Abstract
Background. Treatment and management strategies for asthma in children are generally consistent internationally, but prescription of antiasthma drugs differs among countries. The objective of this study was to examine the prescribing patterns of antiasthma drugs, particularly controller medications, in children. Methods. A retrospective cohort study was performed in children with asthma using an administrative claims database in Japan. Results. A total of 1149 preschool-age and 3226 school-age children were identified. Leukotriene receptor antagonists were prescribed for about 80% of the children. Long-acting β-agonists were prescribed for 87.6% and 59.6% of preschool-age and school-age children, respectively, whereas prescriptions of inhaled corticosteroids had lower rates of 8.2% and 16.5%, respectively. In an examination of prescriptions at 1-month intervals, a relatively high number of children were prescribed bronchodilators without anti-inflammatory agents. Conclusion. Our findings suggest that asthma care for children in Japan can be improved through changes in drug prescriptions.
Keywords: antiasthmatic agents, asthma, children, drug utilization, preschool, pharmacoepidemiology
Introduction
Childhood asthma is a common chronic condition characterized by airway hyperresponsiveness and remodeling. The estimated prevalence of asthma is 6.5% in children aged 6 to 12 years in Japan.1 Antiasthma agents are commonly prescribed drugs to children as outpatients,2 and optimization of antiasthma medications is important in terms of utilization of health care resources. These medications are classified as controllers or relievers. Controllers are used daily on a long-term basis to control asthma, whereas relievers are used on an as-needed basis for reversing bronchoconstriction and relieving symptoms.3
The Japanese Pediatric Guideline for the Treatment and Management of Asthma (JPGL) was first established in 2000 independent of the guidelines for adults and has subsequently been updated about every 3 years.4 A stepwise approach for long-term management based on asthma severity is commonly recommended, as also found in the Global Initiative for Asthma (GINA) guidelines.3,4 The importance of anti-inflammatory agents is stressed in both guidelines because asthma is recognized as a chronic inflammatory disorder in the airways. There are some differences in the severity evaluation in the 2 sets of guidelines. Severity of asthma is evaluated in a strict way according to the JPGL since most children with asthma are classified as mild or moderate based on the adult guidelines in Japan or the GINA guidelines.3
Considering the differences in the severity evaluation, management approach is similar between the JPGL4 and the GINA guidelines.3 In Step 1 in the JPGL, relievers are recommended as basic therapy, but additional leukotriene receptor antagonists (LTRAs) and/or inhaled disodium cromoglycate (DSCG) are optional. In Step 2, anti-inflammatory agents, such as inhaled corticosteroids (ICSs), LTRAs, and/or DSCG, are recommended, and sustained release theophylline (SRT) is listed as an additional treatment option for children aged ≥2 years. In Steps 3 and 4, long-acting β-agonists (LABAs) for children aged ≥2 years and oral corticosteroids (OCSs) for children aged ≥6 years are also treatment options.
Drug utilization studies are useful to identify therapeutic problems in a large pediatric population in real-world practice.5 However, since drug utilization can be influenced by various factors, including the health care system, access to health care, available medications, economics, and culture, the results of drug utilization obtained from one country generally cannot be extrapolated to other countries. Thus, although treatment and management strategies for childhood asthma are generally consistent internationally, prescription of antiasthma agents for children tends to differ among countries.6-8
Several studies have investigated antiasthma medications for children in Japan. The Asthma Insight & Reality study, a nationwide survey, suggested a need for widespread use of ICSs and for enhancement of adherence to asthma controller medications.9 However, longitudinal information on drug utilization in a large sample of children with asthma remains sparse in Japan. In recent years, an environment for epidemiological research using secondary databases has been established in Japan. We have shown that Japanese claims databases are useful for understanding drug utilization in some disease areas.10,11 The objective of this study was to examine the prescribing patterns of asthma controller medications using a large-scale administrative claims database in Japan.
