Abstract
Background
To observe the effect of QiShenYiQi pill (QSYQ) on experimental autoimmune myocarditis rats, and to explore its mechanism of action.
Material/methods
Lewis rats underwent the injection of myocardial myosin mixed with Freund’s complete adjuvant were randomized into 3 groups: model, valsartan, and QSYQ groups. Rats injected with phosphate-buffered saline (PBS) mixed with Freund’s complete adjuvant were used as the control group. Rats were euthanized at 4 and 8 weeks, and we weighed rat body mass, heart mass, and left ventricular mass. Myocardium sections were stained with hematoxylin and eosin (H&E) and Masson trichrome. Myocardial TGF-β1 and CTGF protein expression was detected by immunohistochemistry, and myocardial TGF-β1 and CTGF mRNA expression was detected by real-time qPCR.
Results
QSYQ reduced HMI and LVMI, as well as the histological score of hearts and CVF, which further decreased over time, and its effect was significantly greater than that of valsartan at 4 and 8 weeks. After 4 weeks, QSYQ inhibited the protein and mRNA expression of TGF-β1 and CTGF, and its effect on lowering CTGF was significantly greater than that of valsartan. In addition, after 8 weeks, QSYQ also inhibited the protein and mRNA expression of CTGF, whereas there was no significant difference in the expression of myocardial TGF-β1.
Conclusions
This study provides evidence that QSYQ can improve cardiac remodeling of experimental autoimmune myocarditis rats. It also effectively improved the degree of myocardial fibrosis, which is related to the mechanism of regulation of TGF-β1 CTGF.
MeSH Keywords: Cardiac Myosins, Endomyocardial Fibrosis, Myocarditis
Background
Myocarditis is one of the main heart diseases, especially the viral myocarditis (VMC). As the disease spectrum changes and virus are spreading, viral disease increases. VMC is a common heart disease, the incidence of which tends to further increase in the 21st century. Heart disease statistics from Shanghai, China show that the number of VMC-hospitalized cases admitted has risen from the 10th most common cause of heart disease in 1940s to the 4th in the 1990s [1]. Generally, about 5% of patients have heart involvement, especially when Coxsackie virus, influenza virus, and polio virus are prevalent, and the percentage may increase to more than 10% in certain areas [2]. Many kinds of anti-myocardial autoantibodies can be found in VMC patients; among these antibodies, anti-cardiac myosin heavy chain antibodies, anti-myocardial mitochondrial ADP/ATP carrier protein antibody, anti-β1 receptor antibody, and anti-cholinergic receptor antibody have higher sensitivity, which suggests that the autoimmune response participates in the inflammation of VMC [3]. Some scholars suggested that VMC can be divided into 3 stages: viral replication, immune activation, and dilated cardiomyopathy [4]. Therefore, in the early stage, virus directly causes myocardial injury and dysfunction, and the later stage is secondary autoimmune response and the main target antigen is myocardial myosin. Virus infection can induce cardiomyopathy, but the autoimmune response is the key to myocardial fibrosis development and further transformation to cardiomyopathy [5].
QiShenYiQi is a traditional Chinese medicine composed of Radix Astragali, Radix Salviae Miltiorrhizae, Radix Notoginseng, and Lignum Dalbergiae Odoriferae [6,7]. QiShenYiQi pill (QSYQ) was approved by China State Food and Drug Administration in 2003 for treatment of coronary heart disease and angina pectoris [8]. QSYQ enables a stable dosage form, of which the main effective ingredients are astragaloside, tanshinol, protocatechualdehyde, and ginsenoside Rg1 and Rb1 [9,10]. Without virus infection, autologous antigen stimulates the body to produce autoimmune response and causes transformation of myocarditis to cardiomyopathy. In the current study, we investigated the effect of QSYQ on experimental autoimmune myocarditis rats, and explored its mechanism of action.
Material and Methods
Animals
Male Lewis rats weighing 180–200 g were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China; certificate No. SCXK(Jing)2012–0001). Rats were housed in groups (4/cage) with free access to a normal rat diet and clean drinking water. The study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of Institutional Animal Care and Use Committee of Tianjin University of Traditional Chinese Medicine. The protocol was approved by the Animal Ethics Committee of Tianjin University of Traditional Chinese Medicine (No.TCM-2011-015-E03), China.
