Abstract
Background
After successful utilization of diffusion tensor imaging (DTI) in detecting brain pathologies, it is now being examined for use in the detection of peripheral neuropathies. The aim of this meta-analysis was to evaluate the diagnostic potentials of DTI in carpal tunnel syndrome (CTS).
Material/Methods
The literature search was performed in multiple electronic databases using a keyword search and final selection of the studies was based on predetermined inclusion and exclusion criteria. We performed a meta-analyses of mean differences in fractional anisotropy (FA) and apparent diffusion coefficient (ADC) between CTS patient and healthy subjects. Publication bias detection was done with Begg’s test and sensitivity analyses were performed to explore the source/s of higher heterogeneity and the authenticity of results.
Results
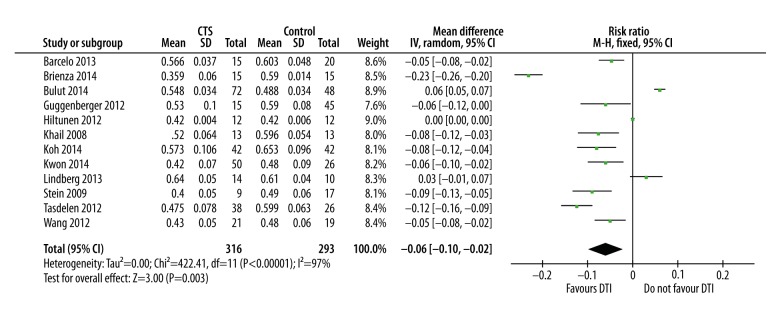
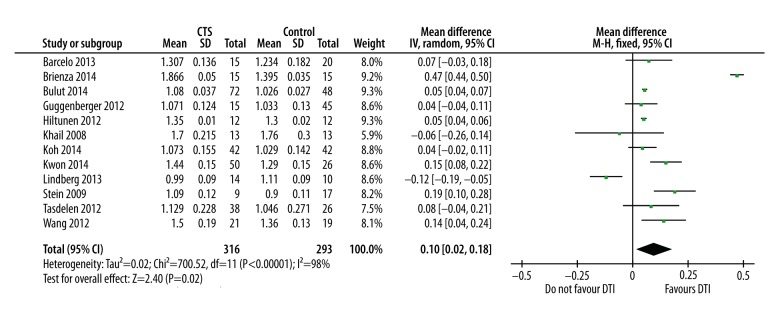
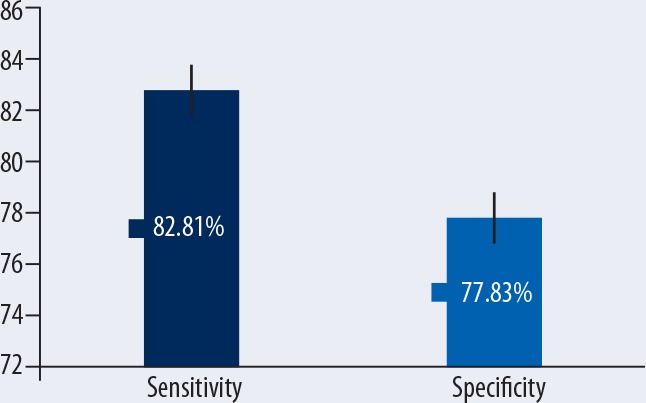
FA was significantly lower in CTS patients in comparison with healthy subjects (mean and the difference [95% confidence interval] was −0.06 [−0.10, −0.02] (p=0.003). The ADC was significantly higher in CTS patients (mean difference [95% CI] was 0.10 [0.02, 0.18], p=0.02). Overall sensitivity of FA-based diagnosis was 82.82%, with 77.83% specificity.
Conclusions
DTI can be a valuable tool in diagnosing CTS.
MeSH Keywords: Carpal Tunnel Syndrome, Diagnosis, Diffusion Tensor Imaging, Magnetic Resonance Imaging, Peripheral Nervous System Diseases
Background
Diffusion tensor imaging (DTI) is an advanced form of magnetic resonance imaging (MRI) that reads diffusion of water in tissues in accordance with the microstructural architecture of tissues [1]. The cytoarchitecture of tissues changes from developmental to aged stage, as well as in the pathological conditions. This physical property has provided the basis for the development of diffusion-weighted MRI methods, of which DTI is a highly sensitive and potentially powerful technique to detect the effects of disease and aging on tissue microstructure [2].
