Abstract
We report that intravenous injection (3 mg/kg of body weight twice daily) of a diastereomer (containing 33% d amino acids) of an antimicrobial peptide, K6L9 (LKLLKKLLKKLLKLL-NH2), but not the all-l-amino-acid parental peptide, cures neutropenic mice infected with gentamicin-sensitive Pseudomonas aeruginosa and gentamicin-resistant Acinetobacter baumannii bacteria. Various biophysical experiments suggest a membranolytic-like effect.
Antimicrobial peptides are natural antibiotics that constitute a major part of the innate immunity in all organisms (33). Many are positively charged, adopt an amphipathic structure when they are in contact with biological membranes (13, 16, 27, 29), and act through a non-receptor-mediated membrane lytic mechanism (2, 30). However, recent studies suggest other targets as well (9). Furthermore, because they act rapidly against the bacterial membrane, with a direct and destructive mode of action, they can escape the mechanisms involved in drug resistance, although there is some evidence that specific resistance might occur (14, 31). Therefore, antimicrobial peptides have been extensively studied for their potential use as antibiotics with new targets (3, 6, 13, 22, 25, 33).
Despite their advantages, systemic administration of antimicrobial peptides is difficult because they are inactivated by blood components. Therefore, the in vivo activities of membrane-active antimicrobial peptides have been reported in only a limited number of studies, and they were mainly administered intraperitoneally (4, 5, 7, 11, 15, 18, 23, 28). To overcome this limitation, we have developed a new family of nonhemolytic dl-amino acid antimicrobial peptides (diastereomers) which maintain their full activities in serum (17, 20, 26). In the present study we compared the in vitro activities of an all-l-amino-acid antimicrobial peptide and its diastereomer after systemic administration to bacteria-infected mice.
We synthesized an amphipathic all-l-amino-acid peptide (amphipathic-l) and its diastereomer (amphipathic-d) with the sequences LKLLKKLLKKLLKLL-NH2 (molecular weight, 1,804) and LKLLKKLLKKLLKLL-NH2 (underlined amino acids are the d enantiomers), respectively. The synthesis protocol was described previously (20). Treatment with trypsin (2 h) completely cleaved amphipathic-l, whereas amphipathic-d was protected by ∼50%. Furthermore, serum inactivated amphipathic-l, and therefore, most experiments were performed only with the diastereomer.
The peptides were first investigated in vitro for their antibacterial and bactericidal activities against gentamicin-sensitive Pseudomonas aeruginosa ATCC 27853 and a gentamicin-resistant strain of Acinetobacter baumannii resistant primarily due to extended-spectrum beta-lactamase production (8) isolated from the blood cultures of a patient with nosocomial pneumonia, as well as against their corresponding spheroplasts. The assay was done in sterile 96-well plates by a dilution assay protocol described in detail elsewhere (17, 20). The antibacterial activities were expressed as the MICs, i.e., the concentrations at which 100% inhibition of growth was observed after 18 to 20 h of incubation. A similar protocol was used, however, with the peptides dissolved in 30% human blood serum. Table 1 shows the biological activities of the peptides. Time-kill experiments (19) of amphipathic-d at its MIC against A. baumannii were performed and showed fast kinetics (Fig. 1A). Similar results were obtained with P. aeruginosa.
TABLE 1.
MICs of the peptides and their hemolytic activities against human red blood cells
| Peptide | MIC (μg/ml)a
|
% Hemolysis at 180 μg/ml | |
|---|---|---|---|
| P. aeruginosa ATCC 27853 | Gentamicin-resistant A. baumannii | ||
| Amphipathic-l | 45.0 (41.4) | 11.2 (8.9) | 100 |
| Amphipathic-d | 5.6 (3.6) | 5.6 (3.6) | 0 |
| Gentamicin | 3.12 | >100 | Not determined |
The activities of the peptides against bacterial spheroplasts are indicated in parentheses.
FIG. 1.
(A) In vitro time-kill curve of amphipathic-d at its MIC; (B and C) membrane depolarization in bacteria (filled triangles) and bacterial spheroplasts (filled circles) of gentamicin-resistant A. baumannii induced by amphipathic-d as a function of the peptide concentration (B) and time (C). The diastereomer at its MIC was added to bacteria or spheroplasts that had been preequilibrated with the fluorescent dye diS-C3-5 for 60 min. Fluorescence recovery was measured 1 to 120 min (at 5-min intervals) after the diastereomer was mixed with the bacteria, and its maximum was recorded. The arrows in panel B indicate the peptide's MICs.
In order to test whether amphipathic-d obtained its biological function through bacterial plasma membrane perturbation, we performed transmembrane potential-depolarizing experiments with the intact bacteria and their spheroplasts using the potential sensitive dye diS-C3-5 (the protocol has been described in detail elsewhere [20, 32]). Figure 1B and C shows the concentration- and time-dependent dissipations of the transmembrane potential of A. baumannii, respectively. These data reveal a direct correlation between the kinetics of potential depolarization and killing of bacteria (Fig. 1A), which supports the notion that the bacterial membrane is a major target for the peptides. Furthermore, the finding of similar activities against the cell wall-deficient spheroplasts suggests a minor role for the bacterial wall in the killing mechanism. Similar results were obtained with P. aeruginosa and therefore are not shown.
Confocal fluorescence microscopy (Olympus FV500 confocal laser scanning microscope) was used to visualize the localization of rhodamine-labeled amphipathic-d (labeled specifically at the N terminus by a protocol described before [21]) when it was bound to P. aeruginosa (data not shown). Interestingly, the peptide initially bound primarily mostly to the septums of two separating bacteria, but it finally became equally distributed within the bacterial cytoplasm. Transmission electron microscopy (the protocol is described elsewhere [1]) revealed damage to the bacterial cell wall (data not shown).
