Abstract
Background
Genetic variations in the IL-27 gene have been proven to be associated with various types of human cancers and diseases. The purpose of the current study was to clarify the associations of the IL-27 rs153109 A>G and rs181206 T>C variants with human diseases using a meta-analysis study.
Material/Methods
A comprehensive electronic and manual search was carried out to find potential eligible studies. The effect size was represented by the unadjusted odds ratios (ORs). A 95% confidence interval (95%CI) was tested for the pooled OR using the Z test.
Results
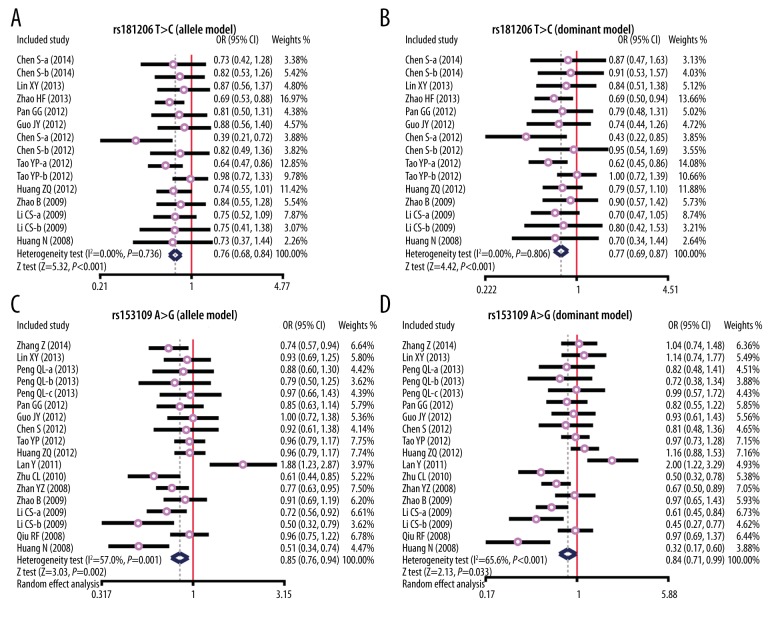
A total of 17 case-control studies (cases=4185, healthy controls=4077) were included in our study. Our study showed that the carriers of the rs181206 T>C and rs153109 A>G polymorphism in the IL-27 gene have elevated risks of diseases in the allele model (rs181206 T>C: OR=0.76, 95%CI=0.69~0.84, P<0.001; rs153109 A>G: OR=0.85, 95%CI=0.76~0.94, P=0.002) and dominant model (rs181206 T>C: OR=0.77, 95%CI=0.69~0.87, P<0.001; rs153109 A>G: OR=0.84, 95%CI=0.71~0.99, P=0.033). Disease type-stratified subgroup analysis yielded increased risk of related diseases in IL-27 rs181206 T>C carriers in the allele model in immune thrombocytopenia (ITP), asthma, and esophageal cancer (EC) subgroups (ITP: OR=0.69, 95%CI=0.53~0.88, P=0.004; asthma: OR=0.60, 95%CI=0.41~0.89, P=0.010; EC: OR=0.79, 95%CI=0.64~0.97, P=0.026); and IL-27 rs153109 A>G polymorphism was remarkably associated with the increased risk of related diseases in the allele model in ovarian cancer (OC), systemic lupus erythematosus (SLE), tuberculosis (TB), ulcerative colitis (UC), and chronic obstructive pulmonary disease (COPD) subgroups (all P<0.05).
Conclusions
Our results indicate that the genetic polymorphisms of IL-27 rs153109 and rs181206 may be involved in the progression of human cancers and diseases, especially of TB, UC, COPD, OC, and ITP.
MeSH Keywords: Interleukin-27; Meta-Analysis; Polymorphism, Genetic
Background
Interleukin-27 (IL-27), which belongs to the IL-12 family, is a newly-discovered protein consisting of 2 subunits, p28 and the Epstein–Barr virus-induced gene 3 protein, and is mainly secreted by activated antigen-presenting cells [1,2]. Human IL-27 gene, located on chromosome 16p11, consists of 5 exons and 4 introns, acting as a mediator between the adaptive and innate immune systems [3]. As a multifunctional gene, IL-27 can regulate the proliferation of naive T-cell and plays an effective role in inducing the production of interferon-gamma [4,5]. In addition, IL-27 is also involved in the up-regulation of Th1 initiation and the down-regulation of Th2 factor GATA binding protein 3, and it is linked to inducing tumor-specific antitumor activity, mediated by CD8+ T cells [1,5]. Mutations of IL-27 were recently reported to be susceptible to a variety of diseases, such as colorectal cancer, asthma, rheumatoid arthritis, ovarian cancer, Crohn’s disease, nasopharyngeal carcinoma, and esophageal cancer [1,6,7]. Specifically, according to previous studies, 2 single-nucleotide polymorphisms (SNPs) of IL-27, 964A/G (rs153109) and 4730 T/C (rs181206), are commonly found to be associated with various diseases [6,8]. For instance, a previous study investigated the association of IL-27 gene polymorphism with Crohn’s disease (CD) risk in a Chinese Han population found remarkable association between IL-27 rs153109 and rs181206 polymorphisms and CD risks [8]. Moreover, previous studies also found that rs153109 polymorphism may be a protective factor for breast cancer in premenopausal women and is associated with decreased risk of lymph node metastasis in papillary thyroid cancer [9,10]. Through the promotion of pro-inflammatory immune response, polymorphisms rs153109 and rs181206 of IL-27 reduce or delay common antitumor activities responded to by Th1 cytotoxic, increasing the susceptibility to diseases [7,11,12]. Therefore, IL-27 could be regarded as a candidate gene related to diverse diseases. Previous studies have also shown that variants of IL-27 are closely correlated with diseases, including numerous common cancers, such as colorectal cancer, nasopharyngeal carcinoma, and ovarian cancer [6,13], while some other studies have presented different results [14,15]. Therefore, the current study was performed to investigate the potential relationships between the polymorphisms of IL-27, rs153109, and rs181206, and their susceptibility to diseases, and to evaluate the role of IL-27 as a biomarker for the diagnosis and prognosis of diseases.
