Abstract
Simple and reliable methods for evaluating the inhibitory effects of drug candidates on complement activation are essential for preclinical development. Here, using an immortalized porcine aortic endothelial cell line (iPEC) as target, we evaluated the feasibility and effectiveness of an in vitro xenoantibody-mediated complement-dependent cytotoxicity (CDC) model for evaluating the complement inhibitory activity of Cp40, a potent analog of the peptidic C3 inhibitor compstatin. The binding of human xenoantibodies to iPECs led to serum dilution-dependent cell death. Pretreatment of the human serum with Cp40 almost completely inhibited the deposition of C3 fragments and C5b-9 on the cells, resulting in a dose-dependent inhibition of CDC against the iPECs. Using the same method to compare the effects of Cp40 on complement activation in humans, rhesus and cynomolgus monkeys, we found that the inhibitory patterns were similar overall. Thus, the in vitro xenoantibody-mediated CDC assay may have considerable potential for future clinical use.
Keywords: SV40-immortalized porcine aortic endothelial, cell, Complement inhibitor, Cp40, Nonhuman primate
1. Introduction
When dysregulated or inadvertently triggered by diseased, injured or foreign cells, the complement system can contribute to a wide spectrum of inflammatory disorders [1]. As a consequence, there is a growing interest in complement therapeutics, particularly in strategies that interfere at the activation of the central complement component C3 [2]. Compstatin, a C3-targeted complement inhibitor, was originally discovered by screening a phage-displayed random peptide library [3]. This peptide specifically binds to native primate C3 and potently inhibits complement activation via all major initiation pathways [4]. Cp40 is a novel analog of compstatin that shows higher serum stability, 5000-fold stronger binding affinity for C3, and improved pharmacokinetic properties when compared to compstatin [1,5,6]. In a clinically relevant study on paroxysmal nocturnal hemoglobinuria (PNH), Cp40 was found to effectively protect PNH erythrocytes from both intravascular and extravascular hemolysis in vitro, thereby showing potential therapeutic advantage over the established anti-C5 therapy [5]. In another preclinical study, Cp40 was able to inhibit complement dysregulation in vitro in C3 glomerulopathy and may therefore offer a novel therapeutic option for affected patients [7]. In addition, Cp40 has been shown to be a potent inhibitor of complement activation in several in vivo and ex vivo animal models, such as a primate model of hemodialysis-induced complement activation [8], a ligature-induced periodontitis model in nonhuman primates (NHP) [9], and a xenogeneic model of interactions between human whole blood and porcine endothelium [10]. This experimental evidence suggests that Cp40 has strong potential as a therapeutic agent for clinical use [4].
Whereas the plasma levels of Cp40 and C3 during in vivo studies can be monitored using analytical methods to estimate the drug-to-target ratio, sensitive ex vivo methods are desired to experimentally confirm the inhibitory efficacy of Cp40 during treatment. The first method described for measuring the inhibitory effect of compstatin on complement activity was based on a hemolytic model in human serum [3]. After the incubation of rabbit erythrocytes and normal human serum pretreated with compstatin, the percentage of red cell lysis was determined by measuring the optical density of supernatant at 414 nm and normalizing the results by considering 100% lysis to be equal to lysis occurring in the absence of the peptide. However, the lack of available standard rabbit erythrocytes and the indirect evaluation by OD value limit the repeatability and accuracy of this method. Within the past decade, an ELISA-based assay was established to quantify the inhibitory effect of Cp40 and other compstatin derivatives on complement activation [11–13]. With this method, complement is activated by antibody–antigen complexes via the classical pathway, and the deposition of C3b is detected by ELISA. Although this method avoided the individual differences inherent in targeting primary cells, OD values were also used as the indicator in this method. The development of a simple and reliable method that can directly evaluate the effects of Cp40 and other complement inhibitors in a clinically relevant context would therefore be valuable for efficacy monitoring.
It has been demonstrated that the majority of preformed natural antibodies contained in human or NHP sera can bind to the Galα1-3Gal (α-Gal) epitope expressed on porcine endothelial cells (PECs), resulting in the activation of complement via the classic pathway and subsequent rapid cell death [14–18], which can be sensitively and accurately detected by flow cytometry via propidium iodide (PI) staining [17,19]. Given that the xenoantibody-mediated cytotoxicity to PECs is well defined as being complement-dependent, the in vitro cell death model may be useful for evaluating the complement inhibitory activity of compstatin and its derivatives.
In the present study, with the use of an SV40-immortalized porcine aortic endothelial cell line iPEC as a target and human or NHP sera as sources of xenoreactive natural antibodies and complement, we have tested the feasibility and effectiveness of the porcine cell lysis model in evaluating the complement inhibitory activity of Cp40 in human serum and have also compared the effects of Cp40 in different primate species.
2. Materials and methods
2.1. Cell line and cell culture
The SV40-immortalized porcine aorta-derived endothelial cell line iPEC was a gift from Dr. J. Holgersson (Karolinska Institute, Huddinge, Sweden). iPECs were maintained in low-glucose Dulbecco's modified Eagle's medium (DMEM; Hyclone, China) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, USA). The cells were cultured in cell culture flasks at 37 °C in a 5% CO2 atmosphere before experimentation.
2.2. Blood and serum preparations
Fresh non-anticoagulated human blood, without any restriction of blood type, was obtained from eight healthy volunteers who had given informed consent in accordance with the Helsinki Protocol and had received no medication for at least 10 days. Complement-active normal human serum (NHS) was collected, pooled, and stored at −80 °C to maintain its complement activity. Pooled normal serum samples from cynomolgus monkeys (n = 8) and rhesus monkeys (n = 8) were obtained separately, following the same procedure as for humans. The donor monkeys (3–5 years old; weight, 3–7 kg) were purchased from Guangzhou Landao Biotechnology Corporation and South China Primate Research Center, Guangzhou, China. They were kept in the primate facility at the Experimental Animal Centre of Tongji Medical College according to the University's Research Animal Resources guidelines. As negative controls, pooled normal serum of each species was heated at 56 °C for 30 min and is referred to as heat-inactivated normal serum.