Methods
Data Source
An administrative claims database established and managed by the Japan Medical Data Center Co Ltd (Tokyo, Japan) was used for this study.12 The database consists of medical and pharmacy claims, and insurance eligibility data from a total of 1 000 000 beneficiaries of corporate employees and their dependent family members, as of 2012. The database represents approximately 1% of the total population in Japan. Data included in the database are as follows: year of birth, sex, year and month (date partly available) of medical services provided or drugs prescribed, diagnoses, drugs, inpatient or outpatient, size and specialty of medical facilities, and types of claims. Diagnoses are coded by the International Classification of Diseases, 10th Revision (ICD-10) and using more specific standardized codes developed by the Medical Information System Development Center (MEDIS-DC). Personal information is removed and anonymous identifiers are assigned to all beneficiaries. The study protocol was approved by the Kyoto University Graduate School and Faculty of Medicine, Ethics Committee (Kyoto, Japan).
Study Design
A retrospective cohort study was performed using data for children with asthma between January 2008 and December 2012. The month when asthma controller(s) was first prescribed for each patient was defined as the index month. The baseline year was defined as 12 months before the index month, and the follow-up year was defined as the index month and the subsequent 11 months. A 12-month observational period was used to minimize seasonal variations in asthma symptoms and drug use.13
Study Cohort
Children with asthma were identified based on diagnosis and drugs prescribed, as illustrated in Figure 1. First, among children diagnosed with asthma based on the ICD-10 J45, those with ≥1 prescription of asthma controller medications were extracted. All asthma controllers listed in the long-term management in the JPGL4 except for OCSs were used for the cohort selection. Next, children receiving antibiotics (ATC J01) prescribed in the same claim with asthma controllers in the index month were excluded to prevent inclusion of children with suspected respiratory tract infections. Then, children with ≥1 diagnosis of asthma based on the MEDIS-DC codes in the follow-up year were selected. Use of diagnoses based on MEDIS-DC codes enabled exclusion of children diagnosed with some specific asthma (eg, exercise-induced asthma and aspirin-induced asthma) or with bronchitis without diagnosis of asthma.
Figure 1.
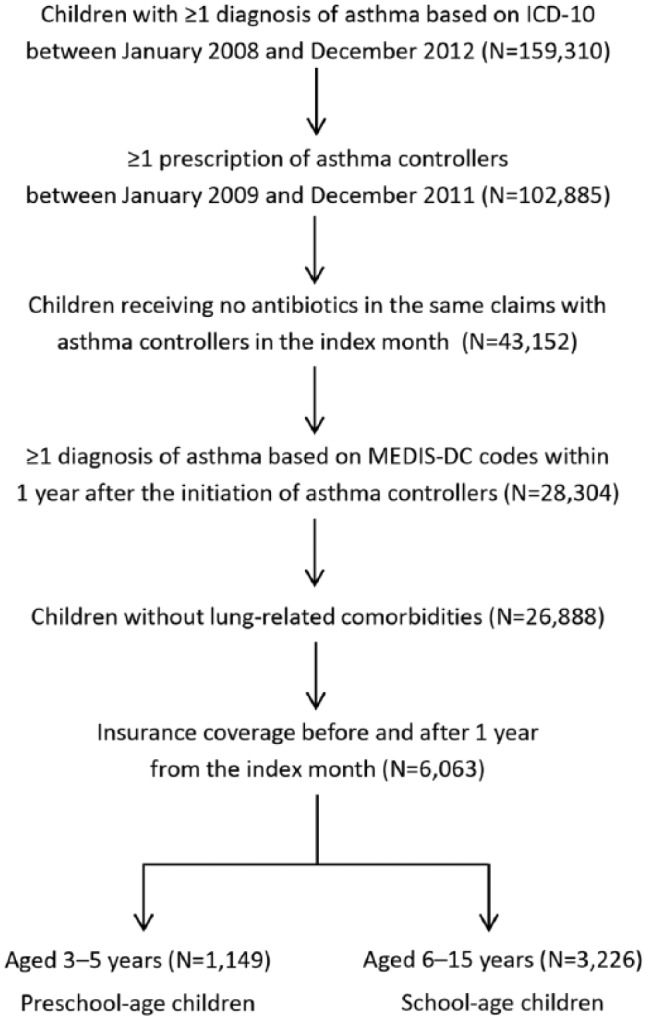
Flow diagram of study cohort selection.