Materials
The reagents used in the study were purchased as follows: Cardiac Myosin (Sigma, USA); Freund’s Complete Adjuvant (Sigma, USA); Pentobarbital Sodium (Baihao Biological Technology CO., LTD., Tianjin, China); Hematoxylin and Eosin Staining Kit (Baihao Biological Technology CO., LTD., Tianjin, China); Trichrome Stain (Masson) Kit (Baihao Biological Technology CO., LTD., Tianjin, China); antibody against TGF-β1, antibody against CTGF, streptavidin – biotin complex with peroxidase (SABC-POD) (rabbit IgG) ready-to-use kits and DAB Chromogenic Reagent Kit (all Boster Biological Engineering CO., LTD., Wuhan, China); Ultrapure RNA Extraction Kit, HiFi-MMLV First Chain cDNA Synthesis Kit, UltraSYBR Mixture (with Rox), and DNase I (RNase-free) (all ComWin Biotech CO., LTD., Beijing, China); QSYQ pill (Tasly Pharmaceutical CO., LTD., Tianjin, China, specification was 500 mg/bag, Lot number: Z20030139); and valsartan capsules (Novartis Pharma Ltd., Beijing, China, specification was 80 mg/tablets, Lot number: H20040217).
Experimental autoimmune myocarditis rats model
We established a rat model of experimental autoimmune myocarditis by injecting myocardial myosin, as described previously by Kodama et al. [11] Purified porcine cardiac myosin (#M0531, Sigma, USA) was emulsified fully at 4°C with an equal volume of Freund’s complete adjuvant (#F5881, Sigma, USA). The concentration of the emulsion made from sticky state of water-in-oil is 3.2 mg/ml. Lewis rats were placed on an adaptive feeding routine for 3–7 days, with free access to drinking water. Then rats were injected in the footpads subcutaneously with 0.312 ml emulsion, producing an immunizing dose of 1.0 mg cardiac myosin. And rats were injected again on the seventh day. The control group rats were injected with phosphate-buffered saline (PBS) mixed with an equal volume of Freund’s complete adjuvant.
Animal groups and treatment
At 4 weeks after the first injection, the rats were randomized into 4 groups, with 16 rats per group: (1) control group (administered isopycnic sterile distilled water by gavage), (2) model group (administered isopycnic sterile distilled water by gavage), (3) valsartan group (7.2 mg/kg by gavage), and (4) QSYQ group (135 mg/kg by gavage). Dosage of valsartan capsules and QSYQ was determined following published pharmacological experiment methodology [12].
Blood and tissue specimens were harvested at 4 (8 rats per group) and 8 (8 rats per group) weeks after treatment started. Each rat was weighed (body mass), then anesthetized with 3% pentobarbital sodium (45 mg/kg) by intraperitoneal injection. Blood was then taken from the abdominal aorta, then the chest was opened, and the heart was irrigated with normal saline at 4°C. The blood sample was centrifuged at 3000 rpm for 10 min and serum was collected. The extracted myocardial tissue was blotted with filter paper, and then we weighed heart mass (HM) and calculated heart mass index (HMI, HMI=HM/BM (mg/g)). We removed the atrium and right ventricle, but retained the left ventricle and interventricular septum, then we weighed left ventricle mass (LVM) and calculated left ventricle mass index (LVMI, LVMI=LWH/BM (mg/g)). Part of the myocardial tissue was fixed in 4% neutral formaldehyde buffer solution and the remainder was flash-frozen in liquid nitrogen for cryopreservation.
Hematoxylin and eosin (H&E) staining of myocardium
Myocardial tissue was embedded in paraffin wax, cut into sections 5-μm thick, and placed onto coverslips coated with poly-L-lysine. Myocardium sections (5 μm) were stained with hematoxylin and eosin (H&E) according to the manufacturer’s instructions for the Hematoxylin and Eosin Staining Kit (Baihao Biological Technology CO., LTD., Tianjin, China). Inflammatory lesions were evaluated using H&E-stained sections, according to the following criteria: grade 1, rare focal inflammatory lesions; grade 2, multiple isolated foci of inflammation; grade 3, diffuse inflammation involving the outer layer of the muscle; grade 4, grade 3 plus focal transmural inflammation; and grade 5, diffuse inflammation with necrosis [13].
Masson trichrome staining of myocardium
The extent of fibrosis was evaluated using Masson Trichrome-stained sections. Myocardium sections (5 μm) were stained with Masson Trichrome according to the manufacturer’s instructions for the Trichrome Stain (Masson) Kit (Baihao Biological Technology CO., LTD., Tianjin, China). Cells were counted in 5 microscopic fields chosen randomly at ×100 magnification under the microscope. Image analysis software (Image-Pro Plus 6.0) was used to calculate collagen volume fraction (CVF, CVF=myocardial collagen fiber area/total area of the image) and the average value was taken.
