Abstract
Despite an increased risk of coronary artery disease (CAD) in persons infected with human immunodeficiency virus (HIV), few data are available on primary prevention of CAD in this population. In this retrospective cohort study, HIV-infected patients treated in an academic medical center HIV Specialty Clinic between 1996 and 2010 were matched by age, gender, and ethnicity to a cohort of presumed uninfected persons followed in an academic medical center Internal Medicine primary care clinic. We compared CAD primary prevention care practices between the two clinics, including use of aspirin, HMG-CoA reductase inhibitors (“statins”), and anti-hypertensive drugs. CAD risk between the two groups was assessed with 10-year Framingham CAD risk scores. In the comparative analysis, 890 HIV-infected persons were compared to 807 controls. Ten-year Framingham CAD Risk Scores were similar in the two groups (median, 3; interquartile range [IQR], 0–5). After adjusting for relevant risk factors, HIV-infected persons were less likely to be prescribed aspirin (odds ratio [OR] 0.53; 95% confidence interval [CI], 0.40–0.71), statins (OR, 0.70; 95% CI, 0.53–0.92), and anti-hypertensive drugs (OR, 0.63; 95% CI, 0.50–0.79) than persons in the control group. In summary, when compared to demographically similar uninfected persons, HIV-infected persons treated in an HIV specialty clinic were less likely to be prescribed medications appropriate for CAD risk reduction. Improving primary preventative CAD care in HIV specialty clinic populations is an important step toward diminishing risk of heart disease in HIV-infected persons.
Keywords: HIV, coronary artery disease, cardiovascular disease, primary care, clinical, outcomes research
Introduction
With the aging of HIV-infected persons in the USA, coronary artery disease (CAD) has emerged as a leading cause of morbidity and mortality in this population (ATCC, 2010; Marin et al., 2009). Recent evidence suggests that persons living with HIV (PLHIV) are at almost 50% higher risk for myocardial infarction than the general population even after controlling for traditional CAD risk factors (Freiberg et al., 2013). Research into the pathophysiology of atherosclerosis in PLHIV has been robust, but less attention has been given to the clinical management of reversible CAD risk factors in HIV-infected persons. Over the last three decades, a high proportion of HIV-infected patients have been treated by providers with infectious diseases specialty training, and the favorable impact of expert HIV care on patient outcomes has been demonstrated previously (Landon et al., 2005). The efficacy of improved antiretroviral therapy has diminished the frequency of opportunistic infections in HIV-infected persons in the USA, and HIV-associated non-AIDS conditions (HANA) have emerged as major causes of morbidity and mortality in this population (Justice and Braithwaite, 2012; Mills, Barnighausen, & Negin, 2012; Rabkin, Kruk, & El-Sadr, 2012) . Among the HANA conditions, CAD is the most prevalent in the general population, causing over 500,000 deaths a year in the USA (Gillespie et al., 2013). The clinical management of coronary artery risk in HIV clinics has not been rigorously studied, nor has such care been compared directly to that of uninfected persons. The present study compares CAD risk factor management among persons cared for in a large, university-based HIV specialty clinic to management of a matched cohort of demographically similar uninfected persons cared for in an academic Internal Medicine clinic.
Methods
Study setting
All HIV-infected persons in the study were treated at the Duke University Adult Infectious Diseases clinic between 1996 and 2010. The Duke ID clinic actively provides medical care to approximately 2000 HIV-infected persons. HIV primary care is provided by 10 full-time infectious diseases faculty with individual panels of 125–150 patients, 2 mid-level providers, and 6 infectious diseases panels with panels of 50–75 patients each. Uninfected persons in the control cohort were treated at the Duke Outpatient Clinic, the medical center’s largest clinic affiliated with the Internal Medicine training program. The clinic provides medical care to approximately 3500 active patients, in a typical resident supervision structure. Three faculty members also care for a full panel of patients at this site. The study was approved by the Institutional Review Board of the academic medical center.
Eligibility criteria
The study protocol was initially approved on 1 March 2010 and all data in the study were obtained from routine clinic visits prior to this date. All patients aged 40 or over (as of 1 March 2010) who attended at least three separate clinic visits over a span of at least 12 months were eligible. Patients with a known history of cardiovascular disease (asymptomatic CAD, stable angina, or acute coronary syndrome), peripheral vascular disease, or stroke were excluded from the study. The exclusion diagnoses were identified by clinic ICD-9 billing data by query of the Duke Enterprise Data Unified Content Explorer and corroborated by chart review of provider-documented problem lists. HIV-infected persons who received ongoing non-HIV outpatient care from providers outside of the academic HIV specialty clinic were also excluded. Only patient encounters that occurred between 1 January 1996 and 1 March 2010.
