Abstract
Objective:
To compare effects of natalizumab on inflammatory and regulatory T cells with regard to expression of α4-integrin (CD49d).
Methods:
Twenty-seven natalizumab-naive and 8 natalizumab-treated patients with multiple sclerosis (MS), 7 patients with neuromyelitis optica (NMO) or NMO spectrum disorder, and 8 healthy controls were included. The positive rate of CD49d was analyzed and compared among T helper 1 (Th1), T helper 17 (Th17), and regulatory T (Treg) cells (CD49d+Th1, CD49d+Th17, and CD49d+Treg, respectively).
Results:
Natalizumab treatment increased CD49d ratios, CD49d+Th1/CD49d+Treg, and CD49d+Th17/CD49d+Treg. This indicates larger reduction of the CD49d+ population in Treg cells than in Th1 or Th17 cells. The CD49d ratios of 2 patients who experienced exacerbation during natalizumab treatment were remarkably higher than those of the other natalizumab-treated patients. Natalizumab treatment increased the expression of TBX21, RORC, interferon (IFN)–γ, and interleukin (IL)–17A, and decreased the expression of FOXP3 in CD49d+ memory CD4 T cells. Natalizumab treatment also increased the amount of IFN-γ and IL-17A secreted by CD49d+ memory CD4 T cells.
Conclusions:
The reduction rate of the CD49d+ population in Treg cells was larger than that in Th1 or Th17 cells. Although the large reduction in CD49d+ population is beneficial for MS, the proinflammatory state of residual CD49d+ cells might, in part, explain the presence of disease activity under natalizumab treatment.
Natalizumab is a humanized monoclonal antibody against α4-integrin (CD49d) that is approved for the treatment of multiple sclerosis (MS). It decreases CD49d expression on circulating lymphocytes and blocks their interaction with vascular cell adhesion molecule–1 (VCAM-1) and fibronectin expressed by vascular endothelial cells. CD49d is the main adhesion molecule involved in lymphocyte migration to the CNS. Therefore, blocking this interaction leads to decreased number of inflammatory lymphocytes with migratory capacity into the CNS.1,2
A clinical trial showed a remarkable reduction in annual relapse rate by natalizumab treatment. More than one third of natalizumab-treated patients achieved complete remission without clinical or radiologic activity.3 Nevertheless, some natalizumab-treated patients experience a clinical relapse or worsening of the Expanded Disability Status Scale.4
CD49d is expressed not only by inflammatory but also by regulatory CD4 T cells. Although natalizumab is supposed to decrease the expression of CD49d on both T-cell subsets, differences in effects on each subset remain unclear.
In this study, we show that natalizumab decreases the CD49d+ population more significantly in regulatory than in inflammatory CD4 T cells. The balance between inflammatory and regulatory CD4 T cells was highly disrupted in 2 patients who experienced exacerbation under natalizumab treatment. Although the large reduction in inflammatory T cells with migratory capacity is beneficial for MS, the disrupted balance may contribute to disease activity during natalizumab treatment.
METHODS
Participants.
Twenty-seven natalizumab-naive and 8 natalizumab-treated patients with MS, 7 patients with neuromyelitis optica (NMO) or NMO spectrum disorder (NMOSD), and 8 healthy controls (HC) were examined. The patient profile was similar between natalizumab-naive and natalizumab-treated MS groups: mean age was 42.5 (range 19–67) and 37.8 (range 33–48) years; ratio of male to female patients was 7/20 and 4/4; ratio of relapsing-remitting MS to secondary progressive MS patients was 18/9 and 5/3; disease duration was 11.0 ± 16.2 and 11.8 ± 7.9 (mean ± 2 SD) years; and annual relapse rate in the year just before the analysis was 0.48 ± 1.4 and 1.0 ± 2.1 (mean ± 2 SD), respectively. All natalizumab-treated patients had occasional disease exacerbations before the start of natalizumab. All natalizumab-treated patients had received more than 3 doses of natalizumab at the time of analysis. Blood samples were obtained just before the infusion of natalizumab. The diagnoses of MS, NMO, and NMOSD were established according to McDonald and Wingerchuk criteria.5–7
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Ethical Committee of the National Institute of Neuroscience and written informed consent was obtained from all participants. This is a cross-sectional study conducted between October 2014 and July 2015 at National Institute of Neuroscience, Japan.
Cell preparation and flow cytometry.