Measurement of target gene in myocardium by RT-qPCR
Total RNA was extracted from myocardial tissue (Ultrapure RNA extraction Kit (#CW0581, ComWin Biotech CO., LTD., Beijing, China)). Absorbance was measured using an ultraviolet spectrophotometer and purification was evaluated by the ratio of absorbance at 260 and 280 nm (OD260/OD280). Ratio ranges between 1.8 and 2.0 indicate that the RNA is highly purified and suitable for real-time quantitative polymerase chain reaction (RT-qPCR). Reverse transcription was carried out using a cDNA First Chain Synthesis Kit HiFi-MMLV (#CW0744, ComWin Biotech CO., LTD., Beijing, China) and amplified using UltraSYBR Mixture (with Rox) (#CW0956, ComWin Biotech CO., LTD., Beijing, China), according to the manufacturer’s instructions. The target gene and housekeeping gene were all purchased from Guangzhou FulenGen CO., LTD., Guangzhou, China: TGF-β1 (Cat#RQP050181), CTGF (Cat#RQP050397), GAPDH (Cat#RQP049537). Relative quantitative analysis of mRNA was carried out using the 2−ΔΔCt method.
Measurement of TGF-β1 and CTGF in myocardium by immunohistochemical staining
Myocardial tissue was embedded in paraffin wax, cut into sections 5-μm thick, and placed onto coverslips coated with poly-L-lysine. Coverslips were incubated for 1 h at 60°C, and then processed by conventional dewaxing. They were then placed in 0.3% H2O2 for 10 min to block endogenous peroxidase activity, and washed 3 times with sterile distilled water. The sections were then placed into 0.02 M PBS (pH 7.2–7.6), and heated in a microwave to boiling point, after which the solution was left to cool naturally. We added 5% bovine serum albumin (BSA) (#AR0004, Boster Biological Engineering CO., LTD., Wuhan, China) dropwise, slides were incubated for 30 min at 37°C, and then the excess liquid was shaken off without washing. The primary antibody (rabbit anti-rat IgG) (antibody against TGF-β1: #BA0290, antibody against CTGF: #BA0752-1, Boster Biological Engineering CO., LTD., Wuhan, China) was added dropwise, then slides were incubated at 4°C overnight (at least 16 h). Slides were washed with PBS (pH 7.2–7.6) 3 times, for 10 min each time. The secondary antibody (biotin-labelled goat anti-rabbit IgG) (#SA1022, Boster Biological Engineering CO., LTD., Wuhan, China) was added dropwise and incubated for 30 min at 37°C, then washed with PBS (pH 7.2–7.6) 3 times, for 10 min each time. SABC reagent (#SA1022, Boster Biological Engineering CO., LTD., Wuhan, China) was then added dropwise, slides were incubated for 30 min at 37°C, and washed with PBS (pH 7.2–7.6) 3 times, 10 min each time. Then diaminobenzidine (DAB) staining (#AR1022, Boster Biological Engineering CO., LTD., Wuhan, China) was performed following the manufacturer’s instructions. The reaction was monitored under a microscope at room temperature until complete, and then washed with sterile distilled water. Slides were counterstained with hematoxylin, dehydrated in ethanol, cleared in xylene, and mounted with neutral rubber sealant, then observed under the microscope. Cells were counted in 5 microscopic fields chosen randomly at ×400 magnification under the microscope. Image analysis software (Image-Pro Plus 6.0) was used to calculate the ratio of positive to all area, and the average value was taken.
Statistical analysis
All parameters were expressed as mean ±S.D. Statistical analysis was performed using one-way ANOVA followed by least significant difference (LSD) test for multiple comparisons. SPSS statistical software (v11.5; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. The level of significance was set at P<0.05.
Results
Effect of QSYQ on HMI and LVMI
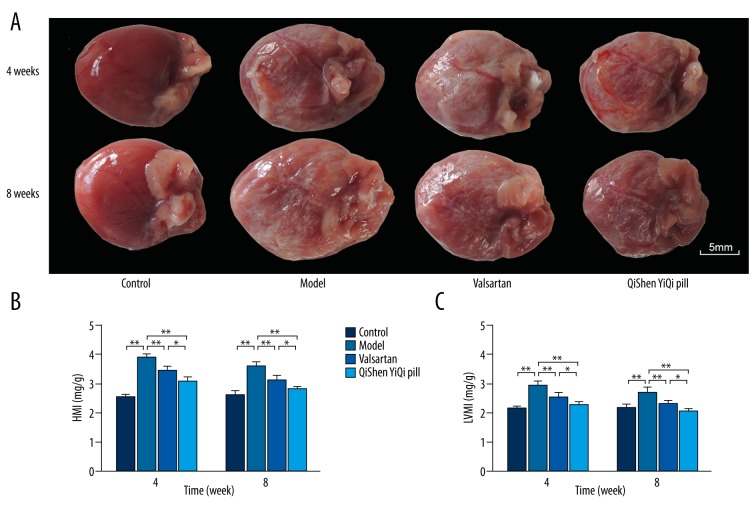
Rat hearts, which were covered with many white infiltration spots, was larger in the model group than in the control group. HMI and LVMI increased significantly (P<0.01) in the model group. With regard to the 2 treatment groups, HMI and LVMI were significantly reduced in the valsartan and the QSYQ group (P<0.01), and this reduction accelerated over time. However, HMI and LVMI were lower in the QSYQ group than in the valsartan group (P<0.05) (Figure 1).
