Abstract
Herein, we show that intraerythrocytic stages of Plasmodium falciparum have an active pathway for biosynthesis of menaquinone. Kinetic assays confirmed that plasmodial menaquinone acts at least in the electron transport. Similarly to Escherichia coli, we observed increased levels of menaquinone in parasites kept under anaerobic conditions. Additionally, the mycobacterial inhibitor of menaquinone synthesis Ro 48-8071 also suppressed menaquinone biosynthesis and growth of parasites, although off-targets may play a role in this growth-inhibitory effect. Due to its absence in humans, the menaquinone biosynthesis can be considered an important drug target for malaria.
Keywords: Malaria, Menaquinone, Apicomplexa, Ubiquinone, Vitamin K, Plasmodium falciparum
1. Introduction
Plasmodium falciparum resistance to current drugs used in the treatment of malaria has led researchers to seek new drug targets, and the study of pathways localized in an apicoplast is particularly important in this context. The shikimate pathway and methylerythritol phosphate pathway (MEP) are localized in this organelle of P. falciparum [1]. In plants and cyanobacteria these pathways are the precursors for biosynthesis of phylloquinone (PhQ) while in bacteria biosynthesizes menaquinone (MQ) [2,3].
Our group demonstrated that the pathway of isoprenoid biosynthesis is functionally active in the intraerythrocytic stages of P. falciparum [4] and that final products of this pathway like dolichol of 11–12 isoprenic units [5], ubiquinones [6], isoprenylated/dolichylated proteins [7,8] and carotenoids [9] are biosynthesized by this parasite when [1-(n)-3H]geranylgeranyl pyrophosphate was used as a metabolic precursor.
During its intraerythrocytic development in the human host, the malaria parasite P. falciparum is submitted to considerable changes in the oxygen concentration due to intermittent cytoadherence in the deep vasculature [10], with consequences for the energy metabolism of the parasite. Facultative anaerobic organisms such as Escherichia coli employ two types of electron carriers, ubiquinone and menaquinone which are tightly regulated depending on the oxygen supply in the environment [11].
The present study shows an active pathway for the biosynthesis of MQ in P. falciparum and shows that MQ could replace the physiological function of ubiquinone (UQ) under anaerobic conditions. This discovery sheds a new light on the P. falciparum metabolism and due to its absence in humans; the MQ biosynthesis can be considered an important drug target against malaria.
2. Materials and methods
2.1. P. falciparum culture
The P. falciparum clone 3D7 was cultured in vitro according to protocol described by Trager and Jensen [12] where human sera was substituted by Albumax I (0.5%) [13]. Parasites were grown in a 40 ml volume under an atmosphere of 5% CO2, 5% O2, and 90% N2 (normal culture). In some experiments parasite were cultured in anaerobic condition in an atmosphere of 5% CO2 and 95% N2. Parasite development and multiplication were monitored by microscopic evaluation of Giemsa-stained thin smears. We conducted the PCR for mycoplasma detection to assure that parasite cultures are not contaminated [14].
2.2. Metabolic labelling
Synchronous cultures of P. falciparum were labelled with [1-(n)-3H]GGPP (3.125 μCi/ml) (Fig. 1) in normal RPMI 1640 medium in ring, trophozoite or schizont stages (20% parasitemia) for 12 h and were then recovered in trophozoite, schizont and ring stages, respectively. After labelling, ring (1–20 h after reinvasion), trophozoite (20–30 h after reinvasion) and schizont (30–48 h after reinvasion) forms were purified on a 40%/70%/80% discontinuous Percoll® gradient [15] and stored in liquid nitrogen until analysis. Protein biosynthesis in synchronous cultures of P. falciparum was monitored as described previously [16].
Fig. 1.
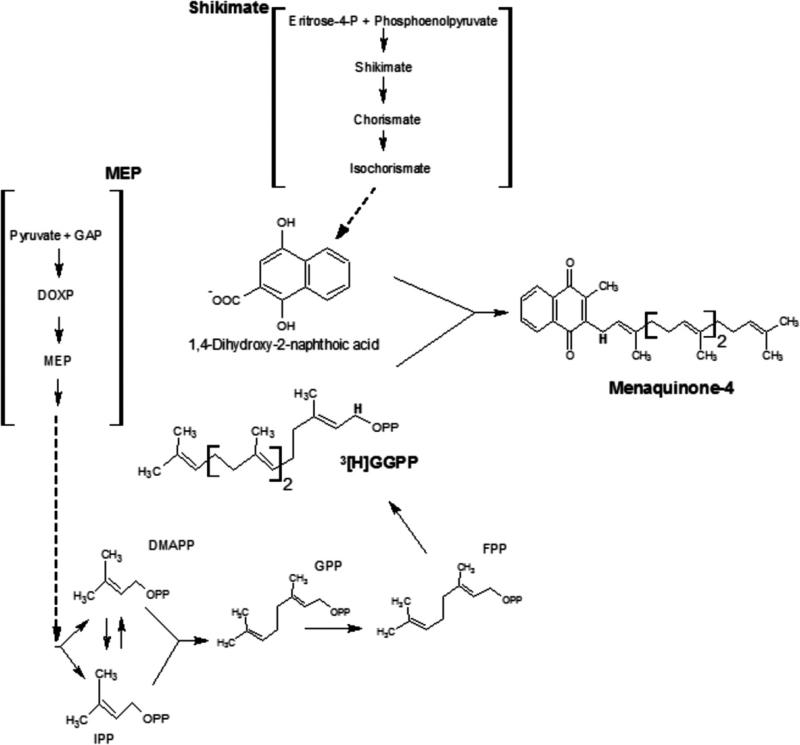
Menaquinone biosynthesis pathway. MQs are biosynthesized by the shikimate and methylerythritol phosphate pathway (MEP). The 4-dihydroxy-2-naphthoate is derived from chorismate formed via the shikimate pathway. The geranylgeranyl-PP is biosynthesized by the MEP pathway. The structure of menaquinone-4 (MQ-4) found in intraerythrocytic stages of P. falciparum was indicated. Labelled MQ-4 was characterized in intraerythrocytic stages of P. falciparum when exogenous [1-(n)-3H]geranylgeranyl pyrophosphate was added in culture medium. The [3H] is indicated in bold.
