Abstract
Since their early 1990s, the chemokine receptor family of G protein-coupled receptors (GPCRs) has been the source of much pharmacological endeavour. Best known for their key roles in recruiting leukocytes to sites of infection and inflammation, the receptors present themselves as plausible drug targets for therapeutic intervention. In this article, we will focus our attention upon CC Chemokine Receptor Four (CCR4) which has been implicated in diseases as diverse as allergic asthma and lymphoma. We will review the discovery of the receptors and their ligands, their perceived roles in disease and the successful targeting of CCR4 by both small molecule antagonists and monoclonal antibodies. We will also discuss future directions and strategies for drug discovery in this field.
Keywords: Chemokine, Receptor, Signaling, Antagonist, Inflammation, Asthma leukaemia
1. Introduction
1.1. The chemokine superfamily
The chemokine superfamily of proteins serves to coordinate a variety of immune system functions that link both innate and adaptive immunity (Zlotnik and Yoshie, 2012) and is best known for its key role in the recruitment and retention of leukocyte populations in both homeostasis and immune responses to pathogens (Viola and Luster, 2008). The superfamily consists of over twenty GPCRs and around 40 chemokine ligands (Table 1) which act as receiver and signal respectively, to guide leukocytes between tissue compartments (Bachelerie et al., 2013). Movement of leukocytes is along a gradient of chemokine, a process known as chemotaxis, which is sensed by the cell surface chemokine receptors. Binding of the chemokine ligand to the extracellular face of the receptor activates multiple intracellular signaling pathways, with Pertussis toxin-sensitive G proteins of the Gαi subset and Phosphatidylinositol-3 kinases (PI3K) playing key roles.
Table 1.
Human chemokine receptors and their cellular expression. Table showing the accepted cellular/tissue distribution of chemokine receptors and their ligands. Abbreviations: B, B-lymphocyte; Bro, Bronchial epithelial cells; Bs, basophil; DC, dendritic cell; Eo, eosinophil; Ker, keratinocytes; Mc, mast cell; Mo, monocyte; MSC, Mesenchymal Stem cell, NK, natural killer cell; No, neutrophil; NT, neuronal tissue; LEC, lymphatic endothelial cell; P, platelets; RBC, red blood cell; SLO, secondary lymphoid organ; Syn, Syncytiotrophoblast; T, T-lymphocytes; VEC, vascular endothelial cell (adapted from Pease, 2011).
| Chemokine receptor | Chemokine ligands | Cellular expression | Chemokine receptor | Chemokine ligands | Cellular expression |
|---|---|---|---|---|---|
| CCR1 | CCL3, CCL4, CCL5, CCL7, CCL8, CCL13, CCL14, CCL15, CCL16, CCL23 | Mo, DC, Eo, Bs, T, PMN, NK | CXCR1 | CXCL5, CXCL6, CXCL8 | No, Mo |
| CCR2 | CCL2, CCL5, CCL7, CCL8, CCL13, CCL16 | Mo, DC, T, Bs | CXCR2 | CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, CXCL8 | No, Mo |
| CCR3 | CCL4, CCL5, CCL7, CCL11, CCL13, CCL15, CCL24, CCL26, CCL28 | Eo, T, Bs, Mc | CXCR3 | CXCL4, CXCL4L1, CXCL9, CXCL10, CXCL11, | T, B |
| CCR4 | CCL17, CCL22 | T, MC, Bro, NK, P | CXCR4 | CXCL12 | T, B, DC, Mo |
| CCR5 | CCL3, CCL4, CCL5, CCL7, CCL14, CCL15 | Mo, DC, T | CXCR5 | CXCL13 | T, B |
| CCR6 | CCL20 | DC, T | CXCR6 | CXCL16 | T |
| CCR7 | CCL19, CCL21 | DC, T, B, NK | |||
| CCR8 | CCL1, CCL18 | Mo, T, NK | XCR1 | XCL1, XCL2 | T, NK |
| CCR9 | CCL25 | T | |||
| CCR10 | CCL27, CCL28 | T | CX3CR1 | CX3CL1 | T, NK, DC, Mo |
| ACKR1 (DARC) | CCL2, CCL5, CCL7, CCL11, CCL13, CCL14, CCL17,CXCL5, CXCL6,CXCL11 | RBC, VEC | ACKR2 (D6) | CCL1,CCL5, CCL7, CCL11, CCL13, CCL14, CCL17, CCL22 | LEC, Syn |
| ACKR3 (CXCR7) | CXCL11, CXCL12 | B, MSC, NT | ACKR4 (CCRL1) | CCL19, CCL21, CCL25 | Ker, SLO |
1.2. Aspects of signaling
Activation of chemokine receptors leads to the recruitment of PI3K to the plasma membrane and the localized production of Phosphatidylinositol (3,4,5)-trisphosphate (PI (3,4,5) P3) from Phosphatidylinositol (4,5)-bisphosphate (PI (4,5) P2). The redistribution of the PI (3,4,5) P3 –specific phosphatase PTEN to the rear of the cell results in the generation of an intracellular gradient of PI (3,4,5) P3, which is highly enriched at the leading edge of the cell. This results in the localization of cytosolic proteins with pleckstrin homology (PH) domains which readily bind PI (3,4,5) P3 (Lemmon, 2007). Such proteins include Akt, GTPase activating proteins (GAPs) and guanine-nucleotide-exchange factors (GEFs). GEFs serve to activate small GTPases belonging to the Rho family by stimulating the exchange of GDP for GTP, thereby activating the protein. Activated Rho GTPases such as Cdc42 and Rac are well known for their contribution to membrane protrusion at the leading edge of the cell and the maintenance of cell polarity (Weiner et al., 2002) in conjunction with members of the Wiskott-Aldrich Syndrome Protein family, such as WASP (Symons et al., 1996). In contrast, GAPs enhance the intrinsic GTPase activity of the Rho family members, hydrolyzing GTP to GDP and turning off the protein function. Accordingly, actin polymerization at the leading edge of the cell, coupled with contraction at the rear, results in movement of the leukocyte in the direction of the stimulus, i.e. along the concentration gradient (Fig. 1).
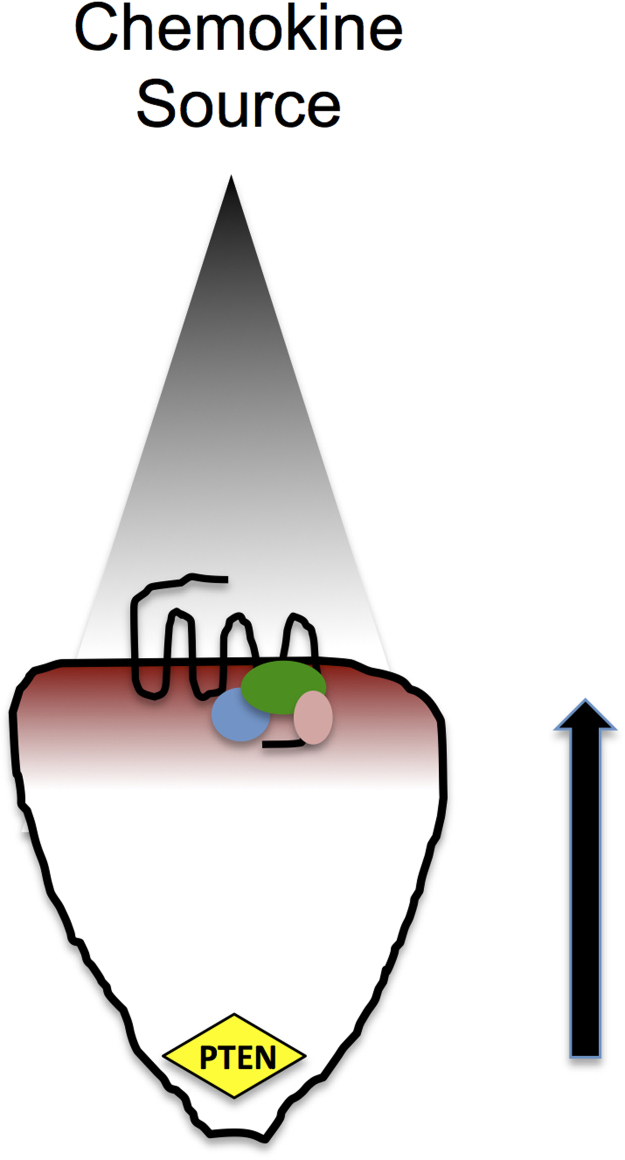
Fig. 1.