Clinical data source/matching
Potential study patients were identified by querying the search engine of the electronic medical record of the institution. Patient demographic data, laboratory results, medication lists and medical histories were manually abstracted from individual patient encounters documented in the electronic medical record. Demographic data were abstracted from the medical center’s HIV clinical case registry, which includes information on over 5000 HIV-infected persons followed since 1995. Data on adverse health behaviors, including smoking, ethanol abuse, and cocaine use, were obtained from a longitudinal review of individual clinic notes. HIV-infected persons were matched 1:1 with presumed uninfected persons by age, sex, race, and ethnicity. Age matching was conducted by grouping individuals into five-year age blocks (each HIV-infected person was directly matched with a person within five years of their age). Race/ethnicity groups were categorized as “White”, “Black” or “Other”, and as “Hispanic” or “non-Hispanic”. Persons of mixed race were recorded as “other”. Data on health insurance status from the index clinical encounter were obtained from the institutional billing database.
Outcomes
The primary outcome measure was prescription of a drug from one of more of three categories of medications associated with CAD risk factor modification: aspirin, HMG-CoA reductase inhibitors (“statins”), or anti-hypertensives. The specific drug or dose and the source of the original prescription were not considered.
Predictor variables
After matching (most patients were able to be matched 1:1 by age, race/ethnicity, and sex), the explanatory variable of interest was HIV status. HIV-seropositivity was defined as having a documented history of HIV-infection prior to 1 March 2010. Predictor variables included diabetes, current or former cigarette smoking, cocaine use, and insurance status at index visit. The five most recent outpatient systolic and diastolic blood pressures, and the three most recent lipid profiles (total cholesterol, LDL-c, and HDL) from the study period were also collected. Ten-year CAD risk scores were calculated using abstracted data and the previously described Framingham Risk Equation (Wilson et al., 1998).
Statistical analysis
The mean and standard deviations are reported for all continuous variables. Categorical variables are reported as frequencies and percentages. Multivariable analyses were done using logistic regression models for crude and adjusted ORs and associated 95% confidence intervals. The two cohorts were matched by age, race/ethnicity, and sex, and thus these variables were excluded from the logistic regression model. Variables included in the final regression model determined a priori include: HIV status, diabetes, former or current tobacco use, and insurance status at index visit (as categorical variables). For regression purposes, individual systolic and diastolic blood pressure, total cholesterol, LDL, and HDL values were all aggregated as mean values across a single observation (continuous variables). A backward reduction approach was used to eliminate non-associated variables from the final model with a significance level (α) of 0.20 (Steyerberg, Eijkemans, Van Houwelingen, Lee, & Habbema, 2000). Covariates known to be associated with the outcome variable were left in the final model regardless of associated p-values. Analyses were performed using SAS statistical software (Cary, North Carolina) version 9.3.
Results
A total of 1152 HIV-infected patients met the pre-specified inclusion criteria, of whom 262 were excluded (receiving concurrent outpatient primary care from non-HIV providers), leaving a final HIV-infected cohort of 890 persons (Figure 1). Matching using the pre-specified criteria was accomplished for 807 of these patients leaving a final analysis cohort of 890 HIV-infected persons and 807 controls. The mean ages (+/− standard deviation) for the infected and uninfected groups were 49.6 ± 7.0 years and 49.0 ± 6.5 years, respectively. A majority of the patients in the study cohorts were African-American (51% in the infected cohort, 55% in the uninfected cohort), and about three-quarters were male. The distribution of health insurance status was similar between the groups: HIV-infected (43% private insurance, 41% public insurance, and 16% uninsured); controls (40% private insurance, 43% public insurance, and 17% uninsured). Among HIV-infected persons, the median CD4 count was 501 cells/mm3 and 47% of the cohort maintained a viral load <50 copies/mL for six months or more. The mean systolic blood pressure (SBP) was lower in the HIV-infected group than in the uninfected group (128.4 ± 12.6 vs. 130.8 ± 14.2 mmHg, p < .001). The mean LDL-c (107.2 ± 34.5 vs. 111.2 ± 35.8 mg/dL, p = .03) and HDL (44.9 ± 19.2 vs. 47.9 ± 16.2, p < .001) were also lower in the infected group than in the uninfected group. There were similar numbers of current/former smokers in the two groups (48% vs. 46%) but fewer persons with a history of cocaine use than in the uninfected group (13 vs. 17%). The median 10-year Framingham risk score for both groups was 3 (Table 1).
Figure 1.
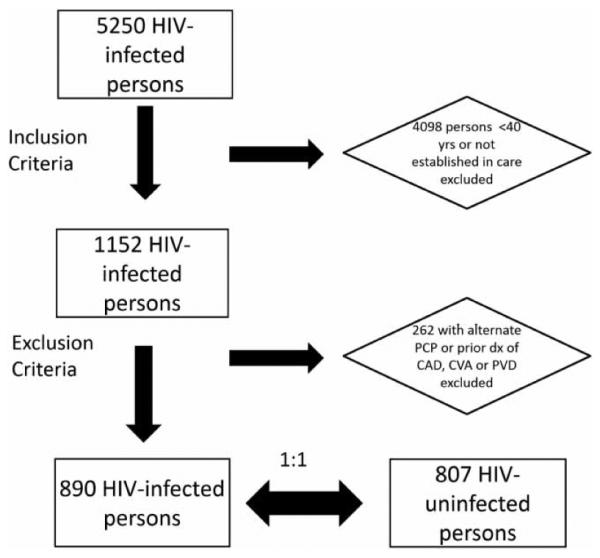
Consort diagram of selection of study cohort from the duke university infectious diseases clinic.
