Abstract
Objective
To determine the effect of exogenous oxytocin (OT) administration on inflammation and atherosclerosis in socially isolated apoE−/− mice. Hyperlipidemic animals housed in isolated or stressful social environments display more extensive atherosclerosis than those in an affiliative social environment. The neurohypophyseal peptide OT may be involved in both affiliative social behavior and cardiovascular homeostasis, suggesting a role in mediating the benefits of positive social interactions on atherosclerosis.
Methods
A total of 43, 12-week-old, apoE−/− mice were surgically implanted with osmotic minipumps containing OT (n = 23) or vehicle (n = 20). Blood samples were taken at baseline and after 6 weeks and 12 weeks of treatment. After 12 weeks of treatment, animals were killed, and samples of adipose tissue were dissected from a subset of OT-treated (n = 12) and vehicle-treated (n = 12) animals and incubated in culture media for 6 hours. Media samples were analyzed for interleukin (IL)-6 concentration corrected by sample dry weight. Aortas were dissected, formalin-fixed, and stained with oil-red O for en face quantification of lesion area. t tests were used to compare group means on measures of percent lesion area and IL-6 concentrations.
Results
There were no group differences in plasma lipids. Adipose tissue samples taken from OT-treated animals secreted significantly less IL-6 over 6 hours (p < .01). OT-treated animals displayed significantly less atherosclerosis in the thoracic aorta (p < .05).
Conclusions
These results indicate that peripheral OT administration can inhibit atherosclerotic lesion development and adipose tissue inflammation, suggesting a potential role for this neuropeptide in mediating the benefits of stable group housing on atherosclerosis.
Keywords: oxytocin, atherosclerosis, inflammation, interleukin-6, visceral adipose tissue
Introduction
It has been well established through numerous animal studies that social isolation, or deprivation of affiliative social interactions, leads to increased atherosclerosis in the presence of hyperlipidemia. In the cholesterol-fed cynomolgus monkey model of atherosclerosis, socially deprived females display increased coronary artery atherosclerosis in comparison with those housed in social groups (1). Watanabe heritable hyperlipidemic rabbits housed in isolation show greater atherosclerotic lesion area than those in an affiliative social environment (2,3). Research has examined the effect of social isolation on apoE−/− mice. These mice lack the lipid carrier protein, apolipoprotein E (apoE), impairing their ability to clear cholesterol from the circulation (4). This results in elevated plasma cholesterol levels (>500 mg/dL) and the development of extensive atherosclerosis throughout the arterial tree (5). A recent study (6) found that apoE−/− mice, housed in isolation, displayed increased atherosclerotic lesion development compared with those housed in social groups.
Unstable or stressful social environment, characterized by antagonistic social interactions, has also been associated with increased atherosclerosis, a finding that has been replicated in cholesterol-fed and normocholesterolemic cynomolgus monkeys (7,8), as well as Watanabe heritable hyperlipidemic rabbits (2,3). Although the effects of social isolation and antagonistic social interactions on atherosclerosis have been well established by the above animal studies, the specific mechanisms responsible for these effects have not been fully clarified. Several studies have investigated the possible roles of physical inactivity (9), sympathetic nervous system (SNS) overactivation (3,9,10), increased vascular oxidative stress (9), and dysregulation of insulin-metabolic variables (2) in the exacerbation of atherosclerosis under these housing conditions. Findings have suggested that animals housed in an unstable, antagonistic social environment exhibit elevated markers of SNS activation, which correlate with the extent of atherosclerosis found in these animals (3,10). Pharmacological blockade of the effects of SNS activation, through administration of β-adrenergic receptor blockers, prevents the exacerbation of atherosclerosis by unstable social environment (10). Animals housed in social isolation also exhibit evidence of increased SNS activity; however, these animals also display a sedentary pattern of behavior and show increased vascular oxidative stress and hyperinsulinemia, compared with animals housed under stable or unstable social conditions (2,9). Thus, although there is substantial evidence to suggest that SNS overactivation is largely responsible for the effects of unstable social environment on atherosclerosis, this mechanism may not fully account for the effect of social isolation on the disease.
Another possibility is that affiliative social interactions associated with stable group housing may convey added protection against atherosclerosis through novel central nervous system (CNS) mechanisms (2). It has been proposed that the neurohypophyseal peptide, oxytocin (OT), may play a role in mediating the beneficial effects of affiliative social interactions on atherosclerosis (11). OT, a nonapeptide produced in the paraventricular and supraoptic nuclei of the hypothalamus, is secreted into circulation through the portal system of the posterior pituitary (12). OT and its receptor (OTR) are traditionally known for their important role in female reproductive functioning, including uterine contraction and milk ejection, but have also been shown to be expressed locally within vascular and cardiac tissues in rats and humans (12–15). Activation of the intrinsic OT system within the heart causes negative inotropic and chronotropic effects through an atrial natriuretic peptide-dependent pathway (12–14).
