Abstract
Background. Cerebral palsy (CP) is the most common cause leading to childhood disability. Human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) transplantation is a promising alternative considering the safety and efficacy in current reports. This report represents a case of hUCB-MSCs transplantation combined with basic rehabilitation treatment beginning as early as age 6 months with follow-up as long as 5 years. Methods. A 6-year-old female patient was diagnosed with CP at age 6 months. The patient accepted 4 infusions of intravenous hUCB-MSCs in each course and received 4 courses of transplantation totally. A series of assessments were performed before the first transplantation, including laboratory tests, CDCC Infant Mental Development Scale, and Gross Motor Function Measure-88 (GMFM-88). Then annual assessments using the GMFM-88, Ashworth spasm assessment, and comprehensive function assessment scale were made in addition to the annual laboratory tests. In addition, electroencephalography and brain magnetic resonance imaging were conducted before transplantation and in the follow-up phase. Rehabilitation and safety follow-up have been ongoing for 5 years up to date. Results. There was no complaint about adverse effects during hospitalization or postoperative follow-up. Motor function recovered to normal level according to the evaluation of scales. Language function improved significantly. Linguistic rehabilitation therapy was enhanced for further improvement. Conclusions. The clinical application of hUC-MSCs combined with basic rehabilitation treatment was effective and safe for improving motor and comprehensive function in a patient with CP.
Keywords: cerebral palsy, human umbilical cord blood mesenchymal stem cells, basic rehabilitation treatment
Background
Cerebral palsy (CP) describes a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to nonprogressive disturbances that occur in the developing fetal or infant brain. The motor disorders of CP are often accompanied by disturbances of sensation, perception, cognition, communication, and behavior; by epilepsy; and by secondary musculoskeletal problems.1 CP is by far the commonest physical neurodisability during childhood and as such has a massive socioeconomic impact.2 The incidence has not changed for decades, despite significant advances in the medical care of neonates. According to data in 2000, overall prevalence in Europe, America, and Australasia is fairly stable at 2 to 3 per 1000 live births, though in susceptible premature infants it rises to up to 100 per 1000 in those born before 28 weeks gestational age.3 The incidence in China is around 1.2 to 2.7 per 1000 live births. The traditional treatments for CP mainly focus on multidisciplinary management based on the comorbidities to minimize the impact of secondary deformities, including, among others, rehabilitation therapy, hyperbaric oxygen treatment, neurotrophic drugs and cytokines, and acupuncture therapy. Stem cells, especially human umbilical cord blood mesenchymal stem cells (hUCB-MSCs) transplantation, have shed a new light on the treatment model for CP. This case report contributes to support the safety and efficacy of hUCB-MSCs in clinical practice with individual features as follows: early diagnosis and treatment (since age of 6 months), long-time follow-up for safety (up to 5 years after initial transplantation infusion), satisfactory safety, and significant improvement.
Methods
Ethics
An informed consent form was signed by the guardians of the patient (with approval of the hospital ethics committee) before stem cell treatment.
General Patient Information
The patient, a 6-year-old girl, was first diagnosed with CP at the age of 6 months due to “neurodevelopmental delay.” The patient was born full-term without any dystocia, asphyxia, or other perinatal risk history. There was no history of familial or hereditary diseases. Around the age of 4 months, her parents found an obvious delay in normal developmental milestones. She could not raise her head, nor sit up when she come to our hospital at the age of 6 months. Development status was as that at age 2 months according to CDCC Infant Mental Development Scale for gross and fine motor, cognizance ability, language competence, and social adaptability, and the development quotient was only 29.9. Brain magnetic resonance imaging (MRI) showing cerebral hypoplasia was also consistent with CP. Any pathologic reflex was not induced. Several scales were used for assessment in scheduled visits including Ashworth spasm assessment, Gross Motor Function Measure-88 (GMFM-88), and comprehensive function assessment. No other significant abnormal signs were observed in vital sign, physical exams, and laboratory tests.