2.3. MQ extraction
Parasites were submitted to MQ extraction following the protocol described by Hirauchi et al. [17] or following the protocol described by Bekker et al. [18].
2.4. Reversed-phase high performance liquid chromatography (RP-HPLC)
For both protocols described below the extracts of each parasite stage obtained from metabolically labelled parasites were analyzed with a Phenomenex Luna C18 column (250 mm × 4.6 mm × 5 μm), maintained at 28 °C. The UV detector was set at 267 nm, and fractions were collected in Gilson HPLC 322 pump (Gilson, Villiers-le-Bel, France) and also a gradient module connected to a 152 UV–visible detector, an 831 temperature regulator and an FC203B fraction collector:
Protocol I
Fractions were collected at 1 min intervals in a isocratic system with methanol:ethanol (95:5, v/v), following the protocol described by Kamao et al. [19] and extraction protocols described by Hirauchi et al. [17].
Protocol II
Fractions were collected at 0.5 min intervals in an isocratic system with methanol:ethanol (50:50, v/v), following the HPLC and extraction protocols described by Bekker et al. [18].
In all HPLC analysis with samples radioactively labelled we co-injected with MQ-4 standard. The resulting fractions were dried using a Speed Vac, resuspended in liquid scintillation cocktail (Perkin Elmer, Turku, Finland) and monitored with a Beckman LS 5000 TB β-counter.
2.5. Mass spectrometry analysis
ESI-MS (electrospray ionization-mass spectrometry) and ESI-MS/MS (electrospray ionization-tandem mass spectrometry) analysis were performed using a LCQ-Duo ion-trap mass spectrometer (ThermoFinnigan, San Jose, CA, USA). For this analysis, 1.0 × 1010 unlabelled schizont extract were purified by RP-HPLC (Protocol I) and the fraction with retention times coincident to the MQ-4 standard was collected in 2.0 ml plastic tubes, dried in a Speed Vac and resuspended in 30 μl of chloroform/methanol (1:1, v/v), 2 mM lithium iodide. The MS spectra were acquired in full ion mode and others parameters were the same as described [20].
2.6. Inhibition tests with Ro 48-8071
Ro 48-8071 was dissolved in 100% DMSO resulting in 10 mM stock solutions [21]. We applied the method proposed by Desjardins et al. and Moneriz et al. [22,23] to determine the 50% inhibitory concentrations (IC50 values). The IC50 values for growth inhibition were calculated by Probit Analysis (Minitab Statistical Software 13.30 TM; Minitab Inc.). The parasites in schizont stage were treated with IC50 value of the drug for a total of 48 h and labelling with [1-(n)-3H]GGPP took place during the last 12 h. MQ was then extracted [17] and analyzed by HPLC (Protocol II) [18] to compare the pattern of MQ-4 synthesized by the treated and untreated parasites.
2.7. MQ biosynthesis in parasites cultivated in anaerobic conditions
The synchronized parasites were maintained in anaerobic conditions for a total time of 48 h. During the last 12 h, parasites were labelled with [1-(n)-3H]GGPP and then harvested. MQ extraction and HPLC purification techniques were conducted as described above (Protocol II). The pattern of MQ-4 and UQ biosynthesis from the parasites submitted to anaerobic conditions was analyzed and compared to patterns of parasites maintained in normal condition.
Parasites exposed to 48 h in anaerobic conditions were returned to normal conditions and cultivated for additional 48 h. During the last 12 h, labelling as before was done and the metabolic profiles of UQ and MQ-4 were analyzed by HPLC (Protocol II) and compared to control.
