Abstract
Apolipoprotein L1 gene (APOL1) nephropathy variants in African American deceased kidney donors were associated with shorter renal allograft survival in a prior single-center report. APOL1 G1 and G2 variants were genotyped in newly accrued DNA samples from African American deceased donors of kidneys recovered and/or transplanted in Alabama and North Carolina. APOL1 genotypes and allograft outcomes in subsequent transplants from 55 U.S. centers were linked, adjusting for age, sex and race/ethnicity of recipients, HLA match, cold ischemia time, panel reactive antibody levels, and donor type. For 221 transplantations from kidneys recovered in Alabama, there was a statistical trend toward shorter allograft survival in recipients of two-APOL1-nephropathy-variant kidneys (hazard ratio [HR] 2.71; p=0.06). For all 675 kidneys transplanted from donors at both centers, APOL1 genotype (HR 2.26; p=0.001) and African American recipient race/ethnicity (HR 1.60; p=0.03) were associated with allograft failure. Kidneys from African American deceased donors with two APOL1 nephropathy variants reproducibly associate with higher risk for allograft failure after transplantation. These findings warrant consideration of rapidly genotyping deceased African American kidney donors for APOL1 risk variants at organ recovery and incorporation of results into allocation and informed-consent processes.
Introduction
The number of individuals wait-listed for kidney transplantation has increased steadily over the past decade without proportionate expansion of the donor pool.(1) As a result, attempts to utilize available donor organs more efficiently and increase allograft-survival years based upon aligning projected allograft survival with recipient life expectancy are being implemented. The impact of donor characteristics on outcomes is now estimated using the kidney donor profile index (KDPI) that incorporates ten factors known to affect the risk of allograft failure. Allocation of deceased donor kidneys based on KDPI is slated for implementation in late 2014.(2) Although African ancestry is a variable in calculating KDPI, donor genetic information is not.(3) Beyond matching for major histocompatibility antigens, employing precision medicine to improve outcomes after solid-organ transplantation has not yet impacted clinical practice.(4)
The G1 and G2 coding variants in the powerful apolipoprotein L1 (APOL1) nephropathy susceptibility gene strongly associate with HIV-associated nephropathy, idiopathic focal segmental glomerulosclerosis, focal global glomerulosclerosis with interstitial fibrosis and vascular changes (hypertension-attributed nephropathy), sickle cell nephropathy, and lupus nephritis-associated end-stage kidney disease (ESKD), exhibiting among the highest odds ratios (ORs) in common disease.(5–12) The G1 and G2 risk variants have been preferentially selected in sub-Saharan Africa, likely due to protection they provide from human African trypanosomiasis and are virtually absent in individuals lacking recent African ancestry. The variation in this single gene accounts for most of the higher risk for non-diabetic nephropathy in African Americans relative to European Americans.(13–15)
A report from the Wake Forest School of Medicine (WFSM) demonstrated that the same APOL1 genetic variants were significantly associated with shorter allograft survival after deceased-donor kidney transplantation (DDKT).(16) This single-center analysis included 136 kidney transplants from 106 unique African American deceased organ donors. Outcomes were based on chart review from kidneys transplanted at WFSM. Kidneys recovered from deceased donors of recent African ancestry with two APOL1-nephropathy variants failed significantly more rapidly after transplant than did those from donors with fewer than two variants. In a multivariate analysis, the effect of APOL1 was stronger than that of Human Leukocyte Antigen (HLA) match, cold ischemia time (CIT), and other conventional predictors of outcome in DDKT.
Recipients of kidneys from African American deceased donors are known to experience poorer outcomes.(17–20) Variation in APOL1 may explain a portion of the higher rate of failure in allografts from deceased African American donors, relative to those from European American donors.(16) Conversely, outcomes of kidney transplants do not appear to be adversely impacted by the presence of two APOL1-nephropathy variants in recipients.(21) The initial report on donor APOL1 genotypes and outcomes after DDKT was limited by its single-center design. Before considering whether APOL1 genotyping should be performed broadly in deceased kidney donors with recent African ancestry, it remains critical to validate results in larger numbers of kidneys transplanted at additional centers.
Methods
Samples and Outcomes
Aliquots of stored DNA from deceased African American kidney donors at WFSM and University of Alabama at Birmingham School of Medicine (UAB) were sent to the Center for Human Genomics and Personalized Medicine Research Laboratory at WFSM for APOL1 G1 and G2 risk-variant genotyping. The UAB Institutional Review Board (IRB) allowed participation because materials came from deceased individuals identified solely by United Network of Organ Sharing (UNOS) identification (ID) numbers. The WFSM site received IRB approval for genotyping of donor DNA samples and linkage of outcomes for kidney recipients based on UNOS ID numbers in the Scientific Registry of Transplant Recipients (SRTR).(22) This study used data from the SRTR. The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Analyses were performed for the 221 kidney transplantations with organs recovered by the Alabama Organ Center, 548 kidney transplantations (221 from the Alabama and 327 from the North Carolina centers) not included in the original Wake Forest report (16), updated results in 127 kidney transplantations from the original Wake Forest report with 3 additional years of follow-up, and in the full sample of 675 kidney transplantations from deceased African American donors (including 127 transplantations updated from the original North Carolina report and the new 548 transplantations). Outcomes were determined using SRTR data in DDKTs that were performed at 55 transplant centers.