Asthma controller medications included inhaled corticosteroids, combination inhalers of inhaled corticosteroids and long-acting β-agonists; leukotriene receptor antagonists; inhaled disodium cromoglycate; inhaled, oral, and transdermal long-acting β-agonists; and sustained release theophylline.
Children with asthma with the following diagnoses were excluded: tuberculosis (ICD-10 A15-A19), malignant neoplasms, respiratory system and intrathoracic organs (C30-C39), mesothelioma (C45), emphysema (J43), other chronic obstructive pulmonary disease (J44), bronchiectasis (J47), lung diseases due to external agents (J60-J70), other respiratory diseases principally affecting the interstitium (J80-J84), suppurative and necrotic conditions of lower respiratory tract (J85-J86), other diseases of pleura (J90-J94), other diseases of the respiratory system (J95-J99), systemic connective tissue disorders (M30-M36), and malformations and deformations of respiratory system (Q30-Q34).
Patients with data from the start of the baseline year until the end of the follow-up year were then identified, and those aged 3 to 15 years in the index month were selected. These patients were divided into groups of 3 to 5 years (preschool-age) and 6 to 15 years (school-age). Although children are classified as <2, 2 to 5, and 6 to 15 years in the JPGL,4 the data in the database are limited to year of birth (not month or date), and diagnosis of asthma is more difficult in infants. For these reasons, we did not include children aged ≤2 years in the index month.
Antiasthma Drugs
We examined the following asthma controller medications: ICSs, combination inhalers of ICSs and LABAs (ICS/LABA), LTRAs, DSCG, inhaled, oral and transdermal LABAs, and SRT. These medications were also categorized into anti-inflammatories (ICSs, LTRAs, and DSCG) and bronchodilators (LABAs and SRT). Use of inhaled short-acting β-agonists (SABAs) and OCSs was examined in the baseline and follow-up years. Omalizumab, an anti-IgE antibody, was not included because use of this drug for children was approved in Japan after the study period.
Analysis
Demographics and antiasthma medications were examined separately in the 2 age groups. Combination drugs containing 2 active ingredients (ie, ICS/LABA) were counted separately for each class of drugs (ie, ICSs and LABAs). Categorical variables were compared by χ2 test for demographics and prescriptions of asthma controller medications between preschool-age and school-age children or prescriptions over time, and by McNemar’s test for SABAs or OCSs between the baseline and follow-up years within each age group. All analyses were conducted using R statistical software (version 3.0.1).14
Results
Characteristics of the Study Cohort
A total of 1149 preschool-age and 3226 school-age children with asthma were included in the analysis (Figure 1). There were slightly more boys than girls (Table 1). About 10% of the children had diagnoses of asthma in the baseline year in both age groups. The most frequent allergic comorbidity was allergic rhinitis, with rates of 31.2% and 46.6% in preschool-age and school-age children, respectively. Allergic rhinitis and allergic conjunctivitis were more prevalent in school-age children, whereas atopic dermatitis was more prevalent in preschool-age children. Fewer children had an index month in the summer than in other seasons. Among all asthma controller medications, 98.7% were prescribed on outpatient claims. The medical facilities associated with prescriptions of controllers were mainly clinics (88.7%), and the clinics had declared specialties of internal medicine (41.7%), pediatrics (31.4%), and otorhinolaryngology (22.5%).
Table 1.