In addition to these homeostatic functions, recent research has investigated a potential role for OT in the pathophysiology of atherosclerosis. The OTR is present in several major cell types important in the progression of the disease, including vascular smooth muscle cells, endothelial cells, macrophages, and adipocytes (11–16). During the early stages of atherosclerotic lesion formation within the arteries of hyperlipidemic animals, increased superoxide production by endothelial nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase causes lipid peroxidation, which can lead to the activation, adherence, and chemotaxis of circulating monocytes (16). Once inside the vessel wall, differentiated macrophages continue to secrete large quantities of proinflammatory cytokines that exacerbate endothelial dysfunction and recruitment of additional monocytes (16). Several proinflammatory cytokines are known to play a role in the pathogenesis of atherosclerosis, the most notable of these being interleukin (IL)-1β, tumor necrosis factor-α, and IL-6 (16). Both IL-1β and tumor necrosis factor-α have been found to induce IL-6, which may then modulate their subsequent activity (17). It has been proposed that IL-6 may be of particular importance in linking systemic inflammation with atherosclerosis due to its circulatory function, induction of C-reactive protein, and diverse effects on hemostasis, lipid metabolism, and the acute-phase response (18). In addition to macrophages, activated endothelial cells also produce their own proinflammatory cytokines, including IL-6, which may perpetuate the inflammatory cascade that characterizes early lesion initiation and progression (16). Studies conducted in vitro have revealed that OT modulates these processes that are critical to early lesion formation within vascular and immune tissues (11). Specifically, OT has demonstrated antioxidant effects on vascular smooth muscle cells, aortic endothelial cells, and macrophages through attenuation of NADPH-oxidase-dependent superoxide production. Additional in vitro findings indicate that OT inhibits lipopolysaccharide-induced IL-6 production from endothelial cells and macrophages (11).
Increased visceral adiposity has been independently associated with increased risk of cardiovascular disease, and research suggests that these fat depots may be particularly susceptible to infiltration by macrophages in a proinflammatory state (19). A recent study (20) found that transplantation of epididymal or visceral adipose tissue into subcutaneous pockets leads to increased markers of inflammation and an acceleration of atherosclerotic lesion development in apoE−/−mice. OTRs are present on adipocytes and circulating monocytes and macrophages, but no studies have examined whether OT has an anti-inflammatory effect on visceral adipose tissue depots in vivo (11,12).
Taken together, these findings suggest that increased circulating OT may play a protective role in the development of atherosclerosis in the context of hyperlipidemia. To date, no studies have examined the potential benefits of OT administration on atherosclerosis in an in vivo model. The current study sought to test if peripheral administration of exogenous OT could reduce tissue inflammation and slow the progression of atherosclerosis in hyperlipidemic animals prone to developing the disease.
Methods
Experimental Animals
Forty-three male apoE−/− mice (C57BL/6 background, 11–12 weeks of age, weighing 22–24 g) were purchased from Jackson Laboratory (Bar Harbor, Maine). Mice were randomized to control (n = 20) and OT treatment (n = 23) groups on arrival and were individually housed in a temperature- and humidity-controlled environment on a reverse 12 hours light/dark cycle and given food and water ad libitum. Mice were acclimated for 7 days before initiation of experiments. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami.
Behavioral Monitoring
Videotape samples of home cage individual behavior were obtained at study midpoint (week 6) for a subset of animals from each group (n = 12 per group) to grossly examine whether groups differed in activity levels. During nocturnal behavioral sampling, each animal was videotaped for 5 minutes in its home cage under red light. Tapes were later reviewed and time spent in active (e.g., cage exploration, rearing) and sedentary (e.g., grooming, sitting) behaviors was recorded to examine potential differences in general activity levels. Behaviors were identified, using methods similar to those previously described (9). Time spent in active versus sedentary behaviors was then converted into percent time based on the 5-minute behavioral sampling.
Blood Draws
Mice were fasted for 7 hours before blood draws at treatment baseline (week 0), midpoint (week 6), and end point (week 12) for measurements of plasma total cholesterol, triglycerides, and insulin. At baseline and midpoint, blood samples were drawn from the submandibular vein and at end point by cardiac puncture with heparinized needles. Samples were collected in ethylenediaminetetraacetic acid-coated tubes and centrifuged at 2000 g for 15 minutes, and plasma was aliquoted and stored at −80°C.
Pumps and Surgeries
Osmotic minipumps (Alzet model 2006, DURECT Corporation, Cupertino, California), set to infuse at 0.15 μL/hr for 6 weeks, were maintained under sterile conditions, and to verify load volumes, they were weighed before and after being filled with 200 μL of OT or vehicle solutions. The pumps were then primed in a sterile saline bath at 37°C for 70 hours. Primed osmotic minipumps containing either 200 μg/mL OT (approximate infusion rate of 1 μg/kg/hr) or vehicle, 50 mmol/L sodium citrate, pH = 4, were surgically implanted into each animal.