Genotyping
Two single nucleotide polymorphisms (SNPs) in the APOL1 G1 nephropathy risk allele (rs73885319; rs60910145) and an insertion/deletion for the G2 risk allele (rs71785313) were genotyped using a custom assay designed at WFSM on the Sequenom platform (San Diego, California).(10) G1 and G2 genotype calls were visually inspected for quality control. Genotyping of three blind duplicates resulted in a concordance rate of 100% and the genotyping efficiency for the three SNPs was >99% in both cohorts. As analyses assessed the clinical impact of APOL1 G1 and G2, and African ancestry outside of APOL1 did not significantly impact DDKT outcomes in our initial report, ancestry informative markers were not genotyped. This approach was intentional because the clinical application of APOL1 genotyping in DDKT requires rapid turnaround and ease of interpretation.
Statistical Analysis
The distribution of demographic variables for recipients and African American deceased kidney donors based on donor APOL1-risk genotype group was contrasted using Wilcoxon two-sample tests for continuous variables and chi-square tests for binary variables. The outcome of interest was time to allograft failure, determined by the interval between the date of transplantation and the date of graft loss (return to dialysis, allograft nephrectomy or repeat transplantation). The date of final observation was censored in the event of death with a functioning graft or at the most recent follow-up (before November 30th, 2013) in those with a functioning graft. Cox proportional hazard models were then fitted.(23–25) The sandwich estimator was used to obtain a robust estimation of covariance matrix associated with the parameter estimates to account for the correlation between allograft failure rate and time to failure of kidneys donated by a single individual to two recipients. Lin and Wei reported that this sandwich estimator was consistent and robust to several misspecifications of the Cox model.(26) This coding was later revised such that death with a functioning allograft was treated as a competing risk to allograft loss. In this case, the date of final observation was censored only for individuals who were still alive with a functioning graft at the most recent follow-up (before November 30, 2013). We applied Fine and Gray’s model to test for association between APOL1 and each outcome, either allograft failure or death with function.(27) This model was fitted using the R package crrSC, which uses weighted estimating equations to account for the correlation between kidneys donated by a single individual to 2 recipients.(28) Missing genotype and phenotype data were excluded from the analyses. The variables considered in this analysis have very low counts of missing data (<5%), which limit the appeal for the data imputation techniques, as they are not likely to vastly improve our results.
Results
Table 1 lists demographic characteristics of the recipients and the African American deceased organ donors based on the number of APOL1 risk variants. Both kidneys from 102 donors in Alabama and 205 donors in North Carolina were engrafted separately; one kidney was engrafted from 17 Alabama and 44 North Carolina donors (368 unique organ donors). Of the 675 transplantations, 535 were first kidney transplantations and 68 were re-transplantations. For these transplantations, donor and recipient age and sex, CIT, peak PRA level, degree of HLA mismatch, and terminal donor serum creatinine concentration were strikingly similar at both centers. For Alabama-recovered kidneys, 125 were transplanted between 2006 and 2010, and 96 after 2010. Of the North Carolina-recovered or transplanted kidneys, 8 were transplanted before 2001, 86 between 2001 and 2005, 272 between 2006 and 2010, and 88 after 2010. Fifty-two percent (N=236) of the 454 transplantations of kidneys from the North Carolina center were performed at WFSM; 82% (N=182) of the 221 transplantations resulting from kidneys recovered in Alabama were performed at UAB. Immunosuppression varied from patient to patient and center to center, but generally included antibody induction with calcineurin inhibitor and anti-proliferative agent, with or without corticosteroids. The median (first quartile, third quartile) follow-up duration after engraftment was 36.0 months (12.1, 60.0 months) for the 576 kidneys from donors with less than two APOL1-risk variants and 24.0 months (12.1, 60.1 months) for the 99 kidneys from donors with two APOL1-risk variants.
Table 1.