2.3. Pretreatment of serum samples with CP40
Cp40 (dTyr-Ile-[Cys-Val-Trp(Me)-Gln-Asp-Trp-SAR-His-Arg-Cys]-mIle-NH2) [17,19], a potent compstatin analog, was prepared by solid-phase peptide synthesis [6] and used to block complement activation. Pooled normal serum samples from either humans, cynomolgus monkeys, or rhesus monkeys were separately pretreated for 1 h at 37 °C with various final concentrations of Cp40 (from 1.25 μg/ml to 1280 μg/ml) prior to incubation with iPECs.
2.4. Detection of α-Gal expression on iPECs by FACS
Staining for α-Gal was performed as described previously [20]. In brief, iPECs (2 × 105) were incubated with fluorescein isothiocyanate (FITC)-conjugated Griffonia simplicifolia isolectin B4 (GS-IB4) (5 μg/ml, Sigma-Aldrich Corp., USA) for 30 min at 4 °C. Cells incubated with PBS alone served as a negative control. The stained cells were washed twice and then analyzed by flow cytometry (FACSCalibur, BD Biosciences).
2.5. Binding of xenoreactive antibodies present in human serum
To detect the binding of preformed anti-pig xenoreactive IgG and IgM to iPECs, 2 × 105 non-fixed single cells were incubated with heat-inactivated normal human serum (HINHS, 1:5 dilutions) at 4 °C for 30 min. After two washes with PBS, the cells were incubated with FITC-conjugated rabbit anti-human IgG or IgM (1:100, Zhongshan Biotechnology Co. Ltd., Beijing, China) at 4 °C for 30 min in the dark. iPECs incubated with secondary antibody alone served as negative controls. The stained cells were analyzed by flow cytometry. The geometric mean fluorescence intensity (Gmean) was used to evaluate the degree of xenoantibody binding to iPECs.
Binding of human xenoreactive IgG and IgM to iPECs was also detected by immunofluorescent staining. In brief, iPECs (5 × 104/well) were cultured overnight in 48-well plates with 200 μl of DMEM. Cells were washed four times with PBS, then incubated with 20% HINHS or PBS (negative control) at 37 °C for 20 min. After being washed, the cells were then incubated with FITC-conjugated rabbit anti-human IgG or IgM (1:50, Zhongshan Biotechnology Co. Ltd., Beijing, China) at 37 °C for 30 min in the dark. Fluorescence was visualized with a fluorescence microscope (Nikon ECLIPSE TE2000-U, Japan).
2.6. Detection of C3b/iC3b, C4b/iC4b, and C5b-9 deposited on iPECs
After incubation with 20% pooled NHS at 37 °C for 20 min, the deposition of C3- and C4-derived opsonins, and C5b-9 onto iPECs was detected by flow cytometry and immunofluorescent staining, using methods similar to those we described previously [20]. Cells incubated with HINHS were used as negative controls. To detect deposition of C3c- and C4c-containing fragments (i.e., C3b/iC3b and C4b/iC4b, respectively), cells were incubated for 30 min with FITC-conjugated rabbit anti-human C3c and anti-human C4c antibody, respectively (1:100 for FACS and 1:50 for immunofluorescent staining, Zhongshan Biotechnology, Beijing, China). To detect terminal complement complex (i.e., C5b-9) deposition, cells were incubated for 30 min with mouse anti-human C5b-9 primary antibody (1:100 for FACS and 1:50 for immunofluorescence, clone aE11, DAKO Corporation, CA, USA), followed by the Dlight 488-conjugated goat anti-mouse secondary antibody (1:100, Zhongshan Biotechnology, Beijing, China) for 30 min; all incubations were done at 4 °C for FACS and 37 °C for immunofluorescent staining. The stained cells were analyzed by flow cytometry. Gmean was used to evaluate the degree of complement deposition. Fluorescence was visualized with a fluorescence microscope (Nikon ECLIPSE TE2000-U, Japan).
2.7. Antibody-mediated complement-dependent cytotoxicity (CDC) assay
iPECs (100 μl, 2 × 105) were added to 100 μl of various diluted or pretreated serum samples. Incubation was carried out at 37 °C for 30 min and stopped with 3 ml FACS buffer (PBS containing 2% FBS and 0.02% azide). iPECs incubated with 20% or 40% heat-inactivated normal serum were used as negative controls. After two washes, 250 μl FACS buffer and 5 μl propidium iodide (PI, eBioscience, Inc., CA, USA) were added to each tube and incubated for 15 min in the dark, and then the fluorescence was analyzed by flow cytometry. The percentage of PI-positive cells (compared to total cells) was used to evaluate the degree of cell death. The inhibition percentage was calculated as described elsewhere [7,21,22], using the following equation: % inhibition = {1 – (A – C) / (B – C)} × 100%, where A, B, and C represent the percentage of cell death when incubated with 20% serum pretreated with Cp40 (A), 20% serum without any treatment (B), and 20% heat-inactivated serum (C), respectively.
2.8. Statistical analysis
The data were expressed as means ± SEM. All of the statistical analyses were performed using GraphPad Prism 6.0 software (GraphPad Software, USA). The significance of the differences between selected groups was evaluated using the unpaired t-test or one-way ANOVA followed by Dunnett's multiple comparisons test or two-way ANOVA followed by Tukey's multiple comparisons test. P < 0.05 was considered significant.
