Abstract
Background
Regulated upon activation, normal T cells expressed and secreted (RANTES) is associated with inflammation and atherosclerosis. We investigated the effect of fenofibrate, a peroxisome proliferator-activated receptor-α (PPAR-α) agonist, on RANTES in type 2 diabetes mellitus (T2DM) patients with hypertriglyceridemia.
Material/Methods
This study evaluated cross-sectional and interventional studies of 25 T2DM patients with hypertriglyceridemia (group A) and 32 controls (group B). Group A was treated with fenofibrate (200 mg/day) for 8 weeks. Serum RANTES and clinical characteristics were examined.
Results
Serum RANTES was significantly higher in group A compared with group B (59.04±16.74 vs. 38.57±12.98 ng/ml, P<0.001) and correlated with triglycerides (TG) (r=0.535, P<0.001), fasting blood glucose (FBG) (r=0.485, P<0.001), glycosylated hemoglobin (HbA1c) (r=0.485, P<0.001), homocysteine (Hcy) (r=0.520, P<0.001), and high-sensitivity C-reactive protein (hsCRP) (r=0.701, P<0.001). In multiple regression analysis after controlling for confounders, increased hsCRP levels (β=7.430, P<0.001) and T2DM with hypertriglyceridemia (β=11.496, P=0.002) were independently related to high serum RANTES levels. After 8 weeks of fenofibrate treatment, serum RANTES significantly decreased in group A compared with baseline (52.75±17.41 vs. 59.04±16.74 ng/ml, P=0.018).
Conclusions
Fenofibrate decreased serum RANTES levels in T2DM patients with hypertriglyceridemia, indicating that PPAR-α agonists may play an important role in inhibiting inflammatory responses.
MeSH Keywords: Chemokine CCL5; Diabetes Mellitus, Type 2; Hypertriglyceridemia; PPAR alpha
Background
Many type 2 diabetes mellitus (T2DM) patients suffer from diabetes-related cardiovascular complications, which are the major cause of death in patients with T2DM. Atherosclerosis, a major risk factor for cardiovascular diseases, is a chronic disease characterized by two fundamental hallmarks: lipid accumulation and inflammation [1]. The interaction between these two processes defines the principal pathogenesis and distinguishes atherosclerosis from other chronic inflammatory disorders.
The dyslipidemia in T2DM is characterized by increased low-density lipoprotein cholesterol (LDL-C), elevated triglycerides (TG), and decreased high-density lipoprotein cholesterol (HDL-C), and it is associated with an increased risk of coronary artery disease [2]. Statins, which decrease LDL-C levels and inhibit inflammatory responses, have been conclusively proven to significantly reduce cardiovascular events in many high-risk patients [3]. However, the residual risk remains after patients have achieved their target LDL-C levels through statins treatment [2]. Combined with statins, fenofibrate has been shown to have highly beneficial effects on protecting against cardiovascular events in patients with type 2 diabetes associated with dyslipidemia [4].
Fenofibrate is known as an important peroxisome proliferator-activated receptor-α (PPAR-α) agonist, which is effective at decreasing TG levels, increasing HDL-C levels, and changing LDL particle morphology. Moreover, fenofibrate plays a pivotal role in regulating insulin resistance, fatty acid oxidation, cellular differentiation, and immune responses, such as inflammation or vascularization related to diabetic complications [5]. Thus, PPAR-α agonists may reduce cardiovascular morbidity and mortality [6] through lipid-lowering-dependent and lipid-lowering-independent mechanisms [7,8].
Our previous studies have demonstrated that fenofibrate improved vascular endothelial function and regulated metabolism through comprehensive mechanisms [9–11]. Vascular endothelial dysfunction changes vascular permeability, allowing circulating monocytes and T cells to migrate into the subendothelial space. The continued presence of immune cells, together with migration and proliferation of vascular smooth muscle cells, serves to perpetuate the inflammatory response and promote atherosclerotic plaque formation. An advanced atherosclerotic plaque typically contains a mixture of proliferating smooth muscle cells, macrophages and T cells, and connective tissue, overlaid with a fibrous cap consisting of smooth muscle cells and matrix [12].
Chemokines are inflammatory cytokines, which may lead to atherosclerosis and plaque destabilization not only by recruiting activated leukocytes into the lesion, but also by directly contributing to plaque rupture and thrombus formation. The role of chemokines in atherosclerosis is further supported by several studies suggesting that chemokines may represent a link between lipids and inflammation in atherogenesis [13]. Regulated upon activation, normal T cells expressed and secreted (RANTES), also known as C-C chemokine ligand 5 (CCL5), belongs to the C-C chemokine family, which is secreted by many cell types, such as endothelial cells, smooth muscle cells, macrophages, platelets, and activated T cells. RANTES plays a critical role in chronic inflammatory processes and progression of atherosclerosis [14]. Our previous studies demonstrated that the circulating levels of RANTES were elevated in hyperhomocysteinemia patients compared with controls and that upregulated RANTES from monocytes in hyperhomocysteinemia patients was involved in hyperhomocysteinemia-induced atherosclerosis [15]. RANTES administration has been proposed as a potential therapeutic strategy to protect the cardiovascular system [16–18]; thus, it may have implications for decreasing cardiovascular risk of T2DM.