Demographic data for kidney transplant recipients, based on APOL1 genotype of their African American deceased donors
| Variable | APOL1=0, N=286 | APOL1=1, N=290 | APOL1=2, N=99 | P-value |
|---|---|---|---|---|
|
| ||||
| Panel reactive antibodies (%) | 22.9 (32.0) 4.0 | 24.0 (32.9) 5.0 | 25.9 (34.8) 8.0 | 0.60 |
|
| ||||
| Donor age (years) | 34.5 (17.2) 34 | 35.8 (16.7) 38 | 33.5 (15.6) 36.0 | 0.48 |
|
| ||||
| Donor terminal serum creatinine concentration (mg/dl) | 1.2 (0.6) 1.0 | 1.4 (0.8) 1.2 | 1.2 (0.4) 1.2 | 0.01 |
|
| ||||
| Recipient age at transplant (years) | 48.0 (15.2) 51.0 | 48.2 (16.1) 50.0 | 46.3 (15.2) 47.0 | 0.38 |
|
| ||||
| Recipient body mass index (kg/m2) | 28.3 (5.6) 28.1 | 26.7 (5.6) 26.0 | 28.7 (6.1) 28.0 | <0.001 |
|
| ||||
| Cold ischemia time (hours) | 23.3 (11.3) 21.2 | 24 (11.6) 22.0 | 22.4 (9.6) 22.0 | 0.77 |
|
| ||||
| HLA mismatches (N) | 4.4 (1.4) 5.0 | 4.2 (1.4) 4.0 | 4.3 (1.4) 5.0 | 0.11 |
|
| ||||
| Allograft survival (months) | 39.3 (31.3) 36.0 | 37.7 (28.9) 36.0 | 41.9 (38.9) 24.0 | 0.94 |
|
| ||||
| Recipient most recent serum creatinine (mg/dl) | 1.7 (1.0) 1.4 | 1.7 (0.8) 1.5 | 2.0 (1.7) 1.5 | 0.89 |
|
| ||||
| Recipient type 2 diabetes, N (%) | 50 (17.5%) | 37 (12.8%) | 38 (38.4%) | 0.04 |
|
| ||||
| Recipient gender (male) | 159 (55.6%) | 171 (59%) | 64 (64.6%) | 0.28 |
|
| ||||
| Donor gender (male) | 176 (61.5%) | 175 (60.3%) | 55 (55.6%) | .58 |
|
| ||||
| Recipient ethnicity (African American) | 150 (52.4%) | 172 (59.3%) | 68 (68.7%) | 0.01 |
|
| ||||
| Standard criteria donor (Yes) | 237 (82.9%) | 231 (79.7%) | 87 (87.9%) | 0.17 |
|
| ||||
| Non-heart beating donor (Yes) | 17 (6.0%) | 10 (3.4%) | 5 (5.2%) | 0.35 |
|
| ||||
| Graft failure within 15 days (Yes) | 13 (4.5%) | 21 (7.2%) | 9 (9.1%) | 0.20 |
|
| ||||
| Graft failure within 6 months (Yes) | 43 (15.0%) | 34 (11.7%) | 15 (15.2%) | 0.45 |
|
| ||||
| Graft failure, total (Yes) | 38 (13.3%) | 49 (16.9%) | 24 (24.2%) | 0.04 |
|
| ||||
| Recipient death (Yes) | 46 (16.1%) | 48 (16.6%) | 13 (13.1%) | 0.72 |
|
| ||||
| Recipient acute rejection (Yes) | 45 (15.7%) | 50 (17.2%) | 13 (13.1%) | 0.62 |
|
| ||||
| Recipient first week dialysis (Yes) | 58 (20.3%) | 72 (24.8%) | 21 (21.2%) | 0.41 |
|
| ||||
| Recipient return to dialysis (Yes) | 37 (12.9%) | 49 (16.9%) | 22 (22.2%) | 0.08 |
|
| ||||
| Death with functioning allograft (Yes) | 27 (9.4%) | 21 (7.2%) | 8 (8.1%) | 0.63 |
|
| ||||
| Recipient primary diagnosis | ||||
| Diabetes | 59 (20.6%) | 70 (24.1%) | 19 (19.2%) | 0.47 |
| Hypertension | 81 (28.3%) | 66 (22.8%) | 26 (26.3%) | |
| Glomerulonephritis | 50 (17.5%) | 51 (17.6%) | 26 (26.3%) | |
| Cystic Kidney Disease | 12 (4.2%) | 13 (4.5%) | 4 (4%) | |
| Other | 84 (29.4%) | 90 (31%) | 24 (24%) | |
Data are shown as mean (SD) median unless otherwise stated.
Association analyses between allograft failure after DDKT and APOL1 genotypes (recessive model) are presented in Table 2. For the 221 Alabama-recovered kidneys, results adjusted for recipient age, sex and ancestry [African American vs. other], HLA match, CIT, PRA level [0% vs. >0%], and expanded-criteria donor [ECD] vs. standard-criteria donor [SCD], revealed a statistical trend toward shorter allograft survival for two APOL1-risk variant donor kidneys (hazard ratio [HR] 2.71; p=0.06). Multivariate analyses for the 548 newly analyzed Alabama-recovered and North Carolina-recovered and/or -transplanted kidneys revealed that kidneys from deceased donors with two APOL1-nephropathy alleles failed more rapidly than did those from donors with fewer than two APOL1 nephropathy alleles (HR 2.27; p=0.02). An updated multivariate analysis for the 127 transplants from our prior report with three additional years of follow-up also demonstrated more rapid allograft failure in kidneys from African American donors with two APOL1-nephropathy alleles (HR 2.33; p=0.03). The multivariate analysis including all 675 Alabama-recovered and North Carolina-recovered and/or -transplanted kidneys from African American deceased donors revealed that those from donors with two APOL1-nephropathy alleles failed more rapidly than those from donors with fewer than two nephropathy alleles (HR 2.26; p=0.001). With further adjustment for recipient time on dialysis (dialysis vintage), recipient diabetes mellitus (yes/no), and recipient body mass index (BMI) in the full sample of 675 renal transplantations, APOL1 genotype recessive model (HR 1.99, p=0.02), maximum PRA (HR 1.01, p=0.02, SCD (HR 0.48, p=0.02), and Alabama Transplant Center (HR 0.40, p=0.002) were significantly associated with time to kidney allograft failure. A gender-stratified analysis for the full sample of 675 kidneys revealed significant effects of recipient gender on allograft survival (p=0.0004), potential mechanisms remain unknown.
Table 2.