Stem Cell Source
hUCB-MSCs were provided by Beike Biotechnology, and the material was from the umbilical cord blood of healthy puerperal woman with all the following tests negative: syphilis, HIV, hepatitis B virus, and TORCH 5. The hUCB-MSCs were cultivated, passaged, and collected at the fourth generation for usage in transplantation after separating and extracting from original material. All the quality test results met the ISCT hUCB-MSCs standards, including cell morphology, cell surface marker detection, bacterial detection, mycoplasma detection, and endotoxin detection. Cells were inoculated into 1-mL vials at a concentration of 5 × 107 cells/mL with medium.
Treatment
hUCB-MSCs Transplantation
The patient accepted her first infusion at the age of 6 months, and she received 4 infusions of intravenous hUCB-MSCs in each course with an interval of 7 days between every 2 infusions. Totally 4 courses of transplantation were completed by the age of 2 years with an interval of half a year between courses, and then annual assessments were performed. At each treatment course, the stem cells were fully dispersed and mixed using 30 mL normal saline, and then 5 × 107 cells were intravenously infused. All the treatments were performed in a specific room for stem cell transplantation. Immunosuppressant was not administered since the stem cell resource was from human and the quality was certificated for clinical usage.
Basic Rehabilitation Treatment
Basic rehabilitation treatment was employed by rehabilitation physiatrists for CP, including Bobath therapy and conductive education. The treatment was for 40 minutes each session and twice a day with a 1-day break in a week, lasting for 3 months in a treatment course. The patient received the first rehabilitation treatment at the age of 6 months, and has carried through as scheduled firmly up to date.
Clinical Response
Safety Monitoring
Physical exams and laboratory tests (including routine blood test, liver and kidney function) were conducted before transplantation and in annual assessments. In addition, thyroid function, virus tests (including syphilis, HIV, and hepatitis virus), TORCH tests, and MRI were performed for first diagnosis. Growth hormone was tested in annual assessments. Vital signs were monitored before and after each infusion over 24 hours, including blood pressure, heart rate, blood oxygen, and respiration.
Efficacy Evaluation
Electroencephalography (EEG) was conducted to evaluate the difference of cerebral function before and after transplantation.
Ashworth spasm assessment was performed since age of 2 years. GMFM-88 scales were conducted before transplantation and during the follow-up phase to assess motor function.
CDCC Infant Mental Development Scale assessment was performed to check the general development status before transplantation, which was modified from Bayley developmental scale for better accuracy and reliability in Chinese children from age 0 to 3.
Comprehensive function assessment was employed on treatment completion at the age of 2 years to assess cognizance ability, language competence, motor function, self-dependence, and social adaptability.
MRI scans were taken in scheduled visits to check the cerebral hypoplasia status and for safety monitoring.
Results
Safety Parameters
No adverse effects or complications were observed during treatment and in the follow-up phase. Physical exams and vital signs were normal. In addition, all the laboratory test results turned out to be within normal range. There was no sign of cerebral tumor according to the MRI exam within 5 years after transplantation.
Efficacy Parameters
Ashworth Spasm Assessment
The muscle tension was grade 1+ in hip flexion and adduction according to Ashworth spasm assessment, which was grade 1 since the assessment at the age of 3 up to date. There was no other abnormality in motor pattern in this case.
GMFM-88 Scales
Eighty-eight questions were addressed in the GMFM-88 scale for 5 function areas: “lying and rolling,” “sitting,” “crawling and kneeling,” “standing,” and “walking, running, and jumping.” The patient could not raise her head nor sit before transplantation at the age of 6 months, but the functions with regard to sitting, crawling, kneeling, lying, and rolling were almost recovered to normal level at the age of 2. Standing function was recovered at the age of 4, while walking, running, and jumping functions were recovered at the age of 5 (Figure 1). Besides, a significant improvement was observed in gross motor function according to the total score of GMFM, with a total recovery at the age of 5 (Figure 2), and an obvious improvement was observed at 6 months after the first treatment course according to clinical representation.
