed to data analysis and manuscript revision. All authors read and approved the final manuscript.
Funding/Support:This study was solely supported by the Islamic Azad University of Hamadan branch, biology department as a self-financed project.
References
- 1.Surange PN, Venkata Rama Mohan BC. Comparative evaluation of continuous lumbar paravertebral versus continuous epidural block for post-operative pain relief in hip surgeries. Anesth Pain Med. 2012;1(3):178–83. doi: 10.5812/kowsar.22287523.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akbarzadeh M, Moradi Z, Jowkar A, Zare N, Hadianfard MJ. Comparing the Effects of Acupressure at the Jian Jing-Gall Bladder Meridian (GB-21) Point on the Severity of Labor Pain, Duration and Cesarean Rate in Mono-and Bi-Stage Interventions. Women's Health Bull. 2014;2(1):e32128 [Google Scholar]
- 3.Hosseini Jahromi SA, Sadeghi Poor S, Hosseini Valami SM, Javadi A. Effects of suppository acetaminophen, bupivacaine wound infiltration, and caudal block with bupivacaine on postoperative pain in pediatric inguinal herniorrhaphy. Anesth Pain Med. 2012;1(4):243–7. doi: 10.5812/aapm.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panah Khahi M, Marashi S, Khajavi MR, Najafi A, Yaghooti A, Imani F. Postoperative gabapentin to prevent postoperative pain: a randomized clinical trial. Anesth Pain Med. 2012;2(2):77–80. doi: 10.5812/aapm.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohtadi A, Nesioonpour S, Salari A, Akhondzadeh R, Masood Rad B, Aslani SM. The effect of single-dose administration of dexamethasone on postoperative pain in patients undergoing laparoscopic cholecystectomy. Anesth Pain Med. 2014;4(3):e32128. doi: 10.5812/aapm.17872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fakharian E, Fazel MR, Tabesh H, Masoud SA. Preemptive Effects of Lidocain on Postoperative Pain in Patients Undergoing Disc Operation: A Randomized, Double Blind, Placebo-Controlled Clinical Trial. Iran Red Crescent Med J. 2009;11(1):37–41. [Google Scholar]
- 7.Baker MD, Wood JN. Involvement of Na+ channels in pain pathways. Trends Pharmacol Sci. 2001;22(1):27–31. doi: 10.1016/s0165-6147(00)01585-6. [DOI] [PubMed] [Google Scholar]
- 8.Bazafkan MH, Hardani A, Afzal Zadeh MR, Amir Zargar A, Moradi N, Jalali N. The Effects of Aqueous Extract of Celery Leaves (<i>Apium Gravelens</i>) on the Delivery Rate, Sexual Ratio, and Litter Number of the Female Rats. Jentashapir J Health Res. 2014;5(5):e32128. doi: 10.17795/jjhr-23221. [DOI] [Google Scholar]
- 9.Rezaei A, Pashazadeh M, Pashazadeh M, Moghadam S. Comparative study of sedative and anxiolytic effects of herbal extracts of Hypericum perforatum with nardostachys jatamansi in rats. Zahedan J Res Med Sci. 2014;16(3):40–3. [Google Scholar]
- 10.Tanideh N, Nematollahi SL, Hosseini SV, Hosseinzadeh M, Mehrabani D, Safarpour A, et al. The healing effect of Hypericum perforatum extract on acetic acid-induced ulcerative colitis in rat. Ann Colorecal Res. 2014;2:e32128 [Google Scholar]
- 11.Javan M, Ahmadiani A, Semnanian S, Kamalinejad M. Antinociceptive effects of Trigonella foenum-graecum leaves extract. J Ethnopharmacol. 1997;58(2):125–9. doi: 10.1016/s0378-8741(97)00089-5. [DOI] [PubMed] [Google Scholar]
- 12.Nafisy AT. A review of traditional medicine in iran. Isfahan Univ Publ. 1989;8(11):121. [Google Scholar]
- 13.Neto AG, Costa JM, Belati CC, Vinholis AH, Possebom LS, Da Silva Filho AA, et al. Analgesic and anti-inflammatory activity of a crude root extract of Pfaffia glomerata (Spreng) Pedersen. J Ethnopharmacol. 2005;96(1-2):87–91. doi: 10.1016/j.jep.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Zarei M, Mohammadi S, Abolhassani N, Asgari M. The Antinociceptive Effects of Hydroalcoholic Extract of Bryonia dioica in Male Rats. Avicenna J Neuro Psych Physio. 2015;2(1):e32128. doi: 10.17795/ajnpp-25761. [DOI] [Google Scholar]
- 15.Mohammadi S, Zarei M, Mahmoodi M, Zarei MM, Nematian MA. In Vivo Antinociceptive Effects of Persian Shallot (Allium hirtifolium) in Male Rat. Avicenna J Neuro Psych Physiol. 2015;2(1) [Google Scholar]
- 16.Kossah R, Nsabimana C, Zhao J, Chen H, Tian F, Zhang H, et al. Comparative study on the chemical composition of Syrian sumac (Rhus coriaria L.) and Chinese sumac (Rhus typhina L.) fruits. Pakistan J Nutr. 2009;8(10):1570–4. [Google Scholar]
