Abstract
G-protein-coupled receptors (GPCRs) comprise a large family of cell-surface receptors, which have recently emerged as key players in tumorigenesis, angiogenesis and metastasis. In this review, we discussed our current understanding of the many roles played by GPCRs in general, and particularly Angiotensin II type I receptor (AGTR1), a member of the seven-transmembrane-spanning G-protein coupled receptor superfamily, and its significance in breast cancer progression and metastasis. We have also discussed different strategies for targeting AGTR1, and its ligand Angiotension II (Ang II), which might unravel unique opportunities for breast cancer prevention and treatment. For example, AGTR1 blockers (ARBs) which are already in clinical use for treating hypertension, merit further investigation as a therapeutic strategy for AGTR1-positive cancer patients and may have the potential to prevent Ang II-AGTR1 signalling mediated cancer pathogenesis and metastases.
Keywords: Breast cancer, GPCR, AGTR1, ACE, Metastases
1. Introduction
G-protein-coupled receptors (GPCRs), the largest family of cell-surface receptors has been known to play a critical role in the neoplastic transformation of many cancers including hormone-refractory cancers of breast and prostate. Some of the key functions of GPCRs include regulation of cellular motility, growth and differentiation, which play an important role in understanding the biology of cancer (Spiegelberg and Hamm, 2007). In 1986, the discovery of the MAS oncogene, which encodes a typical GPCR, established a direct connection between neoplastic transformation and GPCRs (Young et al., 1986). Many independent studies have shown that GPCRs are over-expressed in variety of cancer types, and contribute in cell proliferation when activated by their respective circulating or locally available ligands (Even-Ram et al., 1998, Rhodes et al., 2009). Furthermore, wild-type GPCRs could also become oncogenic when exposed to an excess of locally produced or circulating agonists such as gastrin-releasing peptide (GRP), endothelin, bradykinin and Ang II (Gutkind et al., 1991, Julius et al., 1989); in addition mutations in the conserved domain of the GPCRs could also trigger oncogenic transformation (Allen et al., 1991). Moreover, the activation of endothelin receptors, bradykinin receptors, the Angiotensin II type I receptors (AGTR1) (de Gasparo et al., 2000, Rhodes et al., 2009) and gastrin-releasing peptide receptors (GRPR) has been implicated in prostate cancer pathogenesis (Daaka, 2004).
2. G-protein coupled receptors in breast cancer
The role of GPCRs in breast cancer (BCa) has been explored extensively, for example protease-activated receptor 1 (PAR1) is over-expressed in BCa and is responsible for development of metastases in BCa patients (Hernandez et al., 2009). PAR1 is also known to promote growth and invasion by promoting detachment and migration of the epithelial cancer cells, which is a key step in tumour metastases (Boire et al., 2005, Hernandez et al., 2009). PAR1 also couples to multiple G-proteins (Gq/11, Gi/o, G12/13) and activates Rho signalling (McCoy et al., 2012), thereby resulting in changes in cytoskeleton structure and cell shape (Austin et al., 2013), suggesting its critical role in BCa metastases. Another GPCR, GPR116, plays an important role in cell adhesion and is found to be a novel regulator of BCa metastasis. GPR116 regulates morphology and cell motility through the Gαq-p63RhoGEF-RhoA/Rac1 pathway. Activated Rho GTPases are known to induce many downstream signalling pathways such as ROCK1/2 during cancer cell migration. Specifically, p63RhoGEF (GEFT), a guanine nucleotide exchange factor (GEF) acts as an effector of the guanine-nucleotide binding protein Gαq, thus linking GPCRs to the activation of the Rho-GTPases. Knockdown of GPR116 in MDA-MB-231, a triple negative, hormone-insensitive and metastatic breast cancer cell line results in significant decrease in cell migration and invasion, suggesting that GPR116 promotes BCa cell migration and invasion via Gαq signalling and p63RhoGEF (a Gαq effector) mediated activation of the RhoA and Rac1 (Tang et al., 2013). Taken together, we speculate that targeting both PAR1 and GPR116 in triple negative breast cancer (TNBC) may hold great promise in targeting these pathways and combating hormone-refractory breast cancer.
Nevertheless, orphan GPCRs represent a highly active area of research that has already led to the identification of many new ligands for previously orphaned GPCRs. One such orphan GPCR is GPR161, a class A rhodopsin family member. GPR161 was found to be overexpressed specifically in TNBC and is also correlated with poor prognosis. Importantly, knockdown of the GPR161 in basal breast cancer cell lines demonstrated inhibition in cell proliferation. GPR161 forms a signalling complex with two scaffold proteins, namely β-arrestin 2 and Ile Gln motif containing GTPase Activating Protein 1 (IQGAP1). Cells overexpressing GPR161 activate mammalian target of rapamycin (mTOR) signalling thereby decreasing IQGAP1 phosphorylation. Conversely, unphosphorylated IQGAP1 binds mTOR which leads to the activation of downstream signals, one of which is phosphorylation of S6, a ribosomal protein (Feigin et al., 2014). Taken together, we anticipate that tumour-specific gene expression and proteome profiles of the tumour tissues and premalignant lesions, in combination with ‘reverse pharmacology’ techniques, will aid in identifying and targeting new orphan GPCRs that may contribute to cancer initiation, progression and metastases.