2.8. Determination of menaquinone function as a substrate for electron-carrier in respiratory chain
Free parasites were prepared from aliquots of infected erythrocytes (approximately 8 × 109 cells/ml) by adding 5 volumes of 0.15% (w/v) saponin in phosphate-buffered saline (1.76 mM K2HPO4, 8.0 mM Na2HPO4, pH 7.4, 137 mM NaCl, 2.7 mM KCl, 5.5 mM d-glucose) for 5 min, followed by three washes by centrifugation and resuspension in HEPES (25 mM)-buffered RPMI and restored in distilled water containing a protease inhibitor cocktail (Complete Mini; Roche). Cell extracts were prepared by repeated freeze–thawing in liquid N2, followed by sonication (4 pulses of 15 s at 35 W, on ice). Enzyme activity was measured in a buffered solution containing 10 mM Tris–HCl, pH 7.4, 50 mM KCl, 1 mM EDTA, 2 mM KCN, variant concentration of NADH (0–1000 μM) and 10 μM atovaquone with MQ-4 (200 μM). KCN and atovaquone were added to avoid the electron flow through the cytochrome system (complexes III and IV). The reaction was initiated by the addition of cell free P. falciparum extract (approximately 500 μg protein, according to Fisher et al. [24]), and activity was monitored spectrophotometrically by the decrease in absorbance at 340 nm (NADH Σ = 6.22 mM). Enzyme kinetic parameters were calculated using Enzfitter software (Elsevier-Biosoft, Cambridge, UK).
3. Results
3.1. Biosynthesis of MQ-4 by the intraerythrocytic stages of P. falciparum
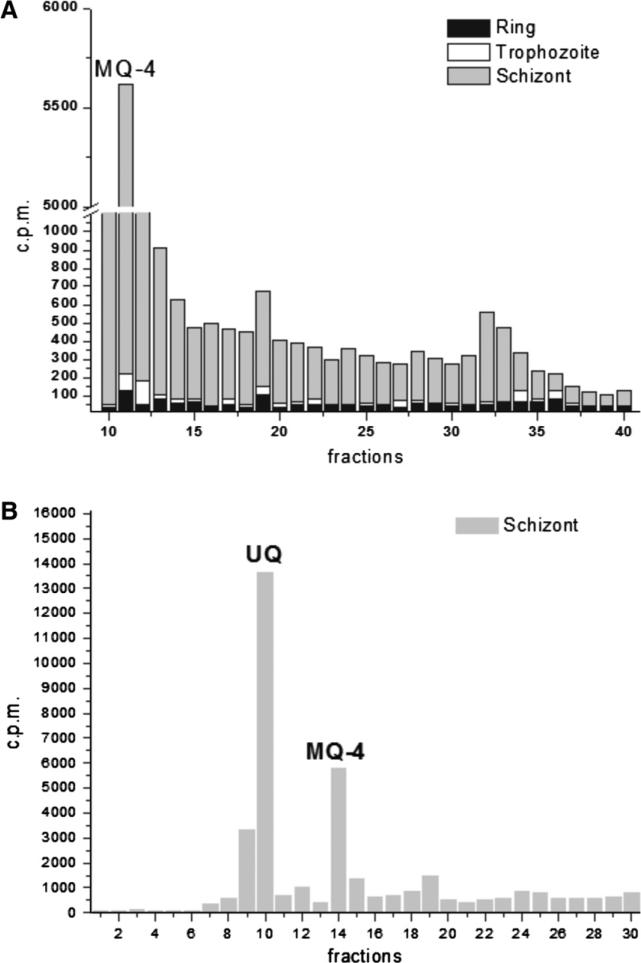
A radioactive fraction with a retention time coincident with the MQ-4 standard (11 min) and other non-characterized were detected in all intraerythrocytic stages (Fig. 2A). Higher quantities of MQ-4 was detected in schizont stages when compared to ring and trophozoite stages, similarly to what was found for the carotenoid synthesis [9].
Fig. 2.
Biochemical evidence for the biosynthesis of menaquinone in intraerythrocytic stages of P. falciparum. (A) Profile of RP-HPLC analysis (C18 column, Protocol I) of P. falciparum extracts [17] from ring, trophozoite and schizont stages (1 × 109 parasites per stage) metabolically labelled with [1-(n)-3H]GGPP. UNIPOINT™ Software (Gilson Inc.) was used as the operational and analytical system. Samples were co-injected with MQ-4 standard. (B) The radioactive peaks corresponding to the retention time of UQ and MQ-4 were plotted from extracts (according to Bekker et al. [18]) of 1 × 109 schizont stages metabolically labelled with [1-(n)-3H]GGPP, followed by RP-HPLC analysis (Protocol II). Sample was co-injected with UQ and MQ-4 standards. MQ-4: Menaquinone; UQ: ubiquinone.
Other method of extraction [18] and HPLC (Protocol II) analysis was used to confirm the presence of MQ-4 biosynthesis in schizont stages of P. falciparum metabolically labelled. Radioactive fraction with retention time coincident with MQ-4 (14 min) was detected (Fig. 2B). Other different HPLC methodologies were performed to confirm the presence of MQ-4 biosynthesis in the parasites (see Supplementary data). Importantly, no radioactive fractions coinciding with the MQ-4 standard were found in the extracts from uninfected erythrocytes labelled with [1-(n)-3H]GGPP in all chromatographic conditions used (data not shown). Under the above chromatographic conditions, polyisoprenoids, ubiquinone and carotenoids have different retention times from the MQ.