Fresh peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation, using Ficoll-Paque PLUS (GE Healthcare Bioscience, Mississauga, Canada). They were stained against cell surface antigens, followed by intracellular staining of transcription factors using Foxp3/transcription factor staining buffer set (eBiosciences, San Diego, CA) according to the manufacturer's instructions. The fluorescent-conjugated monoclonal antibodies (mAb) and isotype controls used in this study were as follows: PE-Cy7-anti-CD3, PerCp-Cy5.5-anti-CD4, BV421-anti-CD127, and BV421-mouse immunoglobulin G (IgG) 1κ (BioLegend, San Diego, CA); FITC-anti-Foxp3, PE-anti-RORγt, eFluor 660-anti-T-bet, FITC-rat IgG2aκ, PE-rat IgG2aκ, and eFluor 660-mouse IgG1κ (eBiosciences); and APC-H7-anti-CD45RA, BV510-anti-CD49d, and BV510-mouse IgG1κ (BD Biosciences, San Jose, CA). The cells were analyzed by a FACS Canto II flow cytometer (BD Biosciences) with FlowJo software (Tree Star, Ashland, OR).
Cell culture.
To analyze cytokine production from CD49d-positive and CD49d-negative memory CD4 T cells in vitro, each population was isolated from PBMC using a FACS Aria II cell sorter (BD Biosciences). Ninety-six-well flat-bottom plates were coated with 2 μg/mL anti-CD3 mAb (OKT3) and 4 μg/mL anti-CD28 mAb (Beckman Coulter, Brea, CA) for 1 hour at 37°C. Isolated cells were cultured in the coated plates at 5 × 104 cells per well with AIM V medium (Life Technologies, Carlsbad, CA) for 72 hours. The amount of cytokines in the supernatant was measured using ELISA kits (interferon [IFN]–γ, interleukin [IL]–10, GM-CSF: BD Biosciences; interleukin [IL]–17A: R&D Systems, Minneapolis, MN). Cell culture was performed in triplicate for each sample.
Quantification of messenger RNA (mRNA).
Total RNA was prepared from the sorted cells using RNeasy Kit and RNase-Free DNase Set (QIAGEN, Germantown, MD), and reverse transcribed to complementary DNA using PrimeScript RT Master Mix (Takara Bio, Shiga, Japan). PCR was performed using SYBR Premix Ex Taq (Takara Bio) on LightCycler 96 (Roche, Basel, Switzerland). The expression level of each mRNA was determined with normalization to β-actin. The following primers were used: IFN-γ-forward, GCAGGTCATTCAGATGTAGCGG, and IFN-γ-reverse, TGTCTTCCTTGATGGTCTCCACAC; IL-17A-forward, CAACCGATCCACCTCACCTT, and IL-17A-reverse, GGCACTTTGCCTCCCAGAT; CSF2-forward, CCATGATGGCCAGCCACTAC, and CSF2-reverse, CTGGCTCCCAGCAGTCAAAG; IL-10-forward, CCGTGGAGCAGGTGAAGAATG, and IL-10-reverse, AGTCGCCACCCTGATGTCTC; TBX21-forward, GGAGGACACCGACTAATTTGGGA, and TBX21-reverse, AAGCAAGACGCAGCACCAGGTAA; RORC-forward, AGAAGGACAGGGAGCCAAGG, and RORC-reverse, GTGATAACCCCGTAGTGGATC; and FOXP3-forward, TTCGAAGAGCCAGAGGACTT, and FOXP3-reverse, GCTGCTCCAGAGACTGTACC.
Data analysis and statistics.
Data were analyzed with FlowJo software (Tree Star) and Prism software (GraphPad Software, San Diego, CA). Unpaired t test or Mann-Whitney U test was used to compare data from 2 groups, as appropriate. Kruskal-Wallis test with Dunn multiple comparison test was used to compare data from more than 2 groups. The difference was considered significant when p value was <0.05.
RESULTS
Natalizumab reduces the CD49d-positive population especially in regulatory T cells.