Table 1.
Characteristics of HIV-infected persons and matched controls in study cohort.
| HIV Infected (n = 890) No. (%)a |
Controls (n = 807) No. (%)a |
Total (n = 1697) No. (%) |
|
|---|---|---|---|
| Age, years, Mean ± SD | 49.6 ± 7.1 | 49.0 ± 6.5 | 49.3 ± 6.8 |
| Male sex | 688 (77) | 614 (76) | 1302 (77) |
| Race/ethnicity | |||
| White/non-Hispanic | 391 (44) | 333 (41) | 724 (43) |
| Black | 450 (51) | 443 (55) | 893 (52) |
| White/Hispanic | 19 (2) | 13 (2) | 32 (2) |
| Other | 30 (3) | 18 (2) | 48 (3) |
| Insuranceb | |||
| Medicare/Medicaid | 362 (41) | 349 (43) | 711 (42) |
| Private | 385 (43) | 321 (40) | 706 (42) |
| None | 140 (16) | 135 (17) | 275 (16) |
| Tobaccob | |||
| Ever | 396 (48) | 368 (46) | 764 (47) |
| Never | 433 (52) | 434 (54) | 867 (53) |
| Current cocaine useb | 112 (13) | 133 (17) | 245 (15) |
| Diabetes mellitusb | 61 (7) | 160 (20) | 221 (13) |
| Systolic blood pressure, mmHg, Mean ± SD | 128.5 ± 12.9 | 130.8 ± 14.2 | 129.6 ± 13.6 |
| Total cholesterol, mg/dL, Mean ± SD | 190.5 ± 56.5 | 189.1 ± 50.6 | 189.8 ± 53.9 |
| LDL, mg/dL, Mean ± SD | 107.2 ± 34.5 | 111.2 ± 35.8 | 109.0 ± 35.2 |
| HDL, mg/dL, Mean ± SD | 44.8 ± 19.2 | 47.9 ± 16.2 | 46.3 ± 17.9 |
| CD4 count, Mean ± SD | 531.4 ± 324.1 | n/a | n/a |
| CD4 Nadir | 227.8 ± 211.1 | n/a | n/a |
| Viral suppression | |||
| <400 copies/mL | 570 (64) | n/a | n/a |
| <50 copies/mL | 423 (48) | n/a | n/a |
| Median (IQR) 10-year risk for CAD event, %c |
3 (0–5) | 3 (0–5) | 3 (0–5) |
| 10-year risk score for CAD event ≥10%c |
34 (4) | 26 (3) | 60 (4) |
Abbreviations: CAD, coronary artery disease; HDL, high density lipoprotein; HIV, human immunodeficiency virus; IQR, interquartile range; LDL, low density lipoprotein; SD, standard deviation.
Column percentages.
Missing data (counts): Cocaine, 15; Insurance, 5; Tobacco, 66.
Based on Framingham Risk Score for Coronary Heart Disease.
Despite similar CAD risk profiles, use of medications in all three CAD prevention categories was significantly less in the HIV-infected population. Aspirin was prescribed significantly less often in the HIV-infected group than in the uninfected group (11.6% vs. 22.7%, p < .001). The same pattern was observed both for statin prescriptions (15.1% vs. 23.6%, p < .001) and for anti-hypertensive drug prescriptions (35.7 vs. 52%, p < .001). In the univariate analysis, diabetes and increased mean SBP were associated with increased odds of aspirin and anti-hypertensive prescribing, as would be anticipated. Increased mean LDL-c, decreased mean HDL, increased mean SBP, and a diagnosis of diabetes were associated with increased odds of statin prescribing. Current tobacco use and having private health insurance were associated with decreased odds of being prescribed a statin (Tables 2, 3 and 4). For all three medications categories, being HIV-infected was associated with decreased likelihood of being prescribed the medication. After adjusting for potential confounding variables in the multivariable regression analysis (DM, mean SBP for ASA and anti-hypertensives, mean LDL, HDL, SBP, and diabetes for statins), HIV-infected persons were significantly less likely to be prescribed aspirin (adjusted OR 0.53 [95% CI, 0.40–0.71]), statins (adjusted OR 0.70 [95% CI, 0.53–0.92]) and anti-hypertensives (adjusted OR 0.63 [95% CI, 0.50–0.79]) than were persons in the control cohort (Table 5).
Table 2.