Figure 1.
Effects of QSYQ on HMI and LVMI. (A) The morphological changes in the heart of rats. (B) HMI of each group. (C) LVMI of each group. Groups: control (n=8), model (n=8), valsartan (n=8), QSYQ (n=8). Data are expressed as mean ±SD. * P<0.05, ** P<0.01.
Effect of QSYQ on Pathomorphism of Myocardium
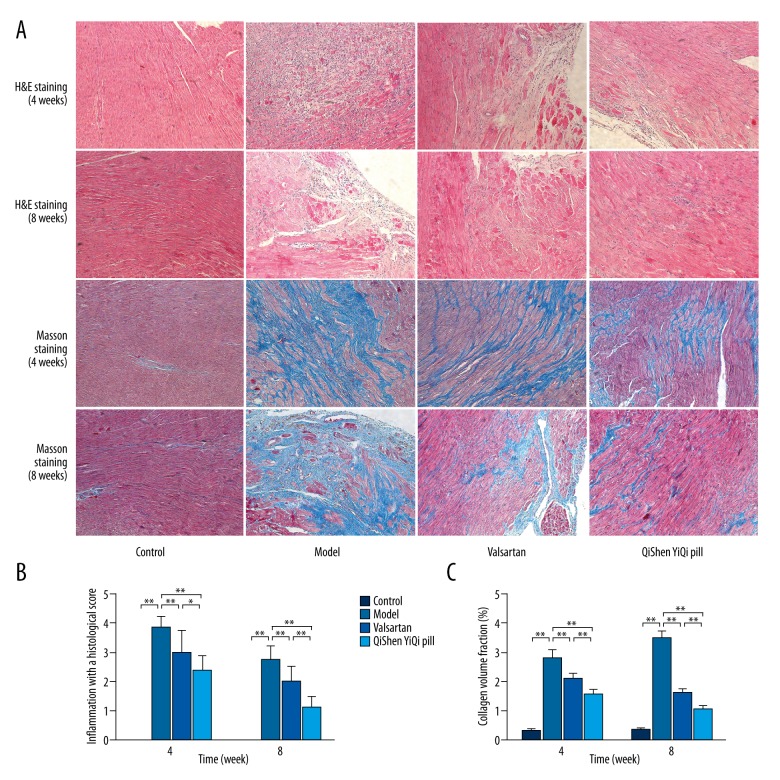
H&E staining in myocardial tissue showed no inflammatory infiltration lesions in the control group, but in the model group, inflammatory infiltration was significantly increased, and the number of myocardial cells was decreased, accompanied with cardiomyocyte hypertrophy and myocardial necrosis. The pathology grading showed that compared with the control group, the histological score was increased significantly (P<0.01) in the model group. After the treatments (valsartan and QYSQ), the histological score was significantly reduced (P<0.01), and this reduction accelerated over time. However, the histological score was lower in the QSYQ group than in the valsartan group (P<0.05 or P<0.01) (Figure 2).
Figure 2.
Effects of QSYQ on pathomorphism of myocardium. (A) Representative photomicrograph of hematoxylin and eosin (H&E) and mason trichrome staining of myocardium. (B) The inflammation with a histological score for each group. (C) The collagen volume fraction for each group. The collagen volume fraction was quantitavely analyzed by using Image-Pro Plus 6.0 (magnification, ×100). Data are expressed as mean ±SD. Groups: control (n=8), model (n=8), valsartan (n=8), QSYQ (n=8). Data are expressed as mean ±SD. * P<0.05, ** P<0.01.
Masson trichrome staining in myocardial tissue showed that in the control group there was a very little blue fiber and no myocardial interstitial fibrosis, but in the model group collagen deposition of myocardial interstitial was evident, and blue fiber was significantly increased and arranged irregularly. Compared with the control group, collagen volume fraction (CVF) was increased significantly (P<0.01) in the model group, and showed a tendency to increase over time. After the treatments (valsartan and QYSQ), CVF was significantly reduced (P<0.01), and this reduction increased over time; however, CVF was lower in the QSYQ group than in the valsartan group (P<0.01) (Figure 2).