Diffusion tensor imaging extracts and characterizes diffusion patterns to provide exquisite details of tissue microstructure and fiber tracking. Because pathological tissue microstructure differs from normal, DTI can also provide quantitative information to differentiate between healthy and pathological states [3]. To quantify the weighted diffusion characterizing the microstructure of the tissues, the 2 most common measures of the diffusion tensor are the trace and anisotropy. Mean diffusivity and apparent diffusion coefficient (ADC) are widely used in DTI to measure compactness of the tissues and intercellular space and provide estimates independent of fiber directionality [4,5]. The fractional anisotropy (FA) estimates the coherence of oriented structures such as myelinated nerve fibers. It is the extent to which water within a voxel diffuses preferentially along 1 axis rather than exhibiting isotropic diffusion (i.e., diffusing equally along all axes) [6,7]. Both these measures complement each other to attain a highly sensitive 3 dimensional diffusion ellipsoid tensor model called DTI [8].
Diffusion tensor imaging has been used to study the white matter architecture and integrity of the normal tissues and pathological conditions, including multiple sclerosis, Alzheimer’s disease, mild cognitive impairment, leukoaraiosis, cervical spondylotic myelopathy, epilepsy, schizophrenia, and aging [9–15]. Furthermore, DTI potentials are also studied in peripheral nervous system in healthy subjects and in patients with peripheral neuropathies.
Carpal tunnel syndrome (CTS) is the most common entrapment peripheral neuropathy which is caused by compression of the median nerve at the wrist [16]. Usually, diagnosis is made either with electrophysiological indices or with non-invasive high-resolution ultrasonography. Electrophysiological studies are associated with painful procedure, discomfort, and incidence of considerably higher false negatives and false positives [17,18]. Ultrasonographic diagnosis is less expensive and quicker but its diagnostic strength is lower in the elderly [19,20]. A number of studies have reported that DTI has shown promising results in diagnosing CTS, but results are not always consistent. The present study systematically reviews trials that used DTI to explore its potentials in diagnosing carpal tunnel syndrome (CTS) and performs a meta-analysis by evaluating the most usual quantitative measures to attain updated evidence regarding the use of DTI for CTS.
Material and Methods
This study was carried out by following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [21].
Literature search
The electronic databases EBSCO, Embase, Google Scholar, Ovid SP, PubMed, Scopus, and Web of Science were used for literature search. The major medical subject headings (MeSH) and keywords – carpal tunnel syndrome, peripheral neuropathy, diffusion tensor imaging, fractional anisotropy, diffusivity, diffusion coefficient, and diffusion-weighted MRI – were used in various logical combinations and phrases. The search encompassed original research papers published before August 2015.
Inclusion and exclusion criteria
The inclusion criteria were: a) trials recruiting CTS patients to study the diagnostic effectiveness of DTI by comparing it with normal controls; and b) measured and provided values of fractional anisotropy and apparent diffusion coefficient. Exclusion criteria were: a) studies examining the effectiveness of DTI either in CTS patients or in healthy subject but not having both the arms or reporting the outcomes of interest in a single arm only; and b) studies providing relevant information without numeric data.
Data extraction, synthesis, and statistical analyses
During data extraction, numerical values regarding the study endpoints, outcome measures, and outcomes, demographic, and clinical characteristics of the patients and healthy subjects and other relevant data were obtained from identified papers and synthesized on datasheets. Eggers and Begg’s tests were performed to estimate the publication bias and fill and trim method was applied to assess the scope of missing studies.
Meta-analyses of inverse variance weighted mean differences were carried out by using RevMan software (Version 5.3.2; Cochrane Collaboration) under the random-effects model. For this purpose, the mean and standard deviation values of the variables of interest were used to calculate individual effects sizes and then an overall effect size was achieved. The significance of differences between CTS patients and healthy controls in DTI indices (FA and ADC) were tested with a 2-tailed z-test. Between-studies statistical heterogeneity was tested by I2 index. Sensitivity analyses were performed to test the authenticity of the results. Meta-regression analyses were performed in Stata (version 12) to identify the effect of age and sex on DTI outcomes.