Material and Methods
Search strategy
Potential studies were retrieved by a thorough literature search, with language restricted to English or Chinese, in the computerized bibliographic databases MEDLINE, Science Citation Index, PubMed, Embase, Current Contents Index, Chinese Biomedical, Chinese Journal Full-Text, and the Weipu Journal (last updated search was October 2014). We used the following highly sensitive search strategy: (“Interleukin-27” or “Interleukin 27” or “IL 27” or “IL-27” or “IL27”) and (“Polymorphism, Genetic” or “polymorphism” or “polymorphisms” or “variants” or “SNP” or “mutation” or “genetic variants”). A manual search was also conducted to find other studies.
Inclusion and exclusion criteria
Retrieved studies were assessed based on the following criteria: (1) studies published in peer-reviewed journal and conducted in human populations; (2) case-control studies investigating the association between SNPs in IL-27 (rs153109 A>G or rs181206 T>C) and susceptibility and human diseases; (3) patients were confirmed by the diagnostic criteria for each disease type; (4) provided sufficient original data on the genotype frequencies of polymorphisms within the IL-27 genes; (5) genotype frequencies of IL-27 met Hardy-Weinberg equilibrium (HWE); (6) studies with overlapping data were only enrolled once. The exclusion criteria were: (1) failed to meet the criteria for study inclusion; (2) letters, abstracts, meta-analysis, reviews, or proceedings; (3) unpublished data; (4) studies without extractable and numerical data; (5) Caucasian population.
Study quality and data extraction
Newcastle-Ottawa Scale (NOS) criteria were applied to assess the methodological quality of all eligible trials [16]. These 3 perspectives were independently scored by 2 reviewers: (1) subject selection: 0~4; (2) subject comparability: 0~2; (3) clinical outcome: 0~3. Study with NOS scores ≥7 were considered as good quality (range, 0~9). Following descriptive information were collected including author name, publication year, journal name, country, detection method, population, language, diseases types, SNP, genotype frequencies, allele frequencies, HWE test, and confirmation of diagnosis. Disagreements during the study inclusion process were settled through consultation with another reviewer.
Statistical analysis
The effect size, as represented by the unadjusted odds ratios (ORs) for the presence of the SNP in IL-27 in patients and controls, was calculated. A 95% confidence interval (95%CI) was tested for the pooled OR using Z test. The summarized ORs were performed for the comparisons in allele model (W allele vs. M allele), dominant model (WW + WM vs. MM), recessive model (WW vs. WM + MM), homozygous model (WW vs. MM), and heterozygous model (WW vs. WM). Cochran’s Q-statistic and I2 tests were carried out to identify the heterogeneity among included trials [17]. Subgroup analyses were also conducted to clarify the substantial heterogeneity. Additionally, the effect of each single study on the overall estimate was determined by application of one-way sensitivity analysis. Further, Egger’s linear regression test and funnel plot were employed to inspect the presence of publication bias [18,19]. Data analyses were performed utilizing STATA software (Version 12.0, Stata Corp., College Station, TX, USA).
Results
Description of included studies
The initial search resulted in a total of 46 potentially eligible articles. After the removal of 1 duplicated study and 16 irrelevant articles, 29 articles remained for further review. Subsequently, 11 articles were regarded as unsuitable and were removed, and 18 articles included qualitative analysis were left. In addition, another study was removed due to absence of data integrity. Finally, 17 case-control studies including 4185 patients and 4077 controls were incorporated into the current analysis [1,4–7,11–15,20–26]. A flow diagram of study selection progress is displayed in Figure 1. As for the diseases involved in the included studies, there were 16 diseases, including rheumatoid arthritis (RA) (Chen S, 2014), ovarian cancer (OC) (Zhang Z), CD (Lin XY), immune thrombocytopenia (ITP) (Zhao HF), chronic hepatitis B (CHB) (Peng QL-a), hepatitis B virus (HBV)-related liver cirrhosis (LC) (Peng QL-b), Hepatocellular carcinoma (HCC) (Peng QL-c), nasopharyngeal carcinoma (NPC) (Pan GG), colorectal cancer (CRC) (Guo JY and Huang ZQ), Asthma (Qiu RF and Chen S, 2012), esophageal cancer (EC) (Tao YP), systemic lupus erythematosus (SLE) (Lan Y), HBV (Zhu CL), Tuberculosis (TB) (Zhan YZ), Glioma (Zhao B), and chronic obstructive pulmonary disease (COPD) (Huang N).
Figure 1.
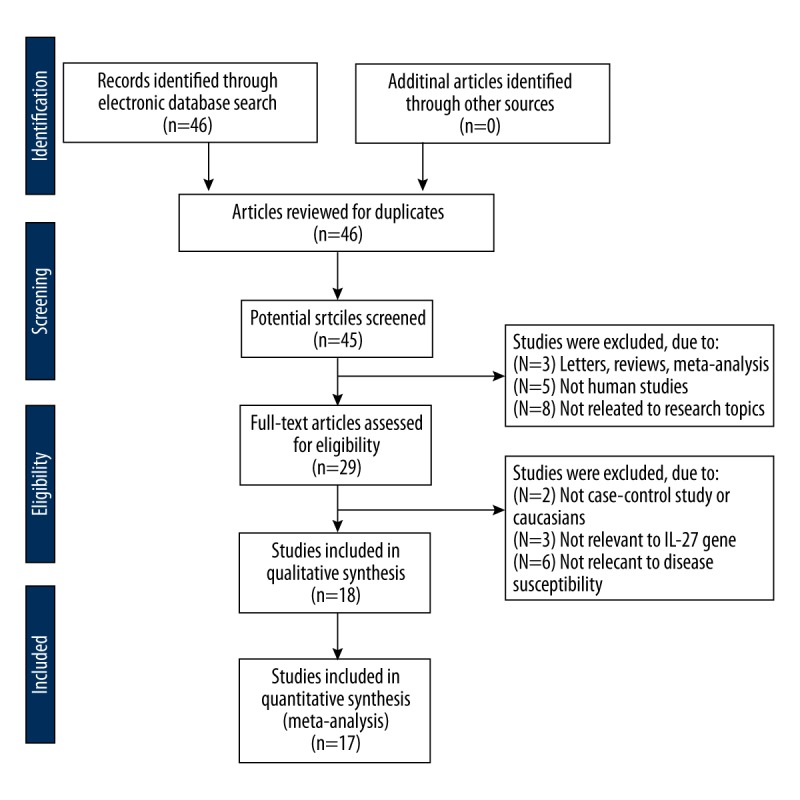
Flow chart shows study selection procedure. Seventeen case-control studies were included in this meta-analysis.