Characteristics of the Study Cohort.
| Characteristics | Preschool-Age (N = 1149)n (%)/Mean ± SD | School-Age (N = 3226)n (%)/Mean ± SD | P Value |
|---|---|---|---|
| Age (years) | 4.2 ± 0.8 | 10.7 ± 2.6 | NA |
| Age group (years) | NA | ||
| 3 | 600 (52.2) | — | |
| 4 | 299 (26.0) | — | |
| 5 | 250 (21.8) | — | |
| 6-9 | — | 1431 (44.4) | |
| 10-12 | — | 1,113 (34.5) | |
| 13-15 | — | 682 (21.1) | |
| Sex | |||
| Male | 591 (51.4) | 1788 (55.4) | .020 |
| Diagnoses of allergic diseases in baseline year | |||
| Asthma | 128 (11.1) | 332 (10.3) | .421 |
| Allergic rhinitis | 358 (31.2) | 1502 (46.6) | <.001 |
| Atopic dermatitis | 226 (19.7) | 360 (11.2) | <.001 |
| Allergic conjunctivitis | 129 (11.2) | 794 (24.6) | <.001 |
| Year of index month | |||
| 2009 | 329 (28.6) | 774 (24.0) | <.001 |
| 2010 | 384 (33.4) | 805 (25.0) | |
| 2011 | 436 (37.9) | 1647 (51.1) | |
| Season of index month | |||
| Spring (March to May) | 304 (26.5) | 1199 (37.2) | <.001 |
| Summer (June to August) | 195 (17.0) | 492 (15.3) | |
| Fall (September to November) | 319 (27.8) | 690 (21.4) | |
| Winter (December to February) | 331 (28.8) | 845 (26.2) |
Asthma Controller Medications: 1-Year Observation
Prescriptions of asthma controller medications in 1 year after the first controller prescription are shown in Table 2. Anti-inflammatories were prescribed to almost 90% of children in both age groups, and among them, 86.2% of preschool-age children and 57.7% of school-age children were also prescribed bronchodilators. LTRAs were the most frequently prescribed anti-inflammatories and were more commonly given to preschool-age children compared to school-age children (83.9% vs 79.1%, P < .001). DSCG was also more commonly prescribed for preschool-age children (22.1% vs 12.9%, P < .001). ICSs were not frequently prescribed, but were given at double the rate in school-age children compared to preschool-age children (16.5% vs 8.2%, P < .001).
Table 2.
Use of Asthma Controller Medications in 1 Year After the First Controller Prescription.
| Preschool-Age (N = 1149)n (%) | School-Age (N = 3226)n (%) | P Value | |
|---|---|---|---|
| Drug category | |||
| Anti-inflammatories only | 139 (12.1) | 1201 (37.2) | <.001 |
| Anti-inflammatories and bronchodilators | 870 (75.7) | 1646 (51.0) | <.001 |
| Bronchodilators only | 140 (12.2) | 379 (11.7) | .695 |
| Drug class | |||
| ICSs | 94 (8.2) | 532 (16.5) | <.001 |
| LTRAs | 964 (83.9) | 2551 (79.1) | <.001 |
| DSCG | 254 (22.1) | 416 (12.9) | <.001 |
| LABAs | 1006 (87.6) | 1924 (59.6) | <.001 |
| SRT | 77 (6.7) | 385 (11.9) | <.001 |
Abbreviations: DSCG, inhaled disodium cromoglycate; ICSs, inhaled corticosteroids; LABAs, long-acting β-agonists (inhaled, oral, and transdermal); LTRAs, leukotriene receptor antagonists; SRT, sustained release theophylline.
LABAs, the main bronchodilators, were more commonly prescribed for preschool-age children (87.6% vs 59.6%). Among all prescriptions of LABAs, the percentages of transdermal, oral, and inhaled formulations were 59.9%, 39.9%, and 0.2% in preschool-age children and 60.6%, 27.5%, and 11.9% in school-age children, respectively. Among the inhaled LABAs for school-age children, combination inhalers with ICSs accounted for 94.2%. SRT was not frequently prescribed.