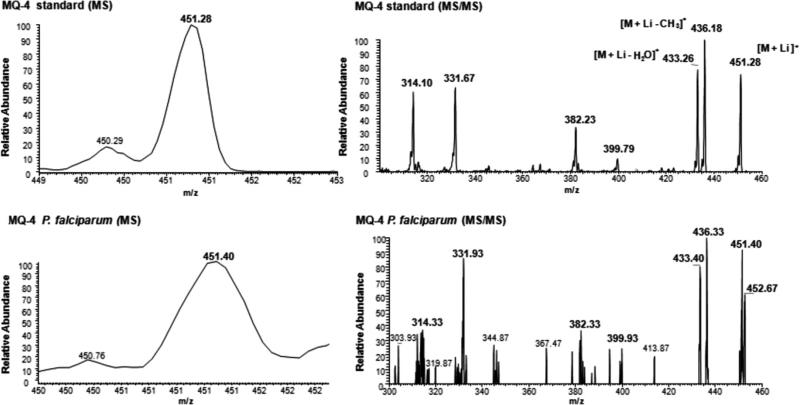
The fraction with retention time of 11 min. (HPLC-Protocol I) corresponding to MQ-4 was collected and analyzed by positive ion ESI-MS and ESI-MS/MS (Fig. 3). Only the schizont stages were submitted to mass spectrometry analysis because this stage exhibited the highest levels of MQ-4. In this analysis, identical ionization profiles were observed in the parasite samples and in the standard, with MQ-4 identified as [M+Li]+ pseudomolecular ions at m/z ratios of 451.40. The molecular structure was confirmed by obtaining matching dissociation patterns when comparing the ESI-MS/MS spectra of the parental ions with m/z 451.28 from P. falciparum with the MQ-4 standard, (Fig. 3). Importantly, this compound was biosynthesized by the parasites since it was not detected by mass spectrometry either in RPMI or in normal red blood cells used in the parasite culture (data not shown).
Fig. 3.
Molecular identification of menaquinone biosynthesis in schizont stages of P. falciparum by positive ion ESI-MS and ESI-MS/MS. (A) ESI-MS and ESI-MS/MS analyses of the MQ-4 standard and unlabelled schizont parasites (1.0 × 1010) without standard co-injection, both previously purified by HPLC (Protocol I). The analysis of parasites sample shows the presence of the m/z+ 451.28 ion, coincident with the predicted molecular mass of MQ-4 standard. The molecular structure was confirmed by comparing the ESI-MS/MS spectra of the parental ions at m/z+ 451.28 from P. falciparum with the standard.
3.2. Ro 48-8071 treatment inhibits the MQ-4 biosynthesis of P. falciparum cultivated in vitro
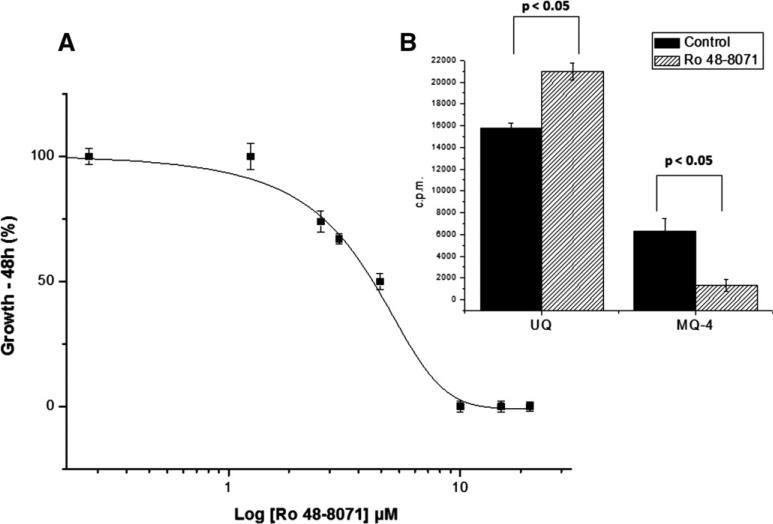
The growth of parasites was inhibited in a concentration-dependent manner (Fig. 4A) with an IC50 value of 4.5 ± 0.3 μM at 48 h of treatment (calculations done as described by Moneriz et al. [23]). At this IC50, no inhibition of protein biosynthesis was observed (data not shown). The effect of Ro 48-8071 on MQ-4 biosynthesis in the schizont stages was further investigated. The treatment of parasites with Ro 48-8071 significantly diminished MQ-4 biosynthesis in 88.2 ± 11%, while UQ increased 30 ± 5% (Fig. 4B). The inhibition assays were performed in three independent experiments. The inhibitory effect of Ro 48-8071 was not reversed by the addition of MQ from 1 nM to 1 μM (data not shown).