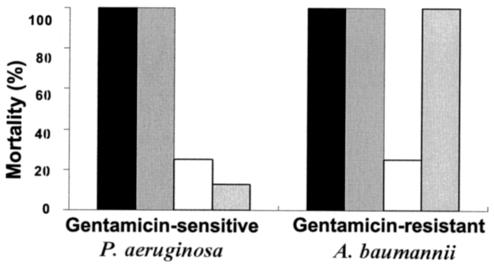
Most importantly, the peptides were tested intravenously for their activities against female CD1 mice that were infected with inocula of 106 CFU of bacteria (n = 8). Treated mice were systemically injected with a daily dose of 6 mg/kg of body weight, divided into two injections of 3 mg/kg at 12-h intervals, starting 1 h after inoculation. The results, shown in Fig. 2, demonstrate that only the diastereomer cured mice infected with both types of bacteria. Inoculated mice became weak and less active as early as 1 day after inoculation (and treatment). The maximum loss of body weight occurred on day 2. Physical improvement of diastereomer-treated mice was noted on day 3, and 5 days after the bacterial challenge, all the survivors displayed normal activity and had recovered their initial weight. Nearly all deaths occurred between days 2 and 3 postinoculation with P. aeruginosa and between days 3 and 4 postinoculation with gentamicin-resistant A. baumannii. In order to decrease the influence of the immune system, all the mice were rendered transiently neutropenic by intravenously injecting 150 and 100 mg of cyclophosphamide per kg on days 0 and 3, respectively (12, 15).
FIG. 2.
Mortality of infected mice after systemic treatment with phosphate-buffered saline (black bars), amphipathic-l (gray bars), amphipathic-d (empty bars), and gentamicin (dotted [rightmost] bars). Mortality was monitored for at least 10 days posttreatment.
The acute toxicities of the two peptides were also examined by intravenously injecting each mouse (n = 8) with two doses per day of a 0.25-ml solution containing peptide at 3 mg/kg (no mortality), 6 mg/kg (20% mortality), or 9 mg/kg (80% mortality) for 5 days. Note that no mortality was observed after doses of 6 mg/kg dissolved in 0.9 ml of buffer were injected. Blood samples were taken from the mice a week after the injection of amphipathic-d (3 mg/kg twice daily). The results of all differential and biochemistry tests (i.e., tests for neutrophil, lymphocyte, monocyte, eosinophil, and basophil counts and creatine phosphokinase, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and creatinine levels) were within the range of normal values. The Weizmann Institute Animal Protection Committee approved all the protocols used with the animal models. The animals had access to food and water throughout all experiments. Furthermore, the animals were acclimatized to our animal facilities for 1 week before the experiments began.
In summary, this study demonstrates that the activity of a diastereomer, but not an all-l-amino-acid antimicrobial peptide, was preserved in blood and that the peptide cured animals infected with bacteria (including a gentamicin-resistant strain). The data suggest that although the diastereomer initially interacts with the bacterial cell wall, it is mainly targeted toward the plasma membrane because (i) the potencies toward intact bacteria and spheroplasts were similar (Table 1), (ii) the diastereomer depolarized the transmembrane potential of the bacteria at the same rate and concentration at which it showed biological activity, and (iii) the diastereomer acted similarly against both resistant and nonresistant bacteria. Although our data suggest that the protection of the mice was due to killing of the bacteria, previous studies have shown that positively charged antimicrobial peptides can bind to lipopolysaccharide (LPS) and neutralize the LPS-stimulated inflammatory response by macrophages (i.e., cytokine production) (10, 24). It is possible, therefore, that the diastereomer further binds to and neutralizes the LPS released from the damaged bacteria. Further advances need to be made in order to overcome the toxicities of such compounds. These may include modification of their sequences, injection of the peptide at a low concentration, or the use of delivery systems.
Acknowledgments
This research was supported by the European Community. Y.S. is the Harold S. and Harriet B. Brady Professorial Chair in Cancer Research.
REFERENCES
- 1.Avrahami, D., Z. Oren, and Y. Shai. 2001. Effect of multiple aliphatic amino acids substitutions on the structure, function, and mode of action of diastereomeric membrane active peptides. Biochemistry 40:12591-12603. [DOI] [PubMed] [Google Scholar]
- 2.Bessalle, R., A. Kapitkovsky, A. Gorea, I. Shalit, and M. Fridkin. 1990. All-d-magainin: chirality, antimicrobial activity and proteolytic resistance. FEBS Lett. 274:151-155. [DOI] [PubMed] [Google Scholar]
- 3.Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 4.Chen, J., T. J. Falla, H. Liu, M. A. Hurst, C. A. Fujii, D. A. Mosca, J. R. Embree, D. J. Loury, P. A. Radel, C. Cheng Chang, L. Gu, and J. C. Fiddes. 2000. Development of protegrins for the treatment and prevention of oral mucositis: structure-activity relationships of synthetic protegrin analogues. Biopolymers 55:88-98. [DOI] [PubMed] [Google Scholar]
- 5.Cirioni, O., A. Giacometti, R. Ghiselli, F. Mocchegiani, A. Fineo, F. Orlando, M. S. Del Prete, M. Rocchi, V. Saba, and G. Scalise. 2002. Single-dose intraperitoneal magainins improve survival in a gram