Abstract
Cognitive remediation therapy is effective for improving cognition, symptoms and social functioning in individuals with schizophrenia; however, the impact on visual episodic memory remains unclear. The objectives of this feasibility study were: (1) to explore whether or not CIRCuiTS—a new computerised cognitive remediation therapy programme developed in England—improves visual episodic memory and other cognitive domains in young adults with early course schizophrenia; and (2) to evaluate acceptability of the CIRCuiTS programme in French-Canadians. Three participants with visual episodic memory impairments at baseline were recruited from clinical settings in Canada, and consented to participate. Neuropsychological, clinical and social functioning was evaluated at baseline and post-treatment. Intervention involved 40 sessions of cognitive remediation. First, the reliable change index (RCI) revealed that each participant demonstrated significant post-therapy change in episodic memory and in other cognitive domains. The response profile was characterised by the use of organisational strategies. Second, the treatment was considered acceptable to participants in terms of session frequency (number of sessions per week), intensity (hours per week; total hours), and number of missed sessions and total completed sessions. This preliminary study yielded encouraging data demonstrating the feasibility of the CIRCuiTS programme in French-Canadian young adults with schizophrenia.
Keywords: Schizophrenia, Cognitive remediation, Young adults, Computerised, Case study, Visual episodic memory
INTRODUCTION
Schizophrenia is characterised by significant heterogeneity in clinical manifestations, course and response to treatment (Tandon, Nasrallah, & Keshavan, 2009). Cognitive deficits are a core feature of schizophrenia. They are present at onset, and relatively stable over the course of the illness (Mesholam-Gately, Giuliano, Goff, Faraone, & Seidman, 2009; Schaefer, Giangrande, Weinberger, & Dickinson, 2013); a subclinical presentation is often observed in relatives (Snitz, MacDonald, & Carter, 2006) and in clinically high-risk populations (Bora, 2014). Few treatments have a significant impact on functional outcomes in individuals with schizophrenia, and patients with cognitive deficits tend to have poorer outcomes overall. Such findings prompted the development of psychological treatments such as cognitive remediation, which are designed to target cognition (Mueser, Deavers, Penn, & Cassisi, 2013).
Cognitive remediation for schizophrenia is described as “a behavioral training-based intervention that aims to improve cognitive processes (attention, memory, executive function, social cognition or metacognition) with the goal of durability and generalization” (Cognitive remediation expert, Florence, Italy, April 2010). The most recent and comprehensive meta-analysis on the effect of cognitive remediation in this population evaluated 40 randomised controlled trials including 2104 patients (mean age: 36 years). Patients were randomised to a cognitive remediation programme, treatment as usual or a treatment controlling for non-specific effects (Wykes, Huddy, Cellard, McGurk, & Czobor, 2011). Significant improvements were observed in global cognition (d = 0.45), symptoms (d = 0.18) and social functioning (d = 0.42). The inclusion of strategic cognitive remediation in a psychological rehabilitation programme significantly impacts social functioning. The studies in previous meta-analyses were also conducted with patients with a mean age of approximately 36 years (McGurk, Twamley, Sitzer, McHugo, & Mueser, 2007; Wykes et al., 2011).
The success of cognitive remediation varies across patients, with the greatest benefits observed in younger patients with cognitive impairments and stable symptoms (Wykes & Spaulding, 2011). Few studies have attempted to evaluate the impact of cognitive remediation in individuals at risk or in individuals with recent-onset schizophrenia (Barlati, Deste, De Peri, Ariu, & Vita, 2013). It has been demonstrated that early intervention in first-episode patients enhances outcome (Tandon, Nasrallah, & Keshavan, 2010) and has neuroprotective effects (Eack et al., 2010); more studies of such interventions in young adults with schizophrenia are therefore warranted.
Few studies have considered baseline characteristics such as neuropsychological impairment as a criterion for inclusion in a randomised controlled trial. Targeting episodic memory in schizophrenia patients is of particular interest because: (1) episodic memory impairments are the most affected domain in patients with recent-onset and chronic schizophrenia (Mesholam-Gately et al., 2009) as well as in clinically high-risk individuals (Bora, 2014); (2) verbal episodic memory is considered to be a predictor of social functioning in high-risk individuals (Niendam et al., 2006), patients with recent-onset schizophrenia (Milev, Ho, Arndt, & Andreasen, 2005) and chronic schizophrenia patients (Green, 1996); and (3) deficits in verbal episodic memory are malleable, as demonstrated by moderately large change subsequent to cognitive remediation therapy (Wykes et al., 2011). Few cognitive remediation studies have explored visual episodic memory. In their recent meta-analysis, Wykes et al. (2011) did not find a significant effect of cognitive remediation therapy on visual episodic memory. Visual episodic memory impairments are either resistant to change or insufficiently studied and deserving of greater research attention.
Visual episodic memory and organisational strategy
Episodic memory refers to the system that allows conscious recollection of episodes within its context—place and time (Strauss, Sherman, & Spreen, 2006; Tulving, 1972). A neuropsychological test evaluating episodic memory usually shows a series of pictures or words to be remembered and the participant has to remember the items from memory in immediate recall, delayed recall or within recognition paradigms (Strauss et al., 2006). One standard and well-known neuropsychological test used to assess visual episodic memory is the Rey Complex Figure Test. In this test, the neuropsychologist shows a complex figure to the participant and asks him to copy it. This test requires organising strategies at the encoding phase. Then, the neuropsychologist asks the participant to recall the complex figure by memory 3 minutes (immediate recall) and 30 minutes (delayed recall) after the copy. Performance on this test not only requires visuospatial perception and visual episodic memory but also executive functioning such as planning and organisational strategies (Heinrichs & Bury, 1991; Shorr, Delis, & Massman, 1992). Poor visual organisation strategies during the encoding phase would lead to a poor performance in memory. Hence, Deckersbach et al. (2004) showed that visual episodic memory problems in bipolar disorder were mediated by poor organisation of non-verbal information during encoding at the Rey-Osterrieth Complex Figure Test. Savage (1998) suggested that organisational strategy at encoding would enhance the representation in memory which would enable recall in memory. Development of organisational strategy within cognitive remediation sessions would be a potential strategy to explore.
Current study
The objectives of this feasibility study were to explore whether or not a new computerised cognitive remediation programme CIRCuiTS (Computerised Interactive Remediation of Cognition Training for Schizophrenia) improves visual episodic memory and other cognitive domains (attention, working memory or executive functioning) in young adults with early onset schizophrenia. CIRCuiTS is a top-down programme that targets various cognitive domains, including visual episodic memory. CIRCuiTS focuses on learning of strategies such as organising the information in order to use it within therapy with massed practice and also in everyday life. For every task, the programme suggests a list of strategies that may be useful to do the task. The hypothesis is that the training of executive functioning—such as organisation strategies—would enhance the episodic memory performance. The programme targets multiple cognitive domains but its focus is on metacognitive improvements so improvement in executive functioning was expected. Improvement on visual episodic memory was also expected because the cases were selected according to a deficit in this specific cognitive domain. The findings will provide information about the breadth of the impact of therapy and about different treatment response profiles. The second objective of the study was to evaluate the applicability and acceptability of the programme in French-Canadian psychiatric care. We used a series of case studies to determine whether or not the same visual episodic memory profile is associated with diverse responses to therapy in young adults with schizophrenia.