Fig. 4.
Menaquinone and Ubiquinone levels change in response to Ro 48-8071 treatment. (A) Ro 48-8071 inhibited parasite growth in a concentration-dependent manner (IC50 = 4.5 ± 0.3 μM; mean ± S.D., n = 3 experiments), calculated according to Moneriz et al. [23]. (B) Decrease of MQ-4 contents in the schizont stages after the treatment with 4.5 μM of Ro 48-8071. The effect of Ro 48-8071 on MQ biosynthesis was analyzed (by RP-HPLC, Protocol II, with the extraction methodology described by Bekker et al. [18]) as the relationship between control and Ro 48-8071 treated parasites (2.7 × 109) metabolically labelled with [1-(n)-3H]GGPP (mean ± S.D., n = 3 experiments). Significant differences comparing control parasites and parasites treated with Ro 48-8071 (One-way ANOVA, P values indicated). UQ: Ubiquinone; MQ-4: menaquinone.
3.3. Biological function of MQ-4 in P. falciparum
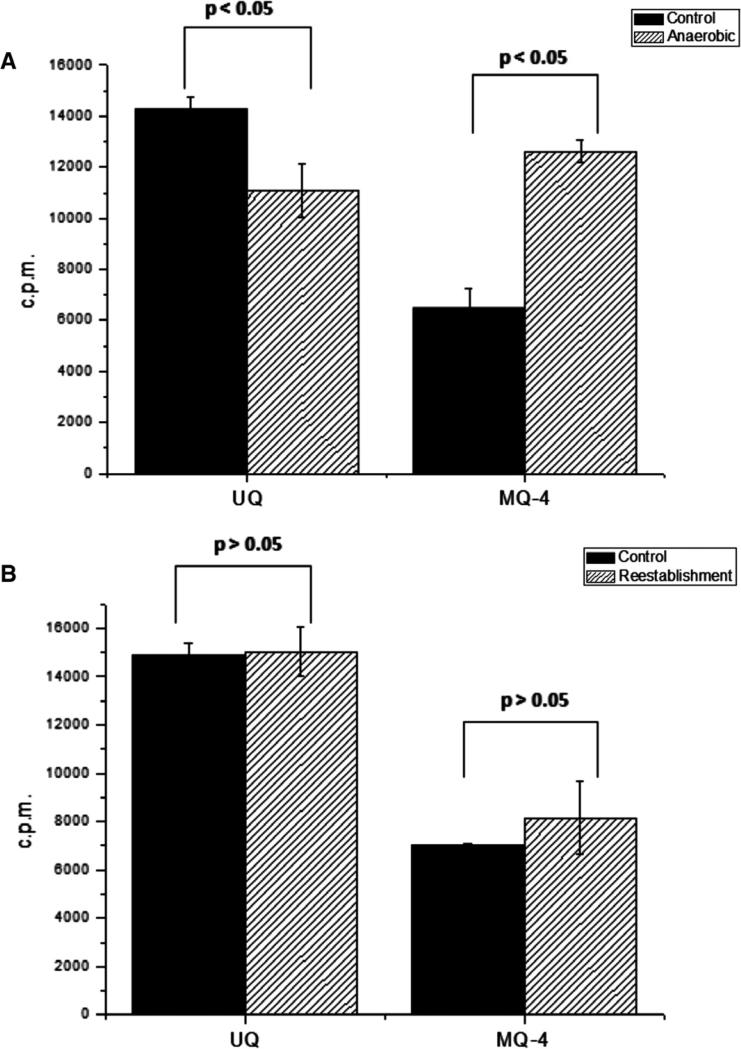
After 10 days under anaerobic conditions, the parasitemia did not change when compared to control conditions indicating that the parasite is adapted to growth in anaerobic conditions (data not shown). Parasites showed an increase of 50 ± 5% in the biosynthesis MQ-4, whereas UQ decreased 15 ± 3% after 48 h in anaerobic conditions when compared to the controls (Fig. 5A). We then tested if the previously observed changes in MQ-4 and UQ contents could be reversed in parasites upon reestablishment of normal conditions. As expected, parasites cultivated for 48 h under anaerobic conditions and then shifted to normal conditions for 48 h, showed the same MQ-4 and UQ levels profile as parasites maintained under normal conditions (Fig. 5B).
Fig. 5.
Menaquinone and ubiquinone levels change in response to oxygen supply. (A) The MQ and UQ extracted by the methodology described by Bekker et al. [18]) from 1 × 109 [1-(n)-3H]GGPP labelled schizonts stages cultivated for 48 h in anaerobic or normal conditions were analyzed by HPLC (Protocol II). The radioactive peaks corresponding to the retention time of UQ and MQ-4 were plotted. (B) Levels of MQ-4 and UQ from parasites upon reestablishment of normal conditions for 48 h after 48 h under anaerobic conditions, compared to parasites maintained in normal conditions (n = 3 experiments). Significant differences in different oxygen-containing atmospheres (One-way ANOVA test P values indicated). Control: (5% O2, 5% CO2 and 90% N2); anaerobic: (5% CO2 and 95% N2). UQ: Ubiquinone; MQ-4: menaquinone.