Cartoon showing a leukocyte migrating along a gradient of chemokine (gray) in the direction of the arrow. Activation of the 7TMRs at the leading edge of the cell activated PI3K to PIP3. The location of the phosphatase PTEN at the rear of the cell results in an internal gradient of PIP3 , which is concentrated in the vicinity of the receptor. This facilitates localized binding of Rho GTPases such as Cdc42 and Rac (blue, green pink ovals) which modulate the actin cytoskeleton and contribute to membrane protrusion in the direction of the chemokine source.
Migration proceeds as long as the chemokine receptor maintains intracellular signaling and actin remodeling at the leading edge. The process is terminated by G protein receptor kinases (GRKs) which induce C-terminal phosphorylation of the chemokine receptor (Penela et al., 2014). This increases the affinity for the scaffold proteins of the arrestin family, which targets the receptor for clathrin-coated pit mediated endocytosis and degradation or recycling. Thus as the cell encounters increasing concentrations of chemokine, fewer and fewer receptors remain at the cell surface to drive the intracellular signals needed for chemotaxis and migration is inhibited. This is one potential explanation for the bell-shaped chemotaxis plots observed in dose–response experiments with increasing concentrations of chemokine. Once thought to function solely as a means of sterically hindering GPCR signaling and promoting receptor endocytosis (Lefkowitz, 1998), arrestin binding to GPCRs is now appreciated to induce additional signaling programs by acting as a scaffold for the recruitment of further signaling molecules such as MAP kinases (DeWire et al., 2007, Lefkowitz and Shenoy, 2005). The significance of this in terms of chemokine signaling was ably shown when mice deficient in arrestin-2 were shown to have impaired chemotaxis to CXCL12 (Fong et al., 2002). This has been translated in part to the human setting by studies of CXCR4 in patients suffereing from WHIM (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis) syndrome where arrestin-2 dependent phosphorylation of ERK1/2 has been reported to account for the hyperresponsiveness of the receptor to its ligand CXCL12 (Lagane et al., 2008).
1.3. Biased agonism at chemokine receptors
Chemokines are typically promiscuous, binding to several receptors, with each receptor often having multiple ligands. This apparent redundancy was originally thought to provide a means by which robust responses to infectious agents could be generated in vivo (Mantovani, 1999, Zlotnik and Yoshie, 2012). In recent years, however, as different aspects of GPCR signaling have become appreciated, it is apparent that different ligands of the same GPCR can transduce signals via distinct cellular pathways leading to distinct signaling outputs. This is termed functional selectivity or biased agonism (Kenakin and Miller, 2010, Kenakin, 2012). The predominant pathway at which ligands diverge appears to be the arrestin-mediated signaling pathway. Several GPCRs exhibit biased agonism with respect to arrestin signaling, including the M3-muscarinic receptor (Poulin et al., 2010), histamine H4 receptor (Rosethorne and Charlton, 2011), vasopressin receptors (Rahmeh et al., 2012) and angiotensin II–type 1 receptors (Saulière et al., 2012). In the chemokine field, the CCR7 ligands CCL19 and CCL21 although equally active in assays of chemotaxis, have been shown to diverge at the level of receptor endocytosis (Bardi et al., 2001, Otero et al., 2006), arrestin-recruitment (DeWire et al., 2007, Kohout et al., 2004) and receptor desensitization (Penela et al., 2014, Zidar et al., 2009). We have recently uncovered aspects of biased signaling at the chemokine receptor CCR4, in both leukocytes and lung epithelial cells, which we believe to be of significance in the setting of allergic inflammation, more of which later (Ajram et al., 2014, Viney et al., 2014).
1.4. Targetting chemokines and their receptors
The inadvertent or over expression of chemokines has been implicated in just about every disease process with an inflammatory component, from diseases as seemingly diverse as asthma, atherosclerosis, multiple sclerosis and rheumatoid arthritis (Charo and Ransohoff, 2006, Viola and Luster, 2008). This has led to the notion that therapeutic intervention, in the form of chemokine receptors blockade may provide a novel therapeutic angle. The discovery that chemokine receptors are portals for the entry of HIV-1 into leukocytes (Alkhatib et al., 1996, Feng et al., 1996) has fueled the drug discovery process further, with inhibitors of the two major receptors, CCR5 (on macrophages) and CXCR4 (on T cells) highly prized. At the time of writing, two small molecule antagonists of CCR5 and CXCR4 have received approval by the relevant agencies. Miraviroc/Selsentri a CCR5 inhibitor from Pfizer has been licensed for the treatment of HIV-1 infection (MacArthur and Novak, 2008). Plerixafor, a CXCR4 antagonist originally developed for similar purposes, has been licensed for its ability to mobilize stem cells from the bone marrow, of use following administration of chemotherapeutics (Brave et al., 2010) and is also showing early promise as a treatment for patients with the immunosuppressive WHIM syndrome, resulting from dysregulation of CXCR4 function (McDermott et al., 2011).
In this article, we will focus upon the chemokine CCR4 and its ligands CCL17 and CCL22, which are postulated to play key roles in the pathogenesis of allergic asthma (Pease and Horuk, 2014), atopic dermatitis (Yamanaka and Mizutani, 2011) and a variety of cancers, including breast cancer (Li et al., 2012), gastric cancer (Yang et al., 2011) renal cell cancer (Liu et al., 2014) and lymphoma (Ishida and Ueda, 2011).
1.5. CCR4 – Discovery and initial characterization
The human coding sequence for CCR4 was first identified by the PCR amplification of a fragment from a cDNA library made from the basophilic cell line KU-812 and found to have around 50% homology to two other CC chemokine receptors identified at that time, CCR1 and CCR2 (Power et al., 1995). The original report assigned CCL3 as a functional ligand for CCR4, inducing Ca2+ influx in Xenopus oocytes although this may be an artifact of the system employed, since the authors subsequently showed that HEK-293 transfectants were unresponsive to CCL3 and its close relative CCL5 (Blanpain et al., 2001). Work from the group of Osamu Yoshie identified a transcript constitutively expressed in thymus and also by PBMCs following activation with phytohaemagglutinin, which they named Thymus and Activation-Regulated Chemokine (TARC) (Imai et al., 1996) and which they subsequently showed to be a high-affinity ligand for CCR4, inducing chemotaxis and Ca2+ influx in CCR4 transfectants (Imai et al., 1997). Northern blot analysis in the same manuscript showed CCR4 mRNA to be expressed by human CD4+ T cells and a handful of T-cell lines including Hut-78 and Jurkat. Expression of CCR4 has subsequently been demonstrated on several T-cell subsets including Th2 and T regulatory (Treg) cells (discussed later) and more recently on Th17 (Acosta-Rodriguez et al., 2007, Lim et al., 2008) and Th22 cells (Trifari et al., 2009), where it is co-expressed with other chemokine receptors , notably CCR6.
Shortly after the discovery of TARC, another CC chemokine was cloned independently by two groups. The group of Patrick Gray at ICOS Corporation named their discovery “monocyte derived chemokine” (MDC) since it was expressed by macrophages and monocyte-derived dendritic cells (Godiska et al., 1997). Andrew and colleagues at Amgen simultaneously cloned an identical CC chemokine by EST sequencing of a cDNA library prepared from activated macrophages which they named STCP-1 (Stimulated T-cell chemotactic protein) since it recruited T-cells in chemotaxis assays (Chang et al., 1997). Both groups subsequently showed that the chemokines bound to the same chemokine receptor, namely CCR4 (Andrew et al., 1998, Imai et al., 1998). TARC and MDC/STCP-1 are now known as CCL17 and CCL22 respectively (Zlotnik and Yoshie, 2000). Both CCL17 and CCL22 bind CCR4 with low nanomolar affinity and have similar potencies in chemotaxis assays, although CCL22 is the slightly more efficacious ligand of the two. The genes for CCL17 and CCL22 reside in close proximity on human chromosome 16q13 suggesting that they arose by gene duplication, although the mature protein sequences are less than 40% identical (Imai et al., 1998). Both CCL22 and CCL17 are expressed in the thymus leading to the notion that one role of the receptor may be to regulate the intrathymic movement of CCR4+CD4+CD8+ thymocytes during the process of T lymphocyte education and differentiation (Annunziato et al., 2000, Chantry et al., 1999).
1.6. CCR4 and its ligands in disease
1.6.1. Asthma
A considerable body of evidence points to a role for CCR4 and its ligands in allergic diseases, notably asthma. Polarization of human T-cells in vitro to Th2 subsets by culture with cytokine and antibody cocktails (IL4, anti-IFNγ and anti-IL-10), has been well documented to generate IL-4 producing Th2 cells, which express CCR4 at both protein and message level (Bonecchi et al., 1998, Sallusto et al., 1998). This facilitates their recruitment by dendritic cells which produce CCL17 and CCL22 during maturation (Tang and Cyster, 1999). Upregulation of CCR4 on T cells is mirrored in vivo, with CCR4 expression a key feature of IL-4 producing T cells recovered from the bronchoalveolar lavage fluid of asthmatic and healthy subjects CCR4 (Morgan et al., 2005, Panina-Bordignon et al., 2001). The CCR4 ligands CCL22 and CCL17 are also upregulated in the lung following allergen challenge (Bochner et al., 2003, Pilette et al., 2004). More recently, a study by Vijayanand and colleagues demonstrated increased CCR4 expression on T cells isolated from patients with asthma. They also notably demonstrated that CCL17 but not CCL22 was significantly upregulated following challenge of ex vivo airway biopsies with house dust mite extract (Vijayanand et al., 2010).