Figure 1.
GMFM-88 subscore.
GMFM-88 subscores showed improvement in sitting, crawling, kneeling, lying, and rolling to normal level at the age of 2. Standing function was recovered at the age of 4, while walking, running, and jumping functions were recovered at the age of 5.
Figure 2.

GMFM-88 total score.
GMFM-88 total score revealed a significant improvement in gross motor function with an overall recovery at the age of 5.
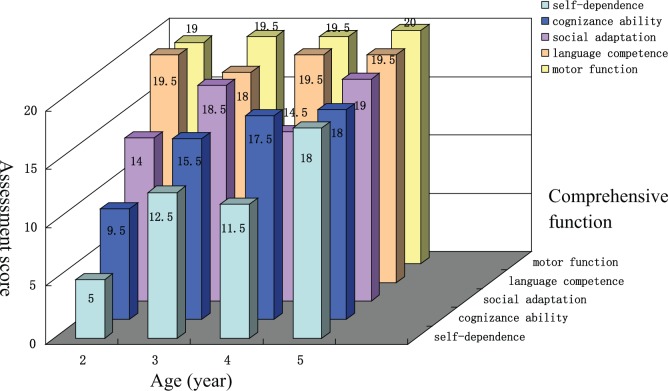
Comprehensive Function Assessment
The CDCC Infant Mental Development Scale was a reference for the baseline status since the comprehensive function assessment was not applicable at the age of 6 months. According to that assessment, the baseline status was at the level of 2 months with regard to gross and fine motor, cognizance ability, language competence, and social adaptability. Motor function and language competence developed well after transplantation, and were almost normal since the age of 2. Significant improvement was noticed regarding self-dependence, cognitive ability, and social adaptation, which were almost normal at the age of 5 (Figure 3). The function impact status was moderate at the age of 2 after transplantation, and recovered to mild since age of 3 according to the total score of comprehensive function assessment (Figure 4).
Figure 3.
Comprehensive function assessment subscores.
Comprehensive function assessment subscores showed motor function and language competence were almost normal since the age of 2, while self-dependence, cognitive ability, and social adaptation were almost recovered at the age of 5.
Figure 4.

Comprehensive function assessment total score.
Comprehensive function assessment total score showed the function impact status was moderate at the age of 2 after transplantation, and recovered to mild since age of 3.
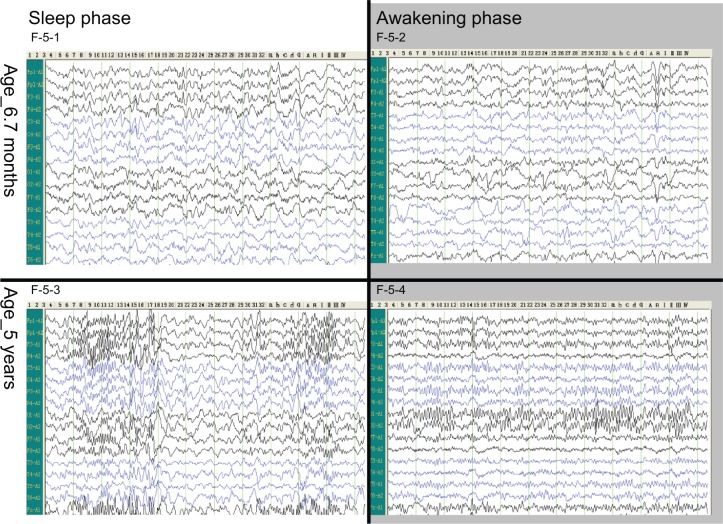
Electroencephalography
Cerebral function impact was revealed in EEC before transplantation at the age of 6 months due to increase in diffuse slow waves in both sleep and awakening stages. And the EEG was normal at the age of 5 years (Figure 5).
Figure 5.
Electroencephalogram.