- 17.Wetherilt H, Pala M. Herbs and spices indigenous to Turkey. Amsterdam: Elsevier Science BV; 1994. p. 764. [Google Scholar]
- 18.Andrade SF, Cardoso LG, Carvalho JC, Bastos JK. Anti-inflammatory and antinociceptive activities of extract, fractions and populnoic acid from bark wood of Austroplenckia populnea. J Ethnopharmacol. 2007;109(3):464–71. doi: 10.1016/j.jep.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 19.James AD. Handbook of Medicinal Herbs. Second ed. Boca Raton, Florida: CRC Press; 2002. [Google Scholar]
- 20.Brunke EJ, Hammerschmidt FJ, Schmaus G, Akgül A. The essential oil of Rhus coriaria L. fruits. Flavour and Fragrance J. 1993;8(4):209–14. doi: 10.1002/ffj.2730080408. [DOI] [Google Scholar]
- 21.Nasar-Abbas SM, Halkman AK. Antimicrobial effect of water extract of sumac (Rhus coriaria L.) on the growth of some food borne bacteria including pathogens. Int J Food Microbiol. 2004;97(1):63–9. doi: 10.1016/j.ijfoodmicro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Özcan M, Haciseferogullari H. A condiment [sumac (Rhus coriaria L.) fruits]: some physicochemical properties. Bulgarian Plant Physiol. 2004;30(3-4):74–84. [Google Scholar]
- 23.Kosar M, Bozan B, Temelli F, Baser KHC. Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.) extracts. Food Chem. 2007;103(3):952–9. doi: 10.1016/j.foodchem.2006.09.049. [DOI] [Google Scholar]
- 24.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 25.Collier HO, Dinneen LC, Johnson CA, Schneider C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother. 1968;32(2):295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'amour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72(1):74–9. [Google Scholar]
- 27.Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4(2):161–74. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 28.Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54(4):275–87. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 29.Russo S. Integrated pain management: using omega 3 fatty acids in a naturopathic model. Tech in Reg Anesthe Pain Manage. 12(2):105–8. doi: 10.1053/j.trap.2008.01.001. [DOI] [Google Scholar]
- 30.Fiorucci S, Antonelli E, Morelli A. Mechanism of non-steroidal anti-inflammatory drug-gastropathy. Dig Liver Dis. 2001;33 Suppl 2:S35–43. doi: 10.1016/s1590-8658(01)80157-2. [DOI] [PubMed] [Google Scholar]
- 31.Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319(2):507–14. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- 32.Koster R, Anderson M, De Beer EJ, editors. Acetic acid-induced analgesic screening, Federation Proceedings.; 1959; Hans Publishing; pp. 412–7. [Google Scholar]
- 33.Jensen TS, Yaksh TL. Comparison of antinociceptive action of morphine in the periaqueductal gray, medial and paramedial medulla in rat. Brain Res. 1986;363(1):99–113. doi: 10.1016/0006-8993(86)90662-1. [DOI] [PubMed] [Google Scholar]
- 34.Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51(1):5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- 35.Shibata M, Ohkubo T, Takahashi H, Inoki R. Modified formalin test: characteristic biphasic pain response. Pain. 1989;38(3):347–52. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- 36.Verma PR, Joharapurkar AA, Chatpalliwar VA, Asnani AJ. Antinociceptive activity of alcoholic extract of Hemidesmus indicus R.Br. in mice. J Ethnopharmacol. 2005;102(2):298–301. doi: 10.1016/j.jep.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 37.Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG, et al. fMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. J Neurophysiol. 2004;91(6):2723–33. doi: 10.1152/jn.00249.2003. [DOI] [PubMed] [Google Scholar]
- 38.Hashemi SR, Zulkifli I, Hair Bejo M, Farida A, Somchit MN. Acute Toxicity Study and Phytochemical Screening of Selected Herbal Aqueous Extract in Broiler Chickens. Int J Pharmacol. 2008;4(5):352–60. doi: 10.3923/ijp.2008.352.360. [DOI] [Google Scholar]
- 39.Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65(4):337–53. doi: 10.1016/s0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- 40.Woodman OL, Chan E. Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol. 2004;31(11):786–90. doi: 10.1111/j.1440-1681.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 41.Starec M, Waitzova D, Elis J. [Evaluation of the analgesic effect of RG-tannin using the "hot plate" and "tail flick" method in mice]. Cesk Farm. 1988;37(7):319–21. [PubMed] [Google Scholar]