Surgeries were performed under sterile conditions, according to pump manufacturer's instructions. Mice were anesthetized with a ketamine/xylazine cocktail and scrubbed with ethanol and betadine sterilization solutions before surgery. A small subcutaneous incision was made in the midscapular region, and a hemostat was used to create a pocket for the pump. The pump was then inserted into the subcutaneous pocket, and the wound was sealed with adhesive (Vetbond, St. Paul, Minnesota) and surgical staples (Kent Scientific, Torrington, Connecticut). Animals were allowed to recover in a 37°C incubator before returning to their home cages. Pump-exchange surgeries performed at midpoint (6 weeks post implantation) were identical, except for the removal of previously implanted pumps.
Blood Pressure
In prior studies, OT has been shown to either increase or decrease blood pressure (21,22), which could directly affect atherosclerosis. Therefore, the effects of OT infusion on blood pressure were examined in a separate cohort of C57BL/6 mice implanted with osmotic pumps to deliver 0 μg/kg/hr, 1 μg/kg/hr, or 5 μg/kg/hr OT, and blood pressure was monitored over a 2-week period. Tail-cuff blood pressure was measured in awake C57BL/6 mice (CODA-6, Kent Scientific). Mice were acclimated daily for 7 days to the tail-cuff system before baseline measurement. Blood pressure was measured 1 day before surgery and at 10 days, 12 days, and 14 days postsurgical pump implantation. The average often measurements per animal were used for each day of recording.
Plasma Assays
Plasma cholesterol and triglyceride levels were measured, using an automated analyzer (Roche Diagnostics, Indianapolis, Indiana). A commercially available enzyme-linked immunosorbent assay kit (Mercodia, Uppsala, Sweden) was used to measure plasma insulin levels.
Tissue Collection
On the date of sacrifice, mice were deeply anesthetized with an IP injection of a ketamine/xylazine cocktail and exanguinated by cardiac puncture. Epididymal fat was dissected, weighed, and prepared for assessment of ex vivo IL-6 secretion. The mice torsos were then fixed in 10% buffered formalin. The heart and aorta were later removed intact, stripped of adventitia, and cut longitudinally for en face preparation. Tissues were stored in 10% buffered formalin for later staining and quantification of atherosclerotic disease.
Ex Vivo IL-6 Secretion
Epididymal fat was sampled randomly from a subset of animals (n = 12 per group), minced for 3 minutes and washed with 5 mL of Dulbecco's Modified Eagle Medium. Tissue was centrifuged for 45 secs at 1000 rpm, and media were removed. Fat tissue was then transferred to preweighed glass tubes and incubated in 1.5 mL of Dulbecco's Modified Eagle Medium culture. After 6 hours of incubation on a water bath shaker at 37°C, culture media were centrifuged, and media samples were aliquoted and stored at −80°C. The adipose tissues were lyophilized overnight, and tissue dry weight was determined. Culture media samples from the ex vivo adipose tissue experiment were used to measure IL-6 concentrations, using a commercially available enzyme-linked immunosorbent assay kit (R & D Systems, Minneapolis, Minnesota), and values were corrected for sample dry weight.
Quantitation of Atherosclerosis
All histomorphometric procedures were performed blinded. The method for preparation of mice aortas and quantification of disease was performed as described previously (23). Formalin-fixed aortas were stained with oil-red-O, and digitally photographed. A reference aorta template, created from the average size and shape of all the aortas in the sample, was overlaid onto each aorta image. Percent lesion area was calculated from the proportional area of pixels stained with oil-red-O for a given aortic section.
Statistical Analyses
Analysis of variance with repeated measures was used to examine treatment effects on weight and all plasma measures (insulin, cholesterol, and triglyceride levels) across three time points. In instances where the sphericity assumption was violated, Greenhouse-Geisser adjusted degrees of freedom were used to determine significance. Welch or Studentized t tests were used to compare groups on all baseline plasma measures, midpoint behavioral measures, and end-point tissue measures, including ex vivo IL-6 secretion and percent lesion area. One animal in the vehicle control group was removed from all lesion area analyses because it had extremely high levels of disease (>5.5 standard deviations above the mean) that substantially influenced all statistical parameters and significance tests.
Correlational analyses were performed to examine which variables significantly predicted extent of atherosclerosis overall and in the arch and thoracic sections of the aorta. Additionally, analyses of covariance were utilized to examine whether group differences in disease were influenced by the covariation of variables that predicted disease. All significance tests were two-tailed and conducted at a significance level of p < .05.