Effect of QSYQ on Protein and mRNA Expression of TGF-β1
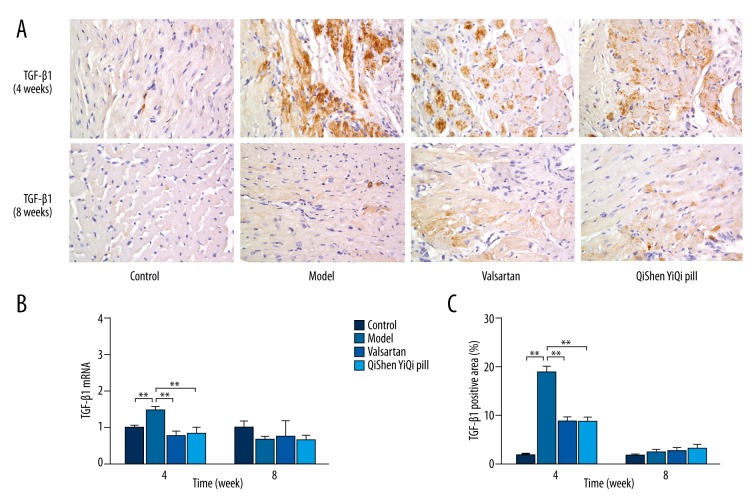
Immunohistochemical staining of TGF-β1 in myocardial tissue after 4 weeks showed that in the control group there were a small number of tiny tan particles scattered throughout the cytoplasm and the color change was weak, but in model group the brown area had expanded, staining was enhanced, and expression of TGF-β1 had increased. After the treatments (valsartan and QYSQ), expression of TGF-β1 was reduced compared with the model group at 4 weeks, whereas at 8 weeks there was no obvious difference in the brown areas between the control (control and model) and treatment (valsartan and QYSQ) groups. Semi-quantitative analysis showed that after 4 weeks the percentage positive area of TGF-β1 protein was significantly increased (P<0.01) in the model group compared with the control group. The percentage of positive area of TGF-β1 significantly (P<0.01) reduced in the valsartan group compared with the model group, and the QSYQ group had a similar result. At 8 weeks there was no significant difference in the expression of myocardial TGF-β1 (P>0.05) (Figure 3).
Figure 3.
Effects of QSYQ on the protein and mRNA expression of TGF-β1. (A) Representative photomicrograph of immunohistochemical staining of myocardium. (B) The TGF-β1 mRNA expression for each group. (C) The TGF-β1 positive area for each group. The percentage of immunohistochemical staining area was quantitavely analyzed by using Image-Pro Plus 6.0 (magnification, ×400). Data are expressed as mean ±SD. Groups: control (n=8), model (n=8), valsartan (n=8), QSYQ (n=8). Data are expressed as mean ±SD. * P<0.05, ** P<0.01.
At 4 weeks, rat myocardial expression of TGF-β1 mRNA was increased in the model group compared with the control group (P<0.01). Valsartan produced a significant reduction in rat myocardial expression of TGF-β1 mRNA (P<0.01) compared with the model group, and QSYQ produced a similar effect. At 8 weeks there was no statistical difference between valsartan and QSYQ regarding mRNA expression of TGF-β1 (P>0.05).
Effect of QSYQ on protein and mRNA expression of CTGF
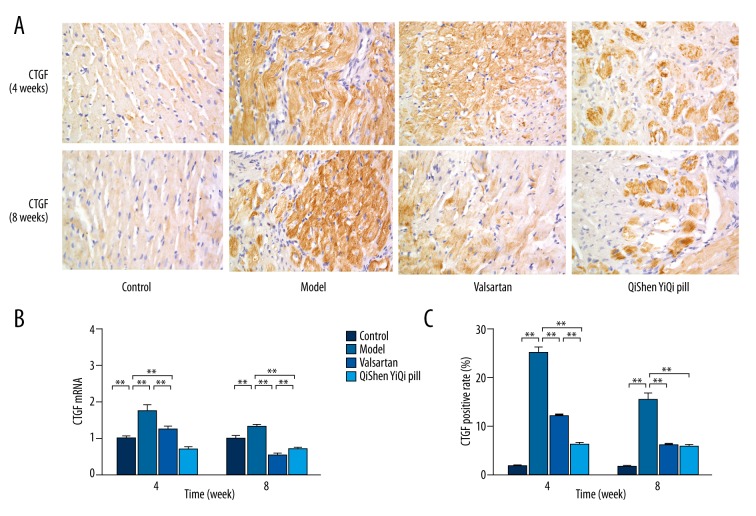
Immunohistochemical staining of CTGF in myocardial tissue showed that in the control group there were a small number of tiny tan particles scattered throughout the cytoplasm and the color change was weak, but in the model group the brown area had expanded, staining was enhanced, and expression of CTGF had increased. After the treatments (valsartan and QYSQ), expression of CTGF was reduced compared with the model group. Semi-quantitative analysis showed that the percentage of positive area of CTGF protein was significantly increased (P<0.01) in the model group compared with the control group at 4 weeks and 8 weeks. The percentage of positive area of CTGF significantly (P<0.01) reduced in the valsartan group compared with the model group, while the QSYQ group had a similar result, except that its effect on lowering CTGF was significantly greater (P<0.01) than that of valsartan at 4 weeks (Figure 4).