METHOD
Participants
This research project was approved by the appropriate ethics committee at the Centre de recherche de l'Institut universitaire en santé mentale de Québec in Quebec City, Canada. Following a full explanation of the study procedures, participants provided informed consent. They were informed that they could withdraw from the study at any time. Four participants with schizophrenia were recruited and consented to participate. One participant was excluded at the beginning of the study because he did not present visual episodic memory impairment. The clinical details of each case study are presented in Table 1. All participants were young adults with schizophrenia.
TABLE 1 . Clinical characteristics of the cases.
| Variables | Case A | Case B | Case C |
|---|---|---|---|
| Age | 26 | 26 | 24 |
| Gender | Male | Male | Male |
| Education (years) | 12 | 11 | 7 |
| Duration of illness (years) | 7 | 10 | 6 |
| Diagnosis | Schizophrenia | Schizophrenia | Schizophrenia |
| Medication | Clozapine (450g) | Clozapine Ativan Olanzapine | Clozapine (350g) |
| Lamotrigine (300g) | |||
| Peak achievement | Full-time at school | Part-time at school, part-time job | Full-time job |
The inclusion criteria for the study were: (1) confirmed DSM-IV diagnosis of schizophrenia within the last 10 years, as per the treating psychiatrist; (2) a clinical status that permitted reliable cognitive assessment (which means that the patient did not show acute psychotic symptoms that may affect the neuropsychological evaluation and that their psychiatrist considered that the collaboration and the medication was considered as optimal for the patient); and (3) cognitive difficulties in visual episodic memory, i.e., immediate recall or delayed recall below the 16th percentile on the Rey Complex Figure Test (RCFT). The exclusion criteria were: (1) brain and metabolic disorders known to cause neuropsychological impairments; (2) substance dependence within the last six months; and (3) IQ below 70.
Materials
Neuropsychological assessments
The neuropsychological battery was selected to address all of the cognitive domains targeted by the CIRCuiTS programme. The selected tests are widely used: California Verbal Learning Test-II (CVLT-II; Delis, Kramer, Kaplan, & Ober, 2000), Rey Complex Figure Test (Meyers & Meyers, 1995), Wechsler Adult Intelligence Scale third edition (WAIS-III; Wechsler, 2005), Continuous Performance Test-II (CPT-II; Conners, 1999), Stroop from the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001), Spatial span (Wechsler, 1997), Digit span (Wechsler, 2005), Wisconsin Card Sorting Test–128 cards (WCST: CV4; Heaton, Chelune, Talley, Kay, & Curtiss, 1993), Verbal Fluency Test (French-Canadian version), Tower of London Test (TOLDX; Culbertson & Zillmer, 2001), and Purdue Pegboard (Tiffin, 1968).
Clinical assessments
Positive and Negative Syndrome Scale (Kay, Opler, & Lindenmayer, 1989): This instrument includes 30 items evaluating psychiatric symptoms. Items are rated on a scale from 1 (absent) to 7 (extreme). This scale is completed by a psychiatrist who knows the patient well and who has interviewed the patient.
Subjective Scale to Investigate Cognition in Schizophrenia (SSTICS; Stip, Caron, Renaud, Pampoulova, & Lecomte, 2003): This scale measures subjective complaints of individuals with cognitive problems; it covers several areas, including attention, language, memory and praxis. The SSTICS is a 21-item scale that was developed specifically for use with individuals with schizophrenia.
Global Assessment of Functioning (GAF; American Psychiatric Association, 1994): This instrument measures social and occupational functioning on a scale from 1 to 100. It is divided into ranges of 10 points, i.e., 1–10, 11–20, etc., up to 91–100. Each range includes a clinical description; GAF was determined by respective participants’ treating psychiatrists.
Self-esteem Rating Scale, short form (Lecomte, Corbière, & Laisné, 2006). This self-report scale measures self-esteem in adults. The short form contains 20 items.
Computerised cognitive remediation therapy package
CIRCuiTS (Reeder & Wykes, 2010) is a computerised cognitive remediation programme designed to improve cognitive (attention, memory, executive functioning) and metacognitive skills. It is based on a metacognitive model which suggests that the transfer of cognitive skills to daily activities depends on adequate metacognitive knowledge and metacognitive regulation (i.e., knowledge and regulation of one's own thinking) (Wykes & Reeder, 2005). It has an integrated focus on the transfer of cognitive skills to daily living, relying on the development of metacognitive regulation and metacognitive knowledge, and using real-world goals, homework to facilitate in vivo use of new strategies, and a formulation-based approach in which the impact of cognitive strengths and difficulties on daily living skills is considered.
A standard programme in CIRCuiTS is one-hour sessions, at least three days per week for a total of 40 sessions. As participant concentration may be limited, session length is often increased gradually with shorter sessions initially. Each sessions includes about four to eight tasks targeting a range of cognitive problems, which become more ecologically valid as the programme progresses. Tasks are rotated to provide variety in the cognitive skills targeted and ensure continued engagement. Most tasks require a wide range of cognitive skills and can be used to practise or to learn to manage multiple cognitive functions.
In England, CIRCuiTS has been shown to be highly acceptable and feasible to both service users and therapists and has included considerable service user involvement in its development (Reeder et al., 2014).
Illustration of one exercise from the CIRCuiTS programme: Plan a day
CIRCuiTS comprises two types of tasks (Reeder & Wykes, Therapist manual): (1) abstract tasks, to improve basic cognitive functions in an abstract context (e.g., remembering word lists), and (2) exercises, which are more complex and ecologically valid tasks, associated with activities which might be carried out in everyday life (e.g., planning a day, planning a journey, following a recipe, remembering a shopping list). Abstract tasks appear throughout the CIRCuiTS programme and exercises are introduced later in the sessions. At the end of the therapy, the sessions generally consist of exercises such as “Plan a day” which is shown in Figure 1. The aim of this exercise is to drag the tasks from the to-do list into the diary; it uses organisational strategies.
Figure 1.