Results
Behavior
After 6 weeks of infusion, OT-treated animals spent an average of 15.0% (standard error of the mean [SEM] = 1.4%) of their time in sedentary behaviors and 85.0% (SEM = 1.4%) of their time in active behaviors. Vehicle control animals spent an average of 13.3% (SEM = 1.7%) of their time in sedentary behaviors and 86.8% (SEM = 1.7%) of their time in active behaviors. There was no significant difference between groups in their level of physical activity.
Risk Factors
No significant group differences were detected between groups on baseline measures of cholesterol, weight, triglycerides, or insulin. Analyses revealed no significant group × time interaction or main effect for group for weight, total cholesterol, triglycerides, or insulin levels across three time points (Table 1).
Table 1. Mean Values of Body Weight, Serum Lipids, and Insulin Across Time.
| Vehicle | Oxytocin | |
|---|---|---|
| Weight (g) | ||
| Baseline | 26.2 ± 0.4 | 26.0 ± 0.4 |
| Midpoint | 29.7 ± 0.4 | 29.8 ± 0.3 |
| End point | 32.6 ± 0.3 | 32.0 ± 0.3 |
| Cholesterol (mg/dL) | ||
| Baseline | 533.4 ± 26.1 | 520.2 ± 25.5 |
| Midpoint | 455.7 ± 19.6 | 482.3 ± 20.5 |
| End point | 539.2 ± 27.6 | 514.1 ± 23.8 |
| Triglyerides (mg/dL) | ||
| Baseline | 80.2 ± 4.2 | 75.8 ± 4.5 |
| Midpoint | 86.0 ± 8.4 | 101.4 ± 7.1 |
| End point | 59.0 ± 5.3 | 51.7 ± 3.2 |
| Insulin (pmol/L) | ||
| Baseline | 139.9 ± 5.1 | 138.9 ± 3.5 |
| Midpoint | 166.3 ± 10.8 | 172.0 ± 4.3 |
| End point | 117.7 ± 8.3 | 99.3 ± 10.9 |
Values are mean ± standard error of the mean. Baseline is pretreatment. Midpoint is after 6 weeks of treatment. End point is after 12 weeks of treatment.
Blood Pressure
Over a 14-day period, there were no changes in blood pressure in mice treated with OT over time, and measurements were similar to the control mice (Fig. 1). Similarly, there were no changes in heart rate between groups (data not shown).
Figure 1.
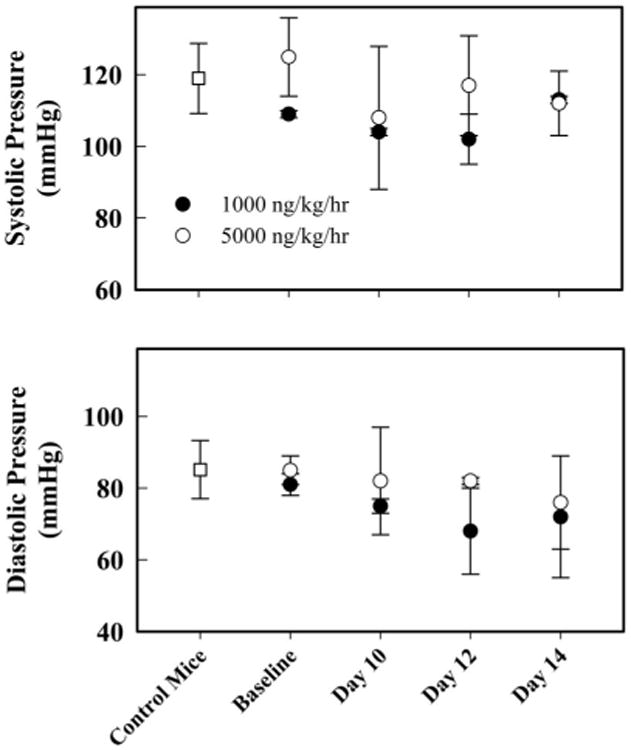
Tail-cuff blood pressure measured in awake C57BL/6 pilot mice. Baseline blood pressure from mice was taken 1 day before surgical osmotic minipump implantation and 10 days, 12 days, and 14 days postsurgical pump implantation. Mice with osmotic minipumps containing vehicle showed no differences in systolic and diastolic blood pressure, and data were pooled. There were no observable differences for systolic and diastolic measurements after surgery between the two groups (n = 2/group). Data represent mean and standard error values.
Atherosclerosis
All animals exhibited evidence of atherosclerotic lesion formation by the end of the study. Aortic atherosclerosis showed a bimodal distribution throughout the aorta, with most disease occurring in the aortic arch and a second area of lesion prevalence within the thoracic aorta. Visual comparison of cumulative lesion prevalence maps indicated an apparent group difference in the extent of atherosclerosis within the thoracic aorta (Fig. 2A). Results of t tests examining group differences in measures of percent lesion area revealed that OT-treated animals displayed significantly less atherosclerosis within the thoracic aorta, t (26) = −2.162, p = .04. Animals in the OT group exhibited 40% less atherosclerotic lesion area within this aortic section (Fig. 2B). No significant group differences were detected within the aortic arch (Fig. 2C).