Although PPAR-α agonists have been proven to reduce diabetic cardiovascular complications [7], its underlying mechanisms are still not fully clear. The concept that atherosclerosis is an inflammatory disease is no longer controversial; nevertheless, the regulation of the inflammatory processes and the pathogenic consequences remains uncertain. PPAR-α has been reported to regulate RANTES expression in vitro and in animal studies [19–22]. However, to the best of our knowledge, the effect of PPAR-α agonist fenofibrate on RANTES in diabetes patients has not been reported. Therefore, in the present study, we aimed to examine whether fenofibrate affected circulating RANTES levels in T2DM patients with hypertriglyceridemia.
Material and Methods
Subjects
All participants (both males and females), ranging in age from 30 to 70 years, were recruited from September 2013 to January 2014.
Twenty-five type 2 diabetes mellitus patients with hypertriglyceridemia (group A) were recruited for this study from a group of outpatients at the Department of Endocrinology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China. Patients diagnosed with type 2 diabetes mellitus, as defined by the World Health Organization (WHO) criteria, and with stable hypoglycemic treatment for at least 3 months, fasting blood glucose (FBG) levels <9 mmol/L, and glycosylated hemoglobin (HbA1c) levels <8%, were eligible for the study. Additionally, the patients had been treated with 20 mg/day atorvastatin for more than 3 months, but their TG levels were still greater than 1.7 mmol/L. The following exclusion criteria for group A were applied: known type 1 and other specific types of diabetes (e.g., genetic defects of the β-cell, genetic defects in insulin action, diseases of the exocrine pancreas, endocrinopathies, drug- or chemical-induced diabetes, infections, uncommon forms of immune-mediated diabetes, or other genetic syndromes associated with diabetes) according to the WHO classification of diabetes mellitus, genetic conditions affecting lipid metabolism (e.g., familial hypercholesterolemia, lipoprotein lipase deficiency), changes in hypoglycemic drugs or lipid-lowering drugs during the 3 months preceding the screening visit, any acute cardiovascular event within last 3 months, and contraindicating treatment with fenofibrate.
Thirty-two healthy people (group B) were recruited as the control group from the community or from the group of people undergoing routine medical check-ups. None of them had a history of prediabetes (including impaired glucose tolerance and impaired fasting glucose), diabetes, hyperlipidemia, or cardiovascular disease.
Moreover, people with hypertension, endocrine disease, systemic inflammatory disease, infectious disease, cancer, chronic kidney disease (i.e., serum creatinine [CR] >120 μmol/L), hepatic enzymes (i.e., aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) >1.5 times the upper normal limits, creatine kinase (CK) >1.5 times the upper normal limit, a history of alcohol abuse, using heparin within last 3 months, pregnancy, and lactation were also excluded from both groups.
Study design
Participants in group A were required to attend 3 study visits: the screening visit, visit 1, and visit 2 (spaced 8 weeks apart), while participants in group B attended the screening visit. Starting at visit 1, the group A participants who fulfilled the inclusion criteria (without any exclusion criterion) were administered fenofibrate 200 mg/day for 8 weeks. The capsules were counted at visit 2, and compliance was considered to be satisfactory if >90% of capsules were taken.
Blood samples and the data on the medical history, height, weight, and blood pressure were collected at the screening visit (groups A and B) and at visit 2 (group A) under fasting conditions, as described below. At visit 1, all participants in group A received instructions to maintain their usual nutritional and exercise habits and to not modify any drug treatment throughout the study. Participants in group A were asked to immediately report the development of unusual muscle soreness or pain throughout the study. In addition, any adverse event in each group A participant was recorded at visit 2.
The study protocol was approved by the Medicine and Pharmacy Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University. Written informed consent was obtained from each participant prior to the performance of any study procedure.
Data collection and laboratory tests
A complete medical history, including duration and treatment of any disease, was obtained from each participant; height and weight were determined using a standardized protocol. Body mass index (BMI) was calculated as weight (kg)/[height (m)]2. Blood pressure was measured using a calibrated standard mercury sphygmomanometer. All readings were measured after a 5-min rest, with the patients in sitting position.
Fasting blood samples were collected in the morning after an 8-h overnight fast. Total cholesterol (TC), HDL-C, TG, FBG, homocysteine (Hcy), high-sensitivity C-reactive protein (hsCRP), AST, ALT, CR, CK, and HbA1c were measured in the central laboratory of Beijing Chao-Yang Hospital, Capital Medical University. LDL-C was calculated using the Friedewald formula (LDL=CHOL−[TG/5+HDL]). Serum samples from all participants were promptly placed on ice after blood collection and were centrifuged at 4°C within 30 min. They were aliquoted and stored at −80°C immediately after centrifugation, and we avoided any repeated thawing and freezing. Serum RANTES concentrations were measured in duplicate at the same time using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) for quantitative detection with an automated ELISA reader (VARIOSKAN FLASH-5250040, Thermo Scientific, USA).
Adverse events were recorded throughout the study. The safety parameters included serum AST, ALT, CR, and CK.