When focused on the SNPs information, 9 studies analyzed rs153109 A>G and rs181206 T>C SNPs, (Lin XY, Chen S, 2014, Chen S, 2012, Tao YP, Huang ZQ, Zhao B, Li CS, Pan GG and Huang N) and 8 studies only investigated rs153109 A>G SNPs (Zhang Z, Zhao HF, Peng QL, Guo JY, Lan Y, Zhu CL, Zhan YZ and Qiu RF). All 17 studies were performed in Asians. Regarding the detection methods, direct sequencing was used in 2 studies [Chen S (2012) and Chen S (2014)]; PCR-RFLP was used in 14 studies [Zhang Z, Zhao HF, Peng QL (rs153109 A>G), Pan GG, Guo JY, Tao YP, Huang ZQ, Lan Y, Zhun CL, Zhan YZ-a, Zhao B, Qiu RF and Huang N]; PCR-SSP was used in 2 studies [Peng QL and Zhan YZ-b]; PCR-LDR was used in 1 study (Lin XY); and SBE was used in 1 study (Li CS). The basic information for 17 eligible studies is presented in Table 1.
Table 1.
Characteristics of included studies in this meta-analysis.
| First author | Year | Country | Disease type | Number | Gender (M/F) | Age (years) | Genotyping method | SNP | NOS score | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | Case | Control | |||||||
| Chen S [14] | 2014 | China | RA | 103 | 104 | 28/75 | 43/61 | 40 (40~76) | 57 (35~71) | Direct sequencing | rs153109 A>G, rs181206 T>C | 7 |
| Zhang Z [7] | 2014 | China | OC | 229 | 320 | 0/229 | 0/320 | 48.9±10.8 | 48.3±10.1 | PCR-RFLP | rs153109 A>G | 8 |
| Lin XY [15] | 2013 | China | CD | 145 | 238 | 94/51 | 155/83 | 34.3±12.9 | 36.4±11.8 | PCR-LDR | rs153109 A>G, rs181206 T>C | 7 |
| Zhao HF [12] | 2013 | China | ITP | 120 | 280 | 50/70 | 101/179 | 11 (0.8~44) | 36 (28~72) | PCR-RFLP | rs153109 A>G | 7 |
| ITP | 210 | 280 | 88/122 | 101/179 | 36 (17~78) | 36 (28~72) | PCR-RFLP | rs153109 A>G | 8 | |||
| Peng QL-a [11] | 2013 | China | CHB | 112 | 105 | 85/27 | 83/22 | 41.3±11.3 | 43.2±11.1 | PCR-RFLP | rs153109 A>G | 8 |
| LC | 65 | 105 | 65/51 | 83/22 | 45.6±11.9 | 43.2±11.1 | PCR-RFLP | rs153109 A>G | 7 | |||
| HCC | 107 | 105 | 107/85 | 83/22 | 45.4±12.4 | 43.2±11.1 | PCR-RFLP | rs153109 A>G | 7 | |||
| Pan GG [23] | 2012 | China | NPC | 190 | 200 | 135/55 | 140/60 | 48.3±8.1 | 46.5±7.6 | PCR-RFLP | rs153109 A>G, rs181206 T>C | 7 |
| Guo JY [20] | 2012 | China | CRC | 170 | 160 | 107/63 | 105/55 | 52.7±10.3 | 48.2±9.4 | PCR-RFLP | rs153109 A>G | 7 |
| Chen S [13] | 2012 | China | Asthma | 200 | 111 | 82/118 | 69/42 | – | – | Direct sequencing | rs153109 A>G, rs181206 T>C | 7 |
| Tao YP [6] | 2012 | China | EC | 426 | 432 | 312/114 | 325/107 | – | – | PCR-RFLP | rs153109 A>G, rs181206 T>C | 7 |
| Huang ZQ [1] | 2012 | China | CRC | 410 | 450 | 315/95 | 324/126 | 57.7±8.3 | 55.2±7.5 | PCR-RFLP | rs153109 A>G, rs181206 T>C | 8 |
| Lan Y [22] | 2011 | China | SLE | 135 | 150 | – | – | – | – | PCR-RFLP | rs153109 A>G | 6 |
| Zhu CL [32] | 2010 | China | HBV | 168 | 152 | 110/58 | 95/57 | 56.3±10.4 | 48.6±18.5 | PCR-RFLP | rs153109 A>G | 7 |
| Zhan YZ [25] | 2009 | China | TB | 385 | 391 | 243/142 | 266/125 | 30.0±15.0 | 24.0±15.0 | PCR-RFLP | rs153109 A>G | 8 |
| Zhao B [4] | 2009 | China | Glioma | 210 | 220 | 127/83 | 125/95 | 42.3±8.2 | 40.9±7.1 | PCR-RFLP | rs153109 A>G, rs181206 T>C | 8 |
| Li CS [5] | 2009 | Korea | UC | 249 | 444 | 179/141 | 278/166 | – | – | SBE | rs153109 A>G, rs181206 T>C | 7 |
| CD | 71 | 444 | – | – | ||||||||
| Qiu RF [24] | 2008 | China | Asthma | 360 | 220 | 165/195 | 160/60 | – | – | PCR-RFLP | rs153109 A>G | 7 |
| Huang N [21] | 2008 | China | COPD | 120 | 100 | 73/47 | 64/36 | 59.1±11.0 | 57.7±10.8 | PCR-RFLP | rs153109 A>G, rs181206 T>C | 6 |
RA – rheumatoid arthritis; OC – ovarian cancer; CD – Crohn’s disease; ITP – immune thrombocytopenia; T1D – type 1 diabetes mellitus; CHB – chronic hepatitis B; LC – hepatitis B virus (HBV)-related liver cirrhosis; HCC – hepatocellular carcinoma; NPC – nasopharyngeal carcinoma; CRC – colorectal cancer; EC – esophageal cancer; SLE – systemic lupus erythematosus; HBV – hepatitis B virus; TB – tuberculosis; COPD – chronic obstructive pulmonary disease; M – male; F – female; NOS – Newcastle-Ottawa Scale; SNP – single-nucleotide polymorphism; PCR-RFLP – polymerase chain reaction-restriction fragment length polymorphism; SBE – single-base extension; PCR-LDR – polymerase chain reaction-ligase detection reaction; UC – ulcerative colitis.