3.4. P. falciparum menaquinone functions as electron-carrier
The previous assays confirmed the kinetic parameters observed with decylubiquinone (Km for NADH of 5.1 μM with a Vmax of 0.12 μmol min−1 mg−1) [24,25]. Menaquinone-dependent NADH oxidation was measured, and the apparent Km for NADH oxidation was 72 ± 15 μM with a Vmax of 3 ± 0.8 μmol min−1 mg−1. Without MQ-4 or decylubiquinone addition no NADH oxidation was observed. The activity was shown to be insensitive to rotenone (50 μM), the inhibitor of mammalian complex I, but sensitive to diphenylene iodonium chloride (DPI), a classical Type II NADH:dehydrogenase inhibitor [26]. Accordingly, DPI inhibited MQ-4 reduction (data not shown).
4. Discussion
The intraerythrocytic stages of P. falciparum biosynthesizes MQ-4, and this fact was showed by metabolic labelling with the direct precursor [1-(n)-3H]GGPP. MQ-4 biosynthesized by P. falciparum was identified by five different chromatographic methods reported for this type of molecule, and confirmed also by ESI-MS/MS analysis, excluding any uncertainty about the molecular nature of the detected compound by metabolic labelling. The difference of profile observed for MQ-4 (Fig. 2A and B) probably was due to the extraction methods. The recovery of MQ in the extraction following the protocol described by Hirauchi et al. [17] is higher when compared the protocol described by Bekker et al. [18].
The inhibition of parasite growth by Ro 48-8071, an inhibitor of 1,4-dihydroxy-2-naphthoate prenyltransferase, was possibly due to the inhibition of MQ biosynthesis, although it may be also active against other targets such as neutral lipid biosynthesis [27]. Also, when MQ-4 was added to the medium, restoration of the parasite growth in the presence of Ro 48-8071 could not be observed. This may point to either a non-competitive type of inhibition in Plasmodium (contrasting to results obtained in Mycobacterium tuberculosis [28]) or an insufficient uptake of external MQ.
In P. falciparum, biochemical data indicate that the mitochondrion is not a source of ATP, but instead maintains an active mitochondrial electron transport chain to serve just one metabolic function: regeneration of UQ required as the electron acceptor [29]. Nonetheless, inhibition of cyt bc1 complex electron transport is lethal to the parasite, presumably by interruption of essential links to the de novo pyrimidine biosynthesis, dihydroorotate dehydrogenase (DHODH) pathway, and to the maintenance of the mitochondrial membrane potential. However, Smilkstein et al. [30] showed that the conventional electron transport became non-essential in a drug-selected parasite clone (SB1-A6). They showed that this clone is pan-resistant to cyt bc1 inhibitors and no mutations were identified to account for this. One possible explanation is that DHODH utilizes an alternative readily-available electron acceptor other than UQ. MQ-4 characterized in this work may serve as electron acceptor in P. falciparum.
Many respiratory enzymes from E. coli can use both MQ and UQ as substrate. Different components of the system can be substituted in the membrane in addition to, or in place of, other components as needed: substrate-specific dehydrogenases; lipoquinones; and terminal oxidoreductases – all of the dehydrogenases are lipoquinone reductases and all of the terminal oxidoreductases are lipoquinol oxidases. The lipoquinones involved in the respiratory chains of bacteria consist of MQs and UQs, while mammals have only UQ. MQs are the predominant lipoquinones of mycobacteria and other gram-positive bacteria, whereas gram-negative bacteria such as E. coli typically utilize both MQ and UQ or solely UQ [31]. Shestopalov et al. showed that E. coli changes the composition of its quinone pool depending on the aeration conditions and presented evidence of post-transcriptional regulation of the quinone biosynthesis [11]. We showed that Type II NADH dehydrogenase obtained from extracts of P. falciparum can use MQ-4 to oxidize NADH, and the reaction was functionally isolated from other respiratory complexes by the action of inhibitors of complexes III and IV. The NADH oxidation by MQ-4 is specific since without MQ-4 addition no oxidation is observed. The difference observed in the kinetic parameters from UQ and MQ may be due to that MQ-4 used in the reaction has a lateral chain with four isoprenic units while UQ used as a substrate was decylubiquinone, without isoprenic unit.
P. falciparum can be regarded as microaerophilic [32] during its asexual intraerythrocytic life cycle. However, living inside a large body of haemoglobin, it is difficult to predict the exact O2 pressure to which the parasite is exposed. We showed data that parasites of P. falciparum change the content of the quinone pools depending on the aeration condition. However, while the changes in the MQ/UQ ratios vary in a matter of seconds in E. coli [18] highest differences were observed only after the parasites were submitted to 48 h in anaerobic conditions where the levels of MQ increase up to 50% compared to control parasites. At the same time, UQ production is decreased by 15% in parasites cultivated in anaerobic conditions. This difference in UQ and MQ contents occurs predominantly in schizont stages probably because at this time, the parasite usually resides in an oxygen-depleted environment, sequestered in deep capillaries. A role for MQ as an electron receptor is also supported by the observation that through inhibition of MQ synthesis by Ro 48-8071 a significant increase of UQ was observed. This may be interpreted as an attempt to compensate for an electron acceptor loss.