Statistical analysis
All analyses were performed with the Statistical Package for Social Sciences version 19.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as the means±SD. Comparisons of the baseline clinical and biochemical markers, as well as the RANTES levels, between groups A and B were performed using an independent samples t test. Comparisons of the pre-treatment and post-treatment (with fenofibrate) clinical and biochemical markers, as well as the RANTES levels, in group A were performed with the paired t test. Proportions were analyzed using the chi-squared test. The association between the baseline values of RANTES and the other baseline parameters was examined using Pearson’s and Spearman’s correlation coefficient analyses and multiple stepwise regression analysis. In all statistical tests, P values <0.05 were considered to be significant, and all tests were two-sided.
Results
Baseline clinical characteristics of the study participants
The baseline clinical characteristics of the study participants are listed in Table 1. The participants in groups A and B were similar in sex, age, BMI, systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels, and TC levels (P>0.05 for all). The LDL-C (P<0.001), TG (P<0.001), FBG (P<0.001), HbA1c (P<0.001), Hcy (P=0.002) and hsCRP (P=0.001) levels were higher and the HDL-C levels (P<0.001) were lower in group A compared with group B. The proportion of subjects of using aspirin (P<0.001), using metformin (P<0.001), using insulin secretagogue (P<0.001), using thiazolidinedione (P<0.001), using acarbose (P<0.001), using insulin (P=0.013), and using atorvastatin (P<0.001) were higher in group A compared with group B.
Table 1.
Baseline clinical characteristics of the study participants.
| Parameters | Group A (N=25) | Group B (N=32) | P value |
|---|---|---|---|
| Sex (M/F) | 19/6 | 15/17 | 0.198 |
| Age (years) | 53.76±8.89 | 50.16±7.80 | 0.109 |
| BMI (kg/m2) | 26.46±4.60 | 24.84±3.60 | 0.142 |
| SBP (mmHg) | 125.00±7.65 | 121.47±10.23 | 0.156 |
| DBP (mmHg) | 74.24±8.74 | 70.53±7.82 | 0.097 |
| TC (mmol/L) | 4.62±0.66 | 4.32±0.63 | 0.080 |
| HDL-C (mmol/L) | 1.25±0.29 | 1.63±0.36 | <0.001 |
| LDL-C (mmol/L) | 2.80±0.53 | 2.31±0.45 | <0.001 |
| TG (mmol/L) | 3.05±0.86 | 0.73±0.28 | <0.001 |
| FBG (mmol/L) | 7.43±1.01 | 4.99±0.35 | <0.001 |
| HbA1c (%) | 6.88±0.72 | 5.46±0.33 | <0.001 |
| Hcy (μmol/L) | 17.08±4.30 | 13.78±2.78 | 0.002 |
| HsCRP (mg/L) | 2.44±1.61 | 1.23±0.69 | 0.001 |
| Drug usage within 3 months (n) | |||
| Aspirin | 9 | 0 | <0.001 |
| Metformin | 19 | 0 | <0.001 |
| Insulin secretagogue | 12 | 0 | <0.001 |
| Thiazolidinedione | 10 | 0 | <0.001 |
| Acarbose | 16 | 0 | <0.001 |
| Insulin | 5 | 0 | 0.013 |
| Atorvastatin | 25 | 0 | <0.001 |
Group A – type 2 diabetes mellitus patients with hypertriglyceridemia; Group B – control subjects; BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; TC – total cholesterol; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; TG – triglycerides; FBG – fasting blood glucose; HbA1c – glycosylated hemoglobin; Hcy – homocysteine; hsCRP – high-sensitivity C-reactive protein.
Baseline serum RANTES levels of the study participants
The fasting serum levels of RANTES were significantly higher in group A than in group B (59.04±16.74 vs. 38.57±12.98 ng/ml, P<0.001) (Figure 1).
Figure 1.
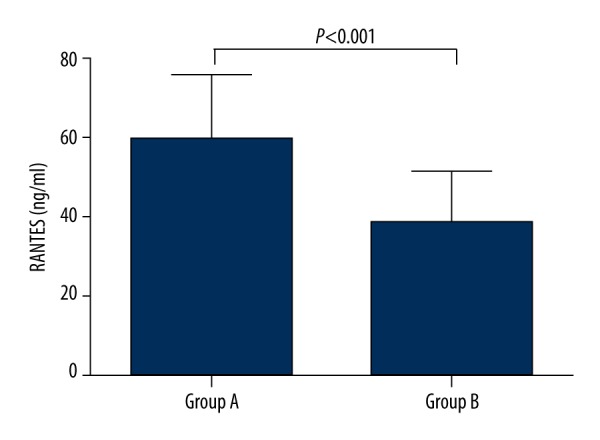
Baseline serum RANTES levels in the study participants. The values are expressed as the means±SD. Group A: type 2 diabetes patients with hypertriglyceridemia (n=25); Group B: control subjects (n=32).
Correlation between serum RANTES levels and the baseline parameters
The following parameters were found to be significantly correlated with the serum RANTES levels: TG (r=0.535, P<0.001), FBG (r=0.485, P<0.001), HbA1c (r=0.485, P<0.001), Hcy (r=0.520, P<0.001), and hsCRP (r=0.701, P<0.001) (Table 2).
Table 2.