A role for CCR4 expression on bronchial epithelial cells has been discovered (Bonner et al., 2013). Interestingly, previous studies reported that bronchial epithelial cells in culture can also produce CCL17 (Sekiya et al., 2000), highlighting the potential for a positive feedback signaling loop. We have recently shown that CCR4 is expressed by both primary bronchial epithelial cells and lines such as BEAS2B, and can bind and internalize CCR4 in response to ligand. Notably, we observed that CCL17 was an extremely efficacious inducer of α-CGRP synthesis and release (Bonner et al., 2013). This, we hypothesize, may play a pathological role in asthma, since α-CGRP production is markedly increased in the airways of asthmatic patients challenged with allergen-derived T-cell peptides (Kay et al., 2007). α-CGRP is known to act synergistically with other mediators of inflammation, including histamine, to produce marked and prolonged edema, thus contributing to pathology (Brain and Williams, 1985). Curiously, in contrast to CCL17, CCL22 is a feeble inducer of α-CGRP release, with an approximately 10,000-fold reduction in activity compared to CCL17, despite both ligands binding CCR4 with similar affinity (Imai et al., 1998, Imai et al., 1997). To our knowledge, this is the first published description of a definitive physiological outcome by an endogenous biased agonist of a chemokine receptor.
Data obtained in humans has been corroborated to a certain extent by rodent models of allergic airways disease. Mikhak et al showed that antigen-specific Th2 cells adoptively transferred from CCR4-deficient mice fail to traffic in significant numbers to the allergic lung (Mikhak et al., 2009). Similarly, antibody neutralization of either CC17 or CCL22 proved to be effective in reducing leukocyte recruitment to the lung and associated parameters of inflammation, following allergen challenge (Kawasaki et al., 2001, Lloyd et al., 2000). In contrast to these studies, ovalbumin challenged CCR4 null mice were not protected against airways inflammation compared with littermates (Chvatchko et al., 2000), nor were ovalbumin challenged guinea pigs protected from lung inflammation when CCR4 was neutralized by an antibody (Conroy et al., 2003). This suggests that the underlying biology of CCR4 may be subtly different in rodents and man. A recent study circumvented this by using a human PBMC-reconstituted SCID mouse model and found that CCR4 blockade via a specific antibody ablated many of the features of inflammation, including airway eosinophilia, goblet cell hyperplasia, IgE synthesis and bronchial hyper-reactivity, thus reinforcing the idea that CCR4 is a viable target in the treatment of asthma (Perros et al., 2009).
1.6.2. CCR4 and its ligands in allergic dermatitis
The discovery by Campbell and colleagues that skin-homing cutaneous lymphocyte antigen (CLA)+ T cells express high levels of CCR4 expression (Campbell et al., 1999), implicated the receptor in the pathology of atopic dermatitis. This was subsequently supported by a study in which the levels of both CCR4 and CLA were shown to be increased on the surface of peripheral blood CD4+ T cells from severe atopic dermatitis subjects compared with control subjects. CCR4 expression levels were also shown to decrease as disease symptoms improved (Wakugawa et al., 2001). In mouse models of cutaneous delayed type hypersensitivity, CCR4 has been shown to support the homing of T cells to skin (Reiss et al., 2001). Several studies have reported elevated serum levels of CCL17 in human atopic dermatitis subjects (Shimada et al., 2004) with CCL17 thought to be produced by keratinocytes (Vestergaard et al., 2000). Serum levels of the chemokine show close correlation with disease severity (Kakinuma et al., 2001) . Indeed, out of a panel of adult biomarkers, CCL17 was found by Kou et al to have the highest odds ratio for the likelihood of having atopic dermatitis (Kou et al., 2012). This is consistent with in vitro studies showing that corneal and dermal fibroblasts stimulated with the Th2-associated cytokines IL-4 and IL-13 are important sources of CCL17 (Fukuda et al., 2003). Human platelets have also been shown to express CCR4 and to undergo aggregation following stimulation with CCL17 and CCL22 (Abi-Younes et al., 2001, Clemetson et al., 2000, Kowalska et al., 2000). This was postulated by Abi-Younes and colleagues to explain the increased serum levels of CXCL4 in a murine model of atopic dermatitis (Watanabe et al., 1999), since CXCL4 is a marker of platelet degranulation. Moreover, since platelets have been shown to contain CCL17 there is potential for positive feedback in this process (Fujisawa et al., 2002).
1.6.3. CCR4 and its ligands in T-cell neoplasms
Working with the hypothesis that the chemokine expression pattern of T-cell neoplasm may give insight into their cellular origins, the group of Osamo Yoshie were first to show that CCR4 was expressed at consistently high levels on the surface of a wide range of human T-cell lines (Yoshie et al., 2002), including Hut87 and Jurkat lines, which they had previously examined by Northern blot analysis (Imai et al., 1997). Using a panel of 24 adult adult T cell lymphoma patients, the vast majority of PBMCs from these subjects (22/24) were shown to express CCR4 by PCR and to respond to CCL17 and CCL22 in chemotaxis assays (Yoshie et al., 2002). Subsequent studies by others confirmed the expression of CCR4 in archived adult T cell lymphoma tissues (Ishida et al., 2003) and also showed CCR4 to be abundantly expressed by other neoplasms including some peripheral T cell lymphoma and NK cell lymphomas (Ishida et al., 2004) and cutaneous T cell lymphomas (Campbell et al., 2010, Ferenczi et al., 2002). Subsequent studies addressing the molecular mechanisms for CCR4 upregulation, found the transcription factor FRA-2 to be significantly upregulated in adult T cell lymphoma and to drive enhanced CCR4 expression and proliferation of both adult T cell lymphomas and cutaneous T cell lymphoma s (Nakayama et al., 2007, Nakayama et al., 2012). A recent study has described mutations in the C-terminus of CCR4 that truncate the receptor and lead to a gain of function, with respect to enhanced cellular signaling, chemotaxis and proliferation (Nakagawa et al., 2014). These are remarkably similar to truncating mutations in CXCR4 that have been described as exacerbating signaling in WHIM syndrome patients (Hernandez et al., 2003).
2. Blockade of CCR4 in the treatment of disease
2.1. Anti-CCR4 biologicals
Monoclonal antibodies targeting CCR4 have been described by several groups. Our own group, in collaboration with scientists at LeukoSite/Millennium Pharmaceuticals characterized a panel of CCR4-specific antibodies which were generated by immunization of mice with transfectants expressing the receptor (Andrew et al., 2001). The most promising of these was a molecule known as 10E4 which recognizes an N-terminal epitope of CCR4 (Jopling et al., 2002) and which we subsequently used to neutralize CCR4 in a guinea pig model of allergic airways disease (Conroy et al., 2003). Scientists at Kyowa Hakko Kogyo generated a murine monoclonal antibody named KM-2160 by immunizing mice with a peptide corresponding to amino acid residues 2–29 of the human CCR4 N-terminus (Ishida et al., 2003). Using this mAb, they showed that increased levels of CCR4 staining on primary adult T cell lymphoma cells correlated with decreased survival of the patient. This finding spurred on the authors to assess the efficacy of this antibody in mediating antibody-dependent cellular cytotoxicity, turning CCR4 into a target by which adult T cell lymphomas could be sought out and destroyed by host NK cells. The antibody underwent subsequent modifications, namely cDNAs encoding the heavy- and light chain variable region of the KM-2160 hybridoma were cloned into an IgG1 antibody expression vector and the construct expressed in the rat myeloma cell line YB2/0. From the supernatant they were able to purify a chimeric anti-CCR4 antibody which they named KM-2760 (Niwa et al., 2004). This antibody has low levels of fucosylation in the Fc region (7%), which they showed corresponded to greater activity in antibody-dependent cellular cytotoxicity assays. KM2760 then underwent full humanization to generate the mAb KW-0761 (also known as mogamulizumab) which demonstrated potent antitumor activity against primary adult T cell lymphomas both in vitro and ex vivo (Ishii et al., 2010).