Results
Twelve studies [22–33] were selected using the inclusion and exclusion criteria. A flowchart of study screening and selection process is given in Figure 1. Significant publication bias was detected by Begg’s test. A funnel plot depicting publication bias and possible missing studies as estimated with fill and trim method is shown in Figure 2. Overall, the included studies recruited 316 CTS patients and 293 healthy subjects. Age of CTS patients and healthy controls was 48.92±9.52 years and 43.56±8.24 years, respectively. Proportion of males among CTS patients was 24.28±10.43% and in healthy subjects it was 27.67±11.1%.
Figure 1.
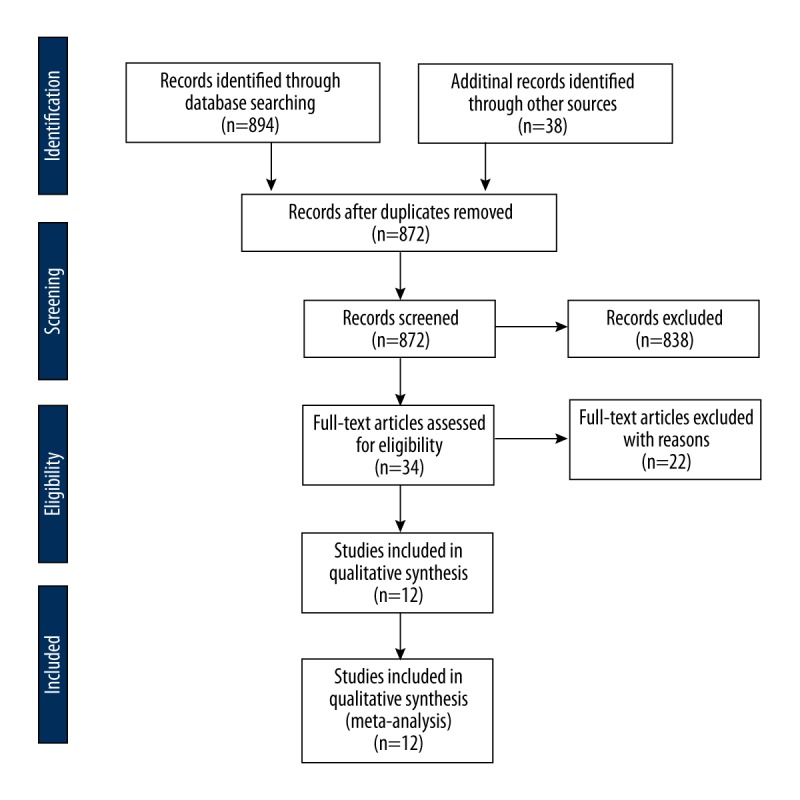
A PRISMA flowchart of study screening and selection.
Figure 2.
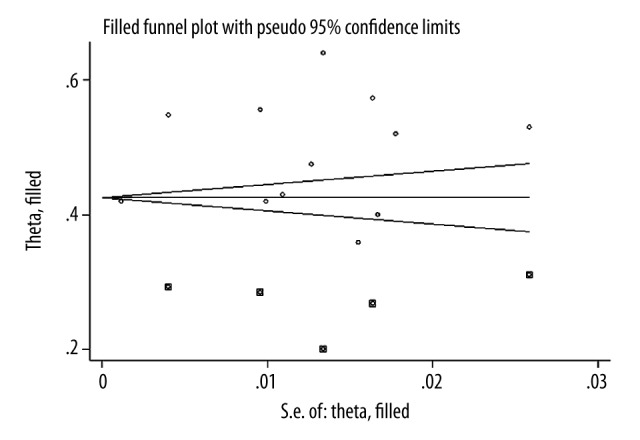
Funnel plot showing a significant publication bias (Begg’s test) and speculated missing studies (square dots) as assessed with trim and fill method.
Fractional anisotropy was significantly lower in CTS patients in comparison with their healthy counterparts. Mean difference and [95% confidence interval; CI] was −0.06 [−0.10, −0.02]; p=0.003 (Figure 3). Statistical heterogeneity was higher (I2=97%) in the overall meta-analysis but sensitivity analysis revealed that I2 could be reduced up to a level of 52% without changing the overall effect size.
Figure 3.
Forest graph showing significantly lower FA in CTS patients in comparison with controls as an overall effect size of 12 studies.