Factors associated with aspirin prescription among HIV-infected persons and matched controls in the study cohort.
| HIV infected |
Controls |
|||
|---|---|---|---|---|
| Unadjusted OR (95% CI)a |
Adjusted OR (95% CI)a,b |
Unadjusted OR (95% CI)a |
Adjusted OR (95% CI)a,b |
|
| Age (per 10 years) |
2.31
(1.78–3.01) |
n/a |
1.95
(1.52–2.50) |
n/a |
| Male sex |
1.99
(1.11–3.58) |
n/a |
1.71
(1.12–2.62) |
n/a |
| Race | ||||
| White | 1.00 (Ref) | n/a | 1.00 (Ref) | n/a |
| Non-White |
0.57
(0.37–0.86) |
n/a | 1.28 (0.91–1.80) |
n/a |
| Insurance | ||||
| Public | 1.19 (0.79–1.80) |
1.28 (0.66–1.81) |
1.00 (0.77–1.50) |
1.19 (0.68–2.08) |
| Private | 0.81 (0.53–1.24) |
0.92 (0.46–1.81) |
1.07 (0.76–1.49) |
1.21 (0.68–2.13) |
| None | 1.05 (0.61–1.84) |
1.09 (0.59–2.02) |
0.77 (0.49–1.23) |
1.20 (0.70–1.89) |
| Tobacco use | 0.70 (0.45–1.08) |
0.80 (0.50–1.30) |
1.41 (1.01–1.96) |
1.28 (0.87–1.89) |
| Cocaine use | 0.80 (0.41–1.56) |
1.11 (0.53–2.33) |
1.27 (0.83–1.95) |
1.15 (0.68–1.93) |
| Diabetes |
2.21
(1.15–4.24) |
2.33
(1.17–4.66) |
6.82
(4.67–9.97) |
5.47
(3.62–8.27) |
| Mean SBP (per 10 mmHg) |
1.13 (0.97–1.32) |
1.16 (0.98–1.39) |
1.27
(1.14–1.43) |
1.21
(1.06–1.37) |
| Mean total cholesterol (per 10 mg/dL) |
1.03 (0.99–1.07) |
1.01 (0.94–1.08) |
0.92
(0.88–0.96) |
0.91 (0.81–1.01) |
| Mean LDL cholesterol (per 10 mg/dL) |
1.07
(1.01–1.14) |
1.06 (0.96–1.17) |
0.93
(0.88–0.98) |
1.08 (0.94–1.23) |
| Mean HDL cholesterol (per 10 mg/dL) |
0.91 (0.80–1.04) |
0.87 (0.74–1.02) |
0.89 (0.79–1.00) |
0.98 (0.85–1.13) |
Note: Bold, meets statistical significance at p-value < 0.05.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; n/ a, not applicable; OR, odds ratio; ref, reference.
Logistic regression.
Adjusting for all relevant clinical risk factors discussed in text.
Table 3.
Factors associated with statin prescription among HIV-infected persons and matched controls in the study cohort.
| HIV infected |
Controls |
|||
|---|---|---|---|---|
| Unadjusted OR (95% CI)a |
Adjusted OR (95% CI)a,b |
Unadjusted OR (95% CI)a |
Adjusted OR (95% CI)a,b |
|
| Age (per 10 years) |
1.90
(1.49–2.41) |
n/a |
2.01
(1.57–2.57) |
n/a |
| Male sex | 1.26 (0.79–1.99) |
n/a |
1.74
(1.14–2.64) |
n/a |
| Race | ||||
| White | 1.00 (Ref) | n/a | 1.00 (Ref) | n/a |
| Non-White |
0.56
(0.39–0.81) |
n/a |
0.84
(0.61–1.18) |
n/a |
| Insurance | ||||
| Public | 1.25 (0.86–1.81) |
0.94 (0.53–1.63) |
1.03 (0.74–1.43) |
0.75 (0.44–1.23) |
| Private |
0.66
(0.46–0.97) |
0.53
(0.30–0.97) |
0.83 (0.59–1.16) |
0.73 (0.43–1.24) |
| None | 1.35 (0.84–2.16) |
1.38 (0.81–2.32) |
1.28 (0.84–1.94) |
1.36 (0.83–2.20) |
| Tobacco use | 0.78 (0.53–1.14) |
0.92 (0.60–1.40) |
0.97 (0.74–1.43) |
0.91 (0.62–1.36) |
| Cocaine use |
0.39
(0.18–0.83) |
0.62 (0.28–1.36) |
0.99 (0.64–1.54) |
1.11 (0.65–1.90) |
| Diabetes |
3.05
(1.73–5.39) |
3.08
(1.62–5.89) |
6.27
(4.31–9.13) |
7.30
(4.72–11.3) |
| Mean SBP (per 10 mmHg) |
0.97 (0.84–1.12) |
0.93 (0.79–1.10) |
1.21 (1.08–1.35) |
1.12 (0.99–1.29) |
| Mean total cholesterol (per 10 mg/dL) |
1.08
(1.04–1.12) |
1.05 (0.99–1.10) |
1.01 (0.98–1.04) |
0.99 (0.94–1.06) |
| Mean LDL cholesterol (per 10 mg/dL) |
1.15
(1.09–1.21) |
1.11
(1.02–1.20) |
1.05 (1.00–1.10) |
1.12
(1.03–1.22) |
| Mean HDL Cholesterol (per 10 mg/dL) |
0.96 (0.86–1.07) |
0.94 (0.82–1.06) |
0.74
(0.65–0.85) |
0.77
(0.66–0.89) |
Note: Bold, meets statistical significance at p-value < 0.05.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; n/ a, not applicable; OR, odds ratio; ref, reference.