Mogalizumab subsequently entered clinical trials for the treatment of both adult T cell lymphoma and peripheral T cell lymphoma and was found to be well tolerated, have a half life of around 18 days and meet preliminary objective responses (Yamamoto et al., 2010). Subsequent phase II studies of relapsed adult T cell lymphoma patients (Ishida et al., 2012) and relapsed peripheral T cell lymphomas and cutaneous T cell lymphomas (Ogura et al., 2014) found mogalizumab to again show efficacy, with significant numbers of objective responses seen in all patient groups. In 2012, mogalizumab was granted approval for the treatment of relapsed or refractory adult T cell lymphoma in Japan.
2.2. Small molecule antagonists of CCR4
Given the importance of CCR4 and its ligands in allergic inflammatory diseases there has been a significant effort over many years to discover small molecule CCR4 antagonists. However, despite all these endeavors, so far only one molecule has made it to human clinical trials and that too appears to have been terminated (Cahn et al., 2013, Solari et al., 2014). Chemokine receptors are GPCRs, which are historically the most successful target class for drug discovery, so this lack of success has been surprising and has been attributed to many factors (Solari et al., 2014).
The patent literature for CCR4 antagonists began to emerge around 2002 and the first comprehensive review of the field in 2006 revealed that these could be divided into four main groups; aryl sulphonamides, substituted amino heterocycles, thiazolidinones and lactams (Purandare and Somerville, 2006). Since then there have been many reports of CCR4 antagonists that can grouped into two main chemical categories. The first is a collection of lipophilic heteroarenes from Bristol Myers Squibb, Astellas and Daiichi Sankyo and the second is a range of aryl sulphonamides from Astra Zeneca, Ono and GlaxoSmithKline (Andrews et al., 2007, Banfield et al., 2010, Burdi et al., 2007, Cahn et al., 2013, Kuhn et al., 2007, Nakagami et al., 2010a, Nakagami et al., 2010b, Nakagami et al., 2009, Procopiou et al., 2013, Procopiou et al., 2012, Purandare et al., 2007, Solari et al., 2014, Yokoyama et al., 2009, Yokoyama et al., 2008, Zhao et al., 2009). Some pertinent structures from these studies are shown in Fig. 2. A number of these compounds looked very promising and showed efficacy in animal models of allergic inflammation however only one, an indazole arylsulfonamide, GSK 2239633 (Slack et al., 2013) appears to have progressed to clinical trials (Cahn et al., 2013).
Fig. 2.
The chemical structures of a handful of small molecule CCR4 antagonists described in the main text.
Clues began to emerge about the complex biology of CCR4 that might explain why drug discovery has been so challenging. The first came from studies by Astra Zeneca on a series of pyrazinyl-sulfonamides that were allosteric antagonists of CCR4 and that appeared to bind to an intracellular site on the receptor, the so-called “Site 2” (Andrews et al., 2007). Furthermore, it appeared that this intracellular allosteric binding site was different to the site bound by the compound BMS-397 (“Site 1”) and both of these antagonist sites were distinct from the binding site for the natural ligands (Ajram et al., 2014). Unpublished mutagenesis work from our groups suggests that “Site 1” resides in a well characterized hydrophobic pocket comprised of resides in transmembrane helix III, whilst “Site 2” is centered around the cytoplasmic Helix VIII thought to run parallel to the lipid bilayer (Fig. 3).
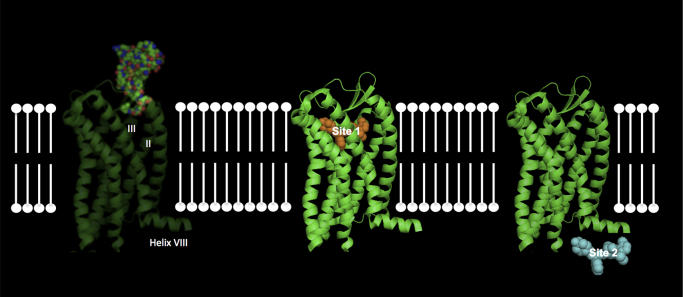
Fig. 3.
A diagram representing various modes of allosteric modulation at CCR4. The left panels shows transmembrane helices II and III of CCR4 being activated by chemokine (space filled model). The centre panel shows a "Site 1" antagonist (yellow) bound to an intrahelical binding site. The right panel shows a lipophillic "Site 2" antagonist (cyan) interacting with C-terminal helix VIII of CCR4.
In addition to this complex pharmacology it appears the receptor displays complex biological responses to its natural ligands. Like most GPCRs, CCR4 is internalised following agonist binding as part of the desensitisation process. However, it appeared that the two natural CCR4 ligands, CCL17 and CCL22, induced different rates of receptor internalisation (Imai et al., 1998, Mariani et al., 2004). Moreover, this difference in receptor trafficking was reflected by small molecule antagonists. Arylsulphonamides that bind to the intracellular allosteric site were unable to induce receptor internalisation whereas lipophilic amine antagonists binding to the extracellular site were (Ajram et al., 2014). The possibility that receptor down regulation by antagonists may contribute to the inhibition of a biological response was highlighted by studies of another CCR4 antagonist, K777 (Sato et al., 2013). Thus this chemokine receptor, and perhaps others, shows complex regulation of cell surface expression and trafficking that may in part reflect the need for accurate control of chemotaxis signals. In addition to differences in receptor trafficking, the two natural ligands also showed differences in receptor coupling with CCL22 coupling to arrestin signaling, whereas CCL17 does not (Ajram et al., 2014).
3. Conclusions
Clearly CCR4 is amenable to the discovery of drug-like antagonists, however the failure of these to translate small molecules into drugs raises the question that perhaps we still do not appreciate the subtle and complex biological controls that regulate the function of this receptor. For example, what are the relative contributions of arrestins to CCR4-mediated signalling? Which members of the GRK family govern CCR4 desentization and trafficking? An obvious potential caveat of total CCR4 blockade as an asthma treatment is the potential for the impairment of regulatory T cell recruitment, since T-regulatory cells (Tregs) have been shown to express CCR4 and to migrate in vitro in response to both CCL17 and CCL22 (Iellem et al., 2001). Blockade of CCR4 function on these cells might therefore be envisaged to worsen rather than dampen allergic inflammation since Tregs have the capacity to suppress Th2-mediated inflammation in vivo (Saito et al., 2008). Indeed, mogalizumab treatment has been associated with several cases of severe skin inflammation, notably Steven–Johnson syndrome, which in one case proved to be fatal (Ishida et al., 2013). Examination of one Steven–Johnson syndrome patient revealed a significant reduction in staining for the Treg marker FOXP3, in both PBMCs and skin lesions, incriminating Treg depletion in the pathogenesis. The efficacy of mogamulizumab in the treatment of CCR4-negative solid cancers, where Treg depletion is desirable, is currently being assessed. A recent report outlining the treatment of four elderly patients with mogalizumab has also suggested that an additional side-effect may be the risk of opportunistic infection with cytomegalovirus (Ohyama et al., 2014).
However, it may be possible to employ small molecules to block the activity of one CCR4 agonist whilst sparing another, since they are biased agonists with respect to arrestin coupling (Ajram et al., 2014) and also with respect to αCGRP induction in bronchial epithelial cells (Bonner et al., 2013). In a proof of principle approach, we have recently shown that CCL22 signaling can be spared whilst ablating CCL17 signaling by the use of a CCR4-specific mAb 10E4 which binds the receptor N-terminus (Viney et al., 2014). This mAb presumably preferentially inhibits a CCR4 conformation required for CCL17 signaling but dispensable for CCL22 signaling. This may be important in dialing out the off-target effects of inhibiting Treg recruitment, since their recruitment by activated dendritic cells appears to be mediated principally via CCL22 (Iellem et al., 2001). Analysis of the structure–activity relationship of existing compounds with attention to the signaling pathways blocked by the compounds could be a fruitful approach to fine-tune these compounds into molecules which block CCL17 but spare CCL22 signaling.
Acknowledgements
We are grateful to the MRC-Asthma UK Centre in Allergic Mechanisms of Asthma for supporting our research in this field.