3. Results
3.1. Human serum-mediated iPEC lysis is a typical model of xenoantibody-mediated complement-dependent cytotoxicity
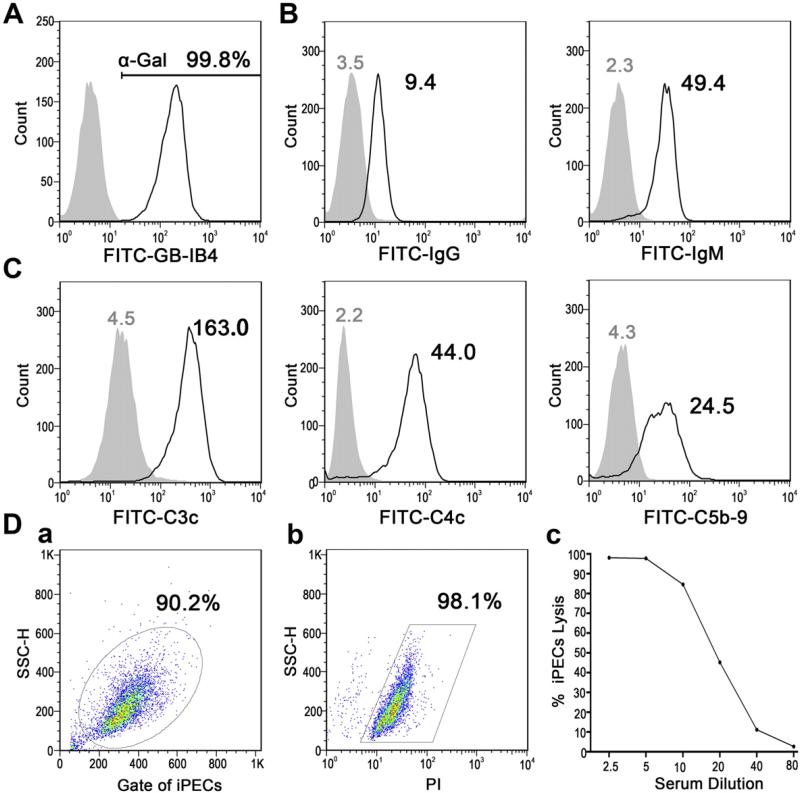
Expression of the major xenoantigen α-Gal on iPECs was detected by flow cytometry. Almost all iPECs were found to be α-Gal-positive (Fig. 1A). After the incubation with NHS, iPECs were found to be able to bind both IgG and IgM xenoantibodies contained in human serum (Fig. 1B), resulting in the activation of complement via the classic pathway, as indicated by the deposition of both C4- and C3-derived complement fragments, and the terminal complement complex C5b-9 (Fig. 1C). To determine whether iPECs could be killed by NHS, the CDC assay was performed by flow cytometry with sequentially diluted NHS (1:2.5 to 1:80) as a source of xenoantibodies and complement. With the higher serum concentrations (i.e., 1:2.5 and 1:5 dilutions), NHS showed a very potent cytotoxic effect on iPECs. After further sequential dilution of NHS (1:10 to 1:80), the cell-killing effect became progressively weaker (Fig. 1D). Serum incubated with 40% HINHS (1:2.5) displayed only background cytotoxic activity for iPECs (2.6%). Taken together, these results demonstrated that the human serum-mediated iPEC lysis represents a typical xenoantibody-mediated complement-dependent cytotoxic model that can be applied to evaluate the activity of complement inhibitors.
Fig. 1.
An in vitro human xenoantibody-mediated complement-dependent cytotoxic model. (A) FACS analysis for α-Gal expression on iPECs with FITC-GS-IB4 staining. Cells incubated with PBS alone were used as a negative control. (B) After incubation with 20% HINHS, the binding of human IgG and IgM on iPECs was analyzed by flow cytometry. The geometric mean fluorescence intensity (Gmean) was used to evaluate the degree of xenoantibody binding to iPECs. Cells incubated with secondary antibody alone served as negative controls. (C) Deposition of C3c- and C4c-containing complement opsonins and C5b-9 on iPECs after incubation with 20% NHS was detected by flow cytometry and evaluated by Gmean. Negative controls were cells incubated with 20% HINHS. (D) Cell death was analyzed by flow cytometry after the incubation of iPECs with sequentially diluted NHS. Representative FACS analyses for the gating of iPECs (a) and PI-positive iPECs (b, 1:5 diluted NHS) are shown. The percentages of iPEC lysis mediated by sequentially diluted NHS (1:2.5 to 1:80) are shown as a line graph (c). All results shown are representative of three independent experiments.