Logistic regression.
Adjusting for all relevant clinical risk factors discussed in text.
Table 4.
Factors associated with anti-hypertensive prescription among HIV-infected persons and matched controls in the study cohort.
| HIV infected |
Controls |
|||
|---|---|---|---|---|
| Unadjusted OR (95% CI)a |
Adjusted OR (95% CI)a,b |
Unadjusted OR (95% CI)a |
Adjusted OR (95% CI)a,b |
|
| Age (per 10 years) |
2.15
(1.74–2.65) |
n/a |
1.58
(1.26–1.96) |
n/a |
| Male sex | 1.08 (0.78–1.50) |
n/a | 1.11 (0.80–1.53) |
n/a |
| Race | ||||
| White | 1.00 (Ref) | n/a | 1.00 (Ref) | n/a |
| Non-White |
1.33
(1.01–1.75) |
n/a |
1.46
(1.11–1.94) |
n/a |
| Insurance | ||||
| Public | 1.24 (0.93–1.65) |
0.79 (0.50–1.26) |
0.81 (0.61–1.07) |
0.98 (0.59–1.61) |
| Private | 0.93 (0.70–1.22) |
0.90 (0.56–1.41) |
1.22 (0.92–1.61) |
0.71 (0.43–1.18) |
| None | 0.96 (0.66–1.38) |
1.18 (0.77–1.80) |
1.28 (0.84–1.94) |
1.19 (0.74–1.90) |
| Tobacco use | 1.22 (0.91–1.61) |
1.28 (0.92–1.78) |
1.04 (1.00–1.75) |
1.10 (0.77–1.60) |
| Cocaine use | 1.08 (0.72–1.64) |
1.20 (0.73–1.97) |
1.12 (0.77–1.62) |
0.93 (0.55–1.58) |
| Diabetes |
3.50
(2.04–5.39) |
3.36
(1.83–6.16) |
5.35
(3.49–8.20) |
5.28
(3.18–8.76) |
| Mean SBP (per 10 mmHg) |
1.77
(1.56–2.00) |
1.77
(1.54–6.16) |
2.48
(2.12–2.89) |
2.62
(2.19–3.13) |
| Mean total cholesterol (per 10 mg/dL) |
1.01 (0.98–1.04) |
1.01 (0.96–1.07) |
0.95
(0.92–0.99) |
0.96 (0.90–1.02) |
| Mean LDL cholesterol (per 10 mg/dL) |
1.02 (0.98–1.06) |
1.01 (0.94–1.08) |
0.94
(0.90–0.99) |
1.00 (0.92–1.08) |
| Mean HDL cholesterol (per 10 mg/dL) |
0.98 (0.91–1.06) |
0.97 (0.89–1.06) |
0.89
(0.81–0.98) |
0.96 (0.86–1.07) |
Note: Bold, meets statistical significance at p-value < 0.05.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; n/ a, not applicable; OR, odds ratio; ref, reference.
Logistic regression.
Adjusting for all relevant clinical risk factors discussed in text.
Table 5.
Odds ratios for aspirin, statin, and anti-hypertensive prescription among HIV-Positive Persons (n = 890) compared to Matched Controls (n = 807)a.
| Medication | Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
p-value |
|---|---|---|---|
| Aspirinb | 0.44 (0.34–0.58) | 0.53 (0.40–0.71) | <0.0001 |
| Statinc | 0.57 (0.45–0.74) | 0.70 (0.53–0.92) | 0.01 |
| Anti- hypertensivesd |
0.51 (0.42–0.62) | 0.63 (0.50–0.79) | <0.0001 |
Reference = uninfected persons.
Covariates in model for aspirin prescription include diabetes, mean systolic blood pressure, mean high density lipoprotein (HDL), and HIV-infection.
Covariates in model for aspirin prescription include diabetes, private insurance, mean total cholesterol, mean LDL-c, mean HDL, and HIV-infection.
Covariates in model for anti-hypertensive prescription include diabetes, mean systolic blood pressure, smoking, and HIV-infection.
Discussion
Dramatic improvements in survival with contemporary antiretroviral treatment regimens have led to the emergence of non-AIDS conditions as major causes of death among HIV-infected persons (Effros et al., 2008; Friis-Moller et al., 2010; Mocroft et al., 2010; Palella et al., 2006). The goal of this study was to determine if patients treated by specialist physicians with expertise in HIV care were managed comparably to persons in the control group treated by general internists in the prevention of one major HANA condition – CAD. As it relates to primary prevention of CAD, these data indicate that HIV-infected persons cared for in an HIV specialty clinic were significantly less likely to be prescribed aspirin, statins, and anti-hypertensive agents, after adjusting for relevant CAD risk factors, than were similar control patients cared for in an academic Internal Medicine practice.