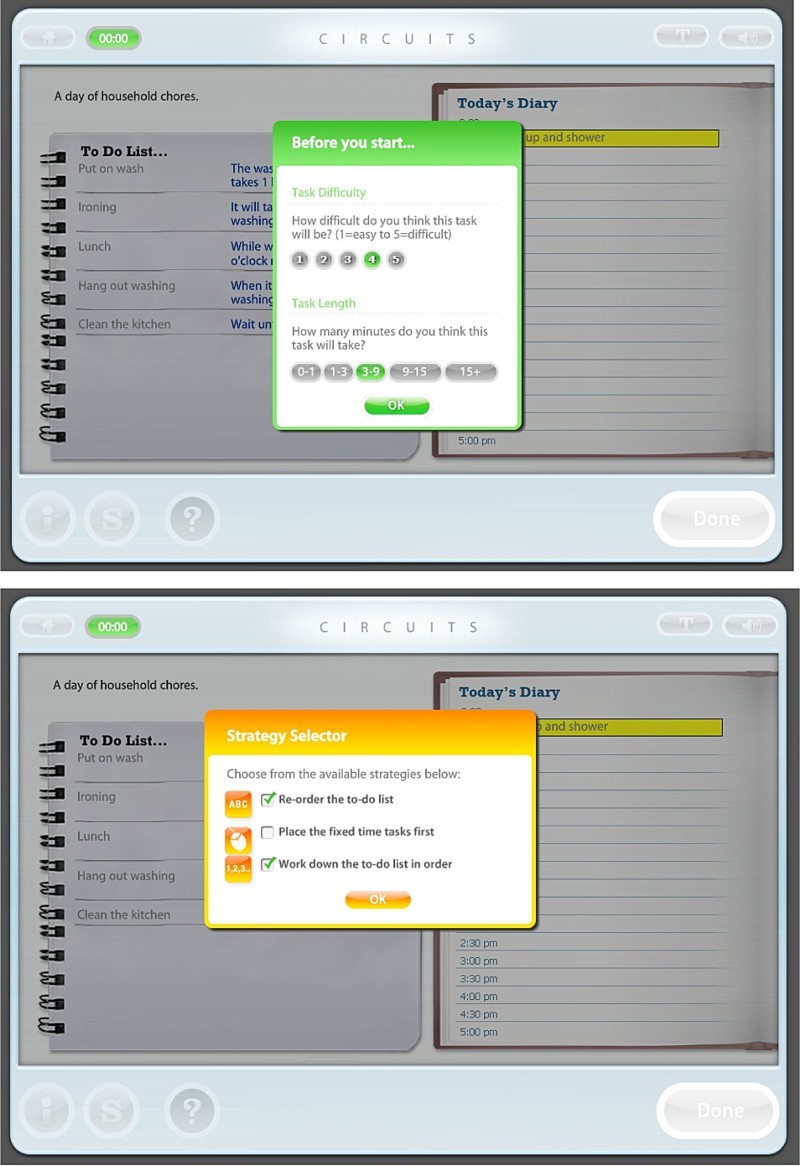
Illustration of one exercise from the CIRCuiTS programme: Plan a day.
Following the task instructions, a metacognitive box appears and the patient has to think about the task difficulty and the task length. Then the Strategy selector appears which suggests different strategies for completing the task efficiently. Then, the task appears. The patient—with the help of the therapist—completes the task using the strategies and, once complete, a feedback and Score page appear. The therapist and patient discuss task difficulty and the usefulness of each strategy during the task. The therapist and the patient also discuss how to use the strategies in everyday life.
Strategies
The therapist underlines the importance of completing a task in an organised and strategic style rather than only achieving a 100% score. For example, the following is a selection from the therapy manual (Reeder & Wykes, Therapist manual):
Verbalisation of cues, prompts and strategies relating to the task in hand. Verbalised prompts are often used very repetitively, in an increasingly independent way. In the Learning a list task, the therapist may encourage the client to repeat the list of words over to him or herself.
Information reduction: In tasks in which participants are confronted with large amounts of information, the risk of overload can be reduced by covering up some of the material. The computer will frequently offer the client the option to reduce what can be seen on the screen.
Breaking the task into smaller steps: For complex tasks, or when participants are easily overwhelmed or are very disorganised, tasks can be broken down according to component parts, so that the participant only partially completes the task, or completes the task a step at a time. The computer frequently offers options to use this as a strategy but the therapist can also help by suggesting only parts of a task are completed or focused upon.
Design
This feasibility study used a case study design. Participants were assessed at baseline (neuropsychological and clinical assessments), received therapy sessions approximately three times a week for approximately three months and were reassessed at post-treatment using the same neuropsychological and clinical assessments.
Procedure
The treating psychiatrist at the Institut universitaire en santé mentale de Quebec identified patients with recent-onset schizophrenia who were motivated to return to school or to work. Consenting participants were included if they had a demonstrated deficit in visual episodic memory at baseline. If the inclusion criteria were met, a research assistant (AA or CJ) completed neuropsychological and clinical evaluations. The therapist did not evaluate the patient. The treating psychiatrist administered the Global Assessment of Functioning (GAF) and Positive and Negative Syndrome Scale (PANSS) (R-HB or M-AR). Once baseline evaluation was complete, therapy was initiated. Participants maintained their usual treatment (e.g., medication) throughout the study. After therapy sessions were completed, a research assistant administered the post-treatment neuropsychological evaluation and the treating psychiatrist conducted the post-treatment clinical assessment.
Statistical analysis
Two complementary approaches were adopted to objectively estimate changes on main outcomes after cognitive remediation. First, the Reliable Change Index (RCI; Jacobson & Truax, 1991) was calculated to determine whether post-therapy change observed for each variable for each participant reached significance level. RCI, similar to a Z-change score, (a) allows an estimation of the extent by which a patient distances himself or herself from a distribution of similar symptomatic patients who were not exposed to the intervention, while (b) controlling for the instrument reliability, and (c) allowing a conclusion about the “statistical significance” of the change. According to the original authors, RCI larger than 1.96 are seen as statistically significant at a two-tailed 5% alpha level. It can be noted that this statistical approach is very conservative: only a change of at least two standard deviations is considered significant.
Normative data, needed to compute the baseline distribution of the data for the RCI, were extracted from a comparison sample of patients recruited in the same clinical settings as the study. The standard deviation for the neuropsychological variables were gathered from our laboratory (Maziade et al., 2011). The results have been published previously (Maziade et al., 2011). However, to have a greater sample size, we used unpublished standard deviations collected from our laboratory since the previous publication. Only one neuropsychological variable, the Stroop from the D-KEFS battery, was gathered from a paper in the literature (Simonsen et al., 2009). The standard deviations for the clinical variables were obtained from published research: Subjective Scale to Investigate Cognition in Schizophrenia (SSTICS; Stip et al., 2003), Self-Esteem Rating Scale short form (SERS; Staring, Van der Gaag, Van den Berge, Duivenvoorden, & Mulder, 2009), Positive and Negative Syndrome Scale (PANSS; Bayard, Capdevielle, Boulenger, & Raffard, 2009), and Global Assessment of Functioning (GAF; Simonsen et al., 2009). The test-retest change is not the same across populations, especially in schizophrenia (Heaton et al., 2001). Thus, we fixed the same test-retest to r = .80 as a standard for each test. The reliability of .80 to .90 is considered as the minimum acceptable for internal consistency and .70 is the minimum for the test-retest reliability (Strauss et al., 2006).
A second more common approach in clinical settings was also used to determine whether or not participants improved in the outcome variables. Patients who exhibited deficits at baseline (i.e., a percentile of 16 or lower on instruments with available clinical normative data, Lezak, Howieson, Bigler, & Tranel, 2012) were compared to themselves at post-treatment to investigate whether or not they continued to exhibit the same deficits. Changes in status were considered to be a clinical improvement.