Quantitative data synthesis
In the current study, 2 SNPs (rs153109 A>G, and rs181206 T>C) within the IL-27 gene were identified. Additionally, since significant heterogeneity existed, studies were stratified by disease type. Our results suggested that the carriers of the rs181206 T>C polymorphism in the IL-27 gene have an elevated risk of diseases in the allele model (OR=0.76, 95%CI=0.69~0.84, P<0.001) and dominant model (OR=0.77, 95%CI=0.69~0.87, P<0.001). With respect to the rs153109 A>G polymorphism, findings in the present study suggested that the population containing rs153109 A>G polymorphism was more likely to develop related diseases in comparison to the control group in both the allele model (OR=0.85, 95%CI=0.76~0.94, P=0.002) and the dominant model (OR=0.84, 95%CI=0.71~0.99, P=0.033) (Figure 2A–2D).
Figure 2.
Forest plots for the influences of IL-27 genetic polymorphism and various human diseases under the allele and dominant models ((A) Allele model of rs1181206 T>C; (B) Dominant model of rs1181206 T>C; (C) Allele model of rs153109 A>G; (D) Dominant model of rs 153109 A>G).
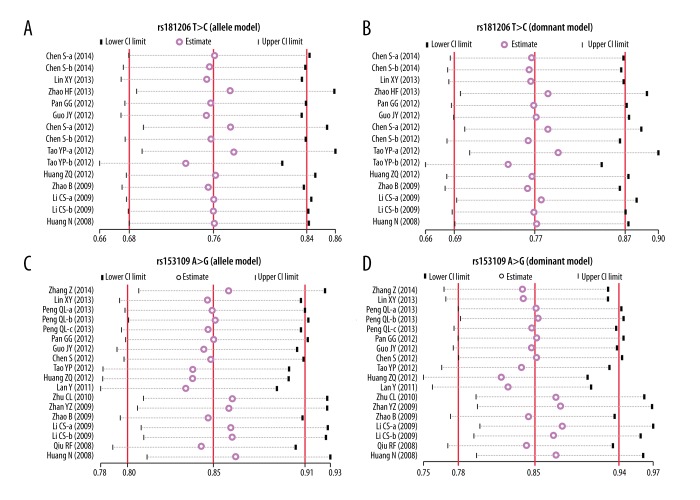
In the disease type-stratified subgroup analysis, our results yielded elevated risk of related diseases in IL-27 rs181206 T>C carriers in allele model in the ITP, Asthma, and EC subgroup (ITP: OR=0.69, 95%CI=0.53~0.88, P=0.004; Asthma: OR=0.60, 95%CI=0.41~0.89, P=0.010; EC: OR=0.79, 95%CI=0.64~0.97, P=0.026), but not in RA, CD, NPC, CRC, Glioma, UC, and COPD (all P > 0.05). It has been suggested that subjects with the IL-27 rs153109 A>G polymorphism had elevated risk of related diseases in the allele model in the OC, SLE, TB, UC and the COPD subgroups (all P<0.05); but not in the CD, CHB, LC, HCC, NPC, CRC, Asthma, EC, or Glioma subgroups (all P>0.05) (Figure 3A, 3B and Table 2).
Figure 3.
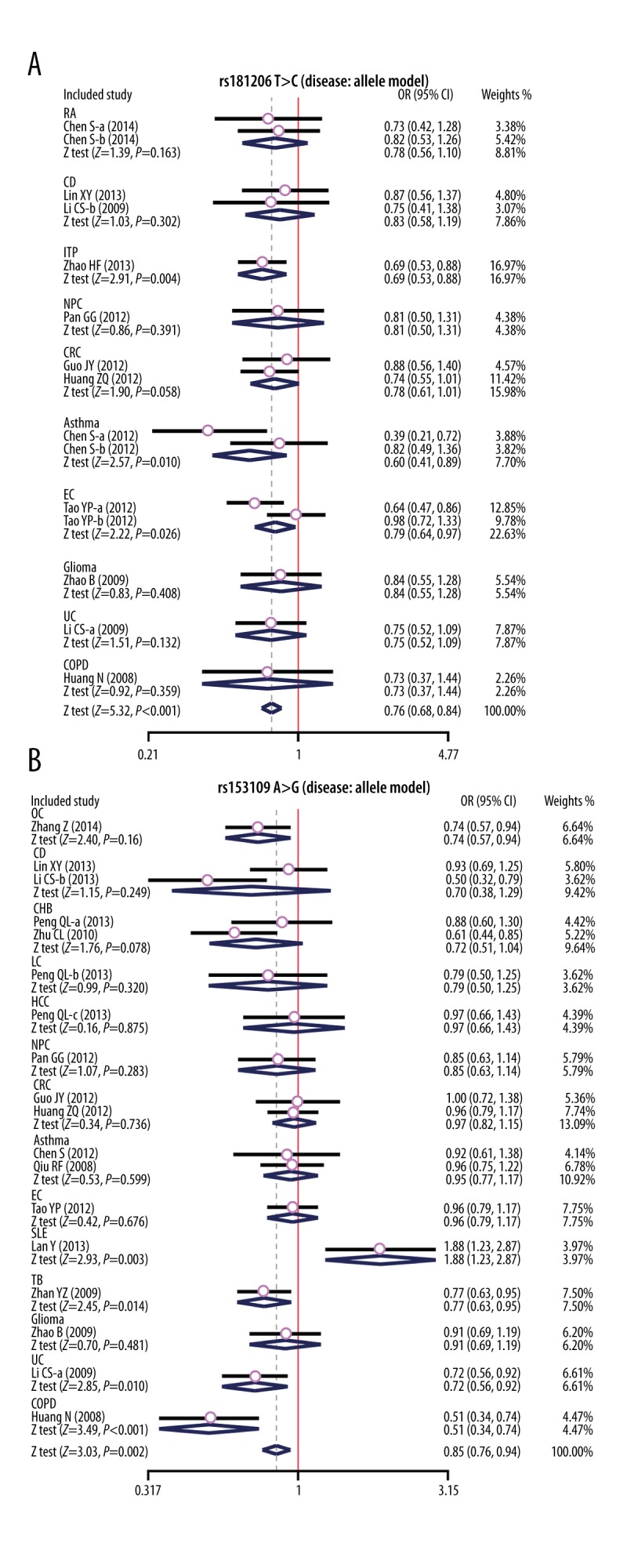
Subgroup analyses for the influences of IL-27 genetic polymorphism and various human diseases under the allele and dominant models ((A) Allele model of rs1181206 T>C; (B) Allele model of rs153109 A>G).