Correlation analyses of the baseline parameters associated with RANTES.
| Parameters | r | P value |
|---|---|---|
| Age (years) | 0.208 | 0.120 |
| BMI (kg/m2) | 0.222 | 0.098 |
| SBP (mmHg) | 0.151 | 0.262 |
| DBP (mmHg) | 0.197 | 0.142 |
| TC (mmol/L) | 0.174 | 0.197 |
| HDL-C(mmol/L) | −0.170 | 0.207 |
| LDL-C (mmol/L) | 0.184 | 0.170 |
| TG (mmol/L) | 0.535 | <0.001 |
| FBG (mmol/L) | 0.485 | <0.001 |
| HbA1c (%) | 0.485 | <0.001 |
| Hcy (μmol/L) | 0.520 | <0.001 |
| HsCRP (mg/L) | 0.701 | <0.001 |
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; TC – total cholesterol; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; TG – triglycerides; FBG – fasting blood glucose; HbA1c – glycosylated hemoglobin; Hcy – homocysteine; hsCRP – high sensitivity C-reactive protein.
Multiple stepwise regression analysis was performed to determine the parameters that were independently associated with serum RANTES. The results showed that increased hsCRP (β=7.430, P<0.001) and T2DM with hypertriglyceridemia (β=11.496, P=0.002) were independently related to high serum RANTES levels after adjusting for all clinical characteristics and drug usage. The model had an adjusted R squared of 0.551, F=35.407 and P<0.001 (Table 3).
Table 3.
Multiple regression analysis of the baseline parameters associated with RANTES.
| Parameters | β | SE | Standardized β | 95% CI | P value |
|---|---|---|---|---|---|
| Constant | 17.930 | 4.856 | 8.194–27.665 | 0.001 | |
| HsCRP (mg/L) | 7.430 | 1.364 | 0.549 | 4.695–10.166 | <0.001 |
| T2DM with hypertriglyceridemia | 11.496 | 3.591 | 0.322 | 4.297–18.696 | 0.002 |
SE – standard error; CI – confidence interval; HsCRP – high sensitivity C-reactive protein; T2DM with hypertriglyceridemia – type 2 diabetes mellitus with hypertriglyceridemia. Adjustment for age, sex, BMI, SBP, DBP, TC, HDL-C, LDL-C, TG, FBG, HbA1c, Hcy, and usage of aspirin, metformin insulin secretagogue, thiazolidinedione, acarbose, insulin, and atorvastatin.
Effects of fenofibrate on the clinical characteristics in group A
The pre-treatment and post-treatment (with fenofibrate) clinical parameters in group A are summarized in Table 4. Compared with baseline, at visit 2, the patients in group A presented significantly lower levels of TG (P<0.001) and hsCRP (P=0.020) but significantly higher levels of HDL-C (P<0.001). In addition, no statistically significant changes were observed in BMI, SBP, DBP, TC, LDL, FBG, AST, ALT, CK, CR, or Hcy after 8 weeks of fenofibrate treatment compared with baseline (P>0.05 for all).
Table 4.
Pre-treatment and post-treatment clinical characteristics of type 2 diabetes patients with hypertriglyceridemia treated with fenofibrate.
| Parameters | Pre-treatment (N=25) | Post-treatment (N=25) | P value |
|---|---|---|---|
| BMI (kg/m2) | 26.46±4.60 | 26.37±4.59 | 0.209 |
| SBP (mmHg) | 125.00±7.65 | 125.48±6.76 | 0.668 |
| DBP (mmHg) | 74.24±8.74 | 73.92±7.30 | 0.831 |
| TC (mmol/L) | 4.62±0.66 | 4.83±0.69 | 0.145 |
| HDL-C(mmol/L) | 1.25±0.29 | 1.47±0.27 | <0.001 |
| LDL -C(mmol/L) | 2.80±0.53 | 2.82±0.66 | 0.845 |
| TG (mmol/L) | 3.05±0.86 | 1.84±0.76 | <0.001 |
| FBG (mmol/L) | 7.43±1.01 | 7.32±1.00 | 0.215 |
| Hcy (μmol/L) | 17.08±4.30 | 16.24±3.88 | 0.283 |
| HsCRP (mg/L) | 2.44±1.61 | 1.59±1.39 | 0.020 |
| AST (U/L) | 22.08±7.33 | 23.84±9.04 | 0.322 |
| ALT (U/L) | 26.00±5.69 | 24.44±11.64 | 0.518 |
| CR (μmol/L) | 71.92±13.74 | 76.10±17.29 | 0.164 |
| CK (U/L) | 84.64±27.69 | 91.20±37.28 | 0.307 |
BMI – body mass index; SBP – systolic blood pressure; DBP – diastolic blood pressure; TC – total cholesterol; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; TG – triglycerides; FBG – fasting blood glucose; Hcy – homocysteine; hsCRP – high-sensitivity C-reactive protein; AST – aspartate aminotransferase; ALT – alanine aminotransferase; CR – creatinine; CK – creatine kinase.
Effect of fenofibrate on the serum levels of RANTES in group A
After 8 weeks of fenofibrate treatment, the serum RANTES levels in group A were significantly decreased compared with the baseline levels (from 59.04±16.74 ng/ml at pre-treatment to 52.75±17.41 ng/ml at post-treatment, P=0.018) (Figure 2).
Figure 2.