Finally, an analysis of strategies was done according to the strategies used within the therapy and the strategies observed during the neuropsychological evaluation.
RESULTS
Changes in episodic memory
Baseline scores for each case are reported in Tables 2 and 4. RCI are reported in Tables 3 and 5. All cases demonstrated deficits (below 16th percentile) in immediate and delayed recall in visual episodic memory as measured by the RCFT. Moreover, the cases demonstrated deficits in delayed recall in verbal episodic memory.
TABLE 2 . Raw scores and percentile rank as compared to age and sex corrected norms at baseline and post-treatment assessments.
| Case A | Case B | Case C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-test | Baseline | Post-test | Baseline | Post-test | |||||||
| Score | PR | Score | PR | Score | PR | Score | PR | Score | PR | Score | PR | |
| Episodic memory | ||||||||||||
| Verbal episodic memory | ||||||||||||
| CVLT-II total recall | 34 | 3 | 49 | 45 | 57 | 77 | 48 | 42 | 34 | 3 | 48 | 42 |
| CVLT-II delayed recall | 7 | 7 | 9 | 16 | 8 | 7 | 11 | 50 | 6 | 2 | 9 | 16 |
| CVLT-II recognition | 13 | 7 | 16 | 70 | 12 | 1 | 12 | 1 | 15 | 50 | 16 | 70 |
| Visual episodic memory | ||||||||||||
| RCFT immediate Recall | 11 | 1 | 6.5 | 1 | 10.5 | 1 | 17.5 | 7 | 8.5 | 1 | 14 | 1 |
| RCFT delayed recall | 6.5 | 1 | 6 | 1 | 11.5 | 1 | 15 | 1 | 7 | 1 | 16.5 | 4 |
| RCFT recognition | 17 | 1 | 19 | 1 | 23 | 84 | 23 | 84 | 18 | 1 | 22 | 62 |
| Other cognitive domains | ||||||||||||
| Intelligence | ||||||||||||
| Global IQa | 78 | 7 | 78 | 7 | 92 | 30 | 94 | 34 | 73 | 4 | 77 | 6 |
| Sustained attention | ||||||||||||
| CPT-hit reaction time Block change | 0.01 | 59 | 0.03 | 21 | 0.01 | 32 | 0.02 | 7 | 0.02 | 23 | 0.01 | 32 |
| CPT-hit standard error Block change | 0.04 | 34 | .12 | 5.67 | .04 | 33 | .21 | 1 | .13 | 5 | .06 | 19 |
| Selective attention | ||||||||||||
| CPT omissions | 0 | 79 | 5 | 24 | 18 | 1 | 7 | 10 | 1 | 56 | 1 | 56 |
| CPT commissions | 19 | 27 | 16 | 41 | 29 | 2.5 | 22 | 14 | 27 | 5 | 17 | 37 |
| CPT detectability d’ | 0.51 | 38 | 0.49 | 32 | 0.04 | 9 | 0.29 | 19 | 0.23 | 18 | 0.58 | 40 |
| Stroop D-KEFS inhibition | 44.6 | 63 | 47.75 | 63 | 61 | 16 | 64 | 16 | 48 | 63 | 40 | 84 |
| Working Memory | ||||||||||||
| Total spatial span | 14 | 25 | 14 | 25 | 17 | 50 | 18 | 63 | 13 | 16 | 13 | 16 |
| Total digit span | 16 | 25 | 18 | 50 | 14 | 16 | 15 | 25 | 12 | 5 | 11 | 5 |
| Executive Function/problem solving | ||||||||||||
| WCST total errors | 43 | 12 | 64 | 2 | 24 | 63 | 20 | 75 | 66 | 5 | 87 | 1 |
| WCST number of categories completed | 3 | 2 | 2 | 1 | 6 | <16 | 6 | <16 | 2 | 1 | 0 | 1 |
| WCST trials 1st category | 11 | >16 | 36 | 1 | 11 | >16 | 11 | >16 | 46 | 1 | 129 | 1 |
| WCST failure to maintain set | 2 | >16 | 0 | >16 | 2 | 11 | 0 | >16 | 1 | >16 | 1 | >16 |
| WCST learning to learn | −10.86 | 6 | 0 | >16 | −0.91 | >16 | −3.18 | >16 | −3.56 | >16 | NA | NA |
| Executive Function/initiation | ||||||||||||
| Letter fluency test | 14 | 53 | 10 | 31 | 8 | 10 | 11 | 27 | 7 | 9 | 7 | 9 |
| Category fluency test | 17 | 25 | 18 | 25 | 23 | 77 | 16 | 19 | 10 | 1 | 11 | 2 |
| Executive Function/planning | ||||||||||||
| Total number of problems solved with minimal movements | 2 | 19 | 1 | 8 | 5 | 60 | 3 | 33 | 2 | 19 | 5 | 60 |
| Total time violations | 0 | 66 | 0 | 66 | 0 | 66 | 0 | 66 | 0 | 66 | 0 | 66 |
| Total rules violations | 0 | 56 | 0 | 56 | 0 | 56 | 2 | 2 | 0 | 56 | 1 | 8 |
| Motor coordination | ||||||||||||
| Purdue-Both hands | 8 | 0.01 | 6 | 0.01 | 14 | 79 | 13 | 53 | 9 | 0.04 | 11 | 5 |
PR = Percentile rank. The score correspond to the raw score obtained at each variable. (CVLT-II) = California Verbal Learning Test-II; RCFT = Rey Complex Figure; Global IQ as measured with the Wechsler Adult Intelligence Scale (WAIS-III); CPT-II = Continuous Performance Test-II; D-KEFS = Delis-Kaplan Executive Function System; WCST = Wisconsin Card Sorting Test-128 cards.
aGlobal IQ was reported in standardised score.
TABLE 4 . Raw scores for each clinical variable at baseline and post-treatment.
| Case A | Case B | Case C | ||||
|---|---|---|---|---|---|---|
| Baseline | Post-test | Baseline | Post-test | Baseline | Post-test | |
| Clinical assessments | ||||||
| GAF | 42 | 59 | 45 | 40 | 38 | 40 |
| PANSS | ||||||
| Positive Symptoms Scale | 17 | 9 | 24 | 28 | 28 | 19 |
| Negative Symptoms Scale | 20 | 12 | 21 | 24 | 24 | 20 |
| General Psychopathological | 34 | 20 | 56 | 47 | 47 | 35 |
| Scale | ||||||
| Self-report questionnaires | ||||||
| SERS | ||||||
| Total | 23 | 43 | 13 | 15 | 22 | 27 |
| Positive scale | 45 | 57 | 40 | 42 | 46 | 46 |
| Negative scale | −22 | −14 | −27 | −27 | −24 | −19 |
| SSTICS | 21 | 18 | 45 | 32 | 15 | 25 |
GAF = Global Assessment of Functioning ; PANSS = Positive and Negative Syndrome Scale; SERS = Self-esteem rating scale short form; SSTICS = Subjective Scale to Investigate Cognition in Schizophrenia.