Table 2.
Meta-analysis of the association between IL-27 polymorphisms and various human diseases.
| Subgroup analysis | W allele vs. M (allele model) | WW + WM vs. MM (dominant model) | WW vs. WM (homozygous model) | WW vs. MM (heterozygous model) | WW vs. WM + MM (recessive model) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| rs181206 T>C | 0.76 | 0.69~0.84 | < 0.001 | 0.78 | 0.69~0.87 | <0.001 | 0.38 | 0.26~0.56 | < 0.001 | 2.16 | 1.46~3.21 | <0.001 | 0.29 | 0.20~0.43 | < 0.001 |
| RA | 0.78 | 0.56~1.10 | 0.163 | 0.89 | 0.59~1.34 | 0.583 | 0.23 | 0.07~0.77 | 0.017 | 4.44 | 1.32~14.96 | 0.016 | 0.15 | 0.05~0.51 | 0.002 |
| CD | 0.83 | 0.58~1.19 | 0.302 | 0.83 | 0.56~1.23 | 0.348 | 0.69 | 0.16~3.00 | 0.625 | 1.22 | 0.28~5.43 | 0.790 | 0.56 | 0.13~2.44 | 0.442 |
| ITP | 0.69 | 0.53~0.88 | 0.004 | 0.69 | 0.50~0.94 | 0.020 | 0.34 | 0.17~0.70 | 0.003 | 2.24 | 1.08~4.63 | 0.030 | 0.24 | 0.12~0.49 | < 0.001 |
| NPC | 0.81 | 0.50~1.31 | 0.391 | 0.79 | 0.48~1.31 | 0.364 | – | – | – | – | – | – | – | – | – |
| CRC | 0.78 | 0.61~1.01 | 0.058 | 0.78 | 0.59~1.03 | 0.081 | 0.60 | 0.26~1.40 | 0.237 | 1.27 | 0.53~3.07 | 0.592 | 0.52 | 0.23~1.21 | 0.130 |
| Asthma | 0.60 | 0.41~0.89 | 0.010 | 0.68 | 0.44~1.05 | 0.082 | 0.10 | 0.02~0.58 | 0.010 | 7.86 | 1.32~46.63 | 0.023 | 0.09 | 0.02~0.50 | 0.006 |
| EC | 0.79 | 0.64~0.97 | 0.026 | 0.79 | 0.63~0.99 | 0.041 | 0.14 | 0.02~1.11 | 0.062 | 5.78 | 0.70~48.01 | 0.104 | 0.11 | 0.01~0.90 | 0.039 |
| Glioma | 0.84 | 0.55~1.28 | 0.408 | 0.90 | 0.57~1.42 | 0.656 | 0.11 | 0.01~2.13 | 0.146 | 8.61 | 0.45~164.64 | 0.153 | 0.09 | 0.00~1.72 | 0.110 |
| UC | 0.75 | 0.52~1.09 | 0.132 | 0.70 | 0.47~1.05 | 0.085 | 1.22 | 0.27~5.49 | 0.798 | 0.56 | 0.12~2.62 | 0.460 | 1.04 | 0.23~4.67 | 0.963 |
| COPD | 0.73 | 0.37~1.44 | 0.359 | 0.70 | 0.34~1.44 | 0.336 | – | – | – | – | – | – | – | – | – |
| rs153109 A>G | 0.85 | 0.76~0.94 | 0.002 | 0.84 | 0.71~0.99 | 0.033 | 0.73 | 0.60~0.89 | 0.002 | 1.21 | 0.98~1.48 | 0.074 | 0.78 | 0.65~0.93 | 0.007 |
| OC | 0.74 | 0.57~0.94 | 0.016 | 1.04 | 0.74~1.48 | 0.810 | 0.19 | 0.09~0.42 | < 0.001 | 7.39 | 3.41~16.04 | <0.001 | 0.16 | 0.07~0.34 | < 0.001 |
| CD | 0.70 | 0.38~1.29 | 0.249 | 0.73 | 0.30~1.80 | 0.495 | 0.51 | 0.17~1.52 | 0.227 | 1.81 | 1.05~3.14 | 0.033 | 0.58 | 0.34~0.98 | 0.040 |
| CHB | 0.72 | 0.51~1.04 | 0.078 | 0.62 | 0.38~1.01 | 0.056 | 0.63 | 0.38~1.04 | 0.069 | 0.99 | 0.59~1.64 | 0.957 | 0.79 | 0.49~1.26 | 0.313 |
| LC | 0.79 | 0.50~1.25 | 0.320 | 0.72 | 0.38~1.34 | 0.300 | 0.70 | 0.29~1.73 | 0.441 | 1.03 | 0.42~2.56 | 0.945 | 0.82 | 0.36~1.90 | 0.648 |
| HCC | 0.97 | 0.66~1.43 | 0.875 | 0.99 | 0.57~1.72 | 0.973 | 0.92 | 0.42~2.01 | 0.843 | 1.10 | 0.51~2.36 | 0.803 | 0.92 | 0.45~1.86 | 0.807 |
| NPC | 0.85 | 0.63~1.14 | 0.283 | 0.82 | 0.55~1.22 | 0.334 | 0.74 | 0.39~1.40 | 0.355 | 1.14 | 0.60~2.16 | 0.685 | 0.80 | 0.44~1.46 | 0.475 |
| CRC | 0.97 | 0.82~1.15 | 0.736 | 1.09 | 0.87~1.38 | 0.457 | 0.83 | 0.58~1.19 | 0.304 | 1.26 | 0.58~2.77 | 0.559 | 0.81 | 0.45~1.46 | 0.487 |