Multivariate association results for time to kidney allograft failure, based on APOL1 genotype (recessive model)
| Dataset | Variable | Hazard Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|---|
| Full dataset (N=675) | APOL1 (recessive model) | 2.26 | (1.37,3.74) | 0.001 |
| Maximum PRA | 1.00 | (1,00,1.01) | 0.17 | |
| Age at Transplant | 1.00 | (0.99,1.01) | 0.96 | |
| Number of HLA mismatches | 0.98 | (0.84,1.13) | 0.76 | |
| Cold Ischemia Time | 1.01 | (0.99,1.02) | 0.39 | |
| Expanded criteria donor (No) | 0.65 | (0.36,1.17) | 0.15 | |
| Recipient gender (Female) | 0.84 | (0.55,1.30) | 0.43 | |
| Recipient ethnicity (African American) | 1.60 | (1.05,2.44) | 0.03 | |
| Transplant Center (Alabama) | 0.33 | (0.19,0.57) | <0.001 | |
| Alabama transplants (N=221) | APOL1 (recessive model) | 2.71 | (0.95,7.69) | 0.06 |
| Maximum PRA | 1.02 | (1.00,1.04) | 0.07 | |
| Age at Transplant | 0.99 | (0.96,1.02) | 0.55 | |
| Number of HLA mismatches | 1.25 | (0.79,1.97) | 0.33 | |
| Cold Ischemia Time | 1.01 | (0.96,1.07) | 0.61 | |
| Expanded criteria donor (No) | 0.31 | (0.07,1.37) | 0.12 | |
| Recipient gender (Female) | 0.20 | (0.05,0.83) | 0.03 | |
| Recipient ethnicity (African American) | 3.52 | (0.68,18.34) | 0.13 | |
| Original North Carolina transplants (N=127) with additional 3 year follow-up | APOL1 (recessive model) | 2.33 | (1.10,4.90) | 0.03 |
| Maximum PRA | 1.00 | (0.99,1.01) | 0.96 | |
| Age at Transplant | 1.02 | (0.99,1.05) | 0.23 | |
| Number of HLA mismatches | 0.97 | (0.79,1.20) | 0.80 | |
| Cold Ischemia Time | 1.00 | (0.97,1.03) | 0.94 | |
| Expanded criteria donor (No) | 1.55 | (0.47,5.13) | 0.47 | |
| Recipient gender (Female) | 1.36 | (0.68,2.73) | 0.38 | |
| Recipient ethnicity (African American) | 1.21 | (0.60,2.45) | 0.59 | |
| All new transplants from North Carolina & Alabama (N=548) | APOL1 (recessive model) | 2.27 | (1.11–4.64) | 0.02 |
| Maximum PRA | 1.01 | (1.00,1.02) | 0.01 | |
| Age at Transplant | 0.99 | (0.97,1.01) | 0.31 | |
| Number of HLA mismatches | 1.08 | (0.81,1.34) | 0.50 | |
| Cold Ischemia Time | 1.01 | (0.99,1.03) | 0.26 | |
| Expanded criteria donor (No) | 0.39 | (0.21,1.00) | 0.01 | |
| Recipient gender (Female) | 0.56 | (0.40,1.45) | 0.05 | |
| Recipient ethnicity (African American) | 1.96 | (0.97,3.32) | 0.02 | |
| Transplant Center (Alabama) | 0.39 | (0.22,0.69) | 0.001 |
Multivariable adjustment reflects recipient age, recipient sex, recipient ancestry, HLA mismatch, cold ischemia time, panel reactive antibodies (0% vs. >0%), and standard-criteria vs. expanded-criteria donor. Recipient diabetes status was not included in the adjustment due to incomplete data in SRTR.
Adjusted for the Multivariable model + Center (Alabama or North Carolina).