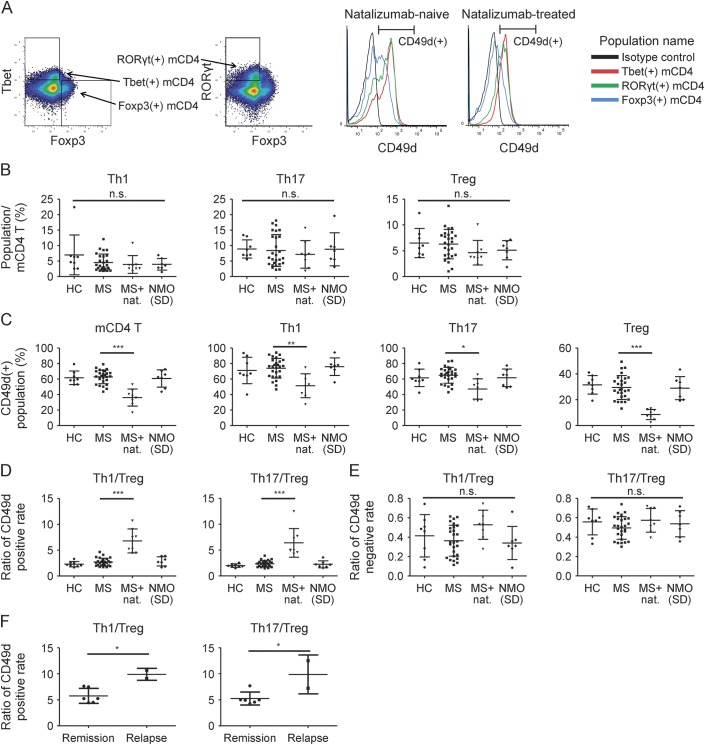
To clarify differences in effects of natalizumab on inflammatory and regulatory T cells, T-cell subsets were defined by flow cytometry. After memory CD4 (mCD4) T cells were identified as CD3+CD4+CD45RA− lymphocytes, T helper 1 (Th1), T helper 17 (Th17), and regulatory T (Treg) cells were defined according to the expression of transcription factors, Tbet, RORγt, and Foxp3, respectively (figure 1A). The CD49d-positive population was assessed in each T-cell subset. The frequency of Th1, Th17, and Treg cells in mCD4 T cells was similar among patient subgroups (natalizumab-naive MS, natalizumab-treated MS, NMO/NMOSD, and HC) (figure 1B). The expression of CD49d was higher in Th1 and Th17 cells than in Treg cells and was reduced in all subsets with natalizumab treatment (figure 1, A and C). Unexpectedly, natalizumab reduced the CD49d+ population especially in regulatory T cells. The ratio of CD49d positivity of Th1 cells to that of Treg cells (CD49d+Th1/CD49d+Treg) was significantly higher in natalizumab-treated patients than in the other patients with MS. CD49d+Th17/CD49d+Treg was also significantly higher in natalizumab-treated patients (figure 1D). As CD49d+ T cells are considered to have migratory capacity into the CNS, this result indicates a disrupted balance between inflammatory and regulatory CD4 T cells in the CNS. The CD49d ratios were not different among natalizumab-naive MS, NMO/NMOSD, and HC. In contrast, the ratios of CD49d negativity of inflammatory T cells to that of regulatory T cells (CD49d-Th1/CD49d-Treg and CD49-Th17/CD49d-Treg) were not different among patient subgroups. They were much lower than the CD49d-positive counterparts (CD49d+Th1/CD49d+Treg and CD49d+Th17/CD49d+Treg, respectively) (figure 1E). This suggests the predominance of inflammatory T cells in the CD49d+ population irrespective of disease or treatment.
Figure 1. Natalizumab induces larger reduction of CD49d+ population in regulatory T (Treg) than in T helper 1 (Th1) or T helper 17 (Th17) cells.
(A) Representative dot plot and histogram by flow cytometry. CD3+ CD4+ CD45RA− mCD4 T cells were divided into Th1, Th17, and Treg cells based on the expression of each characteristic transcription factor. The CD49d+ population was defined based on the fluorescence intensity of isotype control. (B) Frequencies of Th1, Th17, and Treg cells among memory CD4 (mCD4) T cells. (C) CD49d+ population among mCD4 T, Th1, Th17, and Treg cells. (D) Ratio of CD49d-positive rate of inflammatory cells to that of Treg cells (CD49d+Th1/CD49d+Treg, CD49d+Th17/CD49d+Treg). (E) Ratio of CD49d-negative rate of inflammatory cells to that of Treg cells (CD49d-Th1/CD49d-Treg, CD49d-Th17/CD49d-Treg). (F) Ratios (as described in D) of natalizumab-treated patients during remission and during relapse. Kruskal-Wallis test with Dunn multiple comparison test was used in B, C, D, and E. Statistical data were described only between MS and MS + natalizumab groups in C and D. Unpaired t test was used in F. Error bars represent the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. NC = healthy controls; MS = multiple sclerosis; NMO = neuromyelitis optica.