Another interesting member of the seven-transmembrane-spanning G-protein coupled receptor superfamily is AGTR1, which was prioritized as second ranked meta-outlier by employing a bioinformatics tool named meta-Cancer Outlier Profile Analysis (MetaCOPA) using multiple independent breast cancer profiling studies (Rhodes et al., 2009). As anticipated, HER2/neu was identified as the most significant meta-outlier and AGTR1 as the second most consistently high-scoring gene in BCa, which is also known for its role in Ang II‐dependent vasoconstriction (Luft, 2001, Schmieder et al., 2007). While AGTR1 is found in a variety of normal tissues, increased expression is often observed in the corresponding neoplastic tissues, suggesting that it’s over-expression is involved in carcinogenesis (Marsigliante et al., 1996, Takeda and Kondo, 2001). AGTR1 has also been linked to pancreatic, renal and ovarian cancers (Fujimoto et al., 2001, Miyajima et al., 2002, Rivera et al., 2001, Suganuma et al., 2005, Timmermans, 1999, Uemura et al., 2003) and cancer-related signalling pathways (Amaya et al., 2004, Muscella et al., 2003). AGTR1 is over-expressed in oestrogen receptor positive (ER+) tumours and mutually exclusive with HER2/neu, indicating the possibility that over-expression of these two genes may represent alternative pathways in breast cancer pathogenesis (Ateeq et al., 2009, Rhodes et al., 2009). Most importantly, AGTR1 mediated oncogenic effects could be antagonized by commonly prescribed antihypertensive agents, such as losartan (Rhodes et al., 2009, Timmermans, 1999). It is interesting to note that the BCa prevalence was reported low in hypertensive patients who received angiotensin converting enzyme inhibitors (ACEi) previously, which blocks the conversion of Ang I to Ang II, thereby reducing activation of AGTR1 by Ang II (Lever et al., 1998). Similarly, ARBs have been reported to inhibit cell proliferation and angiogenesis in prostate cancer cells (Uemura et al., 2003). Ang II mediates its complex physiological effects by binding to two pharmacologically distinct receptors; AGTR1 and Angiotensin II Type 2 Receptor (AGTR2) (Timmermans et al., 1992). The stimulatory actions of Ang II on angiogenesis, cell growth, and cell proliferation in tissues are mediated via AGTR1 (De Paepe et al., 2001, Egami et al., 2003) and opposed via AGTR2 (Goto et al., 2002, Silvestre et al., 2002). Moreover, Ang II activates AGTR1, which couples to the heterotrimeric G proteins Gq/11 (to stimulate phospholipase C mediated calcium mobilization), Gi/o, G12/13 and Gs, as well as the other monomeric G proteins (de Gasparo et al., 2000). It has been shown that Arhgef1, a RhoA guanine exchange factor is specifically responsible for Ang II-induced activation of RhoA signalling and as a result Jak2 phosphorylates Tyr738 of Arhgef1 (Guilluy et al., 2010). In addition, activated AGTR1 also activates soluble and receptor tyrosine kinases, the mitogen-activated protein kinases (MAPK pathway), the JAK–STAT pathway, the generation of reactive oxygen species and various ion channels (de Gasparo et al., 2000, Hunyady and Catt, 2006, Mehta and Griendling, 2007). Taken together, AngII–AGTR1 signalling pathways play a critical role in the pathogenesis of AGTR1-positive breast and prostate cancer.
3. Single nucleotide polymorphisms in AngII–AGTR1 pathway
Recent genome-wide association studies have revolutionized the field of cancer research and led to the identification of numerous single nucleotide polymorphism (SNP), which are associated with increased risk for breast cancer (Easton et al., 2007). For example, germline mutations in BRCA1 and BRCA2 could predispose women to BCa, as well as to ovarian cancer (King et al., 2003). Somatic mutations in other genes, such as p53, PTEN, or CHEK2, are also associated with increased risk of BCa (Hirshfield et al., 2010, Weischer et al., 2008). Moreover, an association between the genetic polymorphisms in the 5′ region of AGTR1 and the increased risk of BCa has been reported among Chinese women. This study also revealed three genetic polymorphisms A168G, C535T, T825A in the 5′ region of AGTR1. Individuals harbouring genotypes with one or two copies of these allelic variants were found to be associated with 30% lower risk of BCa as compared to the homozygotes (Koh et al., 2005). Conversely, another independent study showed no significant association between A168G polymorphism of AGTR1 and BCa risk, but demonstrated the significance of AGTR2 SNPs (T1247G and A5235G) as a predictor of BCa in Brazilian women (Molina Wolgien Mdel et al., 2014). Nevertheless, deletion of the 5′ flanking region of AGTR1 showed 20-fold increase in chloramphenicol acetyltransferase reporter activity, thus confirming the presence of a negative regulatory element(s) in the upstream region of AGTR1 (Takayanagi et al., 1994). These observations indicate that the genetic variants in the 5′ flaking region of AGTR1 might be associated with an increase in breast cancer risk. Furthermore, increased frequency of a SNP at 1166 position (A/C transversion) in the 3′ UTR of AGTR1 has been associated with hypertension (Bonnardeaux et al., 1994), cardiac hypertrophy (Osterop et al., 1998), myocardial infarction (Tiret et al., 1994) and increased oxidative stress levels in human heart failure (Cameron et al., 2006).
An association between Angiotensin I converting enzyme (ACE), which converts Ang I into a physiologically active form Ang II and BCa risk has been demonstrated (Lever et al., 1998). The SNP of ACE (A240T and I/D) regulates its level in the plasma, for example homozygotic individuals for D or T alleles have higher ACE levels than in the homozygotic individuals for I or A alleles. Therefore, the individuals with ACE genotype (II or AA) have a lower risk for BCa in comparison to the ones with high activity (DD or TT) alleles (Koh et al., 2003, Koh et al., 2005). Furthermore, a SNP (A1166C) in the AGTR1 has been associated with higher tumour node metastases (TNM) stage of the BCa as compared to the individuals harbouring A1166A (Namazi et al., 2010). However, in a follow-up study, no association between this polymorphism and three years disease free survival was found (Namazi et al., 2013). Conversely, reduced plasma levels of the ACE were not always observed in the individuals with I or A allele (Freitas-Silva et al., 2004, Haiman et al., 2003), suggesting that the association of ACE genotype with BCa risk depends on the ethnicity of the population. We speculate that the genetic polymorphisms in AngII–AGTR1 pathway may have racial disparity. Therefore, additional studies exploring SNPs in the AngII–AGTR1 pathways are warranted on the populations of different ethnicities. Moreover, a population specific genetic profile could be created for evaluating cancer survival based on prognosis markers, which would eventually help in understanding the differences reported for the BCa incidence and outcomes, based on geography and ethnicity.