References
- Abi-Younes S., Si-Tahar M., Luster A.D. The CC chemokines MDC and TARC induce platelet activation via CCR4. Thromb. Res. 2001;101:279–289. doi: 10.1016/s0049-3848(00)00402-3. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- Ajram L., Begg M., Slack R., Cryan J., Hall D., Hodgson S., Ford A., Barnes A., Swieboda D., Mousnier A., Solari R. Internalization of the chemokine receptor CCR4 can be evoked by orthosteric and allosteric receptor antagonists. Eur. J. Pharmacol. 2014;729:75–85. doi: 10.1016/j.ejphar.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G., Combadiere C., Broder C.C., Feng Y., Kennedy P.E., Murphy P.M., Berger E.A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Andrew D.P., Chang M.S., McNinch J., Wathen S.T., Rihanek M., Tseng J., Spellberg J.P., Elias C.G. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J. Immunol. 1998;161:5027–5038. [PubMed] [Google Scholar]
- Andrew D.P., Ruffing N., Kim C.H., Miao W., Heath H., Li Y., Murphy K., Campbell J.J., Butcher E.C., Wu L. C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J. Immunol. 2001;166:103–111. doi: 10.4049/jimmunol.166.1.103. [DOI] [PubMed] [Google Scholar]
- Andrews G., Jones C., Wreggett K.A. An Intracellular Allosteric Site for a Specific Class of Antagonists of the CC Chemokine G Protein-Coupled Receptors CCR4 and CCR5. Mol. Pharmacol. 2007;73:855–867. doi: 10.1124/mol.107.039321. [DOI] [PubMed] [Google Scholar]
- Annunziato F., Romagnani P., Cosmi L., Beltrame C., Steiner B.H., Lazzeri E., Raport C.J., Galli G., Manetti R., Mavilia C., Vanini V., Chantry D., Maggi E., Romagnani S. Macrophage-derived chemokine and EBI1-ligand chemokine attract human thymocytes in different stage of development and are produced by distinct subsets of medullary epithelial cells: possible implications for negative selection. J. Immunol. 2000;165:238–246. doi: 10.4049/jimmunol.165.1.238. [DOI] [PubMed] [Google Scholar]
- Bachelerie F., Ben-Baruch A., Burkhardt A.M., Combadiere C., Farber J.M., Graham G.J., Horuk R., Sparre-Ulrich A.H., LOCATI M., Luster A.D., Mantovani A., Matsushima K., Murphy P.M., Nibbs R., Nomiyama H., Power C.A., Proudfoot A.E.I., Rosenkilde M.M., Rot A., Sozzani S., Thelen M., Yoshie O., Zlotnik A. International Union of Pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for at ypical chemokine receptors. Pharmacol. Rev. 2013;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield G., Watanabe H., Scadding G., Jacobson M.R., Till S.J., Hall D.A., Robinson D.S., Lloyd C.M., Nouri-Aria K.T., Durham S.R. CC Chemokine Receptor 4 (CCR4) in human allergen-induced late nasal responses. Allergy. 2010 doi: 10.1111/j.1398-9995.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi G., Lipp M., Baggiolini M., Loetscher P. The T cell chemo–kine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur. J. Immunol. 2001;31:3291–3297. doi: 10.1002/1521-4141(200111)31:11<3291::aid-immu3291>3.0.co;2-z. doi:10.1002/1521-4141(200111)31:11& #60;3291::AID-IMMU3291& #62;3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Blanpain C., Buser R., Power C.A., Edgerton M., Buchanan C., Mack M., Simmons G., Clapham P.R., Parmentier M., Proudfoot A.E. A chimeric MIP-1α/RANTES protein demonstrates the use of different regions of the RANTES protein to bind and activate its receptors. J. Leukoc. Biol. 2001;69:977–985. [PubMed] [Google Scholar]
- Bochner B.S., Hudson S.A., Xiao H.Q., Liu M.C. Release of both CCR4-active and CXCR3-active chemokines during human allergic pulmonary late-phase reactions. J. Allergy Clin. Immunol. 2003;112:930–934. doi: 10.1016/j.jaci.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Bonecchi R., Bianchi G., Bordignon P.P., D'Ambrosio D., Lang R., Borsatti A., Sozzani S., Allavena P., Gray P.A., Mantovani A., Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner K.T., Pease J.E., Corrigan C.J., Clark P.C., Kay A.B. CCL17/thymus and activation-regulated chemokine induces calcitonin gene–related peptide in human airway epithelial cells through CCR4. J. Allergy Clin. Immunol. 2013;132:942–950. doi: 10.1016/j.jaci.2013.04.015. e3. [DOI] [PubMed] [Google Scholar]
- Brain S.D., Williams T.J. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br. J. Pharmacol. 1985;86:855–860. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brave M., Farrell A., Ching Lin S., Ocheltree T., Pope Miksinski S., Lee S.-L., Saber H., Fourie J., Tornoe C., Booth B., Yuan W., He K., Justice R., Pazdur R. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology. 2010;78:282–288. doi: 10.1159/000315736. [DOI] [PubMed] [Google Scholar]
- Burdi D.F., Chi S., Mattia K., Harrington C., Shi Z., Chen S., Jacutin-Porte S., Bennett R., Carson K., Yin W., Kansra V., Gonzalo J.-A., Coyle A., Jaffee B., Ocain T., Hodge M., LaRosa G., Harriman G. Small molecule antagonists of the CC chemokine receptor 4 (CCR4) Bioorg. Med. Chem. Lett. 2007;17:3141–3145. doi: 10.1016/j.bmcl.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Cahn A., Hodgson S., Wilson R., Robertson J., Watson J., Beerahee M., Hughes S.C., Young G., Graves R., Hall D., van Marle S., Solari R. Safety, tolerability, pharmacokinetics and pharmacodynamics of GSK2239633, a CC-chemokine receptor 4 antagonist, in healthy male subjects: results from an open-label and from a randomised study. BMC Pharmacol. Toxicol. 2013;14:14. doi: 10.1186/2050-6511-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.J., Clark R.A., Watanabe R., Kupper T.S. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116:767–771. doi: 10.1182/blood-2009-11-251926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.J., Haraldsen G., Pan J., Rottman J., Qin S., Ponath P., Andrew D.P., Warnke R., Ruffing N., Kassam N., Wu L., Butcher E.C. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- Chang M.S., McNinch J., Elias C., Manthey C.L., Grosshans D., Meng T., Boone T., Andrew D.P. Molecular cloning and functional characterization of a novel CC chemokine, stimulated T cell chemotactic protein (STCP-1) that specifically acts on activated T lymphocytes. J. Biol. Chem. 1997;272:25229–25237. doi: 10.1074/jbc.272.40.25229. [DOI] [PubMed] [Google Scholar]
- Chantry D., Romagnani P., Raport C.J., Wood C.L., Epp A., Romagnani S., Gray P.W. Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3(+), CD4(+), CD8(low) thymocytes. Blood. 1999;94:1890–1898. [PubMed] [Google Scholar]
- Charo I.F., Ransohoff R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Chvatchko Y., Hoogewerf A.J., Meyer A., Alouani S., Juillard P., Buser R., Conquet F., Proudfoot A.E., Wells T.N., Power C.A. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J. Exp. Med. 2000;191:1755–1764. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemetson K.J., Clemetson J.M., Proudfoot A.E., Power C.A., Baggiolini M., Wells T.N. Functional expression of CCR1, CCR3, CCR4, and CXCR4 chemokine receptors on human platelets. Blood. 2000;96:4046–4054. [PubMed] [Google Scholar]
- Conroy D.M., Jopling L.A., Lloyd C.M., Hodge M.R., Andrew D.P., Williams T.J., Pease J.E., Sabroe I. CCR4 blockade does not inhibit allergic airways inflammation. J. Leukoc. Biol. 2003;74:558–563. doi: 10.1189/jlb.0103030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWire S.M., Ahn S., Lefkowitz R.J., Shenoy S.K. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Feng Y., Broder C.C., Kennedy P.E., Berger E.A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996 doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Ferenczi K., Fuhlbrigge R.C., Pinkus J., Pinkus G.S., Kupper T.S. Increased CCR4 expression in cutaneous T cell lymphoma. J. Investig. Dermatol. 2002;119:1405–1410. doi: 10.1046/j.1523-1747.2002.19610.x. [DOI] [PubMed] [Google Scholar]