The apparent diffusion coefficient was significantly higher in CTS patients. Mean difference and [95% CI] was 0.10 [0.02, 0.18]; p=0.02 (Figure 4). Statistical heterogeneity was higher (I2=98%) in the overall meta-analysis but sensitivity analysis revealed that I2 could be reduced up to a level of 55% without changing the overall effect size.
Figure 4.
Forest graph showing significantly higher ADC in CTS patients in comparison with controls as an overall effect size of 12 studies.
Six studies also mentioned sensitivity and specificity of DTI in diagnosing CTS (Table 1). Overall sensitivity of FA-based diagnosis was 82.82% with 77.83% specificity. In these 6 studies, the FA sensitivity ranged from 72% to 94.4% and FA specificity ranged from 67% to 91%. However, the correlation coefficient between the sensitivities and specificities of the included studies was 0.039.
Table 1.
Sensitivities and specificities of DTI fractional anisotropy in diagnosing CTS observed in 6 studies.
| Study | Cut-off | Sensitivity | Specificity | |
|---|---|---|---|---|
| Barcelo et al. 2013 | – | 93.0% | 91.0% |
 Overall FA sensitivity and specificity in diagnosing CTS at various cut-offs |
| Bulut et al. 2014 | 0.532 | 94.4% | 70.8% | |
| Guggenberger et al. 2012 | 0.47 | 83.0% | 67.0% | |
| Koh et al. 2014 | 0.536 | 73.8% | 76.2% | |
| Kwon et al. 2014 | 0.44 | 72.0% | 82.0% | |
| Tasdelen et al. 2012 | 0.554 | 80.7% | 80.0% |
Results of meta-regression analyses revealed that neither age (coefficient: 0.007; p=0.131) nor sex (coefficient: −0.00013; p=0.960) had any significant relationship with FA or with ADC (coefficient: −0.00692; p=0.674 for age and coefficient: −0.0056; p=0.515 for sex).
Discussion
In the present meta-analysis, we found that in comparison with healthy control subjects, CTS patients have significantly lower fractional anisotropy and significantly higher apparent diffusion coefficient when subjected to diffusion tensor imaging. Sensitivity and specificity of fractional anisotropy in distinguishing CTS patients from healthy subjects were 83% and 78%, respectively. These results reveal the potentials of DTI in the diagnosis of chronic nerve compression characteristic of CTS.
Carpal tunnel syndrome is a peripheral neuropathy, with symptoms of pain, numbness, and paresthesia in the hand due to blockade of median nerve conduction that develops by the partial deafferentation after the compression of the median nerve in the carpal tunnel, and is also associated with altered function of the entire somatosensory system, from the peripheral nerves to the brain [34,35]. CTS affects about 2.7% of the general population and has a multifactorial etiology with risk factors such as age, sex, obesity, diabetes, thyroid conditions, rheumatoid arthritis, gout, smoking, late pregnancy, and rapid weight loss [36,37]. Diagnosis of CTS is usually based on electrophysiology and sonography but both have limitations; therefore, the search for more reliable methods continues. Although, DTI had been used for brain pathologies for years [9–15], it has only recently being used to detect pathological states in the peripheral nerves [38–40], and even more recently, interest has developed in exploring its use in CTS diagnosis [22–33].
Pathophysiologically, when a nerve is chronically compressed, local venous compression causes intrafascicular edema. Excessive water in the tissues and extracellular matrix creates an isotropic environment that leads to decreased FA [31]. Indeed, nerve fibers start undergoing demyelination due to mechanical tension created by nerve compression. This initially occurs at the paranodal site of compression and then progresses throughout the internodal segment, causing a reduction in the random movement of water molecules and a consequent reduction in FA [41].
Significant correlations are observed between DTI indices and electrophysiological indices. Lower FA values and higher ADC values are found in patients with more severe CTS according to electrophysiological indices, indicating a strong association between anatomical alterations and functional changes [23,33]. The usefulness of DTI has also been reported in a rodent models used to examine the Wallerian degeneration and peripheral nerve regeneration; the FA values correlated well with histological and functional changes observed when the sciatic nerve is transected [42,43]. A close correlation has also been observed between DIT and high-resolution ultrasonography in identifying normal nerve fascicles within or around peripheral nerve sheath tumors [44]. Moreover, ultrasound elastic tensor imaging, which assesses the shear wave speed, is demonstrated to have stronger correlation (r2=0.81, p<0.0001) with DTI in detecting the myocardial fiber orientation [45].