4. AngII–AGTR1 signalling mediated epithelial-to-mesenchymal transition
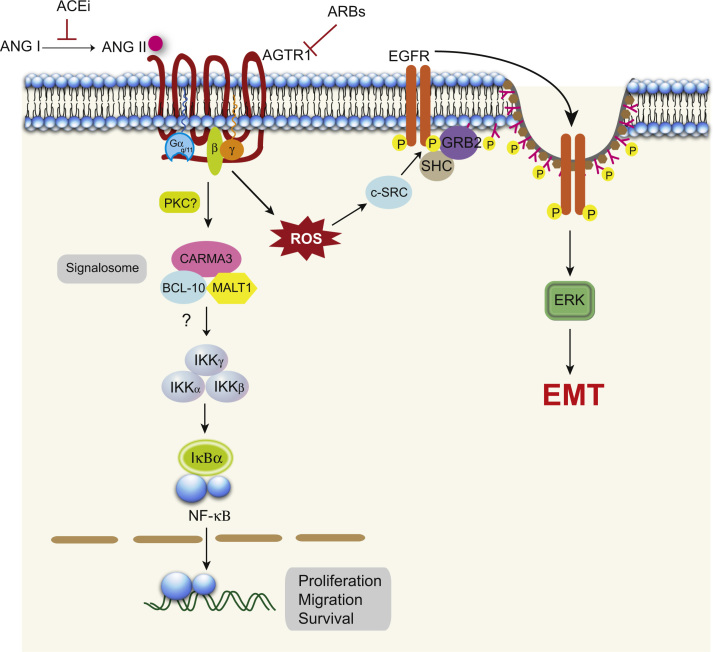
Various cellular responses such as cell proliferation, differentiation or dedifferentiation are triggered by a variety of external stimuli, which involves the transcriptional regulation in cancer cells through intracellular signalling cascades, including multitude of signalling pathways that activate kinases of the mitogen-activated protein kinase (MAPK) family (Treisman, 1996) either through receptor tyrosine kinase (RTK)- or through GPCR-triggered signals (Faure et al., 1994, Pages et al., 1993, van Biesen et al., 1996). It has been known that AGTR1 hijacks epidermal growth factor receptor (EGFR) signalling machinery, which is critical for the AGTR1 mediated downstream signalling and phenotypic effects, such as cellular hypertrophy and proliferation (Asakura et al., 2002, Eguchi et al., 2001, Mifune et al., 2005). Ang II-induced platelet derived growth factor receptor β (PDGFR-β) and thrombin stimulated insulin-like growth factor-1 receptor (IGF-1R) tyrosine phosphorylation have been reported in primary rat smooth muscle cells (Linseman et al., 1995, Rao et al., 1995), suggesting that transactivation of distinct RTKs might contribute in a cell-type specific manner to GPCR mediated mitogenic signalling. Moreover, Ang II-activated EGFR signalling in renal proximal tubule epithelial cells results mostly by the non-ligand-mediated receptor transactivation mediated by ROS-dependant Src activation, which leads to phosphorylation of both EGFR and Caveolin-1 and their association in the lipid rafts (Fig. 1) (Chen et al., 2012, George et al., 2013). Thus, the constant activation of the EGFR serves as a scaffold for SHC/GRB2-mediated ERK activation, subsequently resulting in the dedifferentiation or epithelial-to-mesenchymal transition (EMT) of renal proximal tubule epithelial cells (Chen et al., 2012). These studies indicate that AngII–AGTR1 prolonged signalling activity in the AGTR1-positive cancers may lead to alterations in gene expression and consequently elicit a phenotypic change to EMT, which promotes aggressive phenotype and distant metastases. Interestingly, using functional siRNA screen of the human kinome, new signalling targets such as TRIO, BMX or CHKA have been revealed, which upon knockdown attenuate tyrosine phosphorylation of the EGFR by Ang II stimulation, but failed to directly stimulate EGFR via EGF, suggesting that these proteins are involved in AGTR1–EGFR transactivation (George et al., 2013). Nevertheless, a deeper and comprehensive understanding of AngII–AGTR1 axis and AGTR1–EGFR crosstalk in the context of AGTR1 positive cancers may direct future studies, which may lead to the development of novel drug targets against these pathways as an alternative to existing cancer therapies.
Fig. 1.
Major AGTR1 signalling pathways linked to cancer cell proliferation, angiogenesis and EMT. AngII activated AGTR1 recruits a CARMA3-Bcl10-MALT1 (CBM) signalosome, which activates NFκB downstream signalling. CARMA3 protein might act as a scaffold in recruiting Bcl 10, MALT 1 and IKKγ, the regulatory subunit of the IKK complex. Wherein MALT1 plays a key role in stimulating IKK activity by K63 linked polyubiquitination utilizing IKKγ as a substrate. The activation of this pathway leads to cell proliferation, survival and migration. AngII activation of AGTR1 also leads to EGFR transactivation via ROS‐ dependent Src kinase activation, phosphorylating EGFR and the adaptor proteins GRB2 and SHC, resulting in prolonged EGFR–ERK signalling. Continuous Ang II stimulation may direct alterations in the gene expression and induce phenotypic change from epithelial-to-mesenchymal transition (EMT).
5. Targeting AGTR1 for enhanced drug delivery and improved chemotherapy
Several FDA-approved ARBs, which are orally active have been synthesized and are widely prescribed for the treatment of hypertension, such as losartan, irbesartan, olmesartan, candesartan, valsartan and telmisartan. Previously, we have shown that ectopic over-expression of AGTR1 in immortalized normal breast epithelial cells, confers an invasive phenotype upon AngII stimulation, which was attenuated by losartan (Rhodes et al., 2009). Losartan has also been shown to inhibit many growth factors, including vascular endothelial growth factor (VEGF) (Arrieta et al., 2005). Interestingly, preclinical mice experiments with control or AGTR1 overexpressing breast cancer xenografts showed differential sensitivity to losartan treatment, resulting in 30% decrease in tumour growth in AGTR1 overexpressing group, whereas no effect was observed in the control group (Rhodes et al., 2009). Another ARB, candesartan has been reported to reduce lung metastases, vascularization and tumour growth in sarcomas and melanoma xenografts (Fujita et al., 2002; Egami et al., 2003). On the other hand, telmisartan which is a structurally unique ARB, renders more effective inhibition of the AGTR1 mediated pro-tumorigenic effects and the unique structural characteristics provide partial agonistic response for a member of nuclear receptor family peroxisome proliferator-activated receptor-γ (PPARγ) (Benson et al., 2004). Losartan and telmisartan, both demonstrate higher tissue penetration as compared to candesartan (Michel et al., 2013), which could be a possible reason for selecting Losartan over other ARBs for a pancreatic cancer clinical trial study (Chauhan et al., 2013).