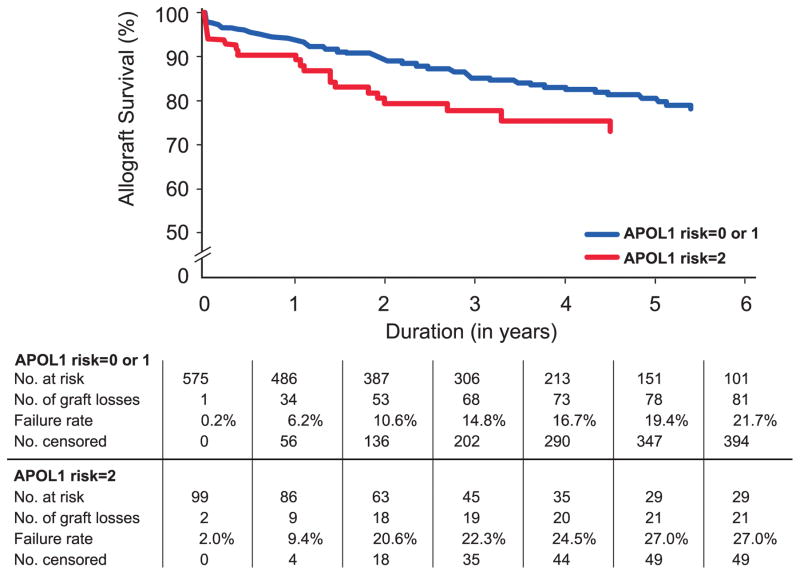
Results of the competing risk model for the association between time to allograft failure and death with a functioning allograft revealed that only APOL1 genotype (recessive model), African American recipient and transplant center significantly impacted allograft failure (Table 3). In contrast, recipient age at transplant was nominally associated with time to death post-transplant, whereas APOL1 genotype was not. Transplant center (North Carolina or Alabama) was also adjusted for to account for possible differences. In this analysis, donor APOL1 genotype and recipient race/ethnicity independently impacted allograft failure. Figure 1 shows the Kaplan-Meier allograft-survival curves for kidneys from African American donors with two or less than two APOL1-nephropathy alleles in the full sample. The number of allograft losses and deaths with a functioning allograft (censored outcomes) are shown. The survival curves separated early after transplantation. Despite early allograft failures from kidney donors with two APOL1 risk variants, many such transplants functioned for extended durations. One-, five-, and ten-year allograft survival for the 99 APOL1 two-risk-variant kidneys in this study were 89.3%, 73.0%, and 54.5%, respectively. The percentage of allograft failures within 15 days were also similar in those transplantations from African American donors with zero APOL1 risk variants (4.5%, N=286), one risk variant (7.2%, N=290) or two risk variants (9.1%, N=99; p=0.20); this finding suggests that technical failures were unlikely to have impacted results. The same pattern was observed with the number of allograft failures within six months post transplantation; failures were observed in 15.0%, 11.7%, and 15.2%, respectively, of individuals carrying zero, one, or two copies of APOL1 risk variants (p=0.45).
Table 3.
Competing risk model for association between time to allograft failure and death in the full sample, based on APOL1 genotype (recessive model)
| Event | Label | Hazard Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|---|
| Allograft failure | APOL1 (recessive model) | 2.16 | (1.26, 3.73) | 0.005 |
| Panel reactive antibodies | 1.00 | (1.00, 1.01) | 0.18 | |
| Age at transplant | 1.00 | (0.98, 1.01) | 0.80 | |
| Number of HLA mismatches | 0.96 | (0.83, 1.11) | 0.57 | |
| Cold ischemia time | 1.01 | (0.99, 1.02) | 0.40 | |
| Expanded criteria donor (No) | 1.58 | (0.88, 2.82) | 0.12 | |
| Recipient gender (Female) | 0.84 | (0.55, 1.29) | 0.43 | |
| Recipient ethnicity (African American) | 1.67 | (1.12, 2.50) | 0.01 | |
| Transplant center (Alabama) | 0.35 | (0.21, 0.58) | <0.001 | |
| Death with functioning allograft | APOL1 (recessive model) | 1.21 | (0.52, 2.81) | 0.66 |
| Panel reactive antibodies | 1.01 | (1.00, 1.02) | 0.09 | |
| Age at transplant | 1.03 | (1.00, 1.06) | 0.03 | |
| Number of HLA mismatches | 1.16 | (0.94, 1.43) | 0.17 | |
| Cold ischemia time | 0.98 | (0.96, 1.01) | 0.17 | |
| Expanded criteria donor (No) | 0.91 | (0.40, 2.05) | 0.81 | |
| Recipient gender (Female) | 0.94 | (0.51, 1.73) | 0.84 | |
| Recipient ethnicity (African American) | 0.69 | (0.40, 1.20) | 0.19 | |
| Transplant Center (Alabama) | 0.68 | (0.33, 1.39) | 0.29 |
Figure 1.
Renal allograft survival according to APOL1 genotype. Kaplan-Meier renal allograft survival curves for recipients of deceased donor kidneys with (red line) and without (blue line) two APOL1-risk alleles (p=0.001).
In multivariate analyses in all 675 renal transplantations, APOL1 genotype (recessive model) was not significantly associated with acute rejection (p=0.26), delayed graft function assessed as recipient first week dialysis (p=0.65), or most recent [log] serum creatinine concentration (p=0.42).
Discussion
African American donor ancestry is known to be a risk factor for poorer outcomes following DDKT.(1;17–20) In this report, the outcomes of 221 kidney transplants recovered from deceased African American donors in Alabama strongly supports the observation that recipients of kidneys from individuals with two APOL1-nephropathy variants (G1G1; G1G2; G2G2) have earlier allograft failure.(16) This result, coupled with outcomes of an additional 454 kidney transplantations from organs recovered and/or engrafted at WFSM in North Carolina, reveals that the presence of two APOL1-nephropathy variants in deceased African American donors independently predicts shorter renal allograft survival. The majority of allograft losses occurred early, many within two years after transplantation. Although this could suggest technical failure, we believe this is unlikely based on non-significant differences in allograft survival at 15 days and 6 months post-transplant. Most of the APOL1 two-risk-variant kidneys that failed shortly after transplantation in our initial report displayed renal histologic lesions compatible with APOL1-associated nephropathy (donor-acquired nephron scarring with arteriosclerosis, FSGS, and FSGS-collapsing variant); however, the present SRTR-based report lacks phenotypic information.(16)
A multivariable analysis in the full sample demonstrated that the magnitude of the HR for allograft failure was 2.26 for APOL1-two-risk-variant kidneys and 1.60 for African American recipient ancestry. Sensitization based on PRA level, recipient age, recipient sex, CIT, standard-criteria (vs. expanded-criteria) donor, and degree of HLA match did not significantly impact allograft survival in these analyses. However, it is also important to emphasize that most transplanted kidneys with two APOL1-risk variants did not fail early after engraftment; 54.5% functioned beyond ten years. As in native-kidney disease, APOL1 risk variants may contribute to nephropathy susceptibility in persons with two risk variants who encounter second hits or modulating factors.(29–31) Two-risk-variant transplant (and native) kidneys may not universally suffer progressive kidney disease in the absence of modulating factors. Studies to identify these factors remain critical to determine the mechanisms behind premature loss that may occur in recipients of two-risk-variant kidneys.