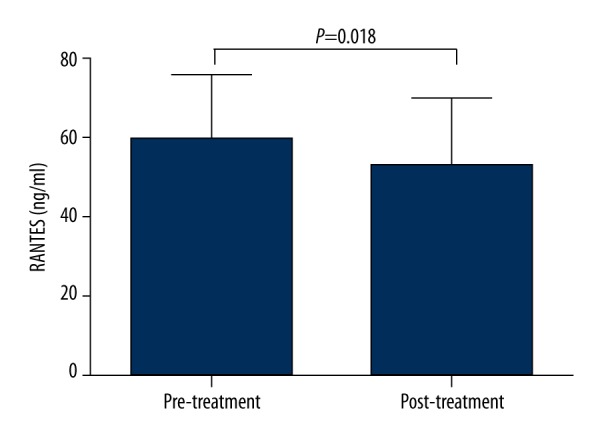
Serum RANTES levels in type 2 diabetes patients with hypertriglyceridemia after 8 weeks of fenofibrate treatment compared with the baseline levels. The values are expressed as the means±SD (n=25).
Safety parameters
All participants completed the study, and no serious adverse effects were observed during the study.
Discussion
In this study we demonstrated that serum RANTES levels were significantly higher in the T2DM patients with hypertriglyceridemia compared with the controls, and that serum RANTES was positively correlated with TG, FBG, HbA1c, Hcy, and hsCRP. After controlling for confounders, increased hsCRP levels and T2DM with hypertriglyceridemia were independently related to high serum RANTES levels. Our findings agree with studies indicating that circulating RANTES was significantly higher in patients with hypertriglyceridemia [23] or with T2DM [24] compared with controls, and was positively related to FBG [25]. Furthermore, our finding that serum RANTES was positively associated with Hcy support our previous result that the circulating RANTES levels were elevated in hyperhomocysteinemia patients compared with controls [15]. In addition, our finding that serum RANTES was positively correlated with hsCRP, a biomarker of inflammation and an independent predictor for cardiovascular events, suggested that RANTES might play an important role in the inflammatory processes and cardiovascular diseases.
Accumulating evidence has demonstrated that PPAR-α is an important modulator of metabolic syndrome and that it might be useful as a therapeutic target for treating some of its features. PPAR-α agonist has been proven to be effective at lowering plasma levels of glucose, insulin, triglycerides, and free fatty acids in ob/ob mice [26]. PPAR-α plays a key role in lipid metabolism. Its known target genes are involved in most aspects of lipid metabolism and lipid transport [27]. In our study, the T2DM patients with hypertriglyceridemia presented significantly lower levels of TG but demonstrated significantly higher levels of HDL-C after 8 weeks of fenofibrate treatment compared with baseline.
Importantly, we report for the first time that fenofibrate treatment administered to T2DM patients with hypertriglyceridemia for 8 weeks resulted in a significant decrease in serum RANTES levels, suggesting that PPAR-α agonists may lead to the inhibition of cardiovascular diseases, independent of their effects on lipid metabolism.
PPAR-α agonists may exert positive effects on cardiovascular diseases. PPAR-α activators decreased atherosclerotic lesions in a mouse model of mixed dyslipidemia [28]. Moreover, activation of PPAR-α protected the heart from ischemia/reperfusion injury [29]. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study revealed that total cardiovascular events were significantly reduced through fenofibrate treatment [7]. Although PPAR-α agonists might protect against cardiovascular diseases, the mechanisms are still not fully understood. It has been reported that PPAR-α agonists decrease the progression of atherosclerosis, mainly by modulating metabolic risk factors and by their anti-inflammatory actions on the level of the vascular wall [30]. Our previous study demonstrated that fenofibrate improved coronary flow velocity reserve and arterial stiffness in patients with hypertriglyceridemia [9], upregulated tetrahydrobiopterin levels by increasing the expression of guanosine triphosphate cyclohydrolase-I in human umbilical vein endothelial cells [10], and decreased circulating irisin levels in type 2 diabetes patients with hypertriglyceridemia [11]. PPAR-α agonists were reported to increase the expression of endothelial nitric oxide synthase (eNOS) and the production of nitric oxide (NO) [31]. PPAR-α agonists have been proven to reduce hsCRP levels [8] and to repress the production of some inflammatory cytokines [8,32]. In our present study, hsCRP levels were also significantly decreased in type 2 diabetes patients with hypertriglyceridemia after 8 weeks of PPAR-α agonist fenofibrate treatment. Based on these results, it appears that PPAR-α agonists have an anti-atherosclerotic effect through anti-inflammation. Activation of PPAR-α interferes with early steps in atherosclerosis by reducing leukocyte adhesion to activated endothelial cells of the arterial vessel wall and by inhibiting subsequent transendothelial leukocyte migration. In addition, in later stages of atherosclerosis, activation of PPAR-α suppresses the formation of macrophage foam cells and reactive oxygen species, and reduces associated-lipoprotein oxidative modification [33]. Therefore, PPAR-α agonists may inhibit the formation of atherosclerotic lessons and may increase the stability of atherosclerotic plaques through suppressing inflammatory responses.