AGTR1 employs the CARMA3-Bcl10-MALT1 (CBM) signalosome for the activation of NF-κB signalling in endothelial and vascular smooth muscle cells (VSMC), thus inducing pro-inflammatory signalling in the vasculature that may lead to atherosclerosis (McAllister-Lucas et al., 2010). Interestingly, Bcl10 deficient mice failed to develop Ang II‐dependent atherosclerotic lesions and abdominal aortic aneurisms (McAllister-Lucas et al., 2010). The decreased rate of arthrosclerosis has also been associated with decrease in the expression of NF-κB responsive genes (Surmi and Hasty, 2010). Interestingly, siRNA mediated knockdown of CARMA3 in AGTR1 over-expressing immortalized VSMC cell lines, showed no response to Ang II dependent ERK activation or TNFα‐dependent pIκB generation (McAllister-Lucas et al., 2010), suggesting that CARMA3 protein might act as a scaffold in recruiting Bcl10, MALT1 and IKKγ, the regulatory subunit of the IKK complex (Stilo et al., 2004). These observations gain much more importance in light of the recent discovery of an inhibitor of MALT1 protease, a component of the signalosome that is enzymatically active and communicates downstream with NF-κB signalling (Rebeaud et al., 2008). Hence we can infer that the CBM signalosome may play a major role in AGTR1 mediated breast cancer pathogenesis, and MALT1 or Bcl10 inhibitors might prove as promising targets.
On another note, proliferating cancer cells are known to consecutively create a new solid substance comprising cells and matrix components, which generate radial and circumferential solid stress (Kharaishvili et al., 2014). This stress in the growing tumour collapses blood vessels and limits perfusion resulting in extensive hypoxia and impaired drug delivery (Griffon-Etienne et al., 1999, Janmey and McCulloch, 2007, Padera et al., 2004). As a result, cancer patients with low tumour perfusion show poor chemotherapy responses and shorter survival versus patients with high perfusion (Park et al., 2009, Sorensen et al., 2011). Interestingly, AGTR1 inhibitors/antagonists have been known to increase vessel perfusion through vascular decompression, thereby reducing stromal activity and production of matrix components responsible for compression. Likewise, AGTR2 agonists or inhibitors of downstream signalling through TGF-β1, CCN2 or ET-1 have been known in reducing solid stress to enhance chemotherapy and overcome challenges associated with chemotherapies (Chauhan et al., 2013). Furthermore, AGTR1 signalling plays an important role in increasing VEGF expression by Cancer Associated Fibroblasts (CAFs) (Fujita et al., 2005), thus both ACE and AGTR1 inhibitors could be used to target VEGF expression and angiogenesis (Suganuma et al., 2005, Yoshiji et al., 2001).
ACE inhibitors (ACEi), which have been successfully used as antihypertensive drugs for the past 20 years, are now being investigated for their possible role as anticancer compounds (Lindberg et al., 2004). Interestingly, epidemiological study suggests that the long-term treatment of ACEi such as captopril, lisinopril and enalapril has reduced the incidence of lung and breast cancer (Lever et al., 1998). However, other epidemiological studies were not in concordance with these results and showed that ACEi treatment had no significant effect on cancer (Friis et al., 2001, Li et al., 2003, Lindholm et al., 2001). One possible explanation might be that the latter studies enroled older patients who underwent treatment for shorter duration. Moreover, use of different ACEi on diverse population of patients along with variability in the dosage, duration of drug prescription as well as patient compliance might be the possible reasons for the contradictory results (Deshayes and Nahmias, 2005). Taken together, ACE and AGTR1 blockers could be used as an adjuvant therapy along with established chemotherapeutic drugs to further potentiate the anti-cancer effects of the conventional cancer therapies. Specifically, AngII–AGTR1 axis could be further explored as a potential therapeutic target for treating AGTR1 positive cancers including AGTR1 and ER-positive BCa. However, in light of the observation of ACE and AGTR1 polymorphisms, more population specific studies need to be carried out to fully understand the role of ACEi and ARBs with respect to anticancer therapy, with an ultimate goal of designing the framework for clinical trials and developing tailored treatment plan for cancer patients.
Acknowledgements
B.A. is an Intermediate Fellow of the Wellcome Trust/ DBT India Alliance and a Young Investigator of the SERB DST-FAST Track scheme, India. This work is supported by the SERB DST-FAST Track Grant, India [SB/YS/LS-35/2013 to BA] and partially by the Wellcome Trust-DBT India Alliance Grant [IA/I(S)/12/2/500635 to BA]. AS is a recipient of the Junior Research Fellowship from the Indian Council of Medical Research, India (ICMR number: 3/1/3/JRF-2011/HRD-88). We thank Indian Institute of Technology, Kanpur for providing infra-structure support.