| Asthma | 0.95 | 0.77~1.17 | 0.599 | 0.92 | 0.69~1.22 | 0.558 | 0.94 | 0.62~1.44 | 0.780 | 1.00 | 0.66~1.53 | 0.993 | 0.96 | 0.65~1.42 | 0.850 |
| EC | 0.96 | 0.79~1.17 | 0.676 | 0.97 | 0.73~1.28 | 0.818 | 0.90 | 0.59~1.37 | 0.623 | 1.10 | 0.73~1.65 | 0.650 | 0.91 | 0.62~1.33 | 0.615 |
| SLE | 1.88 | 1.23~2.87 | 0.003 | 2.00 | 1.22~3.29 | 0.006 | 3.77 | 0.97~14.69 | 0.055 | 0.49 | 0.12~1.99 | 0.320 | 3.09 | 0.80~11.88 | 0.101 |
| TB | 0.77 | 0.63~0.95 | 0.014 | 0.67 | 0.50~0.89 | 0.005 | 0.70 | 0.46~1.05 | 0.087 | 0.93 | 0.62~1.41 | 0.746 | 0.86 | 0.59~1.26 | 0.439 |
| Glioma | 0.91 | 0.69~1.19 | 0.481 | 0.97 | 0.65~1.43 | 0.864 | 0.77 | 0.44~1.35 | 0.363 | 1.36 | 0.79~2.35 | 0.272 | 0.75 | 0.45~1.26 | 0.275 |
| UC | 0.72 | 0.56~0.92 | 0.010 | 0.61 | 0.45~0.84 | 0.002 | 0.72 | 0.40~1.29 | 0.264 | 0.83 | 0.45~1.53 | 0.553 | 0.88 | 0.50~1.57 | 0.670 |
| COPD | 0.51 | 0.34~0.74 | < 0.001 | 0.32 | 0.17~0.60 | <0.001 | 0.24 | 0.10~0.56 | 0.001 | 1.46 | 0.69~3.07 | 0.324 | 0.48 | 0.23~0.98 | 0.043 |
W – wild allele; M – mutant allele; WW – wild homozygote; WM – heterozygote; MM – mutant homozygote; RA – rheumatoid arthritis; OC – ovarian cancer; CD – Crohn’s disease; ITP – immune thrombocytopenia; T1D – type 1 diabetes mellitus; CHB – chronic hepatitis B; LC – hepatitis B virus (HBV)-related liver cirrhosis; HCC – hepatocellular carcinoma; NPC – nasopharyngeal carcinoma; CRC – colorectal cancer; EC – esophageal cancer; SLE – systemic lupus erythematosus; HBV – hepatitis B virus; TB – tuberculosis; IBD – inflammatory bowel disease; UC – ulcerative colitis; COPD – chronic obstructive pulmonary disease; PCR-RFLP – polymerase chain reaction-restriction fragment length polymorphism.
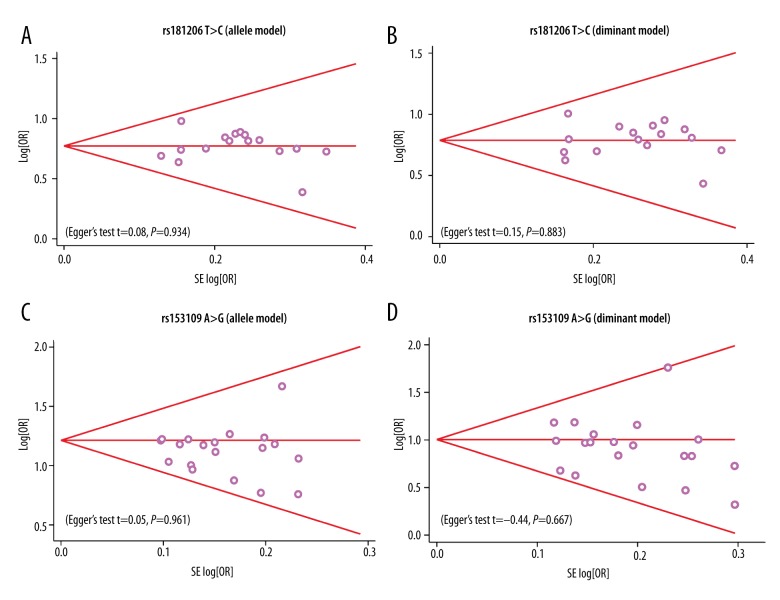
We further conducted sensitivity analyses to determine whether review conclusions were affected by any single study. We found that no single study affected the pooled ORs in the current meta-analysis (Figure 4A–4D). Finally, Egger’s regression test implied no asymmetrical distribution in the funnel plot in the rs181206 T>C (allele model: t=0.08, P=0.934; dominant model: t=0.15, P=0.883, respectively), and the rs153109 A>G (allele model: t=0.05, P=0.961; dominant model: t=−0.44, P=0.667, respectively) (Figure 5A–5D).
Figure 4.
Sensitivity analysis for the influences of IL-27 genetic polymorphism and various human diseases under the allele and dominant models ((A) Allele model of rs1181206 T>C; (B) Dominant model of rs1181206 T>C; (C) Allele model of rs153109 A>G; (D) Dominant model of rs53109 A>G).
Figure 5.
Funnel plot of publication biases on the relationships between IL-27 genetic polymorphism and various human diseases under the allele and dominant models ((A) Allele model of rs1181206 T>C; (B) Dominant model of rs1181206 T>C; (C) Allele model of rs153109 A>G; (D) Dominant model of rs 153109 A>G).