An increase of diffuse slow waves was shown in both sleep and awakening stages in the EEC at the age of 6 months (F-5-1, F-5-2). Almost normal images were shown in EEG at the age of 5 years (F-5-3, F-5-4).
Magnetic Resonance Imaging
Normal cerebral structure was shown in MRI at the age of 5 years.
Discussion
The efficacy of traditional therapies is limited in patients with severe impact or older than 3 years, though patients with mild cerebral impact could benefit from these treatments. Recently, stem cell transplantation has been elevated to a high position for an active intervention, though the complete mechanism of action is not clear enough to focus on critical elements. The major obstacles of the treatment for CP are the lack of proper animal models for mechanism research and the limitation of clinical trials for definite and replicable results. Alternatively, hUCB-MSCs transplantation is a promising approach to cerebral injuries considering the safety in clinical paradigms and the easy accessibility, though the preclinical benefits are controversial.4,5 Regarding the clinical trials registered in ClinicalTrial.gov about UCB-MSC for CP treatment, 3 trials have been completed, while 8 trials were ongoing up to December 9, 2014. A positive result was reported in a double-blind, randomized, placebo-controlled trial with treatment of UCB-MSCs infusion combined with rehabilitation and erythropoietin.6
Similar improvements were observed in our case. In particular, safety evidence in 5-year follow-up made this case different from previous reports. Any hint of cerebral tumor was not present in such a long-term follow-up based on the laboratory tests and MRI results. Transplant rejection was not noticed in the transplantation period without immunosuppressant, which was consistent with the low immunogenicity of hUCB-MSCs. Besides, satisfactory efficacy data were consistent with similar reports.6,7
It was revealed by Min team that the improvements over 6 months on the GMPM, the BSID-II Mental and Motor scales, and the “social cognition” scale in WeeFIM differed significantly among pUCB, erythropoietin, and control groups. Multiple comparison tests for each difference revealed greater improvements in the pUCB group than in the other groups.6
In our case, significant improvements in gross motor function and language competence were shown based on GMFM-88 and comprehensive function assessment, which recovered to normal level at age of 2. Actually, obvious improvement was noticed since the sixth month after the first treatment course, which was similar to the analysis of Min team, that GMFM score differences were significant between the groups at the 3- to 6-month interval, and greater improvement in the pUCB group than in the control group was observed 6. Some expression impediment was observed when the patient repeated a long sentence though she could fully understand and make her expression clear slowly. Linguistic rehabilitation therapy was enhanced for further improvement. Furthermore, benefit was also shown in functional level, which was revealed in the results of EEG before and after transplantation. Finally, the clinical and functional improvements may profit from the normalization of cerebral structure after transplantation according to the MRI results.
The possible mechanisms for the treatment efficacy of stem cell transplantation to cerebral injury are still unclear, especially for chronic injury due to limitation of proper animal models. It did not appear that stem cell replacement of the cerebral lesion would account for the cerebral structure improvement in MRI alone. It was reported that the survival of transplanted cells was too few to repair the lesion,8 and it was also doubted if they could develop normal process and function in neuronal circuitry.9 It seemed more promising for the theory of greater survival of intrinsic cells induced by the transplants, which was reported in an acute injury model.10 Besides, differentiation from transplanted cells to astrocytes9 or microglia might also contribute to the recovery of cerebral structure. Regarding the overall recovery from structure to function, there was no doubt that persistent rehabilitation played an important role in this case. hUCB-MSCs transplantation was treated as a critical factor in the success of the treatment course, since it was impossible to achieve such a significant improvement on motor functions in 6 months relying on the rehabilitation approach alone. Moreover, the recovery of cerebral structure was more likely attributed to the structural repairing induced by hUCB-MSCs transplantation, though the exact mechanism was unknown.