Among patients treated with natalizumab, 2 patients experienced clinical exacerbation confirmed by MRI. Symptoms started within 4 weeks after the final administration of natalizumab in both cases. The CD49d ratios, CD49d+Th1/CD49d+Treg and CD49d+Th17/CD49d+Treg, were significantly higher in these patients than in the other patients under natalizumab treatment (figure 1F). Moreover, the ratios were analyzed repeatedly in one of the exacerbated patients and were found to be lower during remission. CD49d+Th1/CD49d+Treg and CD49d+Th17/CD49d+Treg decreased from 9.08 and 7.23 during exacerbation to 4.67 and 6.78 during remission, respectively. This further supports the clinical importance of the ratios that reflect the balance between inflammatory and regulatory CD4 T cells with migratory capacity.
Natalizumab enhances the expression of inflammatory genes and reduces the expression of regulatory genes in CD49d+ mCD4 T cells.
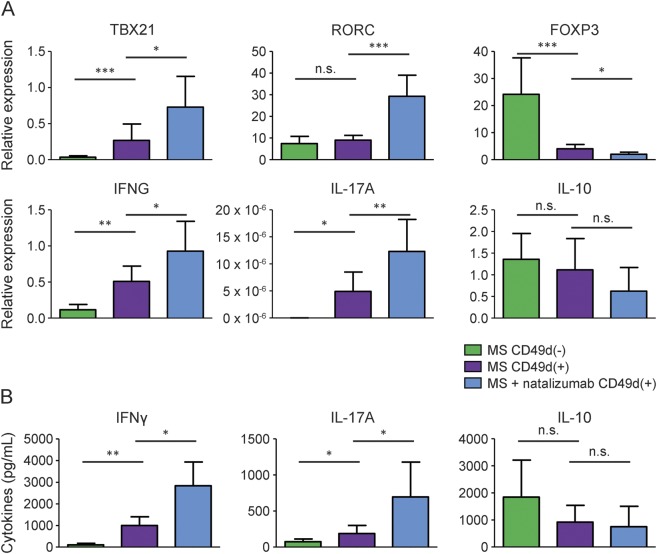
To evaluate the in vivo status of CD49d+ mCD4 T cells, the expression of genes associated with inflammatory and regulatory T cells was measured by quantitative real-time PCR. The expression of mRNA was normalized to that of endogenous β-actin for each sample. Natalizumab enhanced the expressions of proinflammatory genes (TBX21, RORC, IFN-γ, and IL-17A) and reduced the expression of FOXP3 in CD49d+ mCD4 T cells (figure 2A). Although not significant, the expression of IL-10 tended to be reduced by natalizumab treatment. This is consistent with results of flow cytometry, which indicates disrupted balance between inflammatory and regulatory function under natalizumab treatment. Additionally, the expressions of TBX21, IFN-γ, and IL-17A were higher and that of FOXP3 was lower in CD49d+ mCD4 T cells than in CD49d− mCD4 T cells (figure 2A). This is also consistent with the flow cytometry data, which suggests predominance of inflammatory mCD4 T cells among the CD49d+ population irrespective of treatment.
Figure 2. Natalizumab increases expression of proinflammatory genes and cytokines by CD49d+ memory CD4 cells.
(A) Relative expressions of messenger RNA (mRNA) associated with inflammatory and regulatory T cells. The expression level of each mRNA was determined with normalization to β-actin; n = 7 for each group. (B) Amount of cytokines secreted by each population under stimulation with anti-CD3 monoclonal antibodies (mAb) and anti-CD28 mAb. Cell culture was performed in triplicate for each sample. Multiple sclerosis (MS): n = 5; MS + natalizumab: n = 4. Mann-Whitney U test was used. Error bars represent the mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. IFN = interferon; IL = interleukin.
Natalizumab increases the secretion of IFN-γ and IL-17A by CD49d+ mCD4 T cells.
Finally, the in vitro function of CD49d+ mCD4 T cells was evaluated. Those cells were isolated from natalizumab-naive and natalizumab-treated patients, and were cultured under stimulation of anti-CD3 and anti-CD28 mAbs. The amount of IFN-γ and IL-17A in the supernatant was higher in the cell culture from natalizumab-treated patients than in that from natalizumab-naive patients (figure 2B). These data suggest that natalizumab enhances inflammatory function in mCD4 T cells with migratory capacity into the CNS. The amount of IFN-γ and IL-17A was higher in CD49d+ than in CD49d− mCD4 T cells. This again suggests predominance of inflammatory function among the CD49d+ population.