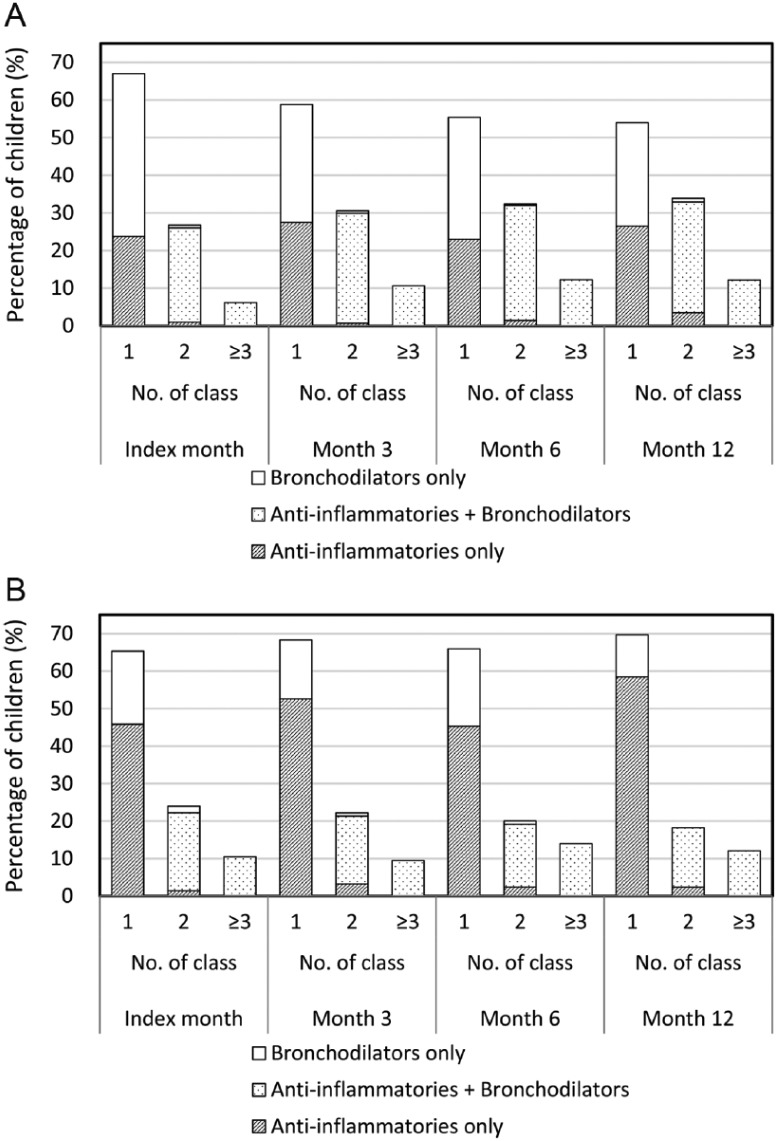
Asthma Controller Medications: Monthly Observations
Prescribing patterns of asthma controller medications were examined by drug category (Figure 2) and drug class (Figure 3). Almost 70% of children were prescribed one class of asthma controller medication in both age groups in the index month. Children receiving ≥2 classes of controllers increased in preschool-age children over time (P < .001), but was unchanged in school-age children (P = .254). In terms of drug category, 44.0% of preschool-age and 21.2% of school-age children were prescribed only bronchodilators in the index month. Subsequently, the percentages of children given bronchodilators only decreased in both age groups (all P < .001).
Figure 2.
Prescribing patterns for asthma controller medications by drug category in (A) preschool-age children and (B) school-age children prescribed these medications each month.
Figure 3.
Prescribing patterns of asthma controller medications by drug class in (A) preschool-age children and (B) school-age children prescribed these medications each month.
Abbreviations: DSCG, inhaled disodium cromoglycate; ICS, inhaled corticosteroid; LABA, long-acting β-agonist (inhaled, oral, and transdermal); LTRA, leukotriene receptor antagonists.
As monotherapy, LABAs (26.8% to 42.9%) followed by LTRAs (17.2% to 25.6%) were the most frequently prescribed controllers in preschool-age children. In school-age children, LTRAs were most commonly prescribed (37.4% to 55.8%), followed by LABAs (11.0% to 23.3%). Most of the 2-class combinations were LTRAs plus LABAs in preschool-age (22.3% to 30.9%) and school-age (10.1% to 18.5%) children. Other common combinations of 2 or more classes included DSCG (preschool-age) and ICSs (school-age), in addition to LTRAs and/or LABAs.