Recent evidence has indicated that RANTES is associated with cardiovascular events. However, the data have been inconsistent. Most studies have supported that elevated RANTES levels are related to high cardiovascular risk. Increased levels of RANTES were reported in patients with acute coronary syndromes compared with controls [34] and were proven in patients with refractory ischemic symptoms compared with stabilized patients [35]. RANTES levels were associated with carotid wall thickness and lipid-core volume [36], suggesting that higher RANTES levels might be related to extensive carotid atherosclerosis and plaque at high risk of rupturing. Therefore, RANTES inhibition is believed to induce cardioprotective effects through its anti-inflammatory properties. By its ability to bind to its chemokine receptors expressed on T cells or monocytes, RANTES might lead to the adhesion and the transmigration of T cells and monocytes through the endothelial wall [37]; thus, blocking these receptors by injecting RANTES receptor antagonists could suppress atherosclerosis [16,17] and might reduce the infarct size [18]. Controversially, there have been some studies suggesting that low RANTES levels might be correlated with atherosclerosis [38,39]. It might be hypothesized that the decreased RANTES levels in patients with high frequency of cardiovascular events could reflect increased deposition of RANTES on the vascular endothelium leading to more RANTES receptor stimulation. It is possible that these conflicting data are caused by the poly-pharmacotherapy or other confounding variables of the study populations, such as age, sex, and species of the subjects. These discrepancies might also be due to differences in the assays used by the different studies.
It has been demonstrated that PPAR-α agonists inhibited RANTES expression in vitro and in animal studies. Fibrates repressed expression of RANTES induced by tumor necrosis factor-α and by chenodeoxycholic acid in human hepatocyte-derived cells [19,20]. WY-14,643, a fibrate class of PPAR-α ligands, significantly inhibited cisplatin-induced upregulation of RANTES in acute renal failure mice [21]. Additionally, after treatment with PPAR-α agonist (R)-K-13675, RANTES levels were significantly suppressed in human coronary endothelial cells [22]. Our present study demonstrated that serum RANTES levels were reduced through fenofibrate treatment in type 2 diabetes mellitus patients with hypertriglyceridemia.
Our findings in the present study may partially explain the beneficial effects of PPAR-α agonist treatment in clinical trials in which the favorable effects were only partly correlated with lipid changes [7], although further animal and clinical studies are still needed to investigate the mechanisms by which PPAR-α agonists protect against the cardiovascular complications of diabetes.
The limitations of our study are identified as follows. Firstly, our study population was limited to Chinese. Therefore, our findings may not be directly applicable to other populations. Secondly, our sample size was relatively small, so our findings might not be powerful enough to account for potentially confounding factors in our analysis, and our results could be improperly influenced by some outliers due to the sample size. However, we performed post hoc sample size calculation (with G*Power 3.1.9.2) showing that the power to compare the baseline RANTES levels between the type 2 diabetes patients with hypertriglyceridemia group and the control group was 1.00, and that the power to compare the pre-treatment and post-treatment (with fenofibrate) RANTES levels in the type 2 diabetes patients with hypertriglyceridemia group was 0.78 (α=0.05, one-sided test, according to previous studies). Additionally, a small sample size may result in a type II error in the statistical analysis, but we have demonstrated that fenofibrate decreased serum RANTES levels in T2DM patients with hypertriglyceridemia in the present study. Therefore, the power of the analyses might be sufficient in our study. Thirdly, we used serum samples rather than platelet-free plasma for detecting RANTES levels. However, given our precautions to prevent degradation of RANTES after blood collection and during storage, exclusion of using heparin, and to compare the difference between the pre-treatment and post-treatment (with fenofibrate) RANTES levels detected by the same ELISA kits at the same time in the same condition, this methodological problem did not obviously affect the outcomes of our study. Moreover, some studies have demonstrated that measuring RANTES levels in serum and in platelet-free plasma presented similar patterns of results [40]. Fourthly, fenofibrate treatment was administered to T2DM patients with hypertriglyceridemia in our present study. Unfortunately, for several reasons this treatment is sometimes not appropriate (e.g., intolerance, adverse effects, and patient preference). Nutraceuticals and functional food ingredients that are also beneficial to vascular health may are useful compounds that might be able to reduce the overall cardiovascular risk induced by dyslipidemia in situations where fenofibrate cannot be used [41]. The mechanisms underlying such actions are not fully understood, and the effect of nutraceuticals and functional food ingredients on RANTES and inflammation remains to be further investigated. Finally, the cross-sectional design of the study prevented us from determining a causal relationship, but it can certainly raise a credible hypothesis to be confirmed and extended by future prospective cohort and mechanistic studies. Despite these limitations, our results still provide strong evidence for the effectiveness of fenofibrate in reducing circulating RANTES levels.
Conclusions
We presented novel data that fenofibrate treatment significantly decreased serum RANTES levels in type 2 diabetes mellitus patients with hypertriglyceridemia. This result indicates that PPAR-α agonists play an important role in preventing inflammatory responses. The physiologic and pathologic significance of our findings remain to be further elucidated.
Footnotes
Competing interests
None.
Ethics approval
The study was approved by the Medicine and Pharmacy Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China.