Concerns regarding the adequacy of primary CAD prevention in HIV-infected patients have been described previously. An analysis of the HIV Outpatient Study Cohort revealed that only about 60% of patients in the cohort met Joint National Commission on Prevention, Detection, Evaluation and Treatment of Hypertension (JNC–VII) guidelines for management of hypertension (Lichenstein et al., 2013). A recent study from the University of Alabama-Birmingham’s 1917 HIV clinic showed that only 17% of HIV-infected patients eligible for aspirin per US Preventative Service Task Force guidelines were receiving it (Burkholder et al., 2012). Although neither study included a comparator group, the relatively low frequency with which guidelines were met is concerning. Whether these observed low levels of adherence to treatment guidelines refiect patient care patterns in general or whether they may be related to provision of care by specialty clinicians rather than generalists has not been examined. Our study highlighting the differences in CAD care in two comparable populations suggests that the problem may be at least in part a reflection of the latter concern.
This concern is amplified by the fact that HIV-infected persons are at higher risk of CAD than the general population (Currier et al., 2003; Durand, Sheehy, Baril, Lelorier, & Tremblay, 2011; Klein, Hurley, Quesenberry, & Sidney, 2002; Obel et al., 2007; Triant, Lee, Hadigan, & Grinspoon, 2007). A recent analysis of the 82,000-person Veterans Aging Cohort Study showed that compared to uninfected persons, HIV-infected veterans were approximately 50% more likely to have a myocardial infarction during the study period after adjusting for traditional risk factors (Freiberg et al., 2013). In addition, there is accumulating evidence that the pathophysiology of CAD may be different in persons with HIV-infection, driven more by immune activation and adverse metabolic conditions (dyslipidemia and hyperglycemia) than is otherwise the case (Deeks and Phillips, 2009; Hsue et al., 2009; Hsue, Deeks, & Hunt, 2012; Kaplan et al., 2011; Oliviero et al., 2009). In that regard, it has been suggested that statins may have the dual role of both improving lipids and diminishing immune activation essential to atheroma formation in HIV-infected persons (Calza et al., 2013; Eckard, Jiang, Debanne, Funderburg, & McComsey, 2014). In a similar vein, aspirin may be important in decreasing markers of T cell and monocyte activation in HIV-infected persons, in addition to its well-known effects on platelet aggregation (O’Brien et al., 2013; Seshasai et al., 2012; Wolff, Miller, & Ko, 2009).
Because of the expertise required to successfully man-age chronic HIV-infection and its complications, many HIV providers may not be as knowledgeable about the finer points of general non-HIV related chronic disease care. Specifically, the differences in CAD prevention care observed in our study perhaps are related to the relative unfamiliarity of the providers with current recommendations for management of CAD risk. For example, a recent study by Fultz and colleagues found that ID physicians treating HIV-infected patients less frequently reported being comfortable treating hypertension, hyperlipidemia, or diabetes than did general internists who cared for HIV-infected persons [HTN: 73 vs. 98%, HL: 71 vs. 98%, DM: 57 vs. 98%] (Fultz et al., 2005). While recent changes in guidelines for treating hyperlipidemia have made things simpler, programs aimed at updating knowledge of chronic disease management customized for the HIV provider are warranted (Stone et al., 2013).
Our study has several limitations. First, this study was conducted in a single academic medical center located in the Southeastern USA and thus may not reflect care in other geographic areas with differing patient populations. Future studies will look to validate our identified predictors for medication prescription in a multi-center cohort. A concerted effort was made to identify a well-matched control population as a comparison group to the HIV-infected patients cared for in the specialty clinic, but the difficulty of this task is illustrated in the imbalance in the size of the final analysis cohorts. To ensure that the major confounder of access-to-care was not a significant factor in our comparative analysis, the comparator cohort was selected from a socioeconomic status (SES) group similar to that of the HIV clinic population, using health insurance status as a surrogate marker. Although not a perfect surrogate for SES, the distribution of insurance status was strikingly similar in the two groups. As mentioned previously, this variable was adjusted for in the multivariable regression analysis. Details of the antiretroviral regimens being administered to HIV-infected persons were not collected in this study. This may be significant for two reasons. First, the impact of pill burden on the provider’s propensity to prescribe additional medication was not taken into consideration, and, second, potential drug interactions between HIV protease inhibitors and many statins are well documented (Busti et al., 2008; Fichtenbaum et al., 2002; Hsyu, Schultz-Smith, Lillibridge, Lewis, & Kerr, 2001). Patients taking HIV protease inhibitors may thus be less likely to be prescribed a statin. Finally, given that this was a casecontrol study, standardizing the longitudinal period of follow up of clinical data was limited by the availability of individual data points. Although we set relatively stringent criteria on the ambulatory clinical data that we incorporated into the final analysis, there may be some variability in the breadth of follow-up time that the clinical data on each individual patient represents.