3.2. No impact of Cp40 on the binding of human xenoreactive antibodies to iPECs
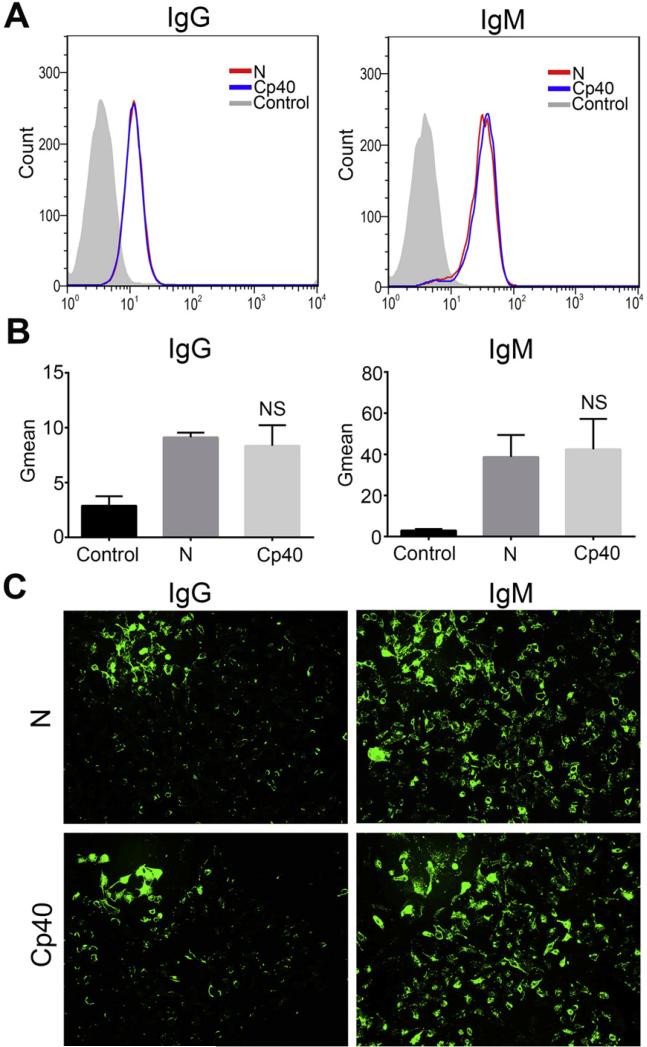
Thus far, no evidence has been reported to indicate whether Cp40 can interfere with the binding of human xenoreactive antibodies to iPECs. To answer this question, we used flow cytometry and immunofluorescent staining to detect the binding of human natural antibodies to iPECs after incubation of the iPECs with 20% HINHS, with or without Cp40 (20 μg/ml) treatment. iPECs incubated with PBS served as negative controls. FACS analysis showed that both xenoreactive IgG and IgM could significantly bind to iPECs, and the degree of binding seen in the Cp40-treated group and the untreated group was almost identical (Figs. 2A,B). Immunofluorescent staining further confirmed these findings (Fig. 2C).
Fig. 2.
No impact of Cp40 on the binding of human xenoreactive antibodies to iPECs. Binding of human xenoreactive antibodies (IgG/IgM) to iPECs was measured by FACS (A and B) and immunofluorescent staining (C, ×100) after the incubation of iPECs with either 20% HINHS (N) or Cp40-pretreated 20% HINHS (Cp40). iPECs incubated with secondary antibody alone served as negative controls (Control). For FACS analysis, the Gmean was used to evaluate the degree of xenoantibody binding to iPECs. (A) FACS results shown are representative of three independent experiments. (B) The extent of human IgG and IgM binding to iPECs is shown in the bar graphs. Data shown are means ± SEM (NS, P > 0.05 vs. N group; n = 3 per group). (C) Images are representative of three independent experiments.
3.3. Cp40 potently inhibits complement C3 activation and the formation of C5b-9
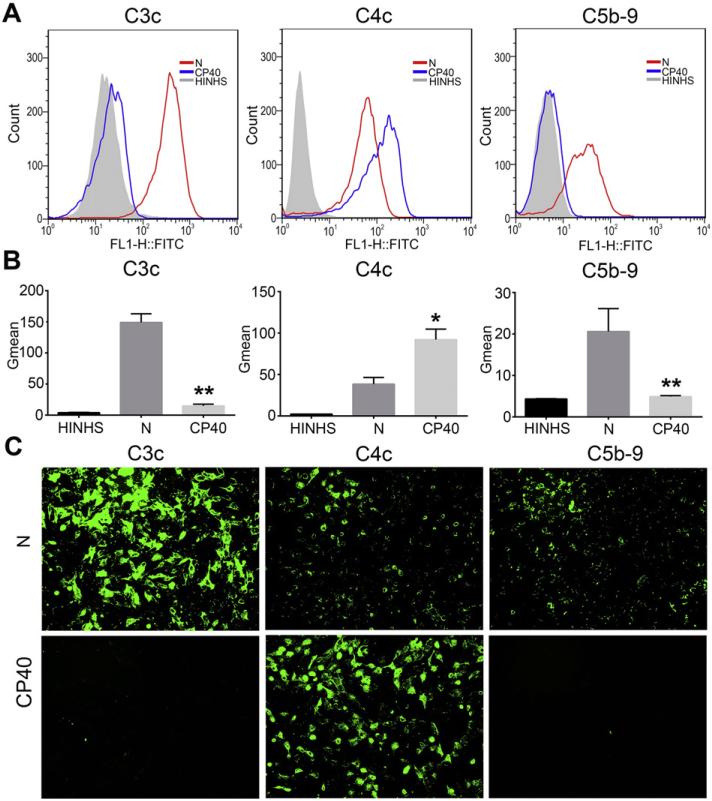
To assess the mechanisms by which Cp40 inhibits complement activation, deposition of the complement opsonins C3b/iC3b and C4b/iC4b (measured using antibodies against the common C3c and C4c parts, respectively) and terminal complement complexes (i.e. C5b-9) onto iPECs was detected by flow cytometry and immunofluorescent staining after incubation of the iPECs with 20% NHS, with or without Cp40 (20 μg/ml) pretreatment. As shown in Figs. 3A and B, FACS analysis did show a marked deposition of C3b/iC3b, C4b/iC4b, and C5b-9 onto the iPECs when the cells were incubated with 20% NHS, indicating that complement was activated via the classic pathway. As expected, Cp40 pretreatment almost completely inhibited the deposition of C3b/iC3b and C5b-9 (P < 0.01 vs. the Cp40-untreated group). Interestingly, increased deposition of C4b/iC4b on iPECs was observed in the Cp40-pretreated group when compared to the Cp40-untreated group (P < 0.05) (Figs. 3A, B). All these results were further confirmed by immunofluorescent staining (Fig. 3C).