SABAs and OCSs
In the baseline year, few children received inhaled SABAs or OCSs in both age groups (≤1.5% each). There was a significant increase in preschool-age children prescribed SABAs or OCSs in the follow-up year (inhaled SABAs, 34.0%; OCSs, 7.6%; all P < .001 vs baseline year). Prescriptions of SABAs or OCSs also significantly increased in school-age children (inhaled SABAs, 21.9%; OCSs, 3.8%; all P < .001 vs baseline year). In addition, these drugs were prescribed more frequently in preschool-age children than school-age children in the follow-up year (all P < .001).
Discussion
In this study, we characterized the utilization of asthma controller medications for children in Japan. Our data indicate that most controllers were prescribed in clinics and written on outpatient claims. Children with asthma are managed by physicians whose specialty is not pediatrics or pulmonology, and this study is useful to understand current clinical practice. In the light of access to health care, patient copayments for asthma as a childhood chronic disease are often compensated by local governments in Japan. This might cause differences in prescribing patterns of antiasthma agents compared to those in other countries with different health care systems.
In the current study, one of the features of utilization of asthma controller medications was the high prescription rate of LTRAs. This may be because most children with asthma have relatively mild symptoms that can be treated in Step 1 in the JPGL (inhaled SABAs as needed and additional LTRAs or DSCG as options).4 There may also be a preference for simple oral application of LTRAs compared to inhaled drugs. LTRAs are also indicated for treatment of allergic rhinitis, but our data refer to use of the drug for asthma because the main drugs for allergic rhinitis are nasal corticosteroids and nonsedative antihistamines.15
Another feature of the data was the frequent use of noninhalation formulations of LABAs, despite this class of drugs being placed in Step 3 or 4 as an additional therapy in the JPGL.4 In particular, more than a half of LABAs were given as a transdermal formulation (tulobuterol patch), which is effective and applicable to all age groups, including infants and children.16 This patch is used extensively in Japan, Korea, and China.16 In our cohort, a relatively high number of children were prescribed LABAs alone despite safety concerns.17 Anti-inflammatory agents should be used or added to achieve remission or prevent progression to difficult-to-treat asthma.
In Europe6,7,18 and the United States,19 ICS monotherapy is the most frequent therapy for treatment of childhood asthma, whereas use of ICSs is much lower in Asian countries, including in Japan.8,9,20,21 This trend was reflected in our study, which showed fewer prescriptions of ICSs. A questionnaire survey using case vignettes of children with asthma revealed some differences among physicians in selection of antiasthma drugs between Europe and Japan.22 These differences might reflect underlying concerns about side effects related to chronic ICSs use,20,21 or physicians and patients might prefer to avoid inhaled drugs. Resistance to the use of corticosteroids affects use of ICSs for children whose parents are concerned about the safety of excessive use of ICSs. We were unable to determine if ICSs should have been prescribed to more children in our cohort, but more widespread use of ICSs may contribute to better management of asthma in children.
In studies using administrative databases, misclassification bias is acknowledged as an important issue.23 In a study using the same database as that used for our study, β-2 agonists were frequently used in children with bronchitis.24 We cannot exclude the possibilities of inclusion of nonasthmatic children and excess exclusion of children with asthma. However, the database has diagnostic codes based on MEDIS-DC, in addition to the ICD-10, and this was helpful to prevent misclassification of nonasthmatic children as children with asthma. These diagnostic codes and the prescription information support the appropriateness of the study cohort selection.