DISCUSSION
CD49d plays a key role in the pathology of MS. CD49d on inflammatory T cells binds VCAM-1 and fibronectin on vascular endothelial cells, which causes T-cell migration into the CNS.1 It has been demonstrated that CD49dhigh encephalitogenic Th1 cells migrate into the brain parenchyma in experimental autoimmune encephalomyelitis, a disease model of MS. In contrast, CD49dlow nonencephalitogenic Th1 cells do not enter the brain parenchyma.8 In MS, CD49dhigh T cells are abundant in the CSF compared to peripheral blood.9 It is supposed that there is an absolute threshold for CD49d on the cell surface that is required for cells to migrate across blood barriers.1 Besides, CD49dhigh mCD4 T cells have proinflammatory function and are associated with relapses in MS.10 As is consistent with these previous reports, our data showed higher expression of proinflammatory genes (TBX21, IFN-γ, and IL-17A) and cytokines (IFN-γ and IL-17A) and lower expression of a regulatory gene (FOXP3) in CD49d+ mCD4 T cells than in CD49d− mCD4 T cells.
Natalizumab is considered to exert its effect by downregulating CD49d on circulating lymphocytes, which in turn inhibits their adhesion to endothelial cells and migration into the CNS.11 Natalizumab binds CD49d on the surface of lymphocytes. CD49d is then internalized and degraded within a few hours.12 CD49d expression on circulating lymphocytes is largely decreased by natalizumab treatment even after the first infusion.13 The reduction rate of the CD49d+ population is known to differ between cell subsets, such as T cells, B cells, and monocytes.14 This varied expression of CD49d is considered to explain different capacities of migration of various types of cells under natalizumab treatment.15 However, differences in effects of natalizumab remain unclear between inflammatory and regulatory T-cell subsets. Although it has been shown that natalizumab increases the frequency of IFN-γ+, IL-17+, and Tbet+ subsets among total CD4 T cells, the expression of CD49d has not yet been determined in these populations.12,16,17
This study was designed to clarify differences in effects of natalizumab on inflammatory and regulatory T cells and the phenotype of CD49d+ T cells under natalizumab treatment. It was shown that natalizumab disrupts the balance between inflammatory and regulatory T cells in the CD49d+ population, which is considered to have migratory capacity. Two ratios comparing CD49d positivity of inflammatory T cells to that of regulatory T cells, CD49d+Th1/CD49d+Treg and CD49d+Th17/CD49d+Treg, were used here to represent the balance. These ratios were shown to be significantly higher in natalizumab-treated patients, as described. Moreover, natalizumab increased expression of genes associated with inflammatory T cells (TBX21, RORC, IFN-γ, and IL-17A) and decreased expression of FOXP3 in CD49d+ mCD4 T cells. Natalizumab also increased the amount of inflammatory cytokines (IFN-γ and IL-17A) secreted by CD49d+ mCD4 T cells. These results indicate that natalizumab enhances inflammatory function among T cells with migratory capacity into the CNS.
Furthermore, the 2 ratios, CD49d+Th1/CD49d+Treg and CD49d+Th17/CD49d+Treg, were correlated with disease activity during natalizumab treatment. Two exacerbated patients had significantly higher ratios compared to the other natalizumab-treated patients. These ratios were analyzed at another time point during remission in one of these exacerbated patients and were shown to be reduced. This suggests the clinical importance of these ratios under natalizumab treatment.
Circulating natalizumab binds residual CD49d on the surface of lymphocytes. However, CD49d is not saturated with natalizumab in vivo. The saturation level is supposed to be approximately 60%–100%, which decreases gradually after the infusion of natalizumab.18–20 Although CD49d measured in our study might not have been fully functional, a certain fraction of CD49d is considered not bound by natalizumab on any CD49d+ lymphocyte. Most importantly, the CD49d ratios based on the residual CD49d expression are shown to have clinical relevance, as discussed above.
