Case Report
Pseudohypoaldosteronism type (PHA) 1 is a rare disease with an incidence of 1:47 000 to 1:80 000.1 The presentation may be confused with other neonatal endocrinological problems like congenital adrenal hyperplasia, hypoaldosteronism, and Barter syndrome. Type I PHA is further subdivided into 2 distinct entities.2 The autosomal dominant trait (adPHA1) results from the mutations in the gene encoding mineralocorticoid receptor (NR3C2).3 The clinical course is mild and is restricted to kidneys. Prognosis for adPHA1 form is good and spontaneous remission is observed over time. The autosomal recessive trait (arPHA1) results from mutations of the genes (SCNN1A, SCNN1B, and SCNN1G) encoding the 3 homologous alpha beta gamma subunits of the amiloride-sensitive epithelial sodium channel (ENaC).4 This causes loss of function or a decrease in ENaC channel activity.4 The arPHA1 not only affects kidneys but also affects colon, lung, sweat, and salivary glands.4 Patients with arPHA1 are reported to have recurrent pulmonary infections with pseudomonas species similar to those seen in cystic fibrosis.5
Patient Presentation
A 7-day-old male was admitted to the neonatal intensive care unit with a day history of poor feeding, reduced urine output, and lethargy. He was born normally after a term and uneventful pregnancy, and his birth weight was 3.3 kg. He was the second child of first-degree consanguineous parents who emigrated from Southeast Asia. There was no family history of significant illness or unexplained death.
On physical examination, he was pale, lethargic, and severely dehydrated. The rest of his systemic examination was unremarkable with normal male external genitalia. He was tachycardic and with normal blood pressure. Electrocardiogram showed wide complex ventricular tachycardia, which resolved after fluid resuscitation and a dose of epinephrine.
Initial labs were serum sodium 127 mEq/L, serum potassium 9.3 mEq/L, serum chloride 103 mmol/L, blood urea nitrogen 25 mg/dL, and creatinine 0.53 mg/dL; capillary blood gas: pH 7.27, PCO2 39.7 mm Hg, HCO3 17.7 mEq/L, base deficit −8.5 mEq/L. Newborn screen showed normal 17-OH progesterone level. Urine sodium was 439 mmol/L, and urine potassium was <2.5 mmol/L. Further laboratory studies subsequently revealed markedly elevated plasma renin (56.32 ng/mL/h; normal range = 0.25-5.82) and serum aldosterone (323 ng/dL; 1-12 months = 2-70 ng/dL). Serum cortisol (AM), ACTH, and 17-OH progesterone were within normal limits. Renal ultrasound and Doppler showed no abnormalities. The study of genes SCNN1B, SCNN1G, and SCNN1A was carried out and a homozygous intronic mutation of the SCNN1A gene was detected.
Antibiotics were started that were later discontinued after 48 hours of negative blood and urine cultures. He was also started on hydrocortisone and fludrocortisone because of the concerns of salt losing form of congenital adrenal hyperplasia. Hydrocortisone was discontinued after normal newborn screen for congenital adrenal hyperplasia. However, fludrocortisone was gradually increased because of increasing sodium requirement but later gradually tapered off, as no improvement in hyponatremia was noted.
Hyperkalemia resolved over the next 16 hours after treatment with calcium gluconate and sodium bicarbonate. He was given scheduled sodium polystyrene sulfonate (SPS). We also pretreated his milk formula with SPS. We determined the potassium level in the milk before adding SPS and noted a decrease from 15.9 mEq/L to 3.9 mEq/L 1 hour after treatment. The top layer of the pretreated milk was given to the patient, and the bottom brown slurry layer was discarded.
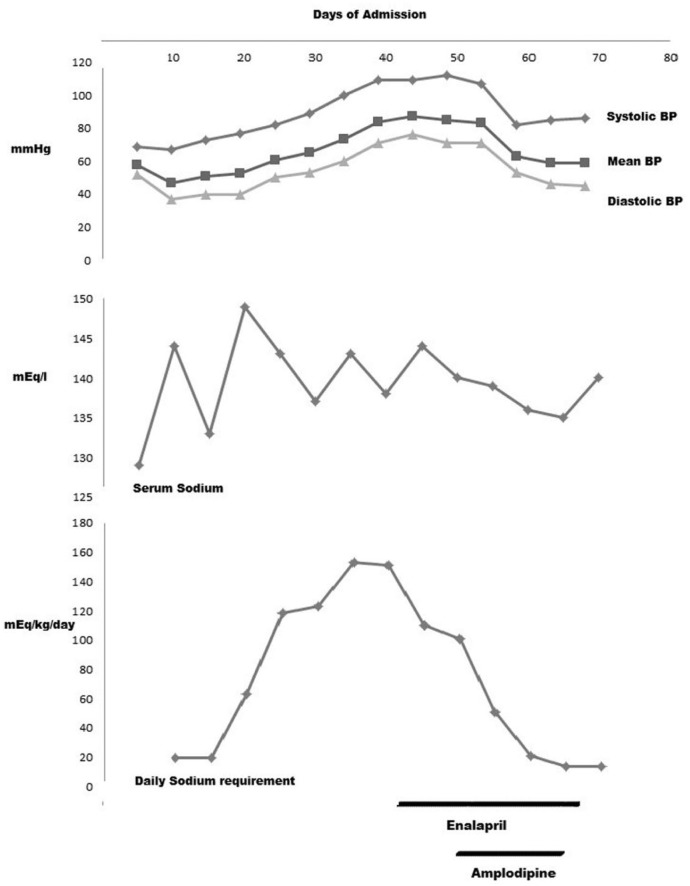
Hyponatremia was initially treated intravenously, and later transitioned to oral sodium supplements. A gastrostomy tube was placed to give high sodium supplement. During the time of increasing sodium intake, he developed systolic hypertension (Figure 1). At this time, he was requiring 100 mEq/kg/day of sodium. He was treated with enalapril (angiotensin-converting enzyme inhibitor) and amlodipine (calcium channel blocker) for a brief period. With time, we observed that the normalization of his blood pressures coincided with decreasing sodium requirement. Both antihypertensive medications were discontinued before discharge. Since discharge from the neonatal intensive care unit our patient’s blood pressure remained within normal limits. He has developed asthma and had several short-term hospitalizations secondary to fluid and electrolyte imbalances and gastrostomy tube infections.