References
- Allen L.F., Lefkowitz R.J., Caron M.G., Cotecchia S. G-protein-coupled receptor genes as protooncogenes: constitutively activating mutation of the alpha 1B-adrenergic receptor enhances mitogenesis and tumorigenicity. Proc. Natl. Acad. Sci. USA. 1991;88:11354–11358. doi: 10.1073/pnas.88.24.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya K., Ohta T., Kitagawa H., Kayahara M., Takamura H., Fujimura T., Nishimura G., Shimizu K., Miwa K. Angiotensin II activates MAP kinase and NF-kappaB through angiotensin II type I receptor in human pancreatic cancer cells. Int. J. Oncol. 2004;25:849–856. [PubMed] [Google Scholar]
- Arrieta O., Guevara P., Escobar E., Garcia-Navarrete R., Pineda B., Sotelo J. Blockage of angiotensin II type I receptor decreases the synthesis of growth factors and induces apoptosis in C6 cultured cells and C6 rat glioma. Br. J. Cancer. 2005;92:1247–1252. doi: 10.1038/sj.bjc.6602483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura M., Kitakaze M., Takashima S., Liao Y., Ishikura F., Yoshinaka T., Ohmoto H., Node K., Yoshino K., Ishiguro H., Asanuma H., Sanada S., Matsumura Y., Takeda H., Beppu S., Tada M., Hori M., Higashiyama S. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- Ateeq B., Tomlins S.A., Chinnaiyan A.M. AGTR1 as a therapeutic target in ER-positive and ERBB2-negative breast cancer cases. Cell Cycle. 2009;8:3794–3795. doi: 10.4161/cc.8.23.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin K.M., Covic L., Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood. 2013;121:431–439. doi: 10.1182/blood-2012-09-355958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S.C., Pershadsingh H.A., Ho C.I., Chittiboyina A., Desai P., Pravenec M., Qi N., Wang J., Avery M.A., Kurtz T.W. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- Boire A., Covic L., Agarwal A., Jacques S., Sherifi S., Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Bonnardeaux A., Davies E., Jeunemaitre X., Fery I., Charru A., Clauser E., Tiret L., Cambien F., Corvol P., Soubrier F. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994;24:63–69. doi: 10.1161/01.hyp.24.1.63. [DOI] [PubMed] [Google Scholar]
- Cameron V.A., Mocatta T.J., Pilbrow A.P., Frampton C.M., Troughton R.W., Richards A.M., Winterbourn C.C. Angiotensin type-1 receptor A1166C gene polymorphism correlates with oxidative stress levels in human heart failure. Hypertension. 2006;47:1155–1161. doi: 10.1161/01.HYP.0000222893.85662.cd. [DOI] [PubMed] [Google Scholar]
- Chauhan V.P., Martin J.D., Liu H., Lacorre D.A., Jain S.R., Kozin S.V., Stylianopoulos T., Mousa A.S., Han X., Adstamongkonkul P., Popovic Z., Huang P., Bawendi M.G., Boucher Y., Jain R.K. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Chen J.K., Harris R.C. Angiotensin II induces epithelial-to-mesenchymal transition in renal epithelial cells through reactive oxygen species/Src/caveolin-mediated activation of an epidermal growth factor receptor-extracellular signal-regulated kinase signaling pathway. Mol. Cell. Biol. 2012;32:981–991. doi: 10.1128/MCB.06410-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daaka Y. G proteins in cancer: the prostate cancer paradigm. Sci. STKE: Signal Transduct. Knowl. Environ. 2004;2004:re2. doi: 10.1126/stke.2162004re2. [DOI] [PubMed] [Google Scholar]
- de Gasparo M., Catt K.J., Inagami T., Wright J.W., Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- De Paepe B., Verstraeten V.L., De Potter C.R., Vakaet L.A., Bullock G.R. Growth stimulatory angiotensin II type-1 receptor is upregulated in breast hyperplasia and in situ carcinoma but not in invasive carcinoma. Histochem. Cell Biol. 2001;116:247–254. doi: 10.1007/s004180100313. [DOI] [PubMed] [Google Scholar]
- Deshayes F., Nahmias C. Angiotensin receptors: a new role in cancer? Trends in endocrinology and metabolism. Trends Endocrinol. Metab. 2005;16:293–299. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Easton D.F., Pooley K.A., Dunning A.M., Pharoah P.D., Thompson D., Ballinger D.G., Struewing J.P., Morrison J., Field H., Luben R., Wareham N., Ahmed S., Healey C.S., Bowman R., Meyer K.B., Haiman C.A., Kolonel L.K., Henderson B.E., Le Marchand L., Brennan P., Sangrajrang S., Gaborieau V., Odefrey F., Shen C.Y., Wu P.E., Wang H.C., Eccles D., Evans D.G., Peto J., Fletcher O., Johnson N., Seal S., Stratton M.R., Rahman N., Chenevix-Trench G., Bojesen S.E., Nordestgaard B.G., Axelsson C.K., Garcia-Closas M., Brinton L., Chanock S., Lissowska J., Peplonska B., Nevanlinna H., Fagerholm R., Eerola H., Kang D., Yoo K.Y., Noh D.Y., Ahn S.H., Hunter D.J., Hankinson S.E., Cox D.G., Hall P., Wedren S., Liu J., Low Y.L., Bogdanova N., Schurmann P., Dork T., Tollenaar R.A., Jacobi C.E., Devilee P., Klijn J.G., Sigurdson A.J., Doody M.M., Alexander B.H., Zhang J., Cox A., Brock I.W., MacPherson G., Reed M.W., Couch F.J., Goode E.L., Olson J.E., Meijers-Heijboer H., van den Ouweland A., Uitterlinden A., Rivadeneira F., Milne R.L., Ribas G., Gonzalez-Neira A., Benitez J., Hopper J.L., McCredie M., Southey M., Giles G.G., Schroen C., Justenhoven C., Brauch H., Hamann U., Ko Y.D., Spurdle A.B., Beesley J., Chen X., Mannermaa A., Kosma V.M., Kataja V., Hartikainen J., Day N.E., Cox D.R., Ponder B.A. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egami K., Murohara T., Shimada T., Sasaki K., Shintani S., Sugaya T., Ishii M., Akagi T., Ikeda H., Matsuishi T., Imaizumi T. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J. Clin. Invest. 2003;112:67–75. doi: 10.1172/JCI16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi S., Dempsey P.J., Frank G.D., Motley E.D., Inagami T. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J. Biol. Chem. 2001;276:7957–7962. doi: 10.1074/jbc.M008570200. [DOI] [PubMed] [Google Scholar]