Figure 2.
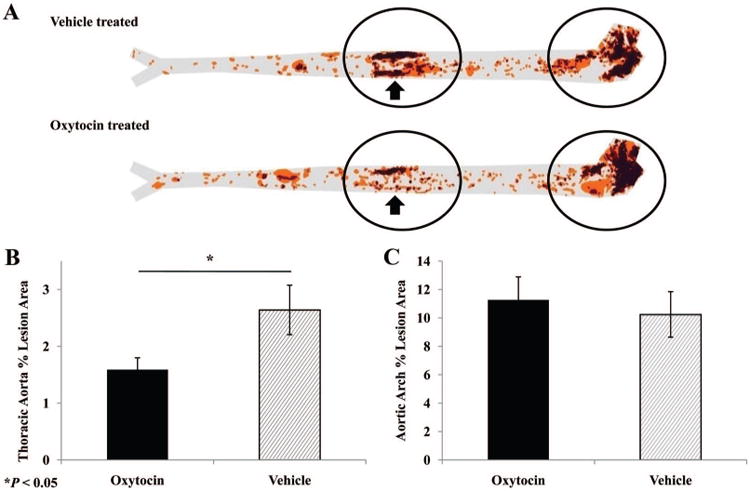
Extent of atherosclerosis in control and oxytocin-treated apoE−/− mice. A) Lesion prevalence maps display the cumulative aortic area covered by atherosclerotic lesions, as indicated by oil-red-O staining, with darker areas representing greater lesion frequency in a given location. Visual inspection of prevalence maps revealed that nearly all of the disease occurred in the aortic arch and the thoracic aorta (circled regions), with an apparent difference in lesion prevalence between groups within the thoracic region (arrows). Quantitative analysis of aortic percent lesion area within the thoracic aorta (B) and the aortic arch (C) confirmed the significant group difference in the thoracic aorta. Data represent mean and standard error values.
Correlational Analyses
Analyses revealed no significant correlation between total lesion area and measures of plasma cholesterol, triglycerides, or insulin. A similar pattern of results was found in the aortic arch; however, end-point triglycerides (R = .308, p = .047) and cholesterol (R = .305, p = .049) both showed modest positive correlations with lesion area in this section of the aorta. There were no significant differences between treatment groups in percent lesion area within the aortic arch after controlling for end-point triglycerides and/or cholesterol.
None of these measures correlated significantly with percent lesion area within the thoracic aorta. The only statistically significant predictor of percent lesion area within the thoracic aorta was end-point insulin, which showed only a modest correlation (R = .331, p = .032). Even after controlling for end-point insulin, animals in the OT treatment group still showed significantly diminished extent of atherosclerosis in this section of the aorta, F(1,39) = 4.476, p = .041.
Ex Vivo IL-6 Secretion
Comparison of a subset of animals from each group on IL-6 secretion from visceral adipose tissue over 6 hours revealed significant group differences, t (15.8) = −2.957, p = .009. Adipose tissue samples taken from OT-treated animals showed a 30% reduction in IL-6 release relative to samples taken from vehicle control animals (Fig. 3).
Figure 3.
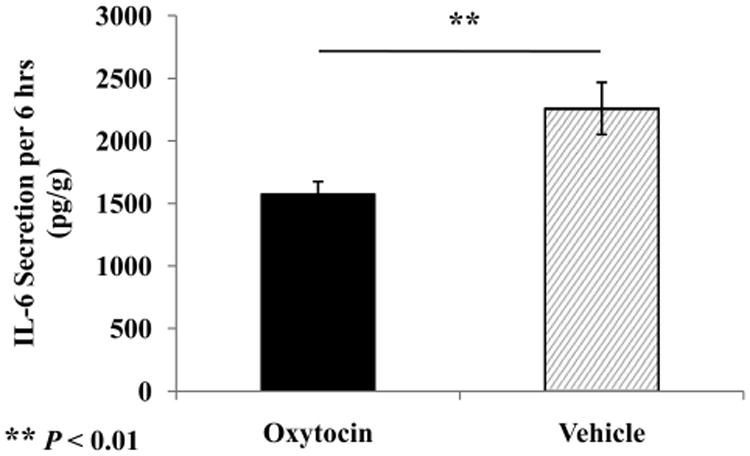
Analysis of interleukin (IL)-6 secretion from visceral adipose tissue samples over 6 hours of ex vivo incubation, revealing that samples taken from oxytocin-treated animals produced less IL-6 than those of the vehicle control group. Values are expressed as picograms IL-6 per gram of sample dry weight. Data represent mean and standard error values.