Fig. 3.
Cp40 significantly inhibits the cleavage of complement C3 and the formation of C5b-9. Deposition of C3c- and C4c-containing complement opsonins and C5b-9 on iPECs was detected by FACS (A and B) and immunofluorescent staining (C, ×100) after the incubation of iPECs with either 20% NHS (N) or Cp40-pretreated 20% NHS (Cp40). Cells incubated with HINHS were used as negative controls. For FACS analysis, the Gmean was used to evaluate the degree of complement deposition. (A) FACS results shown are representative of three independent experiments. (B) The degree of deposition of C3c- and C4c-containing opsonins, and C5b-9 on iPECs is shown in the bar graphs. Data shown are means ± SEM (*P < 0.05, **P < 0.01 vs. the N group; n = 3 per group). (C) Images are representative of three independent experiments.
3.4. Cp40 inhibits human serum-mediated CDC in a dose-dependent manner
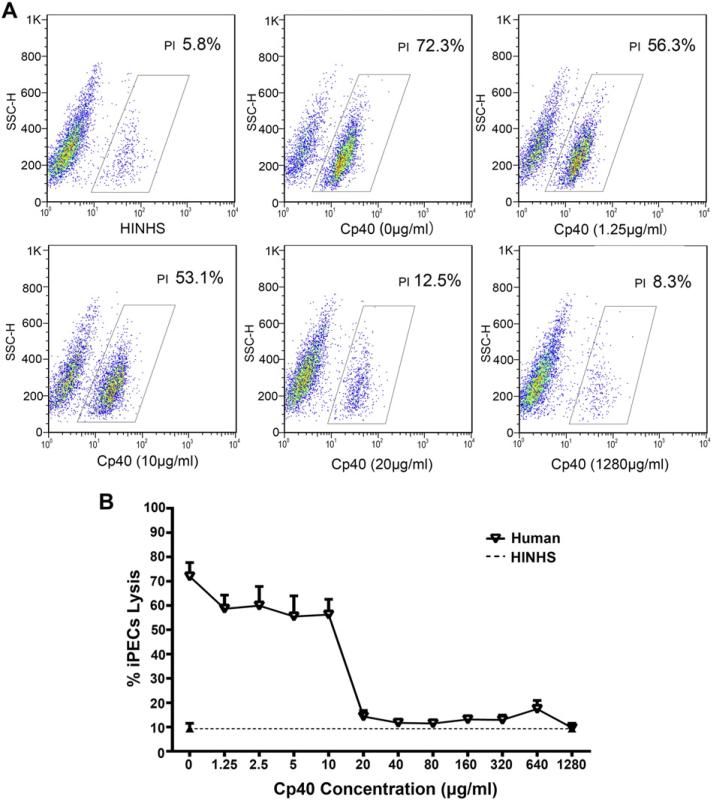
Since human serum-mediated iPEC lysis is complement-dependent, the proportion of dead cells should reflect the severity of complement activation. Therefore, the in vitro CDC model can be used to evaluate the activity of complement inhibitors such as Cp40. To study the effects of Cp40 on human serum-mediated CDC, PI-positive dead cells were detected by flow cytometry after incubation of the iPECs with 20% NHS pretreated with various doses of Cp40 (final concentrations from 1.25 to 1280 μg/ml). As shown in Fig. 4, pretreatment of the NHS with lower doses of Cp40 (1.25 to 10 μg/ml) only slightly inhibited the cell lysis of iPECs. When the concentration of pretreated Cp40 was raised to 20 μg/ml, the cell lysis of the iPECs was dramatically reduced, to a level close to that of the negative control. Further increasing the concentration of Cp40 from 40 μg/ml to 1280 μg/ml did not enhance this inhibitory effect.
Fig. 4.
Cp40 inhibits human serum-mediated CDC in a dose-dependent manner. PI-positive dead cells were detected by FACS after the incubation of iPECs with 20% NHS pretreated with various doses of Cp40 (final concentrations from 1.25 to 1280 μg/ml). iPECs incubated with 20% HINHS were used as negative controls. The percentage of the total cells that were PI-positive was used to evaluate the degree of cell death. (A) Representative FACS results for negative controls and Cp40 pretreatment at 0, 1.25, 10, 20, and 1280 μg/ml. (B) The percentages of iPEC lysis mediated by 20% NHS that had been pretreated with various doses of Cp40 are shown as a line graph. Data shown are means ± SEM (n = 3).
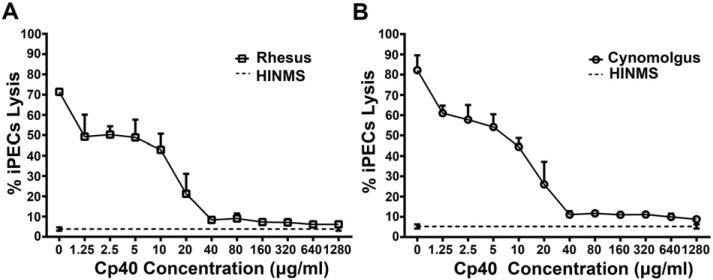
3.5. Effects of Cp40 on NHP serum-mediated CDC
To study the effects of Cp40 on NHP serum-mediated CDC, PI-positive dead cells were detected by flow cytometry after the incubation of iPECs with 20% rhesus or cynomolgus monkey sera pretreated with various doses of Cp40 (final concentrations from 1.25 to 1280 μg/ml; Fig. 5). Cp40 pretreatment in vitro showed a similar dose-dependent inhibition of the cell lysis mediated by both rhesus and cynomolgus monkey sera. Cp40 at higher concentrations (40 μg/ml to 1280 μg/ml) markedly decreased the CDC mediated by NHP sera, to a level close to that of the negative control. However, even with the pretreatment of Cp40 at the highest dose (1280 μg/ml), complete inhibition of NHP sera-mediated CDC to iPECs was not achieved.