It is uncertain whether there is a role for screening APOL1-nephropathy variants in African Americans who are potential live kidney donors.(32;33) The present analyses did not address this issue. While two APOL1-nephropathy variants in deceased donors increased the risk for early allograft failure, this genotype in recipients does not associate with transplant outcomes.(21) Among live kidney donors, African Americans more often develop ESKD compared with non-African Americans.(34–36) However, the risk is fairly low and rates of kidney failure post-donation appear to parallel those in healthy populations of European and African ancestry, respectively.(37) Until there are data on the role of APOL1 variants in live-donor transplantations, we continue to rely on the clinical evaluation. We remain cautious in the setting of child-to-parent donation, where the ultimate renal phenotype may not yet have had a chance to manifest in young potential donors.(38) In African Americans, allografts from deceased donors fail more rapidly than those from living-related donors.(1) Factors other than APOL1 genotype, including prolonged CIT, greater potential for delayed allograft function, less HLA matching, and need for more potent and potentially toxic immunosuppression may contribute to the poorer survival.
It may be possible to improve organ allocation in kidney transplantation based on the genotypes of two SNPs and an insertion/deletion in the APOL1 gene; evaluation of genome-wide African ancestry using ancestry-informative markers may not be required. No differences in global ancestry proportion between African American APOL1 two-risk variant carriers and non-two-risk variant carriers were observed in the initial report from North Carolina (African ancestry proportions were 0.77 ± 0.1 in two risk variant carriers vs. 0.72 ± 0.2 in 0/1 risk variant carriers [p-value=0.56]).(16) Therefore, confounding due to admixture was not likely to have affected our results. In addition, ancestry-informative markers would be impractical to employ in clinical settings. While kidneys from African American donors with two APOL1-risk variants are statistically more likely to fail after transplantation, zero/one-APOL1-risk-variant allografts appear to survive approximately as well as kidneys recovered from European American donors.(1) It is possible that incorporating the impact of APOL1, rather than population ancestry or racial phenotypes, may more appropriately inform clinicians and potential candidates regarding outcome risk associated with accepting kidneys from individual donors. Herein, we observed consistent effects of deceased donor APOL1 risk variants on allograft survival, which in all 675 kidney transplantations from African American deceased kidney donors, as well as in subsets of 221 Alabama transplantations, the original 127 North Carolina transplantations with extended follow-up, and all 548 newly analyzed kidney transplantations, were associated with shorter allograft survival.
Any change in organ allocation must be balanced against the realization that there remains a critical shortage of kidneys for transplantation, particularly in the underserved African American population.(39) African American transplant recipients are more likely to receive kidneys from African American deceased donors, as a consequence of the racial distribution of HLA-DR alleles that contribute to allocation scores, and blood types.(39;40) Thus, transplantation of allografts with two APOL1-risk variants could exacerbate the existing racial disparities in kidney transplantation. Further, rates of discard of recovered kidneys are on the rise and identification of adverse characteristics may increase the rate.(41) In contrast, identification of organs at risk for early failure should result in more appropriate organ allocation, narrowing currently observed racial disparity in allograft outcomes.(19) We anticipate that improved stratification of organs would increase confidence in the clinical assessment of organ quality, and improve the utilization of all organs, with and without APOL1-risk variants.
At present, most clinical laboratories associated with U.S. kidney transplant programs are not equipped to perform APOL1 genotyping. However, polymerase chain reaction methodology is increasingly used for testing donor risk factors, such as nucleic acid testing to screen for infection with human immunodeficiency and hepatitis B and C viruses. Future technological advances will likely permit local genotyping within intervals that allow timely testing of deceased kidney donors.
This report has limitations. Transplant outcomes were captured using the SRTR, whereas the earlier report employed chart review.(16) Large databases are not universally accurate; however, SRTR captures allograft loss because it is linked to the United States Renal Data System, includes initiation of renal replacement therapy (even when not provided by transplant centers), dates of re-transplantation, and date of death.(22) Recipient BMI and time on dialysis were missing in the SRTR for 8% and 9.9% of transplantations, respectively. Additional confounding variables include use of different immunosuppressive regimens at transplant centers, changing patterns of center-specific immunosuppression over time, and lack of causes of allograft failure (or access to renal biopsy diagnoses) due to insufficient data in the SRTR. Adjustments for recipient type of immunosuppressant were not performed due to concerns over missing data in SRTR. Hence, these variables have the potential to have confounded the current results.