Discussion
To date, no studies have examined the effect of chronic OT administration on atherosclerosis and markers of adipose tissue inflammation in an in vivo model of the disease. Findings from the current study suggest that OT may slow the progression of atherosclerosis in a site-specific manner, even in animals with strong genetic determinants. Importantly, these differences were found to be independent of changes in plasma lipids, physical activity levels, or insulin levels. Additional findings indicate that long-term OT treatment reduces inflammation of visceral adipose tissue depots. In hyperlipidemic animals, visceral adipose tissue is susceptible to infiltration by activated macrophages, which produce increased levels of proinflammatory cytokines, such as IL-6 (19). Increased production of proinflammatory cytokines by these inflamed adipose depots has been associated with an acceleration of atherosclerotic lesion development (20). In a recent study, transplantation of epididymal fat pads into apoE−/− mice resulted in acceleration of atherosclerosis relative to sham-operated animals (20). These effects are thought to be mediated by increases in systemic levels of proinflammatory cytokines, which can cause increased endothelial cell dysfunction, macrophage activation, and foam cell formation and transmigration (16,20). However, increased inflammation of visceral adipose tissue is also associated with atherogenic alterations in adipokine secretion, fatty-acid metabolism, and insulin-resistance (24). Future studies examining these secondary effects of visceral adipose tissue inflammation may help explain the significance of the findings of the current study.
Peripheral OT may also attenuate disease by directly affecting oxidative stress and inflammation within cell types present in the vessel wall. Research from our laboratory showed that OT reduces NAD(P)H-oxidase activity and inflammatory cytokine secretion in stimulated macrophages and endothelial cells in vitro (11). This suggests that direct antiinflammatory and antioxidant actions of OT on the endothelial surface and on macrophages could be responsible for the observed decrease in atherosclerosis in OT-treated animals. Future studies examining markers of vascular oxidative stress (e.g., NAD(P)H-oxidase activity) and inflammation after chronic OT administration may further clarify the mechanism by which OT affects lesion formation.
The current study involved apoE−/− mice fed a chow diet for 24 weeks, yielding relatively low levels of disease (∼3% of total aortic area) with nearly all lesions being in the earliest stages of development. This was anticipated, because the focus of the study was on early disease mechanisms. The fact that OT was able to slow the initial development of these lesions in a region of high-lesion prevalence suggests that it may be working through mechanisms important during lesion initiation. This is consistent with the in vitro finding that OT is capable of reducing proinflammatory cytokine release from macrophages, as early lesions are characterized by intravasation of activated macrophages in a proinflammatory state (11,16). Future research is needed to evaluate the effect of OT on later stages of disease.
The present study found that OT treatment led to diminished atherosclerotic lesion area in the thoracic aorta, but no effect was found in the aortic arch. Different atherosclerosis-prone regions have been described in the literature on murine models of hyperlipidemia and frequently vary in their responses to treatment and development over time (5,24). The literature suggests that lesions tend to develop within the aortic root first, then proceed over time to develop within the aortic arch, then around the ostea of the thoracic aorta, and later at the renal and iliac bifurcations within the abdominal aorta (5). This is consistent with the findings of the current study, which examined an early stage of disease when there were few lesions below the thoracic aorta. It has also been shown that differential gene expression profiles exist throughout the aorta and that these differences can convey varying susceptibility to specific atherogenic mechanisms (25). Site-specific differences in hemodynamics, including low shear stress, cyclic reversal of flow direction (i.e., oscillatory stress), stretch stress, and turbulence could also be responsible for differential treatment effects at various locations within the aortic tree (25). OT has a partial affinity for the vasopressin, V1 receptor at high concentrations (12), and it is possible that OT treatment could lead to an increase in blood pressure by directly activating these receptors. Other studies (21,22) have shown that postnatal administration of OT, or reduction of central OT production, can significantly reduce blood pressure in rats. Changes in blood pressure could directly affect atherosclerosis; therefore, the effects of OT infusion on blood pressure were examined under the conditions used in this study. Results indicated that OT treatment did not to alter blood pressure compared with baseline measurement or control mice, suggesting that alterations in blood pressure are not likely to account for the pattern of disease findings from the current study. Nevertheless, differences in flow patterns may have interacted with the pharmacological effects of OT, resulting in differential response to treatment across different regions of the aorta in OT-treated animals.
Another possibility is that the differential treatment effects are due to variation in expression of OTR throughout the aorta, as the distribution of these receptors in the mouse aorta has yet to be investigated. Thus, although no definitive conclusions may be drawn, it is possible that the regional variation in the effects of OT observed in the current study was due to hemodynamic or gene expression differences across these aortic regions.