Source of support: This work was supported by grants from the Major National Basic Research Program of P.R. China (No. 2011CB503904), the Chinese National Natural Science Foundation (No. 81270369, 81070244, and 30770873), and the Beijing Natural Science Foundation (No. 7142060) to Guang Wang
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Fruchart JC, Sacks FM, Hermans MP, et al. The Residual Risk Reduction Initiative: A call to action to reduce residual vascular risk in dyslipidaemic patient. Diab Vasc Dis Res. 2008;5(4):319–35. doi: 10.3132/dvdr.2008.046. [DOI] [PubMed] [Google Scholar]
- 3.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 4.Lella M, Indira K. A comparative study of efficacy of atorvastatin alone and its combination with fenofibrate on lipid profile in type 2 diabetes mellitus patients with hyperlipidemia. J Adv Pharm Technol Res. 2013;4(3):166–70. doi: 10.4103/2231-4040.116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monsalve FA, Pyarasani RD, Delgado-Lopez F, Moore-Carrasco Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediators Inflamm. 2013;2013:549627. doi: 10.1155/2013/549627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robins SJ, Collins D, Wittes JT, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: A randomized controlled trial. JAMA. 2001;285(12):1585–91. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 7.Scott R, O’Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32(3):493–98. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belfort R, Berria R, Cornell J, Cusi K. Fenofibrate reduces systemic inflammation markers independent of its effects on lipid and glucose metabolism in patients with the metabolic syndrome. J Clin Endocrinol Metab. 2010;95(2):829–36. doi: 10.1210/jc.2009-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, He L, Liu J, et al. Coronary flow velocity reserve is improved by PPAR-α agonist fenofibrate in patients with hypertriglyceridemia. Cardiovas Ther. 2013;31(3):161–67. doi: 10.1111/j.1755-5922.2011.00307.x. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Li C, Li F, et al. PPAR α agonist fenofibrate upregulates tetrahydrobiopterin level through increasing the expression of guanosine triphosphate cyclohydrolase-I in human umbilical vein endothelial cells. PPAR Res. 2011;2011:523520. doi: 10.1155/2011/523520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng X, Gao X, Jia Y, et al. PPAR-α agonist fenofibrate decreased serum irisin levels in type 2 diabetes patients with hypertriglyceridemia. PPAR Res. 2015;2015:924131. doi: 10.1155/2015/924131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8(10):802–15. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 13.Terkeltaub R, Banka CL, Solan J, et al. Oxidized LDL induces monocytic cell expression of interleukin-8, a chemokine with T-lymphocyte chemotactic activity. Arterioscler Thromb. 1994;14(1):47–53. doi: 10.1161/01.atv.14.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 15.Sun W, Wang G, Zhang ZM, et al. Chemokine RANTES is upregulated in monocytes from patients with hyperhomocysteinemia. Acta Pharmacol Sin. 2005;26(11):1317–21. doi: 10.1111/j.1745-7254.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 16.Veillard NR, Kwak B, Pelli G, et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94(2):253–61. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 17.Cipriani S, Francisci D, Mencarelli A, et al. Efficacy of the CCR5 antagonist maraviroc in reducing early, ritonavir – induced atherogenesis and advanced plaque progression in mice. Circulation. 2013;127(21):2114–24. doi: 10.1161/CIRCULATIONAHA.113.001278. [DOI] [PubMed] [Google Scholar]
- 18.Braunersreuther V, Pellieux C, Pelli G, et al. Chemokine CCL5/RANTES inhibition reduces myocardial reperfusion injury in atherosclerotic mice. J Mol Cell Cardiol. 2010;48(4):789–98. doi: 10.1016/j.yjmcc.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Hirano F, Kobayashi A, Makino I. Inhibition of TNF-alpha-induced RANTES expression in human hepatocyte-derived cells by fibrates, the hypolipidemic drugs. Int Immunopharmacol. 2003;3(2):225–32. doi: 10.1016/S1567-5769(02)00275-8. [DOI] [PubMed] [Google Scholar]
- 20.Hirano Y, Hirano F, Fujii H, Makino I. Fibrates suppress chenodeoxycholic acid-induced RANTES expression through inhibition of NF-kappaB activation. Eur J Pharmacol. 2002;448(1):19–26. doi: 10.1016/s0014-2999(02)01902-7. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Gokden N, Okusa MD, et al. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. Am J Physiol Renal Physiol. 2005;289(2):F469–80. doi: 10.1152/ajprenal.00038.2005. [DOI] [PubMed] [Google Scholar]
- 22.Kitajima K, Miura S, Mastuo Y, et al. Newly developed PPAR-α agonist (R)-K-13675 inhibits the secretion of inflammatory markers without affecting cell proliferation or tube formation. Atherosclerosis. 2009;203(1):75–81. doi: 10.1016/j.atherosclerosis.2008.05.055. [DOI] [PubMed] [Google Scholar]