TABLE 3 . Reliable Change Index (RCI) for the neuropsychological variables for each case.
| RCI | |||
|---|---|---|---|
| Case A | Case B | Case C | |
| Episodic memory | |||
| Verbal episodic memory | |||
| CVLT-II total recall | 2.40** | −1.44 | 2.24** |
| CVLT-II delayed recall | 1.07 | 1.60 | 1.60 |
| CVLT-II recognition | 2.79** | .00 | .93 |
| Visual episodic memory | |||
| RCFT immediate recall | −1.05 | 1.63 | 1.28 |
| RCFT delayed recall | −.10 | .72 | 1.97** |
| RCFT recognition | 1.60 | .00 | 3.19** |
| Other cognitive domains | |||
| Intelligence | |||
| Global IQ | .00 | .26 | .53 |
| Sustained attention | |||
| CPT-hit reaction time Block change | 1.05 | .53 | −.53 |
| CPT-hit standard error Block change | 1.51 | 3.20** | −1.32 |
| Selective attention | |||
| CPT omissions | .83 | −1.83* | .00 |
| CPT commissions | −.57 | −1.33 | −1.90* |
| CPT detectability d’ | −.08 | .96 | 1.35 |
| Stroop D-KEFS inhibition | .26 | .25 | −.67 |
| Working Memory | |||
| Total spatial span | .00 | .53 | .00 |
| Total digit span | .81 | .45 | −.45 |
| Executive Function/problem solving | |||
| WCST total errors | 1.32 | −.25 | 1.32 |
| WCST number of categories completed | −.73 | .00 | −1.46 |
| WCST trials 1st category | 1.04 | .00 | 3.46** |
| WCST failure to maintain set | −2.96** | −2.96** | .00 |
| WCST learning to learn | 2.56** | 0.53 | .84 |
| Executive Function/initiation | |||
| Letter fluency test | −1.46 | 1.10 | .00 |
| Category fluency test | .28 | −1.96** | .28 |
| Executive Function/planning | |||
| Total number of problems solved with minimal movements | −.63 | −1.26 | 1.89* |
| Total time violations | .00 | .00 | .00 |
| Total rule violations | .00 | 2.17** | 1.08 |
| Motor coordination | |||
| Purdue-Both hands | −1.95* | −.95 | 1.95* |
CVLT-II = California Verbal Learning Test-II; RCFT = Rey Complex Figure; Global IQ as measured with the Wechsler Adult Intelligence Scale (WAIS-III); CPT-II = Continuous Performance Test-II; D-KEFS = Delis-Kaplan Executive Function System; WCST = Wisconsin Card Sorting Test-128 cards.
*Significant with unilateral criteria (cut off = 1.64).
**Significant with bilateral criteria (cut-off = 1.96).
TABLE 5 . Reliable Change Index (RCI) for the clinical variables.
| RCI | |||
|---|---|---|---|
| Case A | Case B | Case C | |
| Clinical assessments | |||
| GAF | 2.59** | −0.76 | .30 |
| PANSS | |||
| Positive symptoms scale | −2.17** | 1.09 | −2.45** |
| Negative symptoms scale | −1.99** | .75 | .99 |
| General psychopathological scale | −.28 | −.18 | −.24 |
| SERS | |||
| Total | 2.72** | .27 | .68 |
| Positive scale | 1.81* | .30 | .00 |
| Negative scale | 1.01 | .00 | .63 |
| SSTICS | −0.77 | −3.32** | 2.55** |
GAF = Global Assessment of Functioning; PANSS = Positive and Negative Syndrome Scale; SERS = Self-esteem rating scale short form; SSTICS = Subjective Scale to Investigate Cognition in Schizophrenia.
*Significant with unilateral criteria (cut off = 1.64).
**Significant with bilateral criteria (cut-off = 1.96).
The first objective of the study was to evaluate whether or not all cases would improve in episodic memory, particularly visual episodic memory (see Table 3). Case A improved marginally on visual episodic memory, RCFT recognition (RCI = 1.60). Case A also improved significantly on verbal episodic memory, CVLT-II total recall (RCI = 2.40) and CVLT-II recognition (RCI = 2.79). Case B improved marginally on RCFT immediate recall (RCI = 1.63) and on CVLT-II delayed recall (RCI = 1.60). Case C improved significantly on visual episodic memory (RCFT delayed recall, RCI = 1.97; RCFT recognition, RCI = 3.19) and verbal episodic memory (CVLT-II total recall, RCI = 2.24); he also improved marginally on CVLT-II delayed recall (RCI = 1.60). Overall, Case C improved significantly on visual episodic memory, whereas Cases A and B improved marginally.
Changes in other cognitive outcomes
We also evaluated whether or not cases improved in other cognitive domains (see Table 3). The domains evaluated included intelligence, attention (sustained and selective), working memory, executive functioning (problem-solving, initiation, planning) and motor coordination.
Case A improved significantly in executive functioning for problem-solving (WCST failure to maintain set, RCI = −2.96).
Case B improved significantly in selective attention (CPT omission, RCI = −1.83) and executive functioning for problem-solving (WCST failure to maintain set, RCI = −2.96).
Case C improved significantly in selective attention (CPT commissions, RCI = −1.90), executive function for problem-solving (total number of problems solved, RCI = 1.89) and motor coordination (Purdue both hands, RCI = 1.95).
None of the cases improved in intelligence or in working memory as evaluated by the RCI, a very conservative test. At the clinical level, it should be noted that working memory was sensitive to change (see Table 2). Case A increased from the 25th percentile (low average) to the 50th (mean) percentile on the total digit span. Case B improved from the 16th percentile (low average/borderline) to the 25th percentile (low average) on the total digit span. No improvement in working memory was observed for Case C.
Overall, all cases demonstrated improvements in episodic memory and in at least two different cognitive domains. The demonstrated improvements in working memory were considered to represent real change in the cases’ abilities.
Analysis of strategies within therapy and during the neuropsychological evaluation
During the therapy, the therapist keeps track of all strategies used by the patient for every task. At the end of the therapy, usually between sessions 37 and 39, the therapist shows a personalised guide in which the strategies used by the patient are written. This guide is completed by the patient and the therapist. At the end of the therapy (session 40), the therapist gives a copy of this guide to the patient. A part of this guide—regarding the strategies—is presented in Table S1 (see Supplementary material). The cases used several strategies during therapy and they were encouraged by the therapist to use them in everyday life by giving homework to the patients. All the patients used visual organisation strategies and organisational strategies at the end of the therapy.
We also looked for evidence for increased strategy use during the neuropsychological evaluation. The neuropsychological tests requiring organisational strategies were analysed: RCFT, CVLT-II, Fluency task and the Tower of London (see Table S2 in Supplementary material). An analysis of the strategies used was conducted in order to provide hypotheses to explain the improvements at post-treatment.