Prior studies have examined acute bolus administration of OT in models of systemic shock and vascular injury (26–28). These studies involving models of acute injury and superphysiological doses (e.g., 1 mg/kg) of OT have found mixed results in terms of the peripheral versus central effects of OT. In the current study, the dose was selected after preliminary pharmacokinetic studies, involving infusion of tritiated OT by the same method, suggested that this dose may produce a more physiologically relevant plasma steady-state concentration of approximately 100 pg/mL to 300 pg/mL (unpublished observations). Peripheral administration of higher doses of OT that are known to cross the blood-brain barrier can produce sedative effects in animals (29). A gross measure of general physical activity showed no differences between treatment groups, suggesting that OT did not affect CNS function. Importantly, this also confirms that the observed differences in inflammation and atherosclerosis were not due to changes in activity levels. Despite these observations, it is still possible that OT exerted its effects on atherosclerosis and inflammation through CNS mechanisms. For example, central administration of OT may reduce sympathetic drive and hypothalamic-pituitary-adrenal axis activation (30). In our experiments, we found that infusion of OT did not significantly affect blood pressure or heart rate in C57BL/6 mice, even at five times the dose used in the current study. Nevertheless, future studies examining markers of hypothalamic-pituitary-adrenal axis or SNS activation, in addition to the ability of OT to cross the blood-brain barrier at lower dosages, may further clarify the question of a peripheral versus central mechanism of action.
Numerous studies (1–3,6) have demonstrated that hyperlipidemic animals housed in a positive social environment develop less extensive atherosclerosis than those housed in isolation or in a stressful social environment. Additional findings (30–35) have shown that positive social and physical contact may lead to increases in peripheral OT. One study (36) found that nurturing physical contact from investigators slowed the progression atherosclerosis in cholesterol-fed rabbits. Together with the current findings, this suggests that increases in peripheral OT, associated with positive social contact, may be partially responsible for the protective effect of positive social environment on atherosclerosis. Although replication and future studies are needed, these findings could suggest that increasing positive social contact in individuals at risk of developing cardiovascular disease may slow lesion progression through increases in OT. These findings may also underscore the importance of including social support in psychosocial interventions targeting this population. However, the relevance of exogenous administration of relatively high doses of OT to endogenously produced responses to prosocial contact remains unclear. Another limitation of the current study is the lack of data on the receptor specificity of the observed effects of OT. The current study sought to determine definitively whether peripheral administration of OT could demonstrate a protective effect on atherosclerosis. Future studies involving the coadministration of OTR antagonists, with OT or positive social environment, may help demonstrate that OTR activation is ultimately responsible for the observed effects on atherosclerosis and inflammation. Future research must demonstrate that positive social behavior is associated with sustained activation of peripheral OT systems to further establish this mechanism as a potential mediating factor in the effect of social environment on atherosclerosis.
Acknowledgments
This study was supported, in part, by Grants HL-36588 and HL-04726 from the National Heart, Lung, and Blood Institute (Principal Investigator: Neil Schneiderman, PhD), and the National Institutes of Health.
Glossary
- OT
oxytocin
- OTR
oxytocin receptor
- SNS
sympathetic nervous system
References
- 1.Shively CA, Clarkson TB, Kaplan JR. Social deprivation and coronary artery atherosclerosis in female cynomolgus monkeys. Atherosclerosis. 1989;77:69–76. doi: 10.1016/0021-9150(89)90011-7. [DOI] [PubMed] [Google Scholar]
- 2.McCabe PM, Gonzales JA, Zaias J, Szeto A, Kumar M, Herron A, Schneiderman N. Social environment influences the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Circulation. 2002;105:354–9. doi: 10.1161/hc0302.102144. [DOI] [PubMed] [Google Scholar]
- 3.Paredes J, Szeto A, Levine JE, Zaias J, Gonzales JA, Mendez AJ, Llabre MM, Schneiderman N, McCabe PM. Social experience influences hypothalamic oxytocin in the WHHL rabbit. Psychoneuroendocrinology. 2006;31:1062–75. doi: 10.1016/j.psyneuen.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–30. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 5.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb Vasc Biol. 1994;14:133–40. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 6.Bernberg E, Andersson IJ, Gan LM, Naylor AS, Johansson ME, Bergstrom G. Effects of social isolation and environmental enrichment on atherosclerosis in apoE−/− mice. Stress. 2008;11:381–9. doi: 10.1080/10253890701824051. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982;2:359–68. doi: 10.1161/01.atv.2.5.359. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM, Miller EW. Social stress and atherosclerosis in normocholesterolemic monkeys. Science. 1983;220:733–5. doi: 10.1126/science.6836311. [DOI] [PubMed] [Google Scholar]