Despite treatment safety and efficacy, the clinical process of transplantation was also explored from specific management in this case regarding the optimal time, dose, and infusion route, which varied in different reports. Early diagnose and transplantation seemed a favorable factor for satisfactory efficacy in this case, although we could not work out an opportune time schedule only based on this case. It was generally accepted that earlier treatment brought more benefit for the patient with CP, since the longer the interval from the injury, the less likely the lesion could recover. Regarding the dose, 5 × 107 cells were sufficient in this case, which could be a reference for other cases. Besides, it was demonstrated in this case that intravenous infusion was effective in achieving satisfactory improvement, which was more feasible compared with intraventricular injection. It supported the notion that the improvement in function might not be directly linked to the implantation of the cells themselves,11 considering the hematoencephalic barrier.
Conclusions
A satisfactory result of safety and efficacy was obtained with hUCB-MSCs transplantation combined with rehabilitation for CP treatment. hUCB-MSCs transplantation is a promising alternative based on the positive results of several cases reports and clinical trials, though the mechanism is still controversial in animal models. Besides, the increasing popularity of postnatal umbilical cord stem cell harvest and storage makes it the most easily accessible stem cells for clinical usage. Regarding the challenges, further efforts are required in mechanism study with proper animal models and the demonstration of clinical safety and efficacy in clinical trials and case reports with definite and replicable results.
Author Contributions
CZ contributed to conception and design; contributed to acquisition and interpretation of data; gave final approval. LH contributed to analysis of data; drafted the manuscript; critically revised the manuscript; gave final approval. JG contributed to acquisition of data; gave final approval. XZ contributed to conception; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Acknowledgments
We acknowledge the assistance of the patient and her family who participated in this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present work is supported by Hubei Province Technology Fund No. 2013BCB002 and is conducted in Children’s Medical Center of Taihe Hospital.
References
- 1. Bax M, Goldstein M, Rosenbaum P, et al. Proposed definition and classification of cerebral palsy. Dev Med Child Neurol. 2005;47:571-576. [DOI] [PubMed] [Google Scholar]
- 2. Sawyer MG, Bittman M, LA Greca AM, et al. Time demands of caring for children with cerebral palsy: what are the implications for maternal mental health? Dev Med Child Neurol. 2011;53:338-343. [DOI] [PubMed] [Google Scholar]
- 3. Surveillance of Cerebral Palsy in Europe (SCPE). Surveillance of cerebral palsy in Europe. A collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. 2000;42:816-824. [DOI] [PubMed] [Google Scholar]
- 4. Pimentel-Coelho PM, Magalhaes ES, Lopes LM, de Azevedo LC, Santiago MF, Mendez-Otero R. Human cord blood transplantation in a neonatal rat model of hypoxic-ischemic brain damage: functional outcome related to neuroprotection in the striatum. Stem Cells Dev. 2009;19:351-358. [DOI] [PubMed] [Google Scholar]
- 5. Dalous J, Pansiot J, Pham H, et al. Use of human umbilical cord blood mononuclear cells to prevent perinatal brain injury: a preclinical study. Stem Cells Dev. 2013;22:169-179. [DOI] [PubMed] [Google Scholar]
- 6. Min K, Song J, Kang JY, et al. Umbilical cord blood therapy potentiated with erythropoietin for children with cerebral palsy: a double-blind, randomized, placebo-controlled trial. Stem Cells. 2013;31:581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papadopoulos KI, Low SS, Aw TC, Chantarojanasiri T. Safety and feasibility of autologous umbilical cord blood transfusion in 2 toddlers with cerebral palsy and the role of low dose granulocytecolony stimulating factor injections. Restor Neurol Neurosci. 2011;29:17-22. [DOI] [PubMed] [Google Scholar]
- 8. Yasuhara T, Hara K, Maki M, et al. Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic-ischemic rats. J Cereb Blood Flow Metab. 2008;28:1804-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711-10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increasers the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma. 2004;21:33-39. [DOI] [PubMed] [Google Scholar]
- 11. Bartley J, Carroll JE. Stem cell treatment for cerebral palsy. Expert Opin Biol Ther. 2003;3:541-549. [DOI] [PubMed] [Google Scholar]