There are some limitations in this study. We focused on use of asthma controllers, and thus we did not cover all children with asthma, including patients receiving SABAs only. We also did not investigate in detail the situations in which inhaled SABAs or OCSs were added to daily controller medications. Given that onset of asthma peaks at age 3 years,1 our cohort might not be incident cases despite confirmation of no use of controller medications in the baseline year. As in other administrative databases, the intention of use of individual drugs was unclear and a mismatch between the disease and drugs might be present. We cannot make judgments whether appropriate prescriptions were given at the patient level because we could not evaluate the severity and control of asthma exactly. We considered concomitant prescriptions based on prescriptions in the same month. This might cause overestimation of use of combination therapy and underestimation of therapy with a single drug class if patients receiving a certain drug switched to another drug. However, this indicates that we did not overestimate the problematic frequent medication with LABA monotherapy.
Conclusions
LTRAs and LABAs are frequently prescribed asthma controller medications for children in Japan. The frequent use of these drugs may be due to the high usability of oral and transdermal formulations of these drugs for children. However, children treated with bronchodilators without anti-inflammatories may be more likely to have disease prolongation and progression to difficult-to-treat asthma. Therefore, a stepwise approach based on exact assessment of asthma severity, as described in the guidelines, is needed to improve asthma management in children, with a particular focus on anti-inflammatory medications and more careful use of bronchodilators. More use of ICSs may also contribute to better management of asthma in children. Further studies are needed to investigate the appropriateness of asthma controller prescriptions in individual children with asthma.
Acknowledgments
We thank the Japan Medical Data Center Co Ltd (Tokyo, Japan) for providing the data set for this study.
Footnotes
Author Contribution: SH contributed to conception and design; contributed to analysis and interpretation; drafted manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. HT contributed to design; contributed to analysis and interpretation; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. AS contributed to design; contributed to analysis and interpretation; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. MI contributed to design; analysis and interpretation; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy. KK contributed to conception and design; contributed to acquisition and interpretation; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: KK has received research funding from Kyowa Hakko Kirin, Bayer, Dainippon Sumitomo Pharma, Olympus, Stella Pharma, and Taiho Pharmaceuticals; honorariums from Daiichi Sankyo, Eisai, Boehringer Ingelheim Japan Inc, Novartis Pharmaceutical K.K., Astra Zeneca, CSL Behring, MSD, Shionogi Pharmaceuticals, Takeda; and consultancy fees from Kyowa Hakko Kirin, Olympus, Kaken Pharmaceutical, Advanced Medical Care, and Otsuka Pharmaceuticals.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Nishima S, Chisaka H, Fujiwara T, et al. ; Allergy Prevalence Survey Group, West Japan Pediatric Allergy Association. Surveys on the prevalence of pediatric bronchial asthma in Japan: a comparison between the 1982, 1992, and 2002 surveys conducted in the same region using the same methodology. Allergol Int. 2009;58:37-53. [DOI] [PubMed] [Google Scholar]
- 2. Clavenna A, Bonati M. Drug prescriptions to outpatient children: a review of the literature. Eur J Clin Pharmacol. 2009;65:749-755. [DOI] [PubMed] [Google Scholar]
- 3. Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143-178. [DOI] [PubMed] [Google Scholar]
- 4. Nishimuta T, Kondo N, Hamasaki Y, Morikawa A, Nishima S. Japanese guideline for childhood asthma. Allergol Int. 2011;60:147-169. [DOI] [PubMed] [Google Scholar]
- 5. Sequi M, Campi R, Clavenna A, Bonati M. Methods in pharmacoepidemiology: a review of statistical analyses and data reporting in pediatric drug utilization studies. Eur J Clin Pharmacol. 2013;69:599-604. [DOI] [PubMed] [Google Scholar]