- Even-Ram S., Uziely B., Cohen P., Grisaru-Granovsky S., Maoz M., Ginzburg Y., Reich R., Vlodavsky I., Bar-Shavit R. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat. Med. 1998;4:909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- Faure M., Voyno-Yasenetskaya T.A., Bourne H.R. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J. Biol. Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- Feigin M.E., Xue B., Hammell M.C., Muthuswamy S.K. G-protein-coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc. Natl. Acad. Sci. USA. 2014;111:4191–4196. doi: 10.1073/pnas.1320239111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Silva M., Pereira D., Coelho C., Bicho M., Lopes C., Medeiros R. Angiotensin I-converting enzyme gene insertion/deletion polymorphism and endometrial human cancer in normotensive and hypertensive women. Cancer Genet. Cytogenet. 2004;155:42–46. doi: 10.1016/j.cancergencyto.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Friis S., Sorensen H.T., Mellemkjaer L., McLaughlin J.K., Nielsen G.L., Blot W.J., Olsen J.H. Angiotensin-converting enzyme inhibitors and the risk of cancer: a population-based cohort study in Denmark. Cancer. 2001;92:2462–2470. doi: 10.1002/1097-0142(20011101)92:9<2462::aid-cncr1596>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Fujimoto Y., Sasaki T., Tsuchida A., Chayama K. Angiotensin II type 1 receptor expression in human pancreatic cancer and growth inhibition by angiotensin II type 1 receptor antagonist. FEBS Lett. 2001;495:197–200. doi: 10.1016/s0014-5793(01)02377-8. [DOI] [PubMed] [Google Scholar]
- Fujita M., Hayashi I., Yamashina S., Fukamizu A., Itoman M., Majima M. Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis. 2005;26:271–279. doi: 10.1093/carcin/bgh324. [DOI] [PubMed] [Google Scholar]
- Fujita M., Hayashi I., Yamashina S., Itoman M., Majima M. Blockade of angiotensin AT1a receptor signaling reduces tumor growth, angiogenesis, and metastasis. Biochem. Biophys. Res. Commun. 2002;294:441–447. doi: 10.1016/S0006-291X(02)00496-5. [DOI] [PubMed] [Google Scholar]
- George A.J., Purdue B.W., Gould C.M., Thomas D.W., Handoko Y., Qian H., Quaife-Ryan G.A., Morgan K.A., Simpson K.J., Thomas W.G., Hannan R.D. A functional siRNA screen identifies genes modulating angiotensin II-mediated EGFR transactivation. J. Cell Sci. 2013;126:5377–5390. doi: 10.1242/jcs.128280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Mukoyama M., Sugawara A., Suganami T., Kasahara M., Yahata K., Makino H., Suga S., Tanaka I., Nakao K. Expression and role of angiotensin II type 2 receptor in the kidney and mesangial cells of spontaneously hypertensive rats. Hypertens. Res. 2002;25:125–133. doi: 10.1291/hypres.25.125. [DOI] [PubMed] [Google Scholar]
- Griffon-Etienne G., Boucher Y., Brekken C., Suit H.D., Jain R.K. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59:3776–3782. [PubMed] [Google Scholar]
- Guilluy C., Bregeon J., Toumaniantz G., Rolli-Derkinderen M., Retailleau K., Loufrani L., Henrion D., Scalbert E., Bril A., Torres R.M., Offermanns S., Pacaud P., Loirand G. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat. Med. 2010;16:183–190. doi: 10.1038/nm.2079. [DOI] [PubMed] [Google Scholar]
- Gutkind J.S., Novotny E.A., Brann M.R., Robbins K.C. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc. Natl. Acad. Sci. USA. 1991;88:4703–4707. doi: 10.1073/pnas.88.11.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman C.A., Henderson S.O., Bretsky P., Kolonel L.N., Henderson B.E. Genetic variation in angiotensin I-converting enzyme (ACE) and breast cancer risk: the multiethnic cohort. Cancer Res. 2003;63:6984–6987. [PubMed] [Google Scholar]
- Hernandez N.A., Correa E., Avila E.P., Vela T.A., Perez V.M. PAR1 is selectively over expressed in high grade breast cancer patients: a cohort study. J. Transl. Med. 2009;7:47. doi: 10.1186/1479-5876-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield K.M., Rebbeck T.R., Levine A.J. Germline mutations and polymorphisms in the origins of cancers in women. J. Oncol. 2010;2010:297671. doi: 10.1155/2010/297671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunyady L., Catt K.J. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- Janmey P.A., McCulloch C.A. Cell mechanics: integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- Julius D., Livelli T.J., Jessell T.M., Axel R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989;244:1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- Kharaishvili G., Simkova D., Bouchalova K., Gachechiladze M., Narsia N., Bouchal J. The role of cancer-associated fibroblasts, solid stress and other microenvironmental factors in tumor progression and therapy resistance. Cancer Cell Int. 2014;14:41. doi: 10.1186/1475-2867-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M.C., Marks J.H., Mandell J.B. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- Koh W.P., Yuan J.M., Sun C.L., van den Berg D., Seow A., Lee H.P., Yu M.C. Angiotensin I-converting enzyme (ACE) gene polymorphism and breast cancer risk among Chinese women in Singapore. Cancer Res. 2003;63:573–578. [PubMed] [Google Scholar]
- Koh W.P., Yuan J.M., Van Den Berg D., Lee H.P., Yu M.C. Polymorphisms in angiotensin II type 1 receptor and angiotensin I-converting enzyme genes and breast cancer risk among Chinese women in Singapore. Carcinogenesis. 2005;26:459–464. doi: 10.1093/carcin/bgh309. [DOI] [PubMed] [Google Scholar]