It has been reported that antibodies against natalizumab are associated with low serum concentration of natalizumab and are also associated with relapses and gadolinium-enhancing lesions observed on MRI.21 The presence of neutralizing antibodies is indeed considered as a cause for some patients being poor responders. Although neutralizing antibodies were not measured in our study, similar or lower expression of CD49d expression by mCD4 T cells in the 2 exacerbated patients (16.8% and 32.3%) than in the other patients suggests that there was sufficient natalizumab in the sera of the 2 patients. CD49d expression is known to be a reliable marker of the presence of neutralizing antibodies and natalizumab concentration.13,21
The lack of longitudinal data including before and after natalizumab treatment is a limitation of this study.
Our study showed that natalizumab enhances proinflammatory function among CD49d+ mCD4 T cells, which are considered to have migratory capacity. The absolute number of CD49d+ cells is largely reduced by natalizumab treatment. Although this is beneficial for MS, the disrupted function might, in part, explain the presence of disease activity during natalizumab treatment. The CD49d ratios described here are candidate biomarkers to monitor patients treated with natalizumab. Further replication on an independent cohort would be needed to establish these findings.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Hiromi Yamaguchi and Yasuko Hirakawa (Department of Immunology, National Institute of Neuroscience, NCNP) for their technical support and expertise.
GLOSSARY
- HC
healthy controls
- IFN
interferon
- IgG
immunoglobulin G
- IL
interleukin
- mAb
monoclonal antibodies
- mCD4
memory CD4
- mRNA
messenger RNA
- MS
multiple sclerosis
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optica spectrum disorder
- PBMC
peripheral blood mononuclear cell
- Th1
T helper 1
- Th17
T helper 17
- Treg
regulatory T
- VCAM-1
vascular cell adhesion molecule–1
AUTHOR CONTRIBUTIONS
K.K.: study concept and design; acquisition, analysis, and interpretation of data; and drafting and revising the manuscript for intellectual content. M.N.: revising the manuscript for intellectual content. W.S.: revising the manuscript for intellectual content. T.O.: revising the manuscript for intellectual content. M.A.: revising the manuscript for intellectual content. Y.L.: revising the manuscript for intellectual content. M.M.: revising the manuscript for intellectual content. R.T.: revising the manuscript for intellectual content. T.Y.: study concept and design; analysis, and interpretation of data; drafting and revising the manuscript for intellectual content.
STUDY FUNDING
Supported by a Research Grant on Super Special Consortia for Supporting the Development of Cutting-Edge Medical Care from Cabinet Office, Health Labour Sciences Research Grants from the Ministry of Health, Labour and Welfare of Japan (H24-nanchito(nan)-ippan-007) and the Practical Research Project for Rare/Intractable Diseases from Japan Agency for Medical Research and development, AMED (15ek0109084h0001).
DISCLOSURE
K. Kimura and M. Nakamura report no disclosures. W. Sato received research support from Novartis. T. Okamoto reports no disclosures. M. Araki received honoraria from Novartis. Y. Lin reports no disclosures. M. Muruta served on the speakers' bureau for Dainippon Sumitomo Pharma Inc., Novartis Pharma KK, Nippon Boehringer Ingelheim Company, and GlaxoSmithKline KK; and received research support from Ministry of Health, Labour, and Welfare, Japan. R. Takahashi served on the scientific advisory board for KAN Research Institute Inc., Dainippon Sumitomo Pharma Inc., and Japan Agency of Medical Research and Development; received travel funding and/or speaker honoraria from Novartis Pharma Japan, Boehringer Ingelheim Japan, GlaxoSmithKline Japan, Dainippon Sumito Pharma, FP Pharmaceuticals, Mitsubishi Tanabe Pharma, Kyowa Hakko Kirin Co., and Otsuka Pharmaceutical Co.; served on the editorial board for Journal of Neural Transmission, Neurology and Clinical Neuroscience, Experimental Neurobiology, Movement Disorders, and Molecular Brain; holds patents for the use of mutant mouse of Chiamaerin for drug screening and drug screening using iPS cells; and received research support from Novartis Pharma Japan, Boehringer Ingelheim Japan, GlaxoSmithKline Japan, Dainippon Sumito Pharma, FP Pharmaceuticals, Ministry of Education, Culture, Sports, Science and Technology, Japan, Ministry of Health, Labor and Welfare, Japan, and