Figure 4.
Effects of QSYQ on the protein and mRNA expression of CTGF. (A) Representative photomicrograph of immunohistochemical staining of myocardium. (B) The CTGF mRNA expression for each group. (C) The CTGF positive area for each group. The percentage of immunohistochemical staining area was quantitavely analyzed by using Image-Pro Plus 6.0 (magnification, ×400). Data are expressed as mean ±SD. Groups: control (n=8), model (n=8), valsartan (n=8), QSYQ (n=8). Data are expressed as mean ±SD. * P<0.05, ** P<0.01.
Rat myocardial expression of CTGF mRNA was increased in the model group compared with the control group (P<0.01) at 4 weeks and 8 weeks. Valsartan produced a significant reduction in rat myocardial expression of CTGF mRNA (P<0.01) compared with the model group at 4 weeks and 8 weeks, and QSYQ produced a similar effect, except that its effect on lowering CTGF was significantly greater (P<0.01) than that of valsartan.
Discussion
Collagen content (collagen volume fraction) in normal rat myocardial tissue is about 3–5%. When the collagen content goes up to 8–12%, the diastolic stress-strain relationship is changed and diastolic function is damaged, but systolic function can be maintained. However, when the content goes up to more than 20%, myocardial systolic function is weakened [14]. In addition, the heart mass index (HMI) and left ventricular mass index (LVMI) can reflect the extent of cardiac enlargement. In the experimental autoimmune myocarditis rats, QSYQ reduced HMI, LVMI, CVF, and the histological score of hearts, which further reduced over time, and its effect was significantly greater than that of valsartan. Therefore, QSYQ can improve the cardiac remodeling of experimental autoimmune myocarditis rats, and had the same effect against myocardial fibrosis.
TGF-β1 is an important cytokine mediating myocardial fibrosis. Myocardial infarction, overload pressure, immune injury, and other stimuli can activate the TGF-β1 signal and initiate the fibrotic response, leading to increased collagen deposition [15,16]. Studies have shown that the increased collagen deposition in chronic viral myocarditis has a close relationship with the sustained high expression of TGF-β1 [17]. Coxsackie B3 virus was inoculated intraperitoneally into Balb/c mice to establish the viral myocarditis model; the type I and III collagen synthesis, the ratio of I/III collagen, and the TGF-β1 expression were significantly increased after 10 days [18]. The myocardial inflammatory injury in autoimmune myocarditis rats was alleviated by inhibition of myocardial TGF-β1 [19]. In the left ventricular myocardium of rats with dilated cardiomyopathy, TGF-β1 expression was significantly increased, suggesting that TGF-β1 plays an important role in myocardial fibrosis [20]. Astragaloside IV had the same effect of against myocardial fibrosis by inhibition of the expression of TGF-β1 and Smad 2–4 in coxsackie virus-induced dilated cardiomyopathy mice [21]. In addition, in a genetic cardiomyopathy hamster model, Losartan inhibited the myocardial fibrosis, which significantly reduced the expression of Smad2, Smad4, and TGF-β1 [22]. In the present study, after 4 weeks of treatment with QSYQ, the mRNA and protein expression of TGF-β1 decreased. After 8 weeks of treatment with QSYQ, there was no significant difference in the expression of myocardial TGF-β1. This indicates that QSYQ can improve cardiac remodeling of experimental autoimmune myocarditis rat by regulating TGF-β1.