Fig. 5.
Effect of Cp40 on NHP serum-mediated CDC. PI-positive dead cells were detected by FACS after incubation of iPECs with 20% rhesus (A) or cynomolgus monkey (B) serum that had been pretreated with various doses of Cp40 (final concentrations from 1.25 to 1280 μg/ml). iPECs incubated with 20% heat-inactivated normal monkey serum (HINMS) were used as negative controls. Data shown are means ± SEM (n = 3).
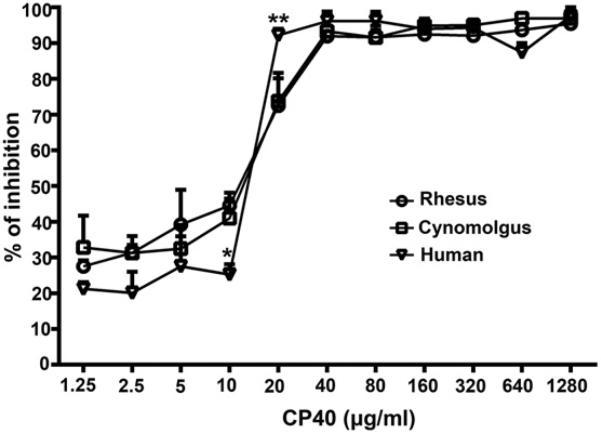
3.6. Comparison of the complement inhibitory activity of Cp40 in humans and NHPs
The percentage of inhibition was calculated to allow us to compare the complement inhibitory activity of Cp40 against human and NHP sera according to the cytotoxicity results. As shown in Fig. 6, the overall inhibitory patterns for human serum and two kinds of NHP serum were similar. However, whereas the percentage of inhibition achieved by Cp40 at 10 μg/ml in the NHP sera was slightly greater than that produced by human serum (P < 0.05), Cp40 at 20 μg/ml showed a stronger inhibition in human serum than in the NHP sera (P < 0.01). No statistical differences were observed between human and NHP sera when Cp40 was administered at the other concentrations.
Fig. 6.
Comparison of the complement inhibitory activity of Cp40 in human and NHP (rhesus and cynomolgus monkey) sera. The percentage of inhibition was calculated according to CDC results to compare the complement inhibitory activity of Cp40 on human and NHP sera. All data are expressed as means ± SEM (*P < 0.05, **P < 0.01 vs. both rhesus and cynomolgus monkeys; n = 3).
4. Discussion
In many clinical situations, comprehensive inhibition of complement activation at the central level of C3 is considered a promising therapeutic strategy [2]. By selectively binding to C3 from humans and NHP, compstatin and its derivatives prevent the essential conversion of C3 to C3b by all C3 convertases and broadly inhibit complement activation. With their high selectivity and potency and their beneficial toxicity and immunogenicity profiles, compstatin derivatives show potential for clinical use in the treatment of complement-mediated diseases [4]. For assessing the efficacy of compstatin derivatives and other complement-targeted drug candidates, it is necessary to develop a simple and reliable method to evaluate the inhibitory effects of these compounds on complement activation. In the present study, the complement inhibitory activity of the clinically developed compstatin analog Cp40 was successfully measured with a method based on an in vitro xenoantibody-mediated complement-dependent cytotoxicity (CDC) model.
Since porcine endothelial cells (PECs) express the major xenoantigen α-Gal, and human or NHP serum contains considerable amounts of natural anti-α-Gal xenoantibodies and normal levels of complement, in vitro incubation of the iPECs with human or NHP serum will activate complement via the classical pathway and cause rapid cell death [14–18]. With the amount and activity of natural xenoantibodies unchanged, the degree of porcine cell death is dependent on the capacity for complement activation. Therefore, this in vitro xenoreactive CDC assay may be used to test the inhibitory effects on complement activation after pretreatment of human or NHP serum with compstatin or its derivatives in vitro or for ex vivo monitoring of residual complement activity in inhibitor-treated animals during preclinical studies. Because a large number of homogeneous PECs can be easily obtained from the SV40-immortalized porcine aorta endothelial cell line (iPEC) [20], these cells were used as target cells in the present study.
As expected, iPECs were found to express a high intensity of α-Gal and to bind large quantities of human xenoreactive antibodies after incubation with human serum, resulting in a serum dilution-dependent cell lysis. The binding of human xenoreactive antibodies to the iPECs was unchanged after pretreatment of the human sera with Cp40, demonstrating that Cp40 did not interfere with binding of the antibodies to the iPECs.
To study the effects of Cp40 on human serum-mediated CDC against iPECs, dead cells were detected by flow cytometry after the incubation of the iPECs with 20% NHS that had been pretreated with various doses of Cp40. Overall, the inhibition of CDC resulting from Cp40 pretreatment was found to be dose-dependent, indicating that the inhibitory effects of compstatin or its derivatives on complement activation can be evaluated by the in vitro xenoreactive CDC assay. As expected, there was a rapid reduction in cell death when the concentration of pretreated Cp40 was raised to 20 μg/ml, whereas no further enhancement of the inhibition of human serum-mediated CDC against iPECs was observed once C3 was saturated with Cp40 at higher concentrations. This assay may therefore be used in guiding dose finding and monitoring drug efficacy in the preclinical and clinical development of Cp40.