- 23.Wooten JS, Nambi P, Gillard BK, et al. Intensive lifestyle modification reduces Lp-PLA2 in dyslipidemic HIV/HAART patients. Med Sci Sports Exerc. 2013;45(6):1043–50. doi: 10.1249/MSS.0b013e3182843961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dworacka M, Krzyżagórska E, Iskakova S, et al. Increased circulating RANTES in type 2 diabetes. Eur Cytokine Netw. 2014;25(3):46–51. doi: 10.1684/ecn.2014.0355. [DOI] [PubMed] [Google Scholar]
- 25.Ueba T, Nomura S, Inami N, et al. Elevated RANTES level is associated with metabolic syndrome and correlated with activated platelets associated markers in healthy younger men. Clin Appl Thromb Hemost. 2014;20(8):813–18. doi: 10.1177/1076029612467845. [DOI] [PubMed] [Google Scholar]
- 26.Ide T, Tsunoda M, Mochizuki T, Murakami K. Enhancement of insulin signaling through inhibition of tissue lipid accumulation by activation of peroxisome proliferator-activated receptor (PPAR) alpha in obese mice. Med Sci Monit. 2004;10(10):BR388–95. [PubMed] [Google Scholar]
- 27.Neve BP, Fruchart JC, Staels B. Role of the peroxisome proliferator-activated receptors (PPAR) in atherosclerosis. Biochem Pharmacol. 2000;60(8):1245–50. doi: 10.1016/s0006-2952(00)00430-5. [DOI] [PubMed] [Google Scholar]
- 28.Hennuyer N, Tailleux A, Torpier G, et al. PPARα, but not PPARγ, activators decrease macrophage-laden atherosclerotic lesions in a nondiabetic mouse model of mixed dyslipidemia. Arterioscler Thromb Vasc Biol. 2005;25(9):1897–902. doi: 10.1161/01.ATV.0000175756.56818.ee. [DOI] [PubMed] [Google Scholar]
- 29.Yue TL, Bao W, Jucker BM, et al. Activation of peroxisome proliferator-activated receptor-alpha protects the heart from ischemia/reperfusion injury. Circulation. 2003;108(19):2393–99. doi: 10.1161/01.CIR.0000093187.42015.6C. [DOI] [PubMed] [Google Scholar]
- 30.van Raalte DH, Li M, Pritchard PH, Wasan KM. Peroxisome proliferatoractivated receptor (PPAR)-alpha: A pharmacological target with a promising future. Pharm Res. 2004;21(9):1531–38. doi: 10.1023/b:pham.0000041444.06122.8d. [DOI] [PubMed] [Google Scholar]
- 31.Yakubu MA, Nsaif RH, Oyekan AO. Peroxisome proliferator-activated receptor α activation-mediated regulation of endothelin-1 production via nitric oxide and protein kinase C signaling pathways in piglet cerebral microvascular endothelial cell culture. J Pharmacol Exper Therapeut. 2007;320(2):774–81. doi: 10.1124/jpet.106.104992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kowalski J, Okopien B, Madej A, et al. Effects of fenofibrate and simvastatin on plasma sICAM-1 and MCP-1 concentrations in patients with hyperlipoproteinemia. Int J Clin Pharmacol Ther. 2003;41(6):241–47. doi: 10.5414/cpp41241. [DOI] [PubMed] [Google Scholar]
- 33.Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochim Biophys Acta. 2007;1771(8):972–82. doi: 10.1016/j.bbalip.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nomura S, Uehata S, Saito S, et al. Enzyme immunoassay detection of platelet-derived microparticles and RANTES in acute coronary syndrome. Thromb Haemost. 2003;89(3):506–12. [PubMed] [Google Scholar]
- 35.Kraaijeveld AO, de Jager SC, de Jager WJ, et al. CC chemokine ligand-5 (CCL5/RANTES) and CC chemokine ligand-18 (CCL18/PARC) are specific markers of refractory unstable angina pectoris and are transiently raised during severe ischemic symptoms. Circulation. 2007;116(17):1931–41. doi: 10.1161/CIRCULATIONAHA.107.706986. [DOI] [PubMed] [Google Scholar]
- 36.Virani SS, Nambi V, Hoogeveen R, et al. Relationship between circulating levels of RANTES (regulated on activation, normal T-cell expressed, and secreted) and carotid plaque characteristics: the Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Eur Heart J. 2011;32(4):459–68. doi: 10.1093/eurheartj/ehq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichel CA, Khandoga A, Anders HJ, et al. Chemokine receptors Ccr1, Ccr2, and Ccr5 mediate neutrophil migration to postischemic tissue. J Leukoc Biol. 2006;79(1):114–22. doi: 10.1189/jlb.0605337. [DOI] [PubMed] [Google Scholar]
- 38.Rothenbacher D, Muller-Scholze S, Herder C, et al. Differential expression of chemokines, risk of stable coronary heart disease, and correlation with established cardiovascular risk markers. Arterioscler Thromb Vasc Biol. 2006;26(1):194–99. doi: 10.1161/01.ATV.0000191633.52585.14. [DOI] [PubMed] [Google Scholar]
- 39.Cavusoglu E, Eng C, Chopra V, et al. Low plasma RANTES levels are an independent predictor of cardiac mortality in patients referred for coronary angiography. Arterioscler Thromb Vasc Biol. 2007;27(4):929–35. doi: 10.1161/01.ATV.0000258789.21585.76. [DOI] [PubMed] [Google Scholar]
- 40.Aukrust P, Müller F, Frøland SS. Circulating levels of RANTES in human immunodeficiency virus type 1 infection: effect of potent antiretroviral therapy. J Infect Dis. 1998;177(4):1091–96. doi: 10.1086/517402. [DOI] [PubMed] [Google Scholar]
- 41.Scicchitano P, Cameli M, Maiello M, et al. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J Funct Foods. 2014;6:11–32. [Google Scholar]