This study of adherence to CAD prevention and management guidelines demonstrated that patients cared for in an HIV specialty clinic were less likely to be treated with aspirin, anti-hypertensive medication, and lipidlowering drugs than were persons in the control group of similar CAD risk cared for in an academic internal medicine primary care clinic. Emerging evidence of HIV-infection as an additional CAD risk factor adds to the importance of good CAD preventive care for PLHIV. Our findings suggest that there is an important need to implement programs to equip all HIV providers with the knowledge base to navigate the increasingly complex demands of comprehensive HIV care, particularly in light of the rising importance of HANA conditions in the aging HIV population. Understanding and optimizing preventive care in PLHIV is essential in maintaining the substantial advances in prognosis for those with HIV-infection.
Acknowledgements
Special thanks to Audrey Lan for assistance with data retrieval. We would also like to thank Susanna Naggie, MD, MHS, Lynn Bowlby, MD and the leadership of the Duke Outpatient Clinic (DOC) for their support of this project.
Funding
This work was supported by the Duke Interdisciplinary Research Training Program in AIDS (T32 AI007392) and the Duke Center for AIDS Research (CFAR) (P30 AI064518).
Footnotes
Disclosure statement
Dr Hicks has received research support from Argos, Bristol-Myers Squibb, Gilead, Merck, Janssen Virology and ViiV. He has also participated in scientific advisory boards for Bristol-Myers Squibb, Gilead, Merck, Janssen Virology and ViiV. Drs. Okeke, Clement and Chow have no potential conflicts of interest to report. Ms. Chin has no potential conflicts of interest to report.
ORCID
Nwora Lance Okeke http://orcid.org/0000-0003-4456-0176
References
- Antiretroviral Therapy Cohort Collaboration [ATCC] Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: Collaborative analysis of 13 HIV cohort studies. Clinical Infectious Disease. 2010;50(10):1387–1396. doi: 10.1086/652283. doi:10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder GA, Tamhane AR, Salinas JL, Mugavero MJ, Raper JL, Westfall AO, Willig JH. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clinical Infectious Disease. 2012;55(11):1550–1557. doi: 10.1093/cid/cis752. doi:10.1093/cid/cis752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busti AJ, Bain AM, Hall RG, 2nd, Bedimo RG, Leff RD, Meek C, Mehvar R. Effects of atazanavir/ritonavir or fosamprenavir/ritonavir on the pharmacokinetics of rosuvastatin. Journal of Cardiovascular Pharmacology. 2008;51(6):605–610. doi: 10.1097/FJC.0b013e31817b5b5a. doi:10.1097/FJC.0b013e31817b5b5a. [DOI] [PubMed] [Google Scholar]
- Calza L, Manfredi R, Colangeli V, Trapani FF, Salvadori C, Magistrelli E, Viale P. Two-year treatment with rosuvastatin reduces carotid intima-media thickness in HIV type 1-infected patients receiving highly active antiretroviral therapy with asymptomatic atherosclerosis and moderate cardiovascular risk. AIDS Research Human Retroviruses. 2013;29(3):547–556. doi: 10.1089/aid.2012.0015. doi:10.1089/aid.2012.0015. [DOI] [PubMed] [Google Scholar]
- Currier JS, Taylor A, Boyd F, Dezii CM, Kawabata H, Burtcel B, Hodder S. Coronary heart disease in HIV-infected individuals. Journal of Acquired Immune Deficiency Syndrome. 2003;33(4):506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. doi:10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: A cohort and nested case-control study using Quebec’s public health insurance database. Journal of Acquired Immune Deficiency Syndrome. 2011;57(3):245–253. doi: 10.1097/QAI.0b013e31821d33a5. doi:10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. Journal of Infectious Disease. 2014;209(8):1156–1164. doi: 10.1093/infdis/jiu012. doi:10.1093/infdis/jiu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, High KP. Aging and infectious diseases: Workshop on HIV infection and aging: What is known and future research directions. Clinical Infectious Disease. 2008;47(4):542–553. doi: 10.1086/590150. doi:10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtenbaum CJ, Gerber JG, Rosenkranz SL, Segal Y, Aberg JA, Blaschke T, Aweeka F. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS. 2002;16(4):569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Internal Medicine. 2013;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728. doi:10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, D:A:D Study Group Predicting the risk of coronary artery disease in HIV-infected patients: The data collection on adverse effects of anti-HIV drugs study. European Journal of Cardiovascuar Prevention Rehabilitation. 2010;17(5):491–501. doi: 10.1097/HJR.0b013e328336a150. doi:10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- Fultz SL, Goulet JL, Weissman S, Rimland D, Leaf D, Gibert C, Justice AC. Differences between infectious diseases-certified physicians and general medicine-certified physicians in the level of comfort with providing primary care to patients. Clinical Infectious Disease. 2005;41(5):738–743. doi: 10.1086/432621. doi:10.1086/432621. [DOI] [PubMed] [Google Scholar]
- Gillespie CD, Wigington C, Hong Y. Coronary heart disease and stroke deaths – United States, 2009. MMWR Surveillance Summaries. 2013;62(Suppl. 3):157–160. [PubMed] [Google Scholar]
- Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. Journal of Infectious Disease. 2012;205(Suppl. 3):S375–S382. doi: 10.1093/infdis/jis200. doi:10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, Deeks SG. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23(9):1059–1067. doi: 10.1097/QAD.0b013e32832b514b. doi:10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsyu PH, Schultz-Smith MD, Lillibridge JH, Lewis RH, Kerr BM. Pharmacokinetic interactions between nelfinavir and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and simvastatin. Antimicrobial Agents and Chemotherapy. 2001;45(12):3445–3450. doi: 10.1128/AAC.45.12.3445-3450.2001. doi:10.1128/AAC.45.12.3445–3450.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Braithwaite RS. Lessons learned from the first wave of aging with HIV. AIDS. 2012;26(Suppl. 1):S11–18. doi: 10.1097/QAD.0b013e3283558500. doi:10.1097/QAD.0b013e3283558500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Hodis HN. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217(1):207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. doi:10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Hurley LB, Quesenberry CP, Jr., Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? Journal of Acquired Immune Deficiency Syndrome. 2002;30(5):471–477. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- Landon BE, Wilson IB, McInnes K, Landrum MB, Hirschorn LR, Marsden RV, Cleary PD. Physician specialization and the quality of care for human immunodeficiency virus infection. Archives of Internal Medicine. 2005;165(10):1133–1139. doi: 10.1001/archinte.165.10.1133. [DOI] [PubMed] [Google Scholar]
- Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi E, Brooks JT. Provider compliance with guidelines for management of cardiovascular risk in HIV-infected patients. Preventing Chronical Disease. 2013;10:E10. doi: 10.5888/pcd10.120083. doi:10.5888/pcd10.120083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin B, Thiebaut R, Bucher HC, Rondeau V, Costagliola D, Dorrucci M, Chene G. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23(13):1743–1753. doi: 10.1097/QAD.0b013e32832e9b78. doi:10.1097/QAD.0b013e32832e9b78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Barnighausen T, Negin J. HIV and aging – Preparing for the challenges ahead. New England Journal of Medicine. 2012;366(14):1270–1273. doi: 10.1056/NEJMp1113643. doi:10.1056/NEJMp1113643. [DOI] [PubMed] [Google Scholar]
- Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, EuroSIDA Study Group Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. Journal of Acquired Immune Deficiency Syndrome. 2010;55(2):262–270. doi: 10.1097/QAI.0b013e3181e9be6b. doi:10.1097/QAI.0b013e3181e9be6b. [DOI] [PubMed] [Google Scholar]
- O’Brien M, Montenont E, Hu L, Nardi MA, Valdes V, Merolla M, Berger JS. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: A pilot study. Journal of Acquired Immune Deficiency Syndrome. 2013;63(3):280–288. doi: 10.1097/QAI.0b013e31828a292c. doi:10.1097/QAI.0b013e31828a292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel N, Thomsen HF, Kronborg G, Larsen CS, Hildebrandt PR, Sorensen HT, Gerstoft J. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: A population-based cohort study. Clinical Infectious Disease. 2007;44(12):1625–1631. doi: 10.1086/518285. doi:10.1086/518285. [DOI] [PubMed] [Google Scholar]
- Oliviero U, Bonadies G, Apuzzi V, Foggia M, Bosso G, Nappa S, Sacca L. Human immunodeficiency virus per se exerts atherogenic effects. Atherosclerosis. 2009;204(2):586–589. doi: 10.1016/j.atherosclerosis.2008.10.012. doi:10.1016/j.atherosclerosis.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Palella FJ, Jr., Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, HIV Outpatient Study Investigators Mortality in the highly active antire-troviral therapy era: Changing causes of death and disease in the HIV outpatient study. Journal of Acquired Immune Deficiency Syndrome. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- Rabkin M, Kruk ME, El-Sadr WM. HIV, aging and continuity care: Strengthening health systems to support services for noncommunicable diseases in low-income countries. AIDS. 2012;26(Suppl. 1):S77–S83. doi: 10.1097/QAD.0b013e3283558430. doi:10.1097/QAD. 0b013e3283558430. [DOI] [PubMed] [Google Scholar]
- Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Erqou S, Sattar N, Ray KK. Effect of aspirin on vascular and nonvascular outcomes: Meta-analysis of randomized controlled trials. Archives of Internal Medicine. 2012;172(3):209–216. doi: 10.1001/archinternmed.2011.628. doi:10.1001/archinternmed.2011.628. [DOI] [PubMed] [Google Scholar]
- Steyerberg EW, Eijkemans MJ, Van Houwelingen JC, Lee KL, Habbema JD. Prognostic models based on literature and individual patient data in logistic regression analysis. Statistics in Medicine. 2000;19(2):141–160. doi: 10.1002/(sici)1097-0258(20000130)19:2<141::aid-sim334>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, Wilson PW. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2013 doi:10.1161/01.cir.0000437738.63853.7a. [Google Scholar]
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. Journal of Clinical Endocrinology Metabolism. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. doi:10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: An update of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2009;150(6):405–410. doi: 10.7326/0003-4819-150-6-200903170-00009. [DOI] [PubMed] [Google Scholar]