Tasdelen et al. [32] reported a significant positive correlation between age and ADC and a negative correlation between age and FA. However, in the present study, meta-regression analyses could not find any significant relationship between age or sex and DTI indices.
The present study identifies ADC as a potentially useful measure of diffusion tensor by virtue of the overall effect size achieved herein, but some of the included studies found that, although, ADC values were positively correlated with severity of damage, association strength was not as strong as with FA [23,31,33]. Kabakci et al. [46] also observed this finding while studying normative diffusion values in the median nerve. Because of this observation, it was suggested that FA is a more reliable indicator than ADC in the diagnosis of CTS [22]. Khalil et al. [27] reported ADC to be non-significantly different in CTS patients in comparison with controls. Lindberg et al. [30] found reduced ADC in the median nerve in recurrent CTS patients, which is also similar to the outcomes reported by Hiltunen et al. [26]. However, both of these studies also reported non-significantly different FA in CTS and control subjects. In the present meta-analysis, the overall specificity and sensitivity of FA in diagnosing CTS were 83% and 78%, respectively, but there was no correlation between the sensitivities and specificities of these 6 studies (r=0.039).
Further research using larger datasets may be needed to refine the evidence. A number of factors need to be considered while analyzing the DTI measurements. Guggenberger et al. [25] reported a decrease in FA from proximal to distal locations. Kabakci et al. [46] observed that FA values at the flexor retinaculum differed from values at the forearm and wrist. Guggenberger et al. [47] compared 3.0 T MR scanners from different vendors to assess the agreement of FA and ADC values of the median nerve and found significant differences; therefore, they suggested that larger studies can minimize such differences.
Conclusions
The most widely used indices of diffusion tensor imaging are the apparent diffusion coefficient and fractional anisotropy. In carpal tunnel syndrome patients, significantly lower fractional anisotropy and significantly higher apparent diffusion coefficient were observed in this meta-analysis, which favors the potential utility of DTI in CTS patients. However, more studies are required to refine this evidence.
Footnotes
Source of support: Self financing
References
- 1.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–67. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–29. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis – A technical review. NMR Biomed. 2002;5:456–67. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu C. The basis of anisotropic water diffusion in the nervous system – A technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 5.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;7–8:333–44. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 6.Cercignani M, Horsfield MA. The physical basis of diffusion weighted MRI. J Neurol Sci. 2001;186(Suppl 1):S11–14. doi: 10.1016/s0022-510x(01)00486-5. [DOI] [PubMed] [Google Scholar]
- 7.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–39. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Jones DK, Lythgoe D, Horsfield MA, et al. Characterization of white matter damage in ischemic leukoaraiosis with diffusion tensor MRI. Stroke. 1999;30(2):393–97. doi: 10.1161/01.str.30.2.393. [DOI] [PubMed] [Google Scholar]
- 10.Assaf Y, Cohen Y. Assignment of the water slowdiffusing component in the central nervous system using q-space diffusion MRS: implications for fiber tract imaging. Magn Reson Med. 2000;43:191–99. doi: 10.1002/(sici)1522-2594(200002)43:2<191::aid-mrm5>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Bammer R, Augustin M, Strasser-Fuchs S, et al. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Mag Res Med. 2000;44:583–91. doi: 10.1002/1522-2594(200010)44:4<583::aid-mrm12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Eichler FS, Itoh R, Barker PB, et al. Proton MR spectroscopic and diffusion tensor brain MR imaging in X-linked adrenoleukodystrophy: initial experience. Radiology. 2002;225(1):245–52. doi: 10.1148/radiol.2251011040. [DOI] [PubMed] [Google Scholar]