Case A improved marginally on visual episodic memory (RCFT recognition) and significantly on verbal episodic memory (total recall and recognition). During the RCFT, Case A recalled a figure that was qualitatively better. Greater details were reported but not at the right place. This may explain why the recognition score was better compared to baseline. During the CVLT-II, Case A used several clustering strategies that may explain his improvement in verbal episodic memory. A recency recall at post-treatment was not observed because he probably preferred the clustering strategies during the CVLT-II task. However, his score in verbal working memory improved. He also improved significantly on problem solving. During the therapy, Case A used strategies to reduce working memory load such as rehearsal and use of mnemonics and several organisational strategies such as planning, organisation, and categorisation. He also used visualisation (mental images) that may have helped to improve his score in visual episodic memory.
Case B improved marginally on visual episodic memory (immediate recall) and on verbal episodic memory (delayed recall). During the RCFT, Case B recalled a figure that was of greater size, in the middle of the page, with greater details. During the CVLT-II, he used several clustering strategies. Of note, he mostly used the serial clustering during recall. Primacy, middle and recency recall were observed. Case B also improved significantly in selective attention and in verbal working memory. Case B's preferred strategies during the therapy were: visualisation (mental images), verbalisation, providing written prompts, the use of mnemonics and information reduction. The pattern of improvement is less characterised by the use of organisational strategies. This may also explain why the improvements in episodic memory were smaller compared to Cases A and C.
Case C improved significantly on visual episodic memory (delayed recall and recognition) and on verbal episodic memory (total recall) and he also improved marginally on verbal episodic memory (delayed recall). During the RCFT, Case C recalled a figure characterised with greater details, the same size as the model and placed in the middle of the page. During the CVLT-II, Case C performance was characterised by several clustering strategies and primacy, middle and recency recalls. He also improved in selective attention, problem solving and motor coordination. Case C used several organisational strategies during the therapy, namely, organisation and planning. He also used mnemonics such as visualisation (making mental images). These strategies may explain the better performance in the neuropsychological evaluation at post-treatment.
Changes in clinical outcomes
The clinical variables were secondary outcomes in the current study. Table 4 presents raw scores at baseline and at post-treatment. RCI are presented in Table 5.
Case A demonstrated significant change in social functioning (GAF, RCI = 2.59) and in symptoms (Positive symptoms scale, RCI = −2.17; Negative symptoms scale, RCI = −1.99). Case A's self-esteem improved significantly at post-treatment (total score, RCI = 2.72; positive score, RCI = 1.81). No change was observed on the SSTICS.
Case B's cognitive complaints were significantly reduced at post-treatment (SSTICS, RCI = −3.32). No significant change was observed on the other clinical outcomes.
Case C's positive symptoms on the PANSS were significantly reduced at post-treatment (Positive symptoms scale, RCI = −2.45); cognitive complaints were significantly increased at post-treatment (RCI = 2.55). This change was encouraging for Case C because he demonstrated very poor insight at baseline and several positive symptoms. No change was observed on social functioning or self-esteem.
The second approach was also used with the clinical scales. The RCI for the PANSS may identify significant change of two standards deviations. Leucht, Davis, Engel, Kissling, and Kane (2009) suggests that a decrease of 50% of symptoms compared to baseline should be used for nonrefractory schizophrenia rather than the 20% usually used in clinical trials. Hence, the cut-off of Leucht et al. (2009) was also used to determine if the clinical change was clinically significant. For Case A, a significant RCI was obtained for the Positive symptoms scale and for the Negative symptoms scale. However, this decrease was not superior to 50% since it was, respectively, 47 and 40%. For Case B, no RCI was significant on the PANSS and the cut-off of 50% was not reached. For Case C, the RCI for the Positive symptoms scale was significant but the decrease at the PANSS was 32%. Of note, if the cut-off of 20% was used, almost all post-treatment changes would be significant except for Case B and for the Negative symptoms scale for Case C (17%). The significant RCIs were only considered as improvements for the PANSS.
The cut-off for functional remission at the GAF is generally between 59 (Bertelsen et al., 2009) and 61 (Boyer et al., 2013). According to this criterion, Case A is the only one who reached the 59–61 points at post-treatment. The RCI for this case was also the only one that was significant.
Acceptability of the CIRCuiTS programme in French-Canadians
The patients were outpatients seeing their psychiatrist in a psychiatric hospital, the Institut universitaire en santé mentale de Québec, about once a month or every two months. Case B was an inpatient at baseline and was then an outpatient during the therapy. These patients also received care from nurses and from other professionals at the Centre local de services communautaires (CLSC), such as a social worker or an educator. CLSCs are public-sector organisations offering general care in Quebec (Canada). This was not a specialised clinic for psychotic disorders.
Four French-Canadian individuals with schizophrenia were asked to participate in this study. One participant was excluded because he did not meet one of the inclusion criteria (episodic memory score below the 16th percentile). Three participants took part in the study and completed the cognitive remediation programme. Number of sessions per week was approximately 3 (range = 2.5–3). The sessions lasted for approximately 50 minutes (range = 41–58), for a total mean of 129 minutes per week (range = 101–155). The mean number of missed sessions was 6 (range = 2–9). The mean total completed sessions was 41 (range = 40–44).
At the end of therapy, we asked the three cases whether or not they believed that the CIRCuiTS programme helped them: “Do you think that CIRCuiTS helped you? If so, how?” Responses included “The programme helped me in my daily life such as solving problems, double-checking a task to do, making sure that I understand what I have to do, following steps”; “Yes, I improved on memory”; “Yes, the brain gets better if you have chance to practise. I now use a notepad.” All participants believed that they had improved.
DISCUSSION
The objectives of this study were (1) to explore whether or not a new computerised cognitive remediation programme (CIRCuiTS) improves visual episodic memory and other cognitive domains (attention, working memory, executive functioning and intelligence) in individuals with early course schizophrenia, and (2) to evaluate its applicability and acceptability in French-Canadian psychiatric care. We conducted a series of case studies. It was hypothesised that using young adults with visual episodic memory impairments at baseline would increase the likelihood of observing a therapeutic response on visual episodic memory. The preliminary results indicated that all cases improve on either verbal or visual episodic memory. All cases seemed to improve in other cognitive domains, with a heterogeneous pattern of response to treatment. The case study design allowed us to identify a visual episodic memory change marked with the use of organisational strategies. However, this study did not permit conclusions about the effectiveness of therapy. The treatment was demonstrated to be acceptable in this series of case studies.
Visual episodic memory may be malleable
The cases in this study reported visual episodic memory impairment at baseline. Two cases (A and B) improved marginally and one case (C) improved significantly on RCFT. It can be hypothesised that all cases improved because they were starting from a deficit. Episodic memory impairments are consistently reported in individuals with recent-onset schizophrenia (Mesholam-Gately et al., 2009) and in those with chronic schizophrenia (Schaefer et al., 2013); however, such deficits are rarely considered as inclusion criteria at baseline. The improvement observed in the participants in the current study may also be partially attributable to their relative youth. Ueland and Rund (2004) explored visual episodic memory as an outcome and reported a significant effect of therapy; their study was conducted with the youngest schizophrenia sample (mean age = 15 years) in the literature on cognitive remediation with this population. Overall, meta-analyses of cognitive remediation studies with individuals with schizophrenia suggest that visual episodic memory is not usually responsive to therapy (McGurk et al., 2007; Wykes et al., 2011). This domain should be included in cognitive remediation trials conducted with young patients.