- Lever A.F., Hole D.J., Gillis C.R., McCallum I.R., McInnes G.T., MacKinnon P.L., Meredith P.A., Murray L.S., Reid J.L., Robertson J.W. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179–184. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- Li C.I., Malone K.E., Weiss N.S., Boudreau D.M., Cushing-Haugen K.L., Daling J.R. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65–79 years. Cancer. 2003;98:1504–1513. doi: 10.1002/cncr.11663. [DOI] [PubMed] [Google Scholar]
- Lindberg H., Nielsen D., Jensen B.V., Eriksen J., Skovsgaard T. Angiotensin converting enzyme inhibitors for cancer treatment? Acta Oncol. 2004;43:142–152. doi: 10.1080/02841860310022346. [DOI] [PubMed] [Google Scholar]
- Lindholm L.H., Anderson H., Ekbom T., Hansson L., Lanke J., Dahlof B., de Faire U., Forsen K., Hedner T., Linjer E., Schersten B., Wester P., Moller T. Relation between drug treatment and cancer in hypertensives in the Swedish Trial in Old Patients with Hypertension 2: a 5-year, prospective, randomised, controlled trial. Lancet. 2001;358:539–544. doi: 10.1016/s0140-6736(01)05704-x. [DOI] [PubMed] [Google Scholar]
- Linseman D.A., Benjamin C.W., Jones D.A. Convergence of angiotensin II and platelet-derived growth factor receptor signaling cascades in vascular smooth muscle cells. J. Biol. Chem. 1995;270:12563–12568. doi: 10.1074/jbc.270.21.12563. [DOI] [PubMed] [Google Scholar]
- Luft F.C. Angiotensin, inflammation, hypertension, and cardiovascular disease. Curr. Hypertens. Rep. 2001;3:61–67. doi: 10.1007/s11906-001-0082-y. [DOI] [PubMed] [Google Scholar]
- Marsigliante S., Resta L., Muscella A., Vinson G.P., Marzullo A., Storelli C. AT1 angiotensin II receptor subtype in the human larynx and squamous laryngeal carcinoma. Cancer Lett. 1996;110:19–27. doi: 10.1016/s0304-3835(96)04449-7. [DOI] [PubMed] [Google Scholar]
- McAllister-Lucas L.M., Jin X., Gu S., Siu K., McDonnell S., Ruland J., Delekta P.C., Van Beek M., Lucas P.C. The CARMA3-Bcl10-MALT1 signalosome promotes angiotensin II-dependent vascular inflammation and atherogenesis. J. Biol. Chem. 2010;285:25880–25884. doi: 10.1074/jbc.C110.109421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K.L., Gyoneva S., Vellano C.P., Smrcka A.V., Traynelis S.F., Hepler J.R. Protease-activated receptor 1 (PAR1) coupling to G(q/11) but not to G(i/o) or G(12/13) is mediated by discrete amino acids within the receptor second intracellular loop. Cell Signal. 2012;24:1351–1360. doi: 10.1016/j.cellsig.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Michel M.C., Foster C., Brunner H.R., Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol. Rev. 2013;65:809–848. doi: 10.1124/pr.112.007278. [DOI] [PubMed] [Google Scholar]
- Mifune M., Ohtsu H., Suzuki H., Nakashima H., Brailoiu E., Dun N.J., Frank G.D., Inagami T., Higashiyama S., Thomas W.G., Eckhart A.D., Dempsey P.J., Eguchi S. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J. Biol. Chem. 2005;280:26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- Miyajima A., Kosaka T., Asano T., Asano T., Seta K., Kawai T., Hayakawa M. Angiotensin II type I antagonist prevents pulmonary metastasis of murine renal cancer by inhibiting tumor angiogenesis. Cancer Res. 2002;62:4176–4179. [PubMed] [Google Scholar]
- Molina Wolgien Mdel C., Guerreiro da Silva I.D., Pinto Nazario A.C., Nakaie C.R., Correa-Noronha S.A., Ribeiro de Noronha S.M., Facina G. Genetic Association Study of Angiotensin II Receptor Types 1 (A168G) and 2 (T1247G and A5235G) Polymorphisms in Breast Carcinoma among Brazilian Women. Breast Care. 2014;9:176–181. doi: 10.1159/000363429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscella A., Greco S., Elia M.G., Storelli C., Marsigliante S. PKC-zeta is required for angiotensin II-induced activation of ERK and synthesis of C-FOS in MCF-7 cells. J. Cell. Physiol. 2003;197:61–68. doi: 10.1002/jcp.10336. [DOI] [PubMed] [Google Scholar]
- Namazi S., Daneshian A., Mohammadianpanah M., Jafari P., Ardeshir-Rouhani-Fard S., Nasirabadi S. The impact of renin-angiotensin system, angiotensin capital I, Ukrainian converting enzyme (insertion/deletion), and angiotensin capital I, Ukrainiancapital I, Ukrainian type 1 receptor (A1166C) polymorphisms on breast cancer survival in Iran. Gene. 2013;532:125–131. doi: 10.1016/j.gene.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Namazi S., Monabati A., Ardeshir-Rouhani-Fard S., Azarpira N. Association of angiotensin I converting enzyme (insertion/deletion) and angiotensin II type 1 receptor (A1166C) polymorphisms with breast cancer prognostic factors in Iranian population. Mol. Carcinog. 2010;49:1022–1030. doi: 10.1002/mc.20685. [DOI] [PubMed] [Google Scholar]
- Osterop A.P., Kofflard M.J., Sandkuijl L.A., ten Cate F.J., Krams R., Schalekamp M.A., Danser A.H. AT1 receptor A/C1166 polymorphism contributes to cardiac hypertrophy in subjects with hypertrophic cardiomyopathy. Hypertension. 1998;32:825–830. doi: 10.1161/01.hyp.32.5.825. [DOI] [PubMed] [Google Scholar]
- Padera T.P., Stoll B.R., Tooredman J.B., Capen D., di Tomaso E., Jain R.K. Pathology: cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- Pages G., Lenormand P., L’Allemain G., Chambard J.C., Meloche S., Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.S., Klotz E., Kim M.J., Song S.Y., Park S.W., Cha S.W., Lim J.S., Seong J., Chung J.B., Kim K.W. Perfusion CT: noninvasive surrogate marker for stratification of pancreatic cancer response to concurrent chemo- and radiation therapy. Radiology. 2009;250:110–117. doi: 10.1148/radiol.2493080226. [DOI] [PubMed] [Google Scholar]
- Rao G.N., Delafontaine P., Runge M.S. Thrombin stimulates phosphorylation of insulin-like growth factor-1 receptor, insulin receptor substrate-1, and phospholipase C-gamma 1 in rat aortic smooth muscle cells. J. Biol. Chem. 1995;270:27871–27875. doi: 10.1074/jbc.270.46.27871. [DOI] [PubMed] [Google Scholar]