In conclusion, kidneys from deceased African American donors possessing two APOL1-nephropathy variants are at increased risk for failure after transplantation. These results suggest that deceased kidney donor APOL1 genotypes could be more informative than considering African American donor ethnicity, the variable included in the KDPI.(2;42) It will be important to test this hypothesis. In an era of profound limitations in kidney availability, proper implementation of these findings (and others likely to emerge in the future) poses challenges. With nucleic acid testing for viral infection now the norm in organ recovery, adding genotyping for APOL1 in donors of self-reported African American ancestry may become feasible. The results could then be incorporated into decisions regarding organ allocation and the informed consent process. This approach may be particularly relevant for African Americans, who are more likely to receive a kidney from a donor of African American ancestry. The powerful APOL1 gene provides an example whereby precision medicine has the potential to improve organ allocation in kidney transplantation.
Acknowledgments
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibilities of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Research support: NIH RO1 DK070941 (BIF), NIH RO1 DK084149 (BIF), NIH RO1 MD009055 (JD, BIF), NIH/NIAD Genomics of Transplantation 5U19-AI070119 (AKI)
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Reference List
- 1.Department of Health and Human Services HRaSAHSBDoT. OPTN / SRTR 2010 Annual Data Report. Rockville, MD: 2011. Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) [Google Scholar]
- 2.Tanriover B, Mohan S, Cohen DJ, Radhakrishnan J, Nickolas TL, Stone PW, et al. Kidneys at higher risk of discard: expanding the role of dual kidney transplantation. Am J Transplant. 2014 Feb;14(2):404–15. doi: 10.1111/ajt.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takemoto S, Terasaki PI, Cecka JM, Cho YW, Gjertson DW. Survival of nationally shared, HLA-matched kidney transplants from cadaveric donors. The UNOS Scientific Renal Transplant Registry. N Engl J Med. 1992 Sep 17;327(12):834–9. doi: 10.1056/NEJM199209173271202. [DOI] [PubMed] [Google Scholar]
- 4.Drawz PE, Sedor JR. Translating associations between common kidney diseases and genetic variation into the clinic. Semin Nephrol. 2010 Mar;30(2):195–202. doi: 10.1016/j.semnephrol.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010 Aug 13;329(5993):841–5. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010 Sep;128(3):345–50. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al. APOL1 Genetic Variants in Focal Segmental Glomerulosclerosis and HIV-Associated Nephropathy. J Am Soc Nephrol. 2011 Nov 1;22(11):2129–37. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashley-Koch AE, Okocha EC, Garrett ME, Soldano K, De Castro LM, Jonassaint JC, et al. MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol. 2011 Nov;155(3):386–94. doi: 10.1111/j.1365-2141.2011.08832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, et al. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013 Jan;83(1):114–20. doi: 10.1038/ki.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol. 2014 Feb;66(2):390–6. doi: 10.1002/art.38220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013 Dec 5;369(23):2183–96. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen CP, Beggs ML, Saeed M, Walker PD. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol. 2013 Apr;24(5):722–5. doi: 10.1681/ASN.2012121180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanhollebeke B, Pays E. The trypanolytic factor of human serum: many ways to enter the parasite, a single way to kill. Mol Microbiol. 2010 May;76(4):806–14. doi: 10.1111/j.1365-2958.2010.07156.x. [DOI] [PubMed] [Google Scholar]
- 14.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, et al. The Apolipoprotein L1 (APOL1) Gene and Nondiabetic Nephropathy in African Americans. J Am Soc Nephrol. 2010 Sep 1;21(9):1422–6. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genovese G, Friedman DJ, Pollak MR. APOL1 variants and kidney disease in people of recent African ancestry. Nat Rev Nephrol. 2013 Feb 12;9(4):240–4. doi: 10.1038/nrneph.2013.34. [DOI] [PubMed] [Google Scholar]
- 16.Reeves-Daniel AM, Depalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. The APOL1 Gene and Allograft Survival after Kidney Transplantation. Am J Transplant. 2011 May;11(5):1025–30. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meier-Kriesche HU, Port FK, Ojo AO, Rudich SM, Hanson JA, Cibrik DM, et al. Effect of waiting time on renal transplant outcome. Kidney Int. 2000 Sep;58(3):1311–7. doi: 10.1046/j.1523-1755.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 18.Swanson SJ, Hypolite IO, Agodoa LY, Batty DS, Jr, Hshieh PB, Cruess D, et al. Effect of donor factors on early graft survival in adult cadaveric renal transplantation. Am J Transplant. 2002 Jan;2(1):68–75. doi: 10.1034/j.1600-6143.2002.020112.x. [DOI] [PubMed] [Google Scholar]
- 19.Chakkera HA, O’Hare AM, Johansen KL, Hynes D, Stroupe K, Colin PM, et al. Influence of race on kidney transplant outcomes within and outside the Department of Veterans Affairs. J Am Soc Nephrol. 2005 Jan;16(1):269–77. doi: 10.1681/ASN.2004040333. [DOI] [PubMed] [Google Scholar]