Improvements in other cognitive domains
Although the pattern of improvement varied across cases, participants in the current study improved in at least two cognitive domains. There are three possible explanations for this result. First, CIRCuiTS is a top-down programme that targets multiple cognitive domains. The programme is classified as “Drill and practise and strategy” (McGurk et al., 2007; Wykes et al., 2011), i.e., participants have several opportunities to practise new cognitive abilities in therapy sessions; they learn new strategies and learn how to apply them in daily life. Second, participants in this study were under 30 years of age, and younger individuals may demonstrate greater improvement (Wykes et al., 2009). Third, participants in this study had baseline cognitive deficits, which made them likely to demonstrate improvement (Wykes & Spaulding, 2011).
Strategies and visual episodic memory improvement
The analysis of strategies used during the therapy and the neuropsychological assessments provide insight about the nature of the improvement at post-treatment. It was hypothesised that since the CIRCuiTS programme focused on executive functioning—such as organisation strategies—improvement would be observed in episodic memory performance, especially on visual episodic memory. The analysis showed that patients used more organisational strategies during therapy and that the performance at post-treatment was characterised by the use of organisational strategies such as clustering and visualisation (mental images). Case B did not use organisational strategies compared to Cases A and C—and his improvement on visual episodic memory was smaller. This analysis may lead to future research to improve visual episodic memory. Of note, one may anticipate that whilst strategy use may have increased, this may not necessarily lead to improved scores at this stage since the participants are relative novices in terms of strategy use.
Capturing recovery process
Subjective scales may capture improvements that would go undetected by neuropsychological tests. In the current study, two cases (B and C) demonstrated improvement on the SSTICS, a measure of subjective cognitive complaints. Case C realised at post-treatment that his cognitive problems were greater than he had previously understood. This was considered to be a therapeutic gain, because Case C had demonstrated several positive symptoms at baseline, as well as less insight. A decrease in positive symptoms (as assessed with the PANSS) and an increase in social functioning on the GAF were observed at post-treatment. Case A did not improve in cognitive complaints; his improvements on neuropsychological measures were less than those observed in the other cases. In contrast to the other participants, however, he reported that his self-esteem had improved. Measurement of subjective effects is critical, as they are often related to patient goals and form a significant part of the recovery model (Mueser et al., 2013).
Acceptability of the treatment package
The new computerised cognitive remediation treatment was demonstrated to be acceptable to participants; they presented themselves for therapy three times per week for approximately one hour at each visit, and each completed 40 sessions of therapy. Few sessions were missed, and participants appeared motivated to attend. Importantly, although therapeutic response differed across participants, all participants reported that their cognitive functioning had improved.
Limitations
Several methodological limitations must be considered when interpreting the results of this feasibility study. First, the lack of control group or baseline control period prevents conclusions about the effectiveness of therapy. Observed improvements may be attributable to non-specific effects rather than to cognitive remediation therapy. Cases all continued treatment as usual, including medication and medical appointments with their respective psychiatrists, and such interventions may have influenced response to therapy. Second, the same neuropsychological tests were administered at baseline and at post-treatment. In the absence of a control group, it is impossible to rule out the possibility that practice effects enhanced the effect of therapy on neuropsychological domains. In particular, tasks designed to measure executive functioning are meant to be novel to participants, which was not the case at post-treatment. Third, our small sample size does not permit generalisations concerning cases’ improvements on visual episodic memory. Finally, the participants in this study were identified by their treating psychiatrist and were motivated to participate in the study; as such, they may not be representative of the larger population of schizophrenia patients. Despite these limitations, this study yielded encouraging data that will inform the design of a randomised controlled trial; such a trial will permit conclusions concerning the effectiveness of therapy.
CONCLUSION
The consideration of baseline characteristics in young adults with schizophrenia allowed us to target key clinical features, increasing the likelihood of a positive response to cognitive remediation. The analysis of strategy use during therapy and neuropsychological evaluations may highlight the nature of the improvement. It has been demonstrated that early interventions in first-episode patients enhances outcome (Tandon et al., 2010), and further studies of young adults with schizophrenia are therefore warranted.
REFERENCES
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders: Diagnostic criteria from DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Barlati S., Deste G., De Peri L., Ariu C., Vita A. Cognitive remediation in schizophrenia: Current status and future perspectives. Schizophrenia Research and Treatment. 2013:12. doi: 10.1155/2013/156084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayard S., Capdevielle D., Boulenger J-P, Raffard S. Dissociating self-reported cognitive complaint from clinical insight in schizophrenia. European Psychiatry. 2009;24(4):251–258. doi: 10.1016/j.eurpsy.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Bertelsen M., Jeppesen P., Petersen L., Thorup A., Øhlenschlæger J., Quach P. L., Nordentoft M. Course of illness in a sample of 265 patients with first-episode psychosis—five-year follow-up of the Danish OPUS trial. Schizophrenia Research. 2009;107(2):173–178. doi: 10.1016/j.schres.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Bora E. Developmental lag and course of cognitive deficits from the premorbid to postonset period in schizophrenia. The American Journal of Psychiatry. 2014;171(3):369. doi: 10.1176/appi.ajp.2013.13091283. [DOI] [PubMed] [Google Scholar]
- Boyer L., Richieri R., Guedj E., Faget-Agius C., Loundou A., Llorca P-M., Lançon C. Validation of a functional remission threshold for the functional remission of general schizophrenia (FROGS) scale. Comprehensive Psychiatry. 2013;54(7):1016–1022. doi: 10.1016/j.comppsych.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Conners K. Continuous performance test II Odessa. North Tonwanda, NY: Multi-Health Systems; 1999. [Google Scholar]
- Culbertson W., Zillmer E. The tower of London DX (TOLDX) manual. North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- Deckersbach T., McMurrich S., Ogutha J., Savage C. R., Sachs G., Rauch S. L. Characteristics of non-verbal memory impairment in bipolar disorder: The role of encoding strategies. Psychological Medicine. 2004;34(5):823–832. doi: 10.1017/S0033291703001685. [DOI] [PubMed] [Google Scholar]