- 6. Bianchi M, Clavenna A, Sequi M, et al. Anti-asthma medication prescribing to children in the Lombardy Region of Italy: chronic versus new users. BMC Pulm Med. 2011;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas M, Murray-Thomas T, Fan T, Williams T, Taylor S. Prescribing patterns of asthma controller therapy for children in UK primary care: a cross-sectional observational study. BMC Pulm Med. 2010;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun HL, Kao YH, Chou MC, Lu TH, Lue KH. Differences in the prescription patterns of anti-asthmatic medications for children by pediatricians, family physicians and physicians of other specialties. J Formos Med Assoc. 2006;105:277-283. [DOI] [PubMed] [Google Scholar]
- 9. Adachi M, Ohta K, Tohda Y, Morikawa A, Nishima S, Mukai I. Asthma insights and reality in Japan: AIR-J 2011. Allergol Immunol. 2012;19:60-68 (in Japanese). [Google Scholar]
- 10. Onishi Y, Hinotsu S, Furukawa TA, Kawakami K. Psychotropic prescription patterns among patients diagnosed with depressive disorder based on claims database in Japan. Clin Drug Investig. 2013;33:597-605. [DOI] [PubMed] [Google Scholar]
- 11. Katada H, Yukawa N, Urushihara H, Tanaka S, Mimori T, Kawakami K. Prescription patterns and trends in anti-rheumatic drug use based on a large-scale claims database in Japan [published online January 14, 2015]. Clin Rheumatol.doi:0.1007/s10067-013-2482-1. [DOI] [PubMed] [Google Scholar]
- 12. Kimura S, Sato T, Ikeda S, Noda M, Nakayama T. Development of a database of health insurance claims: standardization of disease classifications and anonymous record linkage. J Epidemiol. 2010;20:413-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Dole KB, Swern AS, Newcomb K, Nelsen L. Seasonal patterns in health care use and pharmaceutical claims for asthma prescriptions for preschool- and school-aged children. Ann Allergy Asthma Immunol. 2009;102:198-204. [DOI] [PubMed] [Google Scholar]
- 14. R Core Team. R: A language and environment for statistical computing [Computer software]. Vienna, Austria: R Foundation for Statistical Computing; 2013http://www.R-project.org/. Accessed March 5, 2015. [Google Scholar]
- 15. Ebisawa M, Nishima S, Ohnishi H, Kondo N. Pediatric allergy and immunology in Japan. Pediatr Allergy Immunol. 2013;24:704-714. [DOI] [PubMed] [Google Scholar]
- 16. Tamura G, Ichinose M, Fukuchi Y, Miyamoto T. Transdermal tulobuterol patch, a long-acting β2-agonist. Allergol Int. 2012;61:219-229. [DOI] [PubMed] [Google Scholar]
- 17. Chowdhury BA, Dal Pan G. The FDA and safe use of long-acting beta-agonists in the treatment of asthma. N Engl J Med. 2010;362:1169-1171. [DOI] [PubMed] [Google Scholar]
- 18. Moth G, Schiotz PO, Vedsted P. A Danish population-based cohort study of newly diagnosed asthmatic children’s care pathway—adherence to guidelines. BMC Health Serv Res. 2008;8:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arellano FM, Arana A, Wentworth CE, Vidaurre CF, Chipps BE. Prescription patterns for asthma medications in children and adolescents with health care insurance in the United States. Pediatr Allergy Immunol. 2011;22:469-476. [DOI] [PubMed] [Google Scholar]
- 20. Lai CK, De Guia TS, Kim YY; Asthma Insights and Reality in Asia-Pacific Steering Committee. Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific Study. J Allergy Clin Immunol. 2003;111:263-268. [DOI] [PubMed] [Google Scholar]
- 21. Gerez IF, Lee BW, van Bever HP, Shek LP. Allergies in Asia: differences in prevalence and management compared with western populations. Expert Rev Clin Immunol. 2010;6:279-289. [DOI] [PubMed] [Google Scholar]
- 22. Nambu M, Holgate S. Differences in the asthma treatment of children between Europe and Japan: a questionnaire-based survey using model cases. World Allergy Organ J. 2009;2:54-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kent BD, Lane SJ, Moloney ED. Asthma admissions, smoking bans and administrative databases. Thorax. 2013;68:1166. [DOI] [PubMed] [Google Scholar]
- 24. Kamigaki Y, Omori T, Odajima H, Sato T. On prescription of beta 2 agonists for pediatric patients. Jpn J Pharmacoepidemiol. 2012;17:1-12 (in Japanese). [Google Scholar]