- Fong A.M., Premont R.T., Richardson R.M., Yu Y.-R.A., Lefkowitz R.J., Patel D.D. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc. Natl. Acad. Sci. USA. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T., Fujisawa R., Kato Y., Nakayama T., Morita A., Katsumata H., Nishimori H., Iguchi K., Kamiya H., Gray P.W., Chantry D., Suzuki R., Yoshie O. Presence of high contents of thymus and activation-regulated chemokine in platelets and elevated plasma levels of thymus and activation-regulated chemokine and macrophage-derived chemokine in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2002;110:139–146. doi: 10.1067/mai.2002.126079. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Fujitsu Y., Seki K., Kumagai N., Nishida T. Differential expression of thymus- and activation-regulated chemokine (CCL17) and macrophage-derived chemokine (CCL22) by human fibroblasts from cornea, skin, and lung. J. Allergy Clin. Immunol. 2003;111:520–526. doi: 10.1067/mai.2003.59. [DOI] [PubMed] [Google Scholar]
- Godiska R., Chantry D., Raport C.J., Sozzani S., Allavena P., Leviten D., Mantovani A., Gray P.W. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J. Exp. Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez P.A., Gorlin R.J., Lukens J.N., Taniuchi S., Bohinjec J., Francois F., Klotman M.E., Diaz G.A. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat. Genet. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- Iellem A., Mariani M., Lang R., Recalde H., Panina-Bordignon P., Sinigaglia F., D'Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J. Exp. Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T., Baba M., Nishimura M., Kakizaki M., Takagi S., Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor. J. Biol. Chem. 1997;4(272):15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- Imai T., Chantry D., Raport C.J., Wood C.L., Nishimura M., Godiska R., Yoshie O., Gray P.W. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J. Biol. Chem. 1998;273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- Imai T., Yoshida T., Baba M., Nishimura M., Kakizaki M., Yoshie O. Molecular cloning of a novel T cell-directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J. Biol. Chem. 1996;271:21514–21521. doi: 10.1074/jbc.271.35.21514. [DOI] [PubMed] [Google Scholar]
- Ishida T., Inagaki H., Utsunomiya A., Takatsuka Y., Komatsu H., Iida S., Takeuchi G., Eimoto T., Nakamura S., Ueda R. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin. Cancer Res. 2004;10:5494–5500. doi: 10.1158/1078-0432.CCR-04-0371. [DOI] [PubMed] [Google Scholar]
- Ishida T., Ito A., Sato F., Kusumoto S., Iida S., Inagaki H., Morita A., Akinaga S., Ueda R. Stevens-Johnson Syndrome associated with mogamulizumab treatment of adult T-cell leukemia / lymphoma. Cancer Sci. 2013;104:647–650. doi: 10.1111/cas.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Joh T., Uike N., Yamamoto K., Utsunomiya A., Yoshida S., Saburi Y., Miyamoto T., Takemoto S., Suzushima H., Tsukasaki K., Nosaka K., Fujiwara H., Ishitsuka K., Inagaki H., Ogura M., Akinaga S., Tomonaga M., Tobinai K., Ueda R. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J. Clin. Oncol. 2012;30:837–842. doi: 10.1200/JCO.2011.37.3472. [DOI] [PubMed] [Google Scholar]
- Ishida T., Ueda R. Immunopathogenesis of lymphoma: focus on CCR4. Cancer Sci. 2011;102:44–50. doi: 10.1111/j.1349-7006.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- Ishida T., Utsunomiya A., Iida S., Inagaki H., Takatsuka Y., Kusumoto S., Takeuchi G., Shimizu S., Ito M., Komatsu H., Wakita A., Eimoto T., Matsushima K., Ueda R. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin. Cancer Res. 2003;9:3625–3634. [PubMed] [Google Scholar]
- Ishii T., Ishida T., Utsunomiya A., Inagaki A., Yano H., Komatsu H., Iida S., Imada K., Uchiyama T., Akinaga S., Shitara K., Ueda R. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin. Cancer Res. 2010;16:1520–1531. doi: 10.1158/1078-0432.CCR-09-2697. [DOI] [PubMed] [Google Scholar]
- Jopling L.A., Sabroe I., Andrew D.P., Mitchell T.J., Li Y., Hodge M.R., Williams T.J., Pease J.E. The identification, characterization, and distribution of guinea pig CCR4 and epitope mapping of a blocking antibody. J. Biol. Chem. 2002;277:6864–6873. doi: 10.1074/jbc.M109974200. [DOI] [PubMed] [Google Scholar]
- Kakinuma T., Nakamura K., Wakugawa M., Mitsui H., Tada Y., Saeki H., Torii H., Asahina A., Onai N., Matsushima K., Tamaki K. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J. Allergy Clin. Immunol. 2001;107:535–541. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- Kawasaki S., Takizawa H., Yoneyama H., Nakayama T., Fujisawa R., Izumizaki M., Imai T., Yoshie O., Homma I., Yamamoto K., Matsushima K. Intervention of thymus and activation-regulated chemokine attenuates the development of allergic airway inflammation and hyperresponsiveness in mice. J. Immunol. 2001;166:2055–2062. doi: 10.4049/jimmunol.166.3.2055. [DOI] [PubMed] [Google Scholar]
- Kay A.B., Ali F.R., Heaney L.G., Benyahia F., Soh C.P.C., Renz H., Lee T.H., Larche M. Airway expression of calcitonin gene-related peptide in T-cell peptide-induced late asthmatic reactions in atopics. Allergy. 2007;62:495–503. doi: 10.1111/j.1398-9995.2007.01342.x. [DOI] [PubMed] [Google Scholar]
- Kenakin T., Miller L.J. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T.P. Biased signalling and allosteric machines: new vistas and challenges for drug discovery. Br. J. Pharmacol. 2012;165:1659–1669. doi: 10.1111/j.1476-5381.2011.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout T.A., Nicholas S.L., Perry S.J., Reinhart G., Junger S., Struthers R.S. Differential desensitization, receptor phosphorylation, beta-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J. Biol. Chem. 2004;279:23214–23222. doi: 10.1074/jbc.M402125200. [DOI] [PubMed] [Google Scholar]
- Kou K., Aihara M., Matsunaga T., Chen H., Taguri M., Morita S., Fujita H., Yamaguchi Y., Kambara T., Ikezawa Z. Association of serum interleukin-18 and other biomarkers with disease severity in adults with atopic dermatitis. Arch. Dermatol. Res. 2012;304:305–312. doi: 10.1007/s00403-011-1198-9. [DOI] [PubMed] [Google Scholar]
- Kowalska M.A., Ratajczak M.Z., Majka M., Jin J., Kunapuli S., Brass L., Poncz M. Stromal cell-derived factor-1 and macrophage-derived chemokine: 2 chemokines that activate platelets. Blood. 2000;96:50–57. [PubMed] [Google Scholar]
- Kuhn C.F., Bazin M., Philippe L., Zhang J., Tylaska L., Miret J., Bauer P.H. Bipiperidinyl carboxylic acid amides as potent, selective, and functionally active CCR4 antagonists. Chem. Biol. Drug Des. 2007;70:268–272. doi: 10.1111/j.1747-0285.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- Lagane B., Chow K.Y.C., Balabanian K., Levoye A., Harriague J., Planchenault T., Baleux F., Gunera-Saad N., Arenzana-Seisdedos F., Bachelerie F. CXCR4 dimerization and beta-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood. 2008;112:34–44. doi: 10.1182/blood-2007-07-102103. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R.J. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R.J., Shenoy S.K. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Lemmon M.A. Pleckstrin homology (PH) domains and phosphoinositides. Biochem. Soc. Symp. 2007:81–93. doi: 10.1042/BSS0740081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-Y., Ou Z.-L., Yu S.-J., Gu X.-L., Yang C., Chen A.-X., Di G.-H., Shen Z.-Z., Shao Z.-M. The chemokine receptor CCR4 promotes tumor growth and lung metastasis in breast cancer. Breast Cancer Res. Treat. 2012;131:837–848. doi: 10.1007/s10549-011-1502-6. [DOI] [PubMed] [Google Scholar]
- Lim H.W., Lee J., Hillsamer P., Kim C.H. Human Th17 cells share major trafficking receptors with both polarized effector T cells and FOXP3+ regulatory T cells. J. Immunol. 2008;180:122–129. doi: 10.4049/jimmunol.180.1.122. [DOI] [PubMed] [Google Scholar]
- Liu Q., Rexiati M., Yang Y., Wang W.-G., Azhati B., SaiMaiti W., Wang Y.-J. Expression of chemokine receptor 4 was associated with poor survival in renal cell carcinoma. Med. Oncol. 2014;31:882. doi: 10.1007/s12032-014-0882-y. [DOI] [PubMed] [Google Scholar]