Japan Agency for Medical Research and Development. T. Yamaura served on the scientific advisory board for Biogen Idec and Chugai Pharmaceutical Co.; received travel funding and/or speaker honoraria from Novartis Pharma, Nihon Pharmaceutical Co., Santen Pharmaceutical Co., Ltd., Abbott Japan Co., Ltd./Eisai Co., Ltd., Biogen Idec, Dainippon Sumitomo Pharma Co., Ltd., Bayer Holding Ltd., and Astellas Pharma Inc.; and received research support from Ono Pharmaceutical Co., Chigai Pharmaceutical Co., Teva Pharmaceutical KK, Mitsubishi Tanabe Pharma Corporation, Asahi Kasei Kurary Medical Co., Ministry of Health, Labour and Welfare of Japan, and the Health and Labour Sciences Research Grants on Intractable Diseases. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Stuve O, Gold R, Chan A, Mix E, Zettl U, Kieseier BC. alpha4-Integrin antagonism with natalizumab: effects and adverse effects. J Neurol 2008;255(suppl 6):58–65. [DOI] [PubMed] [Google Scholar]
- 2.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 1992;356:63–66. [DOI] [PubMed] [Google Scholar]
- 3.Havrdova E, Galetta S, Hutchinson M, et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol 2009;8:254–260. [DOI] [PubMed] [Google Scholar]
- 4.Prosperini L, Gianni C, Barletta V, et al. Predictors of freedom from disease activity in natalizumab treated-patients with multiple sclerosis. J Neurol Sci 2012;323:104–112. [DOI] [PubMed] [Google Scholar]
- 5.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 7.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. [DOI] [PubMed] [Google Scholar]
- 8.Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA., Jr Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med 1993;177:57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svenningsson A, Hansson GK, Andersen O, Andersson R, Patarroyo M, Stemme S. Adhesion molecule expression on cerebrospinal fluid T lymphocytes: evidence for common recruitment mechanisms in multiple sclerosis, aseptic meningitis, and normal controls. Ann Neurol 1993;34:155–161. [DOI] [PubMed] [Google Scholar]
- 10.Barrau MA, Montalban X, Saez-Torres I, Brieva L, Barbera N, Martinez-Caceres EM. CD4(+)CD45RO(+)CD49d(high) cells are involved in the pathogenesis of relapsing-remitting multiple sclerosis. J Neuroimmunol 2000;111:215–223. [DOI] [PubMed] [Google Scholar]
- 11.Coisne C, Mao W, Engelhardt B. Cutting edge: natalizumab blocks adhesion but not initial contact of human T cells to the blood-brain barrier in vivo in an animal model of multiple sclerosis. J Immunol 2009;182:5909–5913. [DOI] [PubMed] [Google Scholar]
- 12.Benkert TF, Dietz L, Hartmann EM, et al. Natalizumab exerts direct signaling capacity and supports a pro-inflammatory phenotype in some patients with multiple sclerosis. PLoS One 2012;7:e52208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defer G, Mariotte D, Derache N, et al. CD49d expression as a promising biomarker to monitor natalizumab efficacy. J Neurol Sci 2012;314:138–142. [DOI] [PubMed] [Google Scholar]
- 14.Putzki N, Baranwal MK, Tettenborn B, Limmroth V, Kreuzfelder E. Effects of natalizumab on circulating B cells, T regulatory cells and natural killer cells. Eur Neurol 2010;63:311–317. [DOI] [PubMed] [Google Scholar]
- 15.Stuve O, Marra CM, Bar-Or A, et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol 2006;63:1383–1387. [DOI] [PubMed] [Google Scholar]
- 16.Kivisakk P, Healy BC, Viglietta V, et al. Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells. Neurology 2009;72:1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisullo G, Iorio R, Plantone D, et al. CD4+T-bet+, CD4+pSTAT3+ and CD8+T-bet+ T cells accumulate in peripheral blood during NZB treatment. Mult Scler 2011;17:556–566. [DOI] [PubMed] [Google Scholar]
- 18.Harrer A, Pilz G, Einhaeupl M, et al. Lymphocyte subsets show different response patterns to in vivo bound natalizumab: a flow cytometric study on patients with multiple sclerosis. PLoS One 2012;7:e31784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niino M, Bodner C, Simard ML, et al. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol 2006;59:748–754. [DOI] [PubMed] [Google Scholar]
- 20.Khatri BO, Man S, Giovannoni G, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology 2009;72:402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vennegoor A, Rispens T, Strijbis EM, et al. Clinical relevance of serum natalizumab concentration and anti-natalizumab antibodies in multiple sclerosis. Mult Scler 2013;19:593–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.