Figure 1.
Average blood pressure (BP), serum sodium, and daily sodium requirement.
Discussion
Our patient has homozygous intronic mutation of the SCNN1A gene. This mutation was tested for impact on splicing and was found to possibly affect 3 of 5 possible splicing models, thus thought to be consistent with a deleterious effect. A recessive intronic mutation within SCNN1A has been reported to cause arPHA1.6
During the acute phase of electrolyte imbalance, patients with arPHA1 require 25 to 35 mEq/kg/day of sodium supplementations and often require lifelong supplementation.7 However, one case report showed remission without further sodium supplementation.8 Our patient required up to 110 mEq/kg/day of sodium during his hospitalization. He showed improvement in sodium requirements overtime but still requires significant amount of sodium supplements almost 8 months after discharge.
Another challenge in patients with arPHA1 is the management of hyperkalemia. Pretreatment of milk with SPS has been reported as an effective treatment for hyperkalemia in infants with chronic renal insufficiency.9 To our knowledge this is the first reported case of an arPHA1 patient whose hyperkalemia was managed with SPS pretreated milk. Prior to the pretreatment of milk, we gave SPS via oral and per rectal route. This was a cumbersome procedure and caused patient discomfort, rectal leakage, and inadequate retention. Our patient showed no discomfort with SPS pretreated milk, which resulted in improved compliance. The pretreatment of milk with SPS is not without complications such as hypocalcemia, gut necrosis, and increase stooling.
Patients with arPHA1 have hyperreninism and hyperaldosteronism but they do not develop hypertension. This may be due to sustained intravascular volume depletion and not due to peripheral resistance to mineralocorticoid. However, our patient developed hypertension while on high sodium supplement and was treated with 2 different antihypertensive medications for a brief period. We speculate that high sodium requirement in our patient led to retention of intravascular fluid and resulted in hypertension.
In conclusion, arPHA1 should be suspected in any neonate who presents with electrolyte imbalances and arrhythmias. Sodium supplementation and management of hyperkalemia with SPS pretreated milk is a convenient treatment option.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Amin N, Alvi NS, Barth JH, et al. Pseudohypoaldosteronism type 1: clinical features and management in infancy. Endocrinol Diabetes Metab Case Rep. 2013;2013:130010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iwai N, Baba S, Mannami T, Ogihara T, Ogata J. Association of a sodium channel alpha subunit promoter variant with blood pressure. J Am Soc Nephrol. 2002;13:80-85. [DOI] [PubMed] [Google Scholar]
- 3. Hanukoglu A. Type I pseudohypoaldosteronism includes two clinically and genetically distinct entities with either renal or multiple target organ defects. J Clin Endocrinol Metab. 1991;73:936-944. [DOI] [PubMed] [Google Scholar]
- 4. Chang SS, Grunder S, Hanukoglu A, et al. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet. 1996;12:248-253. [DOI] [PubMed] [Google Scholar]
- 5. Marthinsen L, Kornfalt R, Aili M, Andersson D, Westgren U, Schaedel C. Recurrent Pseudomonas bronchopneumonia and other symptoms as in cystic fibrosis in a child with type I pseudohypoaldosteronism. Acta Paediatr. 1998;87:472-474. [PubMed] [Google Scholar]
- 6. Silva N, Costa M, Silva A, et al. A case of systemic pseudohypoaldosteronism with a novel mutation in the SCNN1A gene. Endocrinol Nutr. 2013;60:33-36. [DOI] [PubMed] [Google Scholar]
- 7. Saravanapandian N, Paul S, Matthai J. Pseudohypoaldosteronism type 1: a rare cause of severe dyselectrolytemia and cardiovascular collapse in neonates. J Clin Neonatol. 2012;1:224-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adachi M, Asakura Y, Muroya K, et al. Increased Na reabsorption via the Na-Cl cotransporter in autosomal recessive pseudohypoaldosteronism. Clin Exp Nephrol. 2010;14:228-232. [DOI] [PubMed] [Google Scholar]
- 9. Thompson K, Flynn J, Okamura D, Zhou L. Pretreatment of formula or expressed breast milk with sodium polystyrene sulfonate (Kayexalate®) as a treatment for hyperkalemia in infants with acute or chronic renal insufficiency. J Ren Nutr. 2013;23:333-339. [DOI] [PubMed] [Google Scholar]