- Lloyd C.M., Delaney T., Nguyen T., Tian J., Martinez-A C., Coyle A.J., Gutiérrez-Ramos J.C. CC chemokine receptor (CCR) 3/eotaxin is followed by CCR4/monocyte-derived chemokine in mediating pulmonary T helper lymphocyte type 2 recruitment after serial antigen challenge in vivo. J. Exp. Med. 2000;191:265–274. doi: 10.1084/jem.191.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur R.D., Novak R.M. Reviews of anti-infective agents: maraviroc: the first of a new class of antiretroviral agents. Clin. Infect. Dis. 2008;47:236–241. doi: 10.1086/589289. [DOI] [PubMed] [Google Scholar]
- Mantovani A. The chemokine system: redundancy for robust outputs. Immunol. Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- Mariani M., Lang R., Binda E., Panina-Bordignon P., D'ambrosio D. Dominance of CCL22 over CCL17 in induction of chemokine receptor CCR4 desensitization and internalization on human Th2 cells. Eur. J. Immunol. 2004;34:231–240. doi: 10.1002/eji.200324429. [DOI] [PubMed] [Google Scholar]
- McDermott D.H., Liu Q., Ulrick J., Kwatemaa N., Anaya-O'Brien S., Penzak S.R., Filho J.O., Priel D.A.L., Kelly C., Garofalo M., Littel P., Marquesen M.M., Hilligoss D., DeCastro R., Fleisher T.A., Kuhns D.B., Malech H.L., Murphy P.M. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood. 2011;118:4957–4962. doi: 10.1182/blood-2011-07-368084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhak Z., Fukui M., Farsidjani A., Medoff B.D., Tager A.M., Luster A.D. Contribution of CCR4 and CCR8 to antigen-specific T(H)2 cell trafficking in allergic pulmonary inflammation. J. Allergy Clin. Immunol. 2009;123:67–73. doi: 10.1016/j.jaci.2008.09.049. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A.J., Symon F.A., Berry M.A., Pavord I.D., Corrigan C.J., Wardlaw A.J. IL-4-expressing bronchoalveolar T cells from asthmatic and healthy subjects preferentially express CCR 3 and CCR 4. J. Allergy Clin. Immunol. 2005;116:594–600. doi: 10.1016/j.jaci.2005.03.052. [DOI] [PubMed] [Google Scholar]
- Nakagami Y., Kawase Y., Yonekubo K., Nosaka E., Etori M., Takahashi S., Takagi N., Fukuda T., Kuribayashi T., Nara F., Yamashita M. RS-1748, a novel CC chemokine receptor 4 antagonist, inhibits ovalbumin-induced airway inflammation in guinea pigs. Biol. Pharm. Bull. 2010;33:1067–1069. doi: 10.1248/bpb.33.1067. [DOI] [PubMed] [Google Scholar]
- Nakagami Y., Kawashima K., Etori M., Yonekubo K., Suzuki C., Jojima T., Kuribayashi T., Nara F., Yamashita M. A novel CC chemokine receptor 4 antagonist RS-1269 inhibits ovalbumin-induced ear swelling and lipopolysaccharide-induced endotoxic shock in mice. Basic Clin. Pharmacol. Toxicol. 2010;107:793–797. doi: 10.1111/j.1742-7843.2010.00578.x. [DOI] [PubMed] [Google Scholar]
- Nakagami Y., Kawashima K., Yonekubo K., Etori M., Jojima T., Miyazaki S., Sawamura R., Hirahara K., Nara F., Yamashita M. Novel CC chemokine receptor 4 antagonist RS-1154 inhibits ovalbumin-induced ear swelling in mice. Eur. J. Pharmacol. 2009;624:38–44. doi: 10.1016/j.ejphar.2009.09.058. [DOI] [PubMed] [Google Scholar]
- Nakagawa M., Schmitz R., Xiao W., Goldman C.K., Xu W., Yang Y., Yu X., Waldmann T.A., Staudt L.M. Gain-of-function CCR4 mutations in adult T cell leukemia/lymphoma. J. Exp. Med. 2014;211:2497–2505. doi: 10.1084/jem.20140987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T., Hieshima K., Arao T., Jin Z., Nagakubo D., Shirakawa A.-K., Yamada Y., Fujii M., Oiso N., Kawada A., Nishio K., Yoshie O. Aberrant expression of Fra-2 promotes CCR4 expression and cell proliferation in adult T-cell leukemia. Oncogene. 2007;27:3221–3232. doi: 10.1038/sj.onc.1210984. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Higuchi T., Oiso N., Kawada A., Yoshie O. Expression and function of FRA2/JUND in cutaneous T-cell lymphomas. Anticancer Res. 2012;32:1367–1373. [PubMed] [Google Scholar]
- Niwa R., Shoji-Hosaka E., Sakurada M., Shinkawa T., Uchida K., Nakamura K., Matsushima K., Ueda R., Hanai N., Shitara K. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004;64:2127–2133. doi: 10.1158/0008-5472.can-03-2068. [DOI] [PubMed] [Google Scholar]
- Ogura M., Ishida T., Hatake K., Taniwaki M., Ando K., Tobinai K., Fujimoto K., Yamamoto K., Miyamoto T., Uike N., Tanimoto M., Tsukasaki K., Ishizawa K., Suzumiya J., Inagaki H., Tamura K., Akinaga S., Tomonaga M., Ueda R. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J. Clin. Oncol. 2014;32:1157–1163. doi: 10.1200/JCO.2013.52.0924. [DOI] [PubMed] [Google Scholar]
- Ohyama Y., Kumode T., Eguchi G., Yamaguchi T., Maeda Y. Induction of molecular remission by using anti-CC-chemokine receptor 4 (anti-CCR4) antibodies for adult T cell leukemia: a risk of opportunistic infection after treatment with anti-CCR4 antibodies. Ann. Hematol. 2014;93:169–171. doi: 10.1007/s00277-013-1765-6. [DOI] [PubMed] [Google Scholar]
- Otero C., Groettrup M., Legler D.F. Opposite fate of endocytosed CCR7 and its ligands: recycling versus degradation. J. Immunol. 2006;177:2314–2323. doi: 10.4049/jimmunol.177.4.2314. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P., Papi A., Mariani M., Di Lucia P., Casoni G., Bellettato C., Buonsanti C., Miotto D., Mapp C., Villa A., Arrigoni G., Fabbri L.M., Sinigaglia F. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J. Clin. Invest. 2001;107:1357–1364. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease J.E. Targeting chemokine receptors in allergic disease. Biochem. J. 2011;434:11–24. doi: 10.1042/BJ20101132. [DOI] [PubMed] [Google Scholar]
- Pease J.E., Horuk R. Recent progress in the development of antagonists to the chemokine receptors CCR3 and CCR4. Exp. Opin. Drug Discov. 2014;9:467–483. doi: 10.1517/17460441.2014.897324. [DOI] [PubMed] [Google Scholar]
- Penela P., Nogués L., Mayor F. Role of G protein-coupled receptor kinases in cell migration. Curr. Opin. Cell Biol. 2014;27:10–17. doi: 10.1016/j.ceb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Perros F., Hoogsteden H.C., Coyle A.J., Lambrecht B.N., Hammad H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64:995–1002. doi: 10.1111/j.1398-9995.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- Pilette C., Francis J.N., Till S.J., Durham S.R. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur. Respir. J. 2004;23:876–884. doi: 10.1183/09031936.04.00102504. [DOI] [PubMed] [Google Scholar]
- Poulin B., Butcher A., McWilliams P., Bourgognon J.-M., Pawlak R., Kong K.C., Bottrill A., Mistry S., Wess J., Rosethorne E.M., Charlton S.J., Tobin A.B. The M3-muscarinic receptor regulates learning and memory in a receptor phosphorylation/arrestin-dependent manner. Proc. Natl. Acad. Sci. 2010;107:9440–9445. doi: 10.1073/pnas.0914801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C.A., Meyer A., Nemeth K., Bacon K.B., Hoogewerf A.J., Proudfoot A.E., Wells T.N. Molecular cloning and functional expression of a novel CC chemokine receptor cDNA from a human basophilic cell line. J. Biol. Chem. 1995;270:19495–19500. doi: 10.1074/jbc.270.33.19495. [DOI] [PubMed] [Google Scholar]
- Procopiou P.A., Barrett J.W., Barton N.P., Begg M., Clapham D., Copley R.C.B., Ford A.J., Graves R.H., Hall D.A., Hancock A.P., Hill A.P., Hobbs H., Hodgson S.T., Jumeaux C., Lacroix Y.M.L., Miah A.H., Morriss K.M.L., Needham D., Sheriff E.B., Slack R.J., Smith C.E., Sollis S.L., Staton H. Synthesis and Structure–Activity Relationships of Indazole Arylsulfonamides as Allosteric CC-Chemokine Receptor 4 (CCR4) Antagonists. J. Med. Chem. 2013;56:1946–1960. doi: 10.1021/jm301572h. [DOI] [PubMed] [Google Scholar]
- Procopiou P.A., Ford A.J., Graves R.H., Hall D.A., Hodgson S.T., Lacroix Y.M.L., Needham D., Slack R.J. Lead optimisation of the N1 substituent of a novel series of indazole arylsulfonamides as CCR4 antagonists and identification of a candidate for clinical investigation. Bioorg. Med. Chem. Lett. 2012;22:2730–2733. doi: 10.1016/j.bmcl.2012.02.104. [DOI] [PubMed] [Google Scholar]
- Purandare A.V., Somerville J.E. Antagonists of CCR4 as immunomodulatory agents. Curr. Top Med. Chem. 2006;6:1335–1344. doi: 10.2174/15680266106061335. [DOI] [PubMed] [Google Scholar]