Discussion
A meta-analysis was performed to explore the relationship between polymorphisms of rs153109 (−964 A/G), rs181206 (4730 T/C) in the IL-27 gene and the development of some inflammatory, autoimmune diseases and cancer. The results of our meta-analysis demonstrated the relationship of the polymorphisms of rs153109 with the pathogenesis of TB, SLE, UC, COPD, and OC and the connection between polymorphisms of rs181206 with the development of ITP, asthma, and EC. Interleukins promote the development and differentiation of T and B lymphocytes, and hematopoietic cells and are involved in immune system function. Increasing data demonstrate that many human diseases may be associated with the gene polymorphisms of IL-8, IL-6, IL-1β, and IL-10 [27–30]. IL-27, a heterodimeric cytokine, consists of the Epstein Barr-virus-induced gene 3, which contains 2 cytokine-binding domains without membrane anchoring motifs and a cytoplasmic tail, having no its own activity and IL-27p28, a predicted cytokine-like protein with 4-alpha helix bundle, forming functional IL-27 [31]. IL-27, structurally linked with IL-12 and IL-23, engages a receptor of gp130 and the IL-27Rα, which could activate JAK-signal transducer, an activator of transcription and signaling of mitogen-activated protein kinase [32]. Through promoting the proliferation of T cell and inducing the production of IFN-γ, IL-27 could act as a TH1 type immune response initiator to take part in host defense against pathogen infections and by suppressing effector and memory T cells expansion and inhibiting cytokine secretion, such as TH1, TH2, and TH17, IL-27, which could also act as an immune inflammatory response attenuator to participate in autoimmunity and infection [33]. IL-27 also induces tumor-specific antitumor activity, mainly by mediating CD8+ T cells with enhancement of CTL activity [11]. It has been reported that IL-27 is connected with inflammatory and autoimmunity diseases and cancer by modulating Tr-1 cells, Tfh, Treg, and other linked immune cells [34]. Human IL-27 gene consists of 5 exons, with rs153109 (−964 A/G), rs181206 (4730 T/C) in the promoter of the IL-27 gene, which could modulate the expression of IL-27 [6]. Recently, increasing data found that the rs153109 and rs181206 in IL-27 gene was associated with risk of asthma [35]. One mechanism could be the polymorphism of rs153109 of the IL-27 gene just located in the disease-related locus such as UC and CD, suggesting the association with susceptibility to UC and CD [5]. Another possible mechanism is that different haplotypes and genotypes of the IL-27 gene may vary in the expression of IL-27, thus playing various roles in the progression of diseases. It was reported that the IL-27 964A/G AG genotype and IL-27 gene TGG haplotype could protect the progression of COPD, while the G allele in IL-27 964A/G might also be related to the susceptibility to SLE [21]. rs153109 is located in the promoter of IL-27, which could play a crucial role in initiating the transcription and protein expression regulation, which was associated with epithelial ovarian cancer [7]. In addition, IL-27 could trigger the proliferation of naive T-cells and implicated in the development of EC by inhibiting Th1 cytotoxic response to the delayed acquisition of common antitumor activities [6]. We conclude from the above analysis that the polymorphisms of IL-27 participate in many diseases because of the location in the disease-related area and the promoter, and different genotypes and haplotypes of IL-27. Zhan et al. found that rs153109 variant was susceptible with tuberculosis due to the decreased IFN-γ and IL-12 secretion to improve the survival of tubercle bacilli in the macrophages to abate the immune reaction to tubercle bacilli (http://www.cabdirect.org/abstracts/20103084194.html;jsessionid=43F95B13D04B3CF47861675891E2BCC6).
Considering that possible related influential factors may influence the link of rs153109 and rs181206 in IL-27 gene polymorphisms and the development of diseases, we conducted a stratified analysis based on different diseases and detection methods. Subgroup analysis of different diseases show that the IL-27 gene polymorphisms are related with specific diseases, such as ITP, OC, TB, CD, UC, asthma, EC, and COPD, which may due to different pathogenesis of different diseases. In conclusion, our results are consistent with other studies reporting that the polymorphisms of rs153109 and rs181206 in the IL-27 gene have a close relationship with many diseases, suggesting the IL-27 gene polymorphisms may be important in disease diagnosis and prognosis.
Some limitations of our study need to be noted. Firstly, only 2 SNPs (rs153109 and rs181206), widely discussed in various research, were investigated in our study. Additional genomic loci (such as rs17855750) should be covered in larger samples and with a broader coverage of genetic variation across the human genome [33]. Moreover, although the current study covers a great range of human diseases in Asian populations, the sample size for each disease was rather limited. Finally, further investigations to identify IL-27 rs153109 and rs181206 polymorphisms with human diseases are needed to validate and confirm our results.
Conclusions
Taken together, our findings suggest that the genetic polymorphism of IL-27 rs153109 and rs181206 may be involved in the progression of human cancers and diseases, especially of TB, UC, COPD, OC, and ITP. Clinical studies and functional analyses are required to investigate the mechanisms underlying the association of other genomic loci in the IL-27 gene or the role of the IL-12 cytokine family with the risk of human cancers and diseases.
Acknowledgments
We would like to acknowledge the helpful comments on this paper received from our reviewers.
Footnotes
Competing interests
We declare that we have no conflicts of interest.