- Rebeaud F., Hailfinger S., Posevitz-Fejfar A., Tapernoux M., Moser R., Rueda D., Gaide O., Guzzardi M., Iancu E.M., Rufer N., Fasel N., Thome M. The proteolytic activity of the paracaspase MALT1 is key in T cell activation. Nat. Immunol. 2008;9:272–281. doi: 10.1038/ni1568. [DOI] [PubMed] [Google Scholar]
- Rhodes D.R., Ateeq B., Cao Q., Tomlins S.A., Mehra R., Laxman B., Kalyana-Sundaram S., Lonigro R.J., Helgeson B.E., Bhojani M.S., Rehemtulla A., Kleer C.G., Hayes D.F., Lucas P.C., Varambally S., Chinnaiyan A.M. AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist. Proc. Natl. Acad. Sci. USA. 2009;106:10284–10289. doi: 10.1073/pnas.0900351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera E., Arrieta O., Guevara P., Duarte-Rojo A., Sotelo J. AT1 receptor is present in glioma cells; its blockage reduces the growth of rat glioma. Br. J. Cancer. 2001;85:1396–1399. doi: 10.1054/bjoc.2001.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder R.E., Hilgers K.F., Schlaich M.P., Schmidt B.M. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- Silvestre J.S., Tamarat R., Senbonmatsu T., Icchiki T., Ebrahimian T., Iglarz M., Besnard S., Duriez M., Inagami T., Levy B.I. Antiangiogenic effect of angiotensin II type 2 receptor in ischemia-induced angiogenesis in mice hindlimb. Circ. Res. 2002;90:1072–1079. doi: 10.1161/01.res.0000019892.41157.24. [DOI] [PubMed] [Google Scholar]
- Sorensen A.G., Emblem K.E., Polaskova P., Jennings D., Kim H., Ancukiewicz M., Wang M., Wen P.Y., Ivy P., Batchelor T.T., Jain R.K. Increased survival of glioblastoma patients who respond to antiangiogenic therapy with elevated blood perfusion. Cancer Res. 2011;72:402–407. doi: 10.1158/0008-5472.CAN-11-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg B.D., Hamm H.E. Roles of G-protein-coupled receptor signaling in cancer biology and gene transcription. Curr. Opin. Genet. Dev. 2007;17:40–44. doi: 10.1016/j.gde.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Stilo R., Liguoro D., Di Jeso B., Formisano S., Consiglio E., Leonardi A., Vito P. Physical and functional interaction of CARMA1 and CARMA3 with Ikappa kinase gamma-NFkappaB essential modulator. J. Biol. Chem. 2004;279:34323–34331. doi: 10.1074/jbc.M402244200. [DOI] [PubMed] [Google Scholar]
- Suganuma T., Ino K., Shibata K., Kajiyama H., Nagasaka T., Mizutani S., Kikkawa F. Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin. Cancer Res. 2005;11:2686–2694. doi: 10.1158/1078-0432.CCR-04-1946. [DOI] [PubMed] [Google Scholar]
- Surmi B.K., Hasty A.H. The role of chemokines in recruitment of immune cells to the artery wall and adipose tissue. Vasc. Pharmacol. 2010;52:27–36. doi: 10.1016/j.vph.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi R., Ohnaka K., Sakai Y., Ikuyama S., Nawata H. Molecular cloning and characterization of the promoter for human type-1 angiotensin II receptor gene. Biochem. Biophys. Res. Commun. 1994;200:1264–1270. doi: 10.1006/bbrc.1994.1587. [DOI] [PubMed] [Google Scholar]
- Takeda H., Kondo S. Differences between squamous cell carcinoma and keratoacanthoma in angiotensin type-1 receptor expression. Am. J. Pathol. 2001;158:1633–1637. doi: 10.1016/S0002-9440(10)64119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Jin R., Qu G., Wang X., Li Z., Yuan Z., Zhao C., Siwko S., Shi T., Wang P., Xiao J., Liu M., Luo J. GPR116, an adhesion G-protein-coupled receptor, promotes breast cancer metastasis via the Galphaq-p63RhoGEF-Rho GTPase pathway. Cancer Res. 2013;73:6206–6218. doi: 10.1158/0008-5472.CAN-13-1049. [DOI] [PubMed] [Google Scholar]
- Timmermans P.B. Angiotensin II receptor antagonists: an emerging new class of cardiovascular therapeutics. Hypertens. Res. 1999;22:147–153. doi: 10.1291/hypres.22.147. [DOI] [PubMed] [Google Scholar]
- Timmermans P.B., Benfield P., Chiu A.T., Herblin W.F., Wong P.C., Smith R.D. Angiotensin II receptors and functional correlates. Am. J. Hypertens. 1992;5:221S–235S. doi: 10.1093/ajh/5.12.221s. [DOI] [PubMed] [Google Scholar]
- Tiret L., Bonnardeaux A., Poirier O., Ricard S., Marques-Vidal P., Evans A., Arveiler D., Luc G., Kee F., Ducimetiere P. Synergistic effects of angiotensin-converting enzyme and angiotensin-II type 1 receptor gene polymorphisms on risk of myocardial infarction. Lancet. 1994;344:910–913. doi: 10.1016/s0140-6736(94)92268-3. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Uemura H., Ishiguro H., Nakaigawa N., Nagashima Y., Miyoshi Y., Fujinami K., Sakaguchi A., Kubota Y. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: a possibility of tyrosine kinase inhibitor of growth factor. Mol. Cancer Ther. 2003;2:1139–1147. [PubMed] [Google Scholar]
- van Biesen T., Hawes B.E., Raymond J.R., Luttrell L.M., Koch W.J., Lefkowitz R.J. G(o)-protein alpha-subunits activate mitogen-activated protein kinase via a novel protein kinase C-dependent mechanism. J. Biol. Chem. 1996;271:1266–1269. doi: 10.1074/jbc.271.3.1266. [DOI] [PubMed] [Google Scholar]
- Weischer M., Bojesen S.E., Ellervik C., Tybjaerg-Hansen A., Nordestgaard B.G. CHEK2*1100delC genotyping for clinical assessment of breast cancer risk: meta-analyses of 26,000 patient cases and 27,000 controls. J. Clin. Oncol. 2008;26:542–548. doi: 10.1200/JCO.2007.12.5922. [DOI] [PubMed] [Google Scholar]
- Yoshiji H., Kuriyama S., Kawata M., Yoshii J., Ikenaka Y., Noguchi R., Nakatani T., Tsujinoue H., Fukui H. The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: possible role of the vascular endothelial growth factor. Clin. Cancer Res. 2001;7:1073–1078. [PubMed] [Google Scholar]
- Young D., Waitches G., Birchmeier C., Fasano O., Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986;45:711–719. doi: 10.1016/0092-8674(86)90785-3. [DOI] [PubMed] [Google Scholar]