TGF-β1 has a “double-edged sword” effect: normal expression can inhibit inflammation and cell proliferation (a positive effect), but over-expression can cause negative effects, such as extracellular cell matrix (ECM) accumulation. Connective tissue growth factor (CTGF) is a downstream effector of TGF-β1, which only mediates the negative effect of TGF-β1. Therefore, inhibiting the expression of CTGF may become a new target for myocardial fibrosis therapy. CTGF is an immediate early response gene CCN (CTGF, Cef10/Cyr61 and Nov, CCN) family member, autocrined or paracrined by fibroblasts, smooth muscle cells, epithelial cells, endothelial cells, and infiltrating inflammatory cells, and expressed in a variety of tissues, such as heart, brain, kidney, lung, liver, pancreas, and placenta. CTGF is closely associated with the development of organ fibrosis, and is regulated by a variety of stimulating factors. Studies have confirmed that in cardiac myocytes and cardiac fibroblasts, TGF-β induced the up-regulation of the CTGF, whereas the up-regulation of CTGF was related to high expression of fibronectin, type I collagen, and III collagen [23]. CTGF promoter contains a response element of TGF-β, so CTGF is a downstream effector molecule of TGF-β1. A study of expression of TGF-β1 and CTGF in a rat model of myocardial infarction showed that TGF-β1 was mainly expressed in the initial, acute inflammation and reparative stage of myocardial infarction, whereas CTGF was mainly expressed in the progressive stage of myocardial fibrosis [24]. TGF-β1 can induce the expression of CTGF, and CTGF also enhances the TGF-β1 signaling pathway, forming a vicious cycle resulting in the accumulation of extracellular matrix, and ultimately becoming myocardial fibrosis. In the acute Coxsackie B3-induced viral myocarditis model, the expression of CTGF was significantly increased, suggesting that CTGF mediated the process of myocardial fibrosis in the development of myocarditis [25,26]. In addition, myocardial fibrosis in mdx mice started at the high expression of CTGF, and the up-regulation of CTGF expression was in line with the up-regulation of TIMP-1, then the latter, which increased expression inhibited MMP-2 and MMP-9, showing that CTGF accelerated myocardial fibrosis by promoting collagen synthesis and inhibiting collagen degradation [27]. In our experimental autoimmune myocarditis model, QSYQ inhibited the protein and mRNA expression of CTGF, and its effect on lowering CTGF was significantly greater than that of valsartan at 4 weeks.
Conclusions
QSYQ can adjust the myocardial collagen metabolism in the experimental autoimmune myocarditis rat by regulating the expression of CTGF.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
Source of support: This work was supported by Research Fund for the Doctoral Program of Higher Education of China (20111210110003) and Ministry of Education of People’s Republic of China “Program for Innovative Research Team in University” – Research on TCM for the Prevention and Treatment of Cardiovascular Diseases (No. IRT1276)
References
- 1.Chen HZ, Fan WH, Jin XJ, et al. [Changing trends of etiologic characteristics of cardiovascular diseases among inpatients in Shanghai: a retrospective observational study from 1948 to 1999]. Zhong Hua Nei Ke Za Zhi. 2003;42:829–32. [in Chinese] [PubMed] [Google Scholar]
- 2.Lv S, Rong J, Ren S, et al. Epidemiology and diagnosis of viral myocarditis. Hellenic J Cardiol. 2013;54:382–91. [PubMed] [Google Scholar]
- 3.Xiao N, Zhang J. [The contribution of anti-myocardial autoantibodies in the diagnosis of viral myocarditis]. Shi Jie Zhong Xi Yi Jie He Za Zhi. 2012;7:75–76. [in Chinese] [Google Scholar]
- 4.Liu PP, Mason JW. Advances in the understanding of myocarditis. Circulation. 2001;104:1076–82. doi: 10.1161/hc3401.095198. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Liao Y, Dong J, et al. Experimental study of cardiac myosin-induced autoimmune cardiomyopathy. Zhong Hua Xin Xue Guan Bing Za Zhi. 2003;31:937–40. [in Chinese] [Google Scholar]
- 6.Zhang Y, Shi P, Yao H, et al. Metabolite profiling and pharmacokinetics of herbal compounds following oral administration of a cardiovascular multi-herb medicine (Qishen yiqi pills) in rats. Curr Drug Metab. 2012;13:510–23. doi: 10.2174/1389200211209050510. [DOI] [PubMed] [Google Scholar]