Source of support: Departmental sources
References
- 1.Huang ZQ, Wang JL, Pan GG, Wei YS. Association of single nucleotide polymorphisms in IL-12 and IL-27 genes with colorectal cancer risk. Clin Biochem. 2012;45:54–59. doi: 10.1016/j.clinbiochem.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Wang S, Shan B, et al. IL-27-mediated activation of natural killer cells and inflammation produced antitumour effects for human oesophageal carcinoma cells. Scand J Immunol. 2008;68:22–29. doi: 10.1111/j.1365-3083.2008.02111.x. [DOI] [PubMed] [Google Scholar]
- 3.Pflanz S, Timans JC, Cheung J, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity. 2002;16:779–90. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 4.Zhao B, Meng LQ, Huang HN, et al. A novel functional polymorphism, 16974 A/C, in the interleukin-12-3′ untranslated region is associated with risk of glioma. DNA Cell Biol. 2009;28:335–41. doi: 10.1089/dna.2008.0845. [DOI] [PubMed] [Google Scholar]
- 5.Li CS, Zhang Q, Lee KJ, et al. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J Gastroenterol Hepatol. 2009;24:1692–96. doi: 10.1111/j.1440-1746.2009.05901.x. [DOI] [PubMed] [Google Scholar]
- 6.Tao YP, Wang WL, Li SY, et al. Associations between polymorphisms in IL-12A, IL-12B, IL-12Rbeta1, IL-27 gene and serum levels of IL-12p40, IL-27p28 with esophageal cancer. J Cancer Res Clin Oncol. 2012;138:1891–900. doi: 10.1007/s00432-012-1269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Zhou B, Wu Y, et al. Prognostic value of IL-27 polymorphisms and the susceptibility to epithelial ovarian cancer in a Chinese population. Immunogenetics. 2014;66:85–92. doi: 10.1007/s00251-013-0753-2. [DOI] [PubMed] [Google Scholar]
- 8.Zhou SH, Yang WJ, Liu SW, et al. Gene expression profiling of craniofacial fibrous dysplasia reveals ADAMTS2 overexpression as a potential marker. Int J Clin Exp Pathol. 2014;7:8532–41. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Wang Y, Wang M, Ji Z. IL-27–964A>G polymorphism and the risk of breast cancer: a case-control study. Tumour Biol. 2014;35:12099–102. doi: 10.1007/s13277-014-2512-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S, Gao X, Wang Y, et al. Interleukin 27-964A > G genetic polymorphism and serum IL-27p28 levels in Chinese patients with papillary thyroid cancer. Tumour Biol. 2015 doi: 10.1007/s13277-015-3570-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Peng Q, Qin X, He Y, et al. Association of IL27 gene polymorphisms and HBV-related hepatocellular carcinoma risk in a Chinese population. Infect Genet Evol. 2013;16:1–4. doi: 10.1016/j.meegid.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Zhang Y, Xue F, et al. Interleukin-27 rs153109 polymorphism and the risk for immune thrombocytopenia. Autoimmunity. 2013;46:509–12. doi: 10.3109/08916934.2013.822072. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Lin J, Cui S, Zhang QG. [Relationship between interleukin 27 gene polymorphisms and asthma in the Northeast of Chinease Han population]. Chin J Immunol. 2012;28:552–54. [in Chinese] [Google Scholar]
- 14.Chen S, Zhang QG, Fang F. Relationship between IL-27 gene polymorphisms and rheumatoid arthritis in Chinese Han population. J Jilin Med Col. 2014;30:117–20. [Google Scholar]
- 15.Lin XY, Wang ZT, Zhong J, Zhao M. Association between interleukin-27 gene polymorphisms and Crohn’s disease susceptibility. Journal of Internal Medicine Concepts & Practice. 2013;8:275–78. [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21:3672–73. doi: 10.1093/bioinformatics/bti536. [DOI] [PubMed] [Google Scholar]
- 18.Song F, Gilbody S. Bias in meta-analysis detected by a simple, graphical test. Increase in studies of publication bias coincided with increasing use of meta-analysis. BMJ. 1998;316:471. [PMC free article] [PubMed] [Google Scholar]
- 19.Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 20.Guo JY, Qin AQ, Li RK, et al. Association of interleukin-27 gene polymorphism with genetic susceptibility to colorectal cancer. Chongqing Med. 2012;41:948–50. [Google Scholar]
- 21.Huang N, Liu L, Wang XZ, et al. Association of interleukin (IL)-12 and IL-27 gene polymorphisms with chronic obstructive pulmonary disease in a Chinese population. DNA Cell Biol. 2008;27:527–31. doi: 10.1089/dna.2007.0715. [DOI] [PubMed] [Google Scholar]
- 22.Lan Y, Jiang YW, Tang XS, Wu J. Association of interleukin-27 gene polymorphisms with genetic susceptibility to systematic lupus erythematosus in Guangxi Zhuang population. Chinese Journal of Dermatology. 2011;44:578–80. [Google Scholar]
- 23.Pan GG, Lu D, Liao LN, Huang CC. Association of the genotype and serum level of IL-27 with nasopharyngeal carcinoma. Shandong Med J. 2012;52:18–20. [Google Scholar]
- 24.Qiu RF, Li JS, Gao GM, et al. Association of the -964A > G polymorphism in the IL-27p28 gene promoter region and asthma in a Chinese Han population. J Shandong University. 2008;46:1021–23. 1027. [Google Scholar]
- 25.Zhan YZ, Jiang LP, Wang L, et al. Association between interleukin 27 gene polymorphisms and susceptibility to tuberculosis. J Fourth Military Med University. 2009;30:3100–3. [Google Scholar]
- 26.Zhu CL, Wamg SQ, Zhang R, et al. Study of the relationship between polymorphisms of interleukin 27 gene in promoter region and patients with chronic hepatitis B virus. Int J Labor Med. 2010;31:922–23. 925. [Google Scholar]
- 27.Liang XH, Wai C, Wang de Y. Differential and opposed transcriptional effects of interleukin-18 gene polymorphisms (−137, +113, and +127) in human HepG-2, HeLa, U937, and THP-1 cells. Med Sci Monit. 2008;14(1):BR8–13. [PubMed] [Google Scholar]
- 28.Jia X, Tian Y, Wang Y, et al. Association between the interleukin-6 gene −572G/C and −597G/A polymorphisms and coronary heart disease in the Han Chinese. Med Sci Monit. 2010;16(3):CR103–8. [PubMed] [Google Scholar]
- 29.Hu YY, Liu JH, Jiang GB, et al. Association between Interleukin-1beta gene −511C>T/+3954C>T polymorphisms and aggressive periodontitis susceptibility: Evidence from a meta-analysis. Med Sci Monit. 2015;21:1617–24. doi: 10.12659/MSM.894402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng X, Xu J, Wang P, et al. Interleukin-10-1082A/G polymorphism and diabetic nephropathy: a meta-analysis. Med Sci Monit. 2015;21:890–94. doi: 10.12659/MSM.892972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamopoulos IE, Pflanz S. The emerging role of Interleukin 27 in inflammatory arthritis and bone destruction. Cytokine Growth Factor Rev. 2013;24:115–21. doi: 10.1016/j.cytogfr.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity. 2012;37:960–69. doi: 10.1016/j.immuni.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos AS, Melo ME, Crisostomo LG, et al. Lack of association between IL27 gene variants and type 1 diabetes susceptibility. Cytokine. 2013;61:349–52. doi: 10.1016/j.cyto.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Wojno ED, Hunter CA. New directions in the basic and translational biology of interleukin-27. Trends Immunol. 2012;33:91–97. doi: 10.1016/j.it.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chae SC, Li CS, Kim KM, et al. Identification of polymorphisms in human interleukin-27 and their association with asthma in a Korean population. J Hum Genet. 2007;52:355–61. doi: 10.1007/s10038-007-0123-8. [DOI] [PubMed] [Google Scholar]