In conclusion, we have shown for the first time that P. falciparum has an active biosynthesis of MQ-4. Its absence in the human host makes both pathways (MEP and shikimate) very attractive as potential new target against malaria.
Supplementary Material
Acknowledgements
The authors would like to thank S. Wendel (Sírio Libanês Hospital, NESTA) for the gift of erythrocytes and Maria Belen Cassera for suggestions and critical reading of manuscript. R.T., F.L.D., H.G.B., M.Y.M., R.A.C.S., and R.B. are fellows from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). This research was supported by grants from FAPESP, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and grant number AI049151 from the National Institutes of Health, NIAID, USA (D.C.C.).
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.febslet.2010.10.055.
References
- 1.Kalanon M, McFadden GI. Malaria, Plasmodium falciparum and its apicoplast. Biochem. Soc. Trans. 2010;38:775–782. doi: 10.1042/BST0380775. [DOI] [PubMed] [Google Scholar]
- 2.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb. Haemost. 2008;100:530–547. [PubMed] [Google Scholar]
- 3.Mustafa G, Migita CT, Ishikawa Y, Kobayashi K, Tagawa S, Yamada M. Menaquinone as well as ubiquinone as a bound quinone crucial for catalytic activity and intramolecular electron transfer in Escherichia coli membrane-bound glucose dehydrogenase. J. Biol. Chem. 2008;283:28169–28175. doi: 10.1074/jbc.M804938200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassera MB, et al. The methylerythritol phosphate pathway is functionally active in all intraerythrocytic stages of Plasmodium falciparum. J. Biol. Chem. 2004;279:51749–51759. doi: 10.1074/jbc.M408360200. [DOI] [PubMed] [Google Scholar]
- 5.Couto AS, Kimura EA, Peres VJ, Uhrig ML, Katzin AM. Active isoprenoid pathway in the intra-erythrocytic stages of Plasmodium falciparum: presence of dolichols of 11 and 12 isoprene units. Biochem. J. 1999;341(Pt 3):629–637. [PMC free article] [PubMed] [Google Scholar]
- 6.de Macedo CS, Uhrig ML, Kimura EA, Katzin AM. Characterization of the isoprenoid chain of coenzyme Q in Plasmodium falciparum. FEMS Microbiol. Lett. 2002;207:13–20. doi: 10.1111/j.1574-6968.2002.tb11021.x. [DOI] [PubMed] [Google Scholar]
- 7.D'Alexandri FL, Kimura EA, Peres VJ, Katzin AM. Protein dolichylation in Plasmodium falciparum. FEBS Lett. 2006;580:6343–6348. doi: 10.1016/j.febslet.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Moura IC, Wunderlich G, Uhrig ML, Couto AS, Peres VJ, Katzin AM, Kimura EA. Limonene arrests parasite development and inhibits isoprenylation of proteins in Plasmodium falciparum. Antimicrob. Agents Chemother. 2001;45:2553–2558. doi: 10.1128/AAC.45.9.2553-2558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tonhosolo R, et al. Carotenoid biosynthesis in intraerythrocytic stages of Plasmodium falciparum. J. Biol. Chem. 2009;284:9974–9985. doi: 10.1074/jbc.M807464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 11.Shestopalov AI, Bogachev AV, Murtazina RA, Viryasov MB, Skulachev VP. Aeration-dependent changes in composition of the quinone pool in Escherichia coli. Evidence of post-transcriptional regulation of the quinone biosynthesis. FEBS Lett. 1997;404:272–274. doi: 10.1016/s0014-5793(97)00143-9. [DOI] [PubMed] [Google Scholar]
- 12.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 13.Radfar A, Mendez D, Moneriz C, Linares M, Marin-Garcia P, Puyet A, Diez A, Bautista JM. Synchronous culture of Plasmodium falciparum at high parasitemia levels. Nat. Protoc. 2009;4:1828–1844. doi: 10.1038/nprot.2009.198. [DOI] [PubMed] [Google Scholar]
- 14.Rowe JA, Scragg IG, Kwiatkowski D, Ferguson DJ, Carucci DJ, Newbold CI. Implications of mycoplasma contamination in Plasmodium falciparum cultures and methods for its detection and eradication. Mol. Biochem. Parasitol. 1998;92:177–180. doi: 10.1016/s0166-6851(97)00237-5. [DOI] [PubMed] [Google Scholar]