In the situation of pig-to-human xenotransplantation, the complement cascade is mainly activated via the classical pathway. In our cell-based assay, this is indicated by sequential deposition of the activation fragments C4b/iC4b, C3b/iC3b, and C5b-9 as readouts of the initiating C1 complex, the C3 convertases and the terminal C5 convertases, respectively. By preventing the deposition of C3b, which is a central element of all C3 and C5 convertases, compstatin derivatives are expected to specifically block deposition of C3 and C5-derived fragments. Indeed, it has been reported in an ex vivo model of xenotransplantation that only the concentrations of C3 fragments and C5b-9, but not C1 and C4, are decreased as a result of compstatin treatment [23,24]. In our in vitro CDC model, we monitored the deposition of C3b/iC3b, C4b/iC4b, and C5b-9 on iPECs after incubation with Cp40-treated or untreated NHS. Our results showed that Cp40 pretreatment almost completely inhibited the deposition of C3b/iC3b and C5b-9 but not of C4b/iC4b, confirming inhibition at the level of C3. Interestingly, the deposition of C4 fragments on iPECs was slightly increased in the Cp40-pretreated group when compared to the untreated group. The exact mechanism and potential relevance of this enhanced C4 deposition is not clear and warrants further investigation. Whereas C4b cannot amplify the complement response or induce C5 convertases in the absence of C3b, little is still known about the effector functions of this opsonin. It will therefore be interesting to investigate the effect of compstatin derivatives on C4b levels in different assays and preclinical models.
Due to the narrow species specificity of the compstatin family of C3 inhibitors, nonhuman primate models are frequently used for the preclinical testing of these peptide drugs. In the present study, we therefore compared the effects of Cp40 on complement activation in humans and two NHPs (cynomolgus monkeys and rhesus monkeys) using the in vitro xenoreactive CDC assay. We found that human serum and sera from the two NHP species had an overall similar inhibitory pattern, despite slight differences at a few Cp40 concentrations. These results indicate that testing the effects of Cp40 on disease models in both cynomolgus and rhesus monkeys can provide valuable experimental data for future clinical applications.
The assay used in this study most closely reflects the clinical context of xenotransplantation, in which perfusion of non-human organs with human blood typically induces a strong inflammatory reaction that leads to rejection [25]. To overcome the shortage of human donor organs, a large body of research has been performed to establish pigs as suitable donors. However, the binding of natural antibodies to α-Gal epitopes on endothelial cells with subsequent activation of complement and downstream inflammatory reactions remains a critical complication. Besides the use of genetically modified pigs that do not express α-Gal and/or express human complement regulators, therapeutic intervention at the complement level is considered a promising strategy to enable xenotranplantation. Compstatin analogs, including Cp40, have previously shown efficacy in ex vivo and in vitro models of xenotransplantation [10,24,25], and our current studies underscore and extend the potential of such applications. As even human endothelial cells often become target of complement activation under disease conditions, for example during allotransplantation and ischemia–reperfusion injury but also as part of thrombotic microangiopathies and C3 glomerulopathies [26], our model has broader clinical impact.
In conclusion, we have demonstrated that a simple and reliable method based on an in vitro xenoantibody-mediated complement-dependent cell lysis model with a porcine aortic endothelial cell line as targets can be used to evaluate the complement inhibitory activity of complement-directed drug candidates, as shown with the clinically relevant C3 inhibitor Cp40. The method was found to be highly reproducible and could be successfully translated for evaluating inhibitory efficacy in NHP models. Besides the efficacy monitoring of complement inhibitors, the method may also be used to study functional mechanisms of complement activation and modulation as revealed by the differential effect on C3, C4, and C5 fragment deposition in the presence and absence of Cp40.
Acknowledgments
We thank Dr. Deborah McClellan for editorial assistance. This study was supported by the National High-Tech Researching and Developing Program (Program 863) of the Ministry of Science and Technology of the People's Republic of China (2012AA021010) and the National Natural Science Foundation of China (No. 81172825), grants from the U.S. National Institutes of Health (AI069730 and AI030040) and the National Science Foundation (No. 1423304), and funding from the European Community's Seventh Framework Programme under grant No. 602699 (DIREKT).
Abbreviations
- α-Gal
Galα1-3Gal
- CDC
complement-dependent cytotoxicity
- FACS
fluorescence-activated cell sorter
- Gmean
geometric mean fluorescence intensity
- HINHS
heat-inactivated normal human serum
- HINMS
heat-inactivated normal monkey serum
- iPEC
SV40-immortalized porcine aortic endothelial cell line
- NHS
normal human serum
- NHP
nonhuman primate
- PI
propidium iodide
Footnotes
Disclosure
J.D.L. and D.R. are the inventors of patents and/or patent applications that describe the use of complement inhibitors for therapeutic purposes. J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications. The other authors have no financial conflicts of interest.