- 20.Callender CO, Cherikh WS, Traverso P, Hernandez A, Oyetunji T, Chang D. Effect of donor ethnicity on kidney survival in different recipient pairs: an analysis of the OPTN/UNOS database. Transplant Proc. 2009 Dec;41(10):4125–30. doi: 10.1016/j.transproceed.2009.06.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee BT, Kumar V, Williams TA, Abdi R, Bernhardy A, Dyer C, et al. The APOL1 Genotype of African American Kidney Transplant Recipients Does Not Impact 5-Year Allograft Survival. Am J Transplant. 2012 Jul;12(7):1924–8. doi: 10.1111/j.1600-6143.2012.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, et al. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando ) 2013 Apr;27(2):50–6. doi: 10.1016/j.trre.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Liang K-Y, Zeger SL. Longitudinal data anlysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 24.McCullagh P, Nelder J. Generalized Linear Models. 2. 1989. [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986 Mar;42(1):121–30. [PubMed] [Google Scholar]
- 26.Lin DY, Wei LJ. The robust inference for the Cox Proportional Hazards Model. J Am Stat Assoc. 1989;84:1074–8. [Google Scholar]
- 27.Fine J, Gray A. A proportional Hazards Model for the Subdistribution of a competing risk. J Am Stat Assoc. 1999;(94):496–509. [Google Scholar]
- 28.Zhou B, Latouche A. Competing risks regression for Stratified and Clustered data. R package version 1.1. 2013;2013 http://CRAN.R-project.org/package=crrSC. [Google Scholar]
- 29.Divers J, Nunez M, High KP, Murea M, Rocco MV, Ma L, et al. JC polyoma virus interacts with APOL1 in African Americans with nondiabetic nephropathy. Kidney Int. 2013 Dec;84(6):1207–13. doi: 10.1038/ki.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kopp JB. JC viruria and kidney disease in APOL1 risk genotype individuals: is this a clue to a gene x environment interaction? Kidney Int. 2013 Dec;84(6):1069–72. doi: 10.1038/ki.2013.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Divers J, Palmer ND, Lu L, Langefeld CD, Rocco MV, Hicks PJ, et al. Gene-gene interactions in APOL1-associated nephropathy. Nephrol Dial Transplant. 2014 Mar;29(3):587–94. doi: 10.1093/ndt/gft423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen DM, Mittalhenkle A, Scott DL, Young CJ, Norman DJ. African American living-kidney donors should be screened for APOL1 risk alleles. Transplantation. 2011 Oct 15;92(7):722–5. doi: 10.1097/TP.0b013e31822eec39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kofman T, Audard V, Narjoz C, Gribouval O, Matignon M, Leibler C, et al. APOL1 polymorphisms and development of CKD in an identical twin donor and recipient pair. Am J Kidney Dis. 2014 May;63(5):816–9. doi: 10.1053/j.ajkd.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Gibney EM, King AL, Maluf DG, Garg AX, Parikh CR. Living kidney donors requiring transplantation: focus on African Americans. Transplantation. 2007 Sep 15;84(5):647–9. doi: 10.1097/01.tp.0000277288.78771.c2. [DOI] [PubMed] [Google Scholar]
- 35.Lentine KL, Schnitzler MA, Xiao H, Saab G, Salvalaggio PR, Axelrod D, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010 Aug 19;363(8):724–32. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lentine KL, Schnitzler MA, Xiao H, Axelrod D, Garg AX, Tuttle-Newhall JE, et al. Consistency of racial variation in medical outcomes among publicly and privately insured living kidney donors. Transplantation. 2014 Feb 15;97(3):316–24. doi: 10.1097/01.TP.0000436731.23554.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaston RS, Young CJ. Living donor nephrectomy: understanding long-term risk in minority populations. Am J Transplant. 2010 Dec;10(12):2574–6. doi: 10.1111/j.1600-6143.2010.03324.x. [DOI] [PubMed] [Google Scholar]
- 38.Steiner RW. ‘Normal for now’ or ‘at future risk’: a double standard for selecting young and older living kidney donors. Am J Transplant. 2010 Apr;10(4):737–41. doi: 10.1111/j.1600-6143.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 39.Roberts JP, Wolfe RA, Bragg-Gresham JL, Rush SH, Wynn JJ, Distant DA, et al. Effect of changing the priority for HLA matching on the rates and outcomes of kidney transplantation in minority groups. N Engl J Med. 2004 Feb 5;350(6):545–51. doi: 10.1056/NEJMoa025056. [DOI] [PubMed] [Google Scholar]
- 40.Cannon RM, Brock GN, Marvin MR, Slakey DP, Buell JF. The contribution of donor quality to differential graft survival in African American and Caucasian renal transplant recipients. Am J Transplant. 2012 Jul;12(7):1776–83. doi: 10.1111/j.1600-6143.2012.04091.x. [DOI] [PubMed] [Google Scholar]
- 41.Kasiske BL, Stewart DE, Bista BR, Salkowski N, Snyder JJ, Israni AK, et al. The role of procurement biopsies in acceptance decisions for kidneys retrieved for transplant. Clin J Am Soc Nephrol. 2014 Mar;9(3):562–71. doi: 10.2215/CJN.07610713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Israni AK, Salkowski N, Gustafson S, Snyder JJ, Friedewald JJ, Formica RN, et al. New National Allocation Policy for Deceased Donor Kidneys in the United States and Possible Effect on Patient Outcomes. J Am Soc Nephrol. 2014 May 15; doi: 10.1681/ASN.2013070784. [DOI] [PMC free article] [PubMed] [Google Scholar]