- 7.Yunfei L, Haibin Q, Yiyu C. Identification of major constituents in the traditional Chinese medicine “QI-SHEN-YI-QI” dropping pill by high-performance liquid chromatography coupled with diode array detection-electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2008;47:407–12. doi: 10.1016/j.jpba.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Tang DX, Zhao HP, Pan CS, et al. QiShenYiQi pills, a compound Chinese medicine, ameliorates doxorubicin-induced myocardial structure damage and cardiac dysfunction in rats. Evid Based Complement Alternat Med. 2013;2013:480597. doi: 10.1155/2013/480597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Wei N, Zhao H, et al. Global Chemome Study by LC coupled with DAD and ESI-Q-TOF MS of a composite traditional Chinese medicine Qishenyiqi dropping pills. Chromatographia. 2010;72:431–40. [Google Scholar]
- 10.Fu J, Song S, Jiang M, et al. [Liquid chromatography-mass spectrum determines the contents of astragaloside, tanshinol, protocatechualdehyde, Rg1 and Rb1 ginsenosides in QiShenYiQi pills]. Zhong Guo Yao Xue Za Zhi. 2012;47:61–64. [in Chinese] [Google Scholar]
- 11.Kodama M, Matsumoto Y, Fujiwara M, et al. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1990;57:250–62. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 12.Xu S. Experimental methodology of pharmacology. Beijing: People’s Medical Publishing House; 2002. pp. 200–23. [Google Scholar]
- 13.Matsumoto Y, Tsukada Y, Miyakoshi A, et al. C protein-induced myocarditis and subsequent dilated cardiomyopathy: rescue from death and prevention of dilated cardiomyopathy by chemokine receptor DNA therapy. J Immunol. 2004;173:3535–41. doi: 10.4049/jimmunol.173.5.3535. [DOI] [PubMed] [Google Scholar]
- 14.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–52. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 15.Santibañez JF, Quintanilla M, Bernabeu C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2011;121:233–51. doi: 10.1042/CS20110086. [DOI] [PubMed] [Google Scholar]
- 16.Lei B, Hitomi H, Mori T, et al. Effect of efonidipine on TGF-β1-induced cardiac fibrosis through Smad2-dependent pathway in rat cardiac fibroblasts. J Pharmacol Sci. 2011;117:98–105. doi: 10.1254/jphs.11065fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fairweather D, Frisancho-Kiss S, Yusung SA, et al. Interferon-gamma protects against chronic viral myocarditis by reducing mast cell degranulation, fibrosis, and the profibrotic cytokines transforming growth factor-beta 1, interleukin-1 beta, and interleukin-4 in the heart. Am J Pathol. 2004;165:1883–94. doi: 10.1016/s0002-9440(10)63241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Schwimmbeck PL, Tschope C, et al. Collagen degradation in a murine myocarditis model: relevance of matrix metalloproteinase in association with inflammatory induction. Cardiovasc Res. 2002;56:235–47. doi: 10.1016/s0008-6363(02)00546-1. [DOI] [PubMed] [Google Scholar]
- 19.Shiono T, Kodama M, Hanawa H, et al. Suppression of myocardial inflammation using suramin, a growth factor blocker. Circ J. 2002;66:385–89. doi: 10.1253/circj.66.385. [DOI] [PubMed] [Google Scholar]
- 20.Palaniyandi Selvaraj S, Watanabe K, Ma M, et al. Involvement of mast cells in the development of fibrosis in rats with postmyocarditis dilated cardiomyopathy. Biol Pharm Bull. 2005;28:2128–32. doi: 10.1248/bpb.28.2128. [DOI] [PubMed] [Google Scholar]
- 21.Chen P, Xie Y, Shen E, et al. Astragaloside IV attenuates myocardial fibrosis by inhibiting TGF-β1 signaling in coxsackievirus B3-induced cardiomyopathy. Eur J Pharmacol. 2011;658:168–74. doi: 10.1016/j.ejphar.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 22.Dixon IM, Hao J, Reid NL, et al. Effect of chronic AT(1) receptor blockade on cardiac Smad overexpression in hereditary cardiomyopathic hamsters. Cardiovasc Res. 2000;46:286–97. doi: 10.1016/s0008-6363(00)00035-3. [DOI] [PubMed] [Google Scholar]
- 23.Chen MM, Lam A, Abraham JA, et al. CTGF expression is induced by TGF-beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–19. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 24.Dean RG, Balding LC, Candido R, et al. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem. 2005;53:1245–56. doi: 10.1369/jhc.4A6560.2005. [DOI] [PubMed] [Google Scholar]
- 25.Yun SH, Shin JO, Lim BK, et al. Change in the cells that express connective tissue growth factor in acute Coxsackievirus-induced myocardial fibrosis in mouse. Virus Res. 2007;126:62–68. doi: 10.1016/j.virusres.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Lang C, Sauter M, Szalay G, et al. Connective tissue growth factor: a crucial cytokine-mediating cardiac fibrosis in ongoing enterovirus myocarditis. J Mol Med (Berl) 2008;86:49–60. doi: 10.1007/s00109-007-0249-3. [DOI] [PubMed] [Google Scholar]
- 27.Au CG, Butler TL, Sherwood MC, et al. Increased connective tissue growth factor associated with cardiac fibrosis in the mdx mouse model of dystrophic cardiomyopathy. Int J Exp Pathol. 2011;92:57–65. doi: 10.1111/j.1365-2613.2010.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]