- 9.Nation DA, Gonzales J, Szeto A, Mendez AJ, Paredes J, Brooks L, Schneiderman N, McCabe PM. The effect of social environment on markers of vascular oxidant stress and inflammation. Psychosom Med. 2008;70:269–75. doi: 10.1097/PSY.0b013e3181646753. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan JR, Manuck SB, Adams MR, Weingand KW, Clarkson TB. Inhibition of coronary atherosclerosis by propranolol in behaviorally predisposed monkeys fed an atherogenic diet. Circulation. 1987;76:1364–72. doi: 10.1161/01.cir.76.6.1364. [DOI] [PubMed] [Google Scholar]
- 11.Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM. Oxytocin attenuates NADPH-dependent superoxide activity and IL-6 secretion in macrophages and vascular cells. Am J Physiol Endocrinol Metab. 2008;295:E1495–501. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 13.Gutkowska J, Jankowsi M, Mukaddam-Daher S, McCann SM. Oxytocin is a cardiovascular hormone. Braz J Med Biol Res. 2000;33:625–33. doi: 10.1590/s0100-879x2000000600003. [DOI] [PubMed] [Google Scholar]
- 14.Jankowski M, Wang D, Hajjar F, Mukaddam-Daher S, McCann SM, Gutkowska J. Oxytocin and its receptors are synthesized in the rat vasculature. PNAS. 2000;97:6207–11. doi: 10.1073/pnas.110137497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thibonnier M, Conarty DM, Preston JA, Plesnicher CL, Dweik RA, Erzurum SC. Human vascular endothelial cells express oxytocin receptors. Endocrinology. 1999;140:1301–9. doi: 10.1210/endo.140.3.6546. [DOI] [PubMed] [Google Scholar]
- 16.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 17.Pang G, Couch L, Batey R, Clancy R, Cripps A. GM-CSF, IL-1 alpha, IL-1 beta, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1 alpha and TNF-alpha. Clin Exp Immunol. 1994;96:437–43. doi: 10.1111/j.1365-2249.1994.tb06048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 19.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 20.Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersson M, Uvnas-Moberg K. Postnatal oxytocin treatment of spontaneously hypertensive male rats decreases blood pressure and body weight in adulthood. Neurosci Lett. 2008;440:166–9. doi: 10.1016/j.neulet.2008.05.091. [DOI] [PubMed] [Google Scholar]
- 22.Maier T, Dai WJ, Csikos T, Jirikowski GF, Unger T, Culman J. Oxytocin pathways mediate the cardiovascular and behavioral responses to substance P in the rat brain. Hypertension. 1998;31:480–6. doi: 10.1161/01.hyp.31.1.480. [DOI] [PubMed] [Google Scholar]
- 23.Karra R, Vemullapalli S, Dong C, Herderick EE, Song X, Slosek K, Nevins JR, West M, Goldschmidt-Clermont PJ, Seo D. Molecular evidence for arterial repair in atherosclerosis. Proc Natl Acad Sci U S A. 2005;102:16789–94. doi: 10.1073/pnas.0507718102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–55. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 25.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 26.Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegan BC. Oxytocin ameliorates oxidative colonic inflammation by neutrophil-dependent mechanism. Peptides. 2005;26:483–91. doi: 10.1016/j.peptides.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Petersson M, Lundeberg T, Sohlstrom A, Wilberg U, Uvnas-Moberg U. Oxytocin increases the survival of musculocutaneous flaps. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:701–4. doi: 10.1007/pl00005227. [DOI] [PubMed] [Google Scholar]
- 28.Petersson M, Wiberg U, Lundeberg T, Uvnäs-Moberg K. Oxytocin decreases carrageenan induced inflammation in rats. Peptides. 2001;22:1479–84. doi: 10.1016/s0196-9781(01)00469-7. [DOI] [PubMed] [Google Scholar]
- 29.Uvnas-Moberg, Ahlenius V, Hillegaart V, Alter P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav. 1994;49:101–6. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 30.Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–35. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 31.Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67:531–8. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- 32.Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008;70:976–85. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- 33.Light KC, Grewen KM, Amico JA. More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol Psychol. 2005;69:5–21. doi: 10.1016/j.biopsycho.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Lund I, Ge Y, Yu LC, Uvnas-Moberg K, Wang J, Yu C, Kurosawa M, Agren G, Rosén A, Lekman M, Lundeberg T. Repeated massage-like stimulation induces long-term effects on nociception: contribution of oxytocinergic mechanisms. Eur J Neurosci. 2002;16:330–8. doi: 10.1046/j.1460-9568.2002.02087.x. [DOI] [PubMed] [Google Scholar]
- 35.Uvnäs-Moberg K. Oxytocin linked antistress effects—the relaxation and growth response. Acta Physiol Scand Suppl. 1997;640:38–42. [PubMed] [Google Scholar]
- 36.Nerem RM, Levesque MJ, Cornhill JF. Social environment as a factor in diet-induced atherosclerosis. Science. 1980;208:1475–6. doi: 10.1126/science.7384790. [DOI] [PubMed] [Google Scholar]