- Delis D. C., Kaplan E., Kramer J. H. Delis-Kaplan executive function system (D-KEFS) San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- Delis D. C., Kramer J., Kaplan E., Ober B. CVLT-II. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- Eack S. M., Hogarty G. E., Cho R. Y., Prasad K. M. R., Greenwald D. P., Hogarty S. S., Keshavan M. S. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: Results from a 2-year randomized controlled trial. Archives of General Psychiatry. 2010;67(7):674–682. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. F. What are the functional consequences of neurocognitive deficits in schizophrenia? The American Journal of Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Heaton R. K., Chelune G. J., Talley J. L., Kay G. G., Curtiss G. Wisconsin card sorting test: Manual revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Heaton R. K., Temkin N., Dikmen S., Avitable N., Taylor M. J., Marcotte T. D., Grant I. Detecting change: A comparison of three neuropsychological methods, using normal and clinical samples. Archives of Clinical Neuropsychology. 2001;16(1):75–91. doi: 10.1016/S0887-6177(99)00062-1. [DOI] [PubMed] [Google Scholar]
- Heinrichs R. W., Bury A. Copying strategies and memory on the complex figure test in psychiatric patients. Psychological Reports. 1991;69(1):223–226. doi: 10.2466/PR0.69.5.223-226. [DOI] [PubMed] [Google Scholar]
- Jacobson N. S., Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59(1):12–19. doi: 10.1037/0022-006X.59.1.12. [DOI] [PubMed] [Google Scholar]
- Kay S. R., Opler L. A., Lindenmayer J-P. The positive and negative syndrome scale (PANSS): Rationale and standardisation. The British Journal of Psychiatry. 1989;155(Suppl 7):59–65. [PubMed] [Google Scholar]
- Lecomte T., Corbière M., Laisné F. Investigating self-esteem in individuals with schizophrenia: Relevance of the Self-Esteem rating scale-short Form. Psychiatry Research. 2006;143(1):99–108. doi: 10.1016/j.psychres.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Leucht S., Davis J., Engel R., Kissling W., Kane J. Definitions of response and remission in schizophrenia: Recommendations for their use and their presentation. Acta Psychiatrica Scandinavica. 2009;119(s438):7–14. doi: 10.1111/j.1600-0447.2008.01308.x. [DOI] [PubMed] [Google Scholar]
- Lezak M. D., Howieson D. B., Bigler E. D., Tranel D. Neuropsychological assessment. 5th ed. New York, USA: Oxford University Press; 2012. [Google Scholar]
- Maziade M., Rouleau N., Mérette C., Cellard C., Battaglia M., Marino C., Roy M-A. Verbal and visual memory impairments among young offspring and healthy adult relatives of patients with schizophrenia and bipolar disorder: Selective generational patterns indicate different developmental trajectories. Schizophrenia Bulletin. 2011;37(6):1218–1228. doi: 10.1093/schbul/sbq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk S. R., Twamley E. W., Sitzer D. I., McHugo G. J., Mueser K. T. A meta-analysis of cognitive remediation in schizophrenia. The American Journal of Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately R. I., Giuliano A. J., Goff K. P., Faraone S. V., Seidman L. J. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Meyers J., Meyers K. Rey complex figure test and recognition trial (RCFT) Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- Milev P., Ho B-C., Arndt S., Andreasen N. C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: A longitudinal first-episode study with 7-year follow-up. The American Journal of Psychiatry. 2005;162(3):495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Mueser K. T., Deavers F., Penn D. L., Cassisi J. E. Psychosocial treatments for schizophrenia. Annual Review of Clinical Psychology. 2013;9(1):465–497. doi: 10.1146/annurev-clinpsy-050212-185620. [DOI] [PubMed] [Google Scholar]
- Niendam T. A., Bearden C. E., Johnson J. K., McKinley M., Loewy R., O'Brien M., Cannon T. D. Neurocognitive performance and functional disability in the psychosis prodrome. Schizophrenia Research. 2006;84(1):100–111. doi: 10.1016/j.schres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Reeder C., Harris V., Pickles A., Patel A., Cella M., Wykes T. Does change in cognitive function predict change in costs of care for people with a schizophrenia diagnosis following cognitive remediation therapy? Schizophrenia Bulletin. 2014;40(6):1472–1481. doi: 10.1093/schbul/sbu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder C., Wykes T. Computerised interactive remediation of cognition—Interactive training for schizophrenia (CIRCUITS) London: Kings College London; 2010. [Google Scholar]
- Savage C. Neuropsychology of OCD: research findings and treatment implications. Mosby: St Louis; 1998. [Google Scholar]
- Schaefer J., Giangrande E., Weinberger D. R., Dickinson D. The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizophrenia Research. 2013 doi: 10.1016/j.schres.2013.07.009. No Pagination Specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorr J. S., Delis D. C., Massman P. J. Memory for the rey-osterrieth figure: Perceptual clustering, encoding, and storage. Neuropsychology. 1992;6(1):43–50. doi: 10.1037/0894-4105.6.1.43. [DOI] [Google Scholar]
- Simonsen C., Sundet K., Vaskinn A., Birkenaes A. B., Engh J. A., Færden A., Melle I. 2009 Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophrenia Bulletin, sbp034.
- Snitz B. E., MacDonald A. W., III, Carter C. S. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staring A. B. P., Van der Gaag M., Van den Berge M., Duivenvoorden H. J., Mulder C. L. Stigma moderates the associations of insight with depressed mood, low self-esteem, and low quality of life in patients with schizophrenia spectrum disorders. Schizophrenia Research. 2009;115(2–3):363–369. doi: 10.1016/j.schres.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Stip E., Caron J., Renaud S., Pampoulova T., Lecomte Y. Exploring cognitive complaints in schizophrenia: The subjective scale to investigate cognition in schizophrenia. Comprehensive Psychiatry. 2003;44(4):331–340. doi: 10.1016/S0010-440X(03)00086-5. [DOI] [PubMed] [Google Scholar]
- Strauss E., Sherman E. M., Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- Tandon R., Nasrallah H. A., Keshavan M. S. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophrenia Research. 2009;110(1–3):1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Tandon R., Nasrallah H. A., Keshavan M. S. Schizophrenia, “just the facts” 5. Treatment and prevention past, present, and future. Schizophrenia Research. 2010;122(1–3):1–23. doi: 10.1016/j.schres.2010.05.025. http://dx.doi.org/10.1016/j.schres.2010.05.025 [DOI] [PubMed] [Google Scholar]
- Tiffin J. Purdue pegboard examiner manual. Rosemont, IL: London House; 1968. [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E., Donaldson W., editors. Organization of memory. Oxford, UK: Academic Press; 1972. pp. 382–402. [Google Scholar]
- Ueland T., Rund B. A controlled randomized treatment study: The effects of a cognitive remediation program on adolescents with early onset psychosis. Acta Psychiatrica Scandinavica. 2004;109(1):70–74. doi: 10.1046/j.0001-690X.2003.00239.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-III manual. New York: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 4th ed. San Antonio, TX: The Psychological Corporation; 2005. [Google Scholar]
- Wykes T., Huddy V., Cellard C., McGurk S. R., Czobor P. A meta-analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. The American Journal of Psychiatry. 2011;168(5):472–485. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Wykes T., Reeder C. Cognitive remediation therapy for schizophrenia: Theory and practice. London: Routledge; 2005. [Google Scholar]
- Wykes T., Reeder C., Landau S., Matthiasson P., Haworth E., Hutchinson C. Does age matter? Effects of cognitive rehabilitation across the age span. Schizophrenia Research. 2009;113(2–3):252–258. doi: 10.1016/j.schres.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Wykes T., Spaulding W. D. Thinking about the future cognitive remediation therapy—What works and could we do better? Schizophrenia Bulletin. 2011;37(Suppl 2):S80–S90. doi: 10.1093/schbul/sbr064. [DOI] [PMC free article] [PubMed] [Google Scholar]