References
- 1.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J. Immunol. 2013;190:3839–3847. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricklin D, Lambris JD. Therapeutic Control of Complement Activation at the Level of the Central Component C3. Immunobiology. 2015 doi: 10.1016/j.imbio.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 1996;157:884–891. [PubMed] [Google Scholar]
- 4.Mastellos DC, Yancopoulou D, Kokkinos P, Huber-Lang M, Hajishengallis G, Biglarnia AR, Lupu F, Nilsson B, Risitano AM, Ricklin D, Lambris JD. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur. J. Clin. Investig. 2015;45:423–440. doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risitano AM, Ricklin D, Huang Y, Reis ES, Chen H, Ricci P, Lin Z, Pascariello C, Raia M, Sica M, Del VL, Pane F, Lupu F, Notaro R, Resuello RR, DeAngelis RA, Lambris JD. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094–2101. doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qu H, Ricklin D, Bai H, Chen H, Reis ES, Maciejewski M, Tzekou A, DeAngelis RA, Resuello RR, Lupu F, Barlow PN, Lambris JD. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218:496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Shao D, Ricklin D, Hilkin BM, Nester CM, Lambris JD, Smith RJ. Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology. 2015;220:993–998. doi: 10.1016/j.imbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reis ES, DeAngelis RA, Chen H, Resuello RR, Ricklin D, Lambris JD. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology. 2015;220:476–482. doi: 10.1016/j.imbio.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maekawa T, Abe T, Hajishengallis E, Hosur KB, DeAngelis RA, Ricklin D, Lambris JD, Hajishengallis G. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J. Immunol. 2014;192:6020–6027. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kourtzelis I, Ferreira A, Mitroulis I, Ricklin D, Bornstein SR, Waskow C, Lambris JD, Chavakis T. Complement inhibition in a xenogeneic model of interactions between human whole blood and porcine endothelium. Horm. Metab. Res. 2015;47:36–42. doi: 10.1055/s-0034-1390452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mallik B, Katragadda M, Spruce LA, Carafides C, Tsokos CG, Morikis D, Lambris JD. Design and NMR characterization of active analogues of compstatin containing non-natural amino acids. J. Med. Chem. 2005;48:274–286. doi: 10.1021/jm0495531. [DOI] [PubMed] [Google Scholar]
- 12.Katragadda M, Magotti P, Sfyroera G, Lambris JD. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J. Med. Chem. 2006;49:4616–4622. doi: 10.1021/jm0603419. [DOI] [PubMed] [Google Scholar]
- 13.Qu H, Magotti P, Ricklin D, Wu EL, Kourtzelis I, Wu YQ, Kaznessis YN, Lambris JD. Novel analogues of the therapeutic complement inhibitor compstatin with significantly improved affinity and potency. Mol. Immunol. 2011;48:481–489. doi: 10.1016/j.molimm.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalmasso AP, Vercellotti GM, Fischel RJ, Bolman RM, Bach FH, Platt JL. Mechanism of complement activation in the hyperacute rejection of porcine organs transplanted into primate recipients. Am. J. Pathol. 1992;140:1157–1166. [PMC free article] [PubMed] [Google Scholar]
- 15.Sandrin MS, Vaughan HA, Dabkowski PL, McKenzie IF. Anti-pig IgM antibodies in human serum react predominantly with Gal(alpha 1–3)Gal epitopes. Proc. Natl. Acad. Sci. U. S. A. 1993;90:11391–11395. doi: 10.1073/pnas.90.23.11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holzknecht ZE, Platt JL. Identification of porcine endothelial cell membrane antigens recognized by human xenoreactive natural antibodies. J. Immunol. 1995;154:4565–4575. [PubMed] [Google Scholar]
- 17.Baumann BC, Stussi G, Huggel K, Rieben R, Seebach JD. Reactivity of human natural antibodies to endothelial cells from Galalpha(1,3)Gal-deficient pigs. Transplantation. 2007;83:193–201. doi: 10.1097/01.tp.0000250478.00567.e5. [DOI] [PubMed] [Google Scholar]
- 18.Saethre M, Baumann BC, Fung M, Seebach JD, Mollnes TE. Characterization of natural human anti-non-gal antibodies and their effect on activation of porcine gal-deficient endothelial cells. Transplantation. 2007;84:244–250. doi: 10.1097/01.tp.0000268815.90675.d5. [DOI] [PubMed] [Google Scholar]
- 19.Capey S, van den Berg CW. Porcine complement regulators protect aortic smooth muscle cells poorly against human complement-induced lysis and proliferation: consequences for xenotransplantation. Xenotransplantation. 2005;12:217–226. doi: 10.1111/j.1399-3089.2005.00217.x. [DOI] [PubMed] [Google Scholar]
- 20.Yin Z, Wang L, Xiang Y, Ruan Y, Li J, Wang X, Ichim TE, Chen S, Chen G. Resistance of neonatal porcine Sertoli cells to human xenoantibody and complement-mediated lysis is associated with low expression of alpha-Gal and high production of clusterin and CD59. Xenotransplantation. 2010;17:215–223. doi: 10.1111/j.1399-3089.2010.00581.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt J, Eckardt S, Schlegel PG, Siren AL, Bruttel VS, Mclaughlin KJ, Wischhusen J, Muller AM. Human parthenogenetic embryonic stem cell-derived neural stem cells express HLA-G and show unique resistance to NK cell-mediated killing. Mol. Med. 2015;21:185–196. doi: 10.2119/molmed.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhat R, Rommelaere J. NK-cell-dependent killing of colon carcinoma cells is mediated by natural cytotoxicity receptors (NCRs) and stimulated by parvovirus infection of target cells. BMC Cancer. 2013;13:367. doi: 10.1186/1471-2407-13-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiane AE, Mollnes TE, Videm V, Hovig T, Hogasen K, Mellbye OJ, Spruce L, Moore WT, Sahu A, Lambris JD. Compstatin, a peptide inhibitor of C3, prolongs survival of ex vivo perfused pig xenografts. Xenotransplantation. 1999;6:52–65. doi: 10.1034/j.1399-3089.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- 24.Fiane AE, Mollnes TE, Videm V, Hovig T, Hogasen K, Mellbye OJ, Spruce L, Moore WT, Sahu A, Lambris JD. Prolongation of ex vivo-perfused pig xenograft survival by the complement inhibitor compstatin. Transplant. Proc. 1999;31:934–935. doi: 10.1016/s0041-1345(98)01844-2. [DOI] [PubMed] [Google Scholar]
- 25.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nat. Rev. Immunol. 2012;12:431–442. doi: 10.1038/nri3225. [DOI] [PubMed] [Google Scholar]
- 26.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J. Immunol. 2013;190:3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]