- Purandare A.V., Wan H., Somerville J.E., Burke C., Vaccaro W., Yang X., McIntyre K.W., Poss M.A. Core exploration in optimization of chemokine receptor CCR4 antagonists. Bioorg. Med. Chem. Lett. 2007;17:679–682. doi: 10.1016/j.bmcl.2006.10.091. [DOI] [PubMed] [Google Scholar]
- Rahmeh R., Damian M., Cottet M., Orcel H., Mendre C., Durroux T., Sharma K.S., Durand G., Pucci B., Trinquet E., Zwier J.M., Deupi X., Bron P., Banères J.-L., Mouillac B., Granier S. Structural insights into biased G protein-coupled receptor signaling revealed by fluorescence spectroscopy. Proc. Natl. Acad. Sci. 2012;109:6733–6738. doi: 10.1073/pnas.1201093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss Y., Proudfoot A.E., Power C.A., Campbell J.J., Butcher E.C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosethorne E.M., Charlton S.J. Agonist-biased signaling at the histamine H4 receptor: JNJ7777120 recruits β-arrestin without activating G proteins. Mol. Pharmacol. 2011;79:749–757. doi: 10.1124/mol.110.068395. [DOI] [PubMed] [Google Scholar]
- Saito K., Torii M., Ma N., Tsuchiya T., Wang L., Hori T., Nagakubo D., Nitta N., Kanegasaki S., Hieshima K., Yoshie O., Gabazza E.C., Katayama N., Shiku H., Kuribayashi K., Kato T. Differential regulatory function of resting and preactivated allergen-specific CD4+ CD25+ regulatory T cells in Th2-type airway inflammation. J. Immunol. 2008;181:6889–6897. doi: 10.4049/jimmunol.181.10.6889. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Mackay C.R., Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Iwase M., Miyama M., Komai M., Ohshima E., Asai A., Yano H., Miki I. Internalization of CCR4 and inhibition of chemotaxis by K777, a potent and selective CCR4 antagonist. Pharmacology. 2013;91:305–313. doi: 10.1159/000350390. [DOI] [PubMed] [Google Scholar]
- Saulière A., Bellot M., Paris H., Denis C., Finana F., Hansen J.T., Altié M.-F., Seguelas M.-H., Pathak A., Hansen J.L., Sénard J.-M., Galés C. Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat. Chem. Biol. 2012;8:622–630. doi: 10.1038/nchembio.961. [DOI] [PubMed] [Google Scholar]
- Sekiya T., Miyamasu M., Imanishi M., Yamada H., Nakajima T., Yamaguchi M., Fujisawa T., Pawankar R., Sano Y., Ohta K., Ishii A., Morita Y., Yamamoto K., Matsushima K., Yoshie O., Hirai K. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J. Immunol. 2000;165:2205–2213. doi: 10.4049/jimmunol.165.4.2205. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Takehara K., Sato S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J. Dermatol. Sci. 2004;34:201–208. doi: 10.1016/j.jdermsci.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Slack R.J., Russell L.J., Barton N.P., Weston C., Nalesso G., Thompson S.-A., Allen M., Chen Y.H., Barnes A., Hodgson S.T., Hall D.A. Antagonism of human CC-chemokine receptor 4 can be achieved through three distinct binding sites on the receptor. Pharmacol. Res. Perspect. 2013;1:e00019. doi: 10.1002/prp2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari R., Pease J.E., Begg M. Eur. J. Pharmacol. 2014:1–5. doi: 10.1016/j.ejphar.2014.06.060. [DOI] [PubMed] [Google Scholar]
- Symons M., Derry J.M., Karlak B., Jiang S., Lemahieu V., Mccormick F., Francke U., Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- Tang H.L., Cyster J.G. Chemokine Up-regulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819–822. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- Trifari S., Kaplan C.D., Tran E.H., Crellin N.K., Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat. Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- Vestergaard C., Bang K., Gesser B., Yoneyama H., Matsushima K., Larsen C.G. A Th2 chemokine, TARC, produced by keratinocytes may recruit CLA+CCR4+ lymphocytes into lesional atopic dermatitis skin. J. Investig. Dermatol. 2000;115:640–646. doi: 10.1046/j.1523-1747.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- Vijayanand P., Durkin K., Hartmann G., Morjaria J., Seumois G., Staples K.J., Hall D., Bessant C., Bartholomew M., Howarth P.H., Friedmann P.S., Djukanovic R. Chemokine receptor 4 plays a key role in T Cell recruitment into the airways of asthmatic patients. J. Immunol. 2010;184:4568–4574. doi: 10.4049/jimmunol.0901342. [DOI] [PubMed] [Google Scholar]
- Viney J.M., Andrew D.P., Phillips R.M., Meiser A., Patel P., Lennartz-Walker M., Cousins D.J., Barton N.P., Hall D.A., Pease J.E. Distinct conformations of the chemokine receptor CCR4 with implications for its targeting in allergy. J. Immunol. 2014;192:3419–3427. doi: 10.4049/jimmunol.1300232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A., Luster A.D. Chemokines and their receptors: drug targets in immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- Wakugawa M., Nakamura K., Kakinuma T., Onai N., Matsushima K., Tamaki K. CC chemokine receptor 4 expression on peripheral blood CD4+ T cells reflects disease activity of atopic dermatitis. J. Investig. Dermatol. 2001;117:188–196. doi: 10.1046/j.0022-202x.2001.01430.x. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Natori K., Tamari M., Shiomoto Y., Kubo S., Nakamura Y. Significantly elevated expression of PF4 (platelet factor 4) and eotaxin in the NOA mouse, a model for atopic dermatitis. J. Hum. Genet. 1999;44:173–176. doi: 10.1007/s100380050136. [DOI] [PubMed] [Google Scholar]
- Weiner O.D., Neilsen P.O., Prestwich G.D., Kirschner M.W., Cantley L.C., Bourne H.R. A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 2002;4:509–513. doi: 10.1038/ncb811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Utsunomiya A., Tobinai K., Tsukasaki K., Uike N., Uozumi K., Yamaguchi K., Yamada Y., Hanada S., Tamura K., Nakamura S., Inagaki H., Ohshima K., Kiyoi H., Ishida T., Matsushima K., Akinaga S., Ogura M., Tomonaga M., Ueda R. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J. Clin. Oncol. 2010;28:1591–1598. doi: 10.1200/JCO.2009.25.3575. [DOI] [PubMed] [Google Scholar]
- Yamanaka K.-I., Mizutani H. The role of cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr. Probl. Dermatol. 2011;41:80–92. doi: 10.1159/000323299. [DOI] [PubMed] [Google Scholar]
- Yang Y.-M., Feng A.-L., Zhou C.-J., Liang X.-H., Mao H.-T., Deng B.-P., Yan S., Sun J.-T., Du L.-T., Liu J., Wang Q.-J., Neckenig M.R., Yang Q.-F., Qu X. Aberrant expression of chemokine receptor CCR4 in human gastric cancer contributes to tumor-induced immunosuppression. Cancer Sci. 2011;102:1264–1271. doi: 10.1111/j.1349-7006.2011.01934.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Ishikawa N., Igarashi S., Kawano N., Masuda N., Hamaguchi W., Yamasaki S., Koganemaru Y., Hattori K., Miyazaki T., Ogino S.-I., Matsumoto Y., Takeuchi M., Ohta M. Potent and orally bioavailable CCR4 antagonists: synthesis and structure-activity relationship study of 2-aminoquinazolines. Bioorganic Med. Chem. 2009;17:64–73. doi: 10.1016/j.bmc.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Yokoyama K., Ishikawa N., Igarashi S., Kawano N., Masuda N., Hattori K., Miyazaki T., Ogino S.-I., Orita M., Matsumoto Y., Takeuchi M., Ohta M. Potent CCR4 antagonists: synthesis, evaluation, and docking study of 2,4-diaminoquinazolines. Bioorganic Med. Chem. 2008;16:7968–7974. doi: 10.1016/j.bmc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- Yoshie O., Fujisawa R., Nakayama T., Harasawa H., Tago H., Izawa D., Hieshima K., Tatsumi Y., Matsushima K., Hasegawa H., Kanamaru A., Kamihira S., Yamada Y. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99:1505–1511. doi: 10.1182/blood.v99.5.1505. [DOI] [PubMed] [Google Scholar]
- Zhao F., Xiao J.H., Wang Y., Li S. Synthesis of thiourea derivatives as CCR4 antagonists. Chin. Chem. Lett. 2009;20:296–299. [Google Scholar]
- Zidar D.A., Violin J.D., Whalen E.J., Lefkowitz R.J. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc. Natl. Acad. Sci. USA. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36:705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]