- 15.Braun-Breton C, Jendoubi M, Brunet E, Perrin L, Scaife J, Pereira da Silva L. In vivo time course of synthesis and processing of major schizont membrane polypeptides in Plasmodium falciparum. Mol. Biochem. Parasitol. 1986;20:33–43. doi: 10.1016/0166-6851(86)90140-4. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues Goulart H, Kimura EA, Peres VJ, Couto AS, Aquino Duarte FA, Katzin AM. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob. Agents Chemother. 2004;48:2502–2509. doi: 10.1128/AAC.48.7.2502-2509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirauchi K, Sakano T, Notsumoto S, Nagaoka T, Morimoto A, Fujimoto K, Masuda S, Suzuki Y. Measurement of K vitamins in animal tissues by high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. 1989;497:131–137. doi: 10.1016/0378-4347(89)80012-x. [DOI] [PubMed] [Google Scholar]
- 18.Bekker M, Kramer G, Hartog AF, Wagner MJ, de Koster CG, Hellingwerf KJ, de Mattos MJ. Changes in the redox state and composition of the quinone pool of Escherichia coli during aerobic batch-culture growth. Microbiology. 2007;153:1974–1980. doi: 10.1099/mic.0.2007/006098-0. [DOI] [PubMed] [Google Scholar]
- 19.Kamao M, Suhara Y, Tsugawa N, Okano T. Determination of plasma Vitamin K by high-performance liquid chromatography with fluorescence detection using Vitamin K analogs as internal standards. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2005;816:41–48. doi: 10.1016/j.jchromb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.D'Alexandri FL, Tonhosolo R, Kimura EA, Katzin AM. Mass spectrometry analysis of polyisoprenoids alcohols and carotenoids via ESI(Li(+))-MS/MS. Methods Mol. Biol. 2009;580:109–128. doi: 10.1007/978-1-60761-325-1_6. [DOI] [PubMed] [Google Scholar]
- 21.Kurosu M, Narayanasamy P, Biswas K, Dhiman R, Crick DC. Discovery of 1, 4-dihydroxy-2-naphthoate [corrected] prenyltransferase inhibitors: new drug leads for multidrug-resistant gram-positive pathogens. J. Med. Chem. 2007;50:3973–3975. doi: 10.1021/jm070638m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moneriz C, Marin-Garcia P, Bautista JM, Diez A, Puyet A. Haemoglobin interference and increased sensitivity of fluorimetric assays for quantification of low-parasitaemia Plasmodium infected erythrocytes. Malar J. 2009;8:279. doi: 10.1186/1475-2875-8-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher N, Warman AJ, Ward SA, Biagini GA. Chapter 17 Type II NADH: quinone oxidoreductases of Plasmodium falciparum and Mycobacterium tuberculosiskinetic andhigh-throughput assays. MethodsEnzymol. 2009;456:303–320. doi: 10.1016/S0076-6879(08)04417-0. [DOI] [PubMed] [Google Scholar]
- 25.Biagini GA, Viriyavejakul P, O'Neill PM, Bray PG, Ward SA. Functional characterization and target validation of alternative complex I of Plasmodium falciparum mitochondria. Antimicrob. Agents Chemother. 2006;50:1841–1851. doi: 10.1128/AAC.50.5.1841-1851.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang J, Beattie DS. Rotenone-insensitive NADH dehydrogenase is a potential source of superoxide in procyclic Trypanosoma brucei mitochondria. Mol. Biochem. Parasitol. 2002;123:135–142. doi: 10.1016/s0166-6851(02)00139-1. [DOI] [PubMed] [Google Scholar]
- 27.Morand OH, Aebi JD, Dehmlow H, Ji YH, Gains N, Lengsfeld H, Himber J. Ro 48-8.071, a new 2,3-oxidosqualene:lanosterol cyclase inhibitor lowering plasma cholesterol in hamsters, squirrel monkeys, and minipigs: comparison to simvastatin. J. Lipid Res. 1997;38:373–390. [PubMed] [Google Scholar]
- 28.Dhiman RK, et al. Menaquinone synthesis is critical for maintaining mycobacterial viability during exponential growth and recovery from non-replicating persistence. Mol. Microbiol. 2009;72:85–97. doi: 10.1111/j.1365-2958.2009.06625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Painter HJ, Morrisey JM, Mather MW, Vaidya AB. Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum. Nature. 2007;446:88–91. doi: 10.1038/nature05572. [DOI] [PubMed] [Google Scholar]
- 30.Smilkstein MJ, Forquer I, Kanazawa A, Kelly JX, Winter RW, Hinrichs DJ, Kramer DM, Riscoe MK. A drug-selected Plasmodium falciparum lacking the need for conventional electron transport. Mol. Biochem. Parasitol. 2008;159:64–68. doi: 10.1016/j.molbiopara.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandya KP, King HK. Ubiquinone and menaquinone in bacteria: a comparative study of some bacterial respiratory systems. Arch. Biochem. Biophys. 1966;114:154–157. doi: 10.1016/0003-9861(66)90316-x. [DOI] [PubMed] [Google Scholar]
- 32.Trager W. Cultivation of malaria parasites. Methods Cell Biol. 1994;45:7–26. doi: 10.1016/s0091-679x(08)61844-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.