- 13.Lu S, Ahn D, Johnson G, Law M, et al. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology. 2004;232:221–28. doi: 10.1148/radiol.2321030653. [DOI] [PubMed] [Google Scholar]
- 14.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108(1–3):3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Ellingson BM, Salamon N, Woodworth DC, Holly LT. Correlation between degree of subvoxel spinal cord compression measured with super-resolution tract density imaging and neurological impairment in cervical spondylotic myelopathy. J Neurosurg Spine. 2015;22(6):631–38. doi: 10.3171/2014.10.SPINE14222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mondelli M, Filippou G, Gallo A, Frediani B. Diagnostic utility of ultrasonography versus nerve conduction studies in mild carpal tunnel syndrome. Arthritis Rheum. 2008;59:357–66. doi: 10.1002/art.23317. [DOI] [PubMed] [Google Scholar]
- 17.Nathan PA, Keniston RC, Meadows KD, Lockwood RS. Predictive value of nerve conduction measurements at the carpal tunnel. Muscle Nerve. 1993;16:1377–82. doi: 10.1002/mus.880161217. [DOI] [PubMed] [Google Scholar]
- 18.Lew HL, Date ES, Pan SS, et al. Sensitivity, specificity, and variability of nerve conduction velocity measurements in carpal tunnel syndrome. Arch Phys Med Rehabil. 2005;86:12–16. doi: 10.1016/j.apmr.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Filippucci E, Iagnocco A, Meenagh G, et al. Ultrasound imaging for the rheumatologist II. Ultrasonography of the hand and wrist. Clin Exp Rheumatol. 2006;24:118–22. [PubMed] [Google Scholar]
- 20.Miwa T, Miwa H. Ultrasonography of carpal tunnel syndrome: clinical significance and limitations in elderly patients. Intern Med. 2011;50(19):2157–61. doi: 10.2169/internalmedicine.50.5771. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barcelo C, Faruch M, Lapègue F, et al. 3-T MRI with diffusion tensor imaging and tractography of the median nerve. Eur Radiol. 2013;23(11):3124–30. doi: 10.1007/s00330-013-2955-2. [DOI] [PubMed] [Google Scholar]
- 23.Brienza M, Pujia F, Colaiacomo MC, et al. 3T diffusion tensor imaging and electroneurography of peripheral nerve: a morphofunctional analysis in carpal tunnel syndrome. J Neuroradiol. 2014;41(2):124–30. doi: 10.1016/j.neurad.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Bulut HT, Yildirim A, Ekmekci B, Gunbey HP. The diagnostic and grading value of diffusion tensor imaging in patients with carpal tunnel syndrome. Acad Radiol. 2014;21(6):767–73. doi: 10.1016/j.acra.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Guggenberger R, Markovic D, Eppenberger P, et al. Assessment of median nerve with MR neurography by using diffusion-tensor imaging: normative and pathologic diffusion values. Radiology. 2012;265(1):194–203. doi: 10.1148/radiol.12111403. [DOI] [PubMed] [Google Scholar]
- 26.Hiltunen J, Kirveskari E, Numminen J, et al. Pre- and post-operative diffusion tensor imaging of the median nerve in carpal tunnel syndrome. Eur Radiol. 2012;22(6):1310–19. doi: 10.1007/s00330-012-2381-x. [DOI] [PubMed] [Google Scholar]
- 27.Khalil C, Hancart C, Le Thuc V, et al. Diffusion tensor imaging and tractography of the median nerve in carpal tunnel syndrome: preliminary results. Eur Radiol. 2008;18(10):2283–91. doi: 10.1007/s00330-008-0971-4. [DOI] [PubMed] [Google Scholar]
- 28.Koh SH, Kwon BC, Park C, et al. A comparison of the performance of anatomical MRI and DTI in diagnosing carpal tunnel syndrome. Eur J Radiol. 2014;83(11):2065–73. doi: 10.1016/j.ejrad.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Kwon BC, Koh SH, Hwang SY. Optimal parameters and location for diffusion-tensor imaging in the diagnosis of carpal tunnel syndrome: a prospective matched case-control study. Am J Roentgenol. 2015;204(6):1248–54. doi: 10.2214/AJR.14.13371. [DOI] [PubMed] [Google Scholar]
- 30.Lindberg PG, Feydy A, Le Viet D, et al. Diffusion tensor imaging of the median nerve in recurrent carpal tunnel syndrome – initial experience. Eur Radiol. 2013;23(11):3115–23. doi: 10.1007/s00330-013-2986-8. [DOI] [PubMed] [Google Scholar]
- 31.Stein D, Neufeld A, Pasternak O, et al. Diffusion tensor imaging of the median nerve in healthy and carpal tunnel syndrome subjects. J Magn Reson Imaging. 2009;29(3):657–62. doi: 10.1002/jmri.21553. [DOI] [PubMed] [Google Scholar]
- 32.Taşdelen N, Gürses B, Kiliçkesmez Ö, et al. Diffusion tensor imaging in carpal tunnel syndrome. Diagn Interv Radiol. 2012;18(1):60–66. doi: 10.4261/1305-3825.DIR.3994-10.1. [DOI] [PubMed] [Google Scholar]
- 33.Wang CK, Jou IM, Huang HW, et al. Carpal tunnel syndrome assessed with diffusion tensor imaging: comparison with electrophysiological studies of patients and healthy volunteers. Eur J Radiol. 2012;81(11):3378–83. doi: 10.1016/j.ejrad.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Maeda Y, Kettner N, Sheehan J, et al. Altered brain morphometry in carpal tunnel syndrome is associated with median nerve pathology. Neuroimage Clin. 2013;2:313–19. doi: 10.1016/j.nicl.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopec J, Gadek A, Drozdz M, et al. Carpal tunnel syndrome in hemodialysis patients as a dialysis-related amyloidosis manifestation-incidence, risk factors and results of surgical treatment. Med Sci Monit. 2011;17(9):CR505–9. doi: 10.12659/MSM.881937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodavula VKR, Burke FD, Dubin NH, et al. A prospective, longitudinal outcome study of patients with carpal tunnel surgery and the relationship of body mass index. Hand. 2007;2(1):27–33. doi: 10.1007/s11552-006-9019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komurcu HF, Kilic S, Anlar O. Relationship of age, body mass index, wrist and waist circumferences to carpal tunnel syndrome severity. Neurol Med Chir (Tokyo) 2014;54(5):395–400. doi: 10.2176/nmc.oa2013-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skorpil M, Karlsson M, Nordell A. Peripheral nerve diffusion tensor imaging. Magn Reson Imaging. 2004;22:743–45. doi: 10.1016/j.mri.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 39.Hiltunen J, Suortti T, Arvela S, et al. Diffusion tensor imaging and tractography of distal peripheral nerves at 3 T. Clin Neurophysiol. 2005;116:2315–23. doi: 10.1016/j.clinph.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Meek MF, Stenekes MW, Hoogduin HM, Nicolai JP. In vivo three-dimensional reconstruction of human median nerves by diffusion tensor imaging. Exp Neurol. 2006;198:479–82. doi: 10.1016/j.expneurol.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Pham K, Gupta R. Understanding the mechanism of entrapment neuropathies. Review article. Neurosurg Focus. 2009;26(2):E7. doi: 10.3171/FOC.2009.26.2.E7. [DOI] [PubMed] [Google Scholar]
- 42.Takagi T, Nakamura M, Yamada M, et al. Visualization of peripheral nerve degeneration and regeneration: monitoring with diffusion tensor tractography. Neuroimage. 2009;44(3):884–92. doi: 10.1016/j.neuroimage.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 43.Lehmann HC, Zhang J, Mori S, Sheikh KA. Diffusion tensor imaging to assess axonal regeneration in peripheral nerves. Exp Neurol. 2010;223(1):238–44. doi: 10.1016/j.expneurol.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon NG, Cage T, Narvid J, et al. High-resolution ultrasonography and diffusion tensor tractography map normal nerve fascicles in relation to schwannoma tissue prior to resection. J Neurosurg. 2014;120(5):1113–17. doi: 10.3171/2014.2.JNS131975. [DOI] [PubMed] [Google Scholar]
- 45.Lee WN, Larrat B, Pernot M, et al. Ultrasound elastic tensor imaging: comparison with MR diffusion tensor imaging in the myocardium. Phys Med Biol. 2012;57(16):5075–95. doi: 10.1088/0031-9155/57/16/5075. [DOI] [PubMed] [Google Scholar]
- 46.Kabakci N, Gurses B, Firat Z, et al. Diffusion tensor imaging and tractography of median nerve: normative diffusion values. Am J Roentgenol. 2007;189:923–27. doi: 10.2214/AJR.07.2423. [DOI] [PubMed] [Google Scholar]
- 47.Guggenberger R, Nanz D, Bussmann L, et al. Diffusion tensor imaging of the median nerve at 3.0 T using different MR scanners: agreement of FA and ADC measurements. Eur J Radiol. 2013;82(10):e590–96. doi: 10.1016/j.ejrad.2013.05.011. [DOI] [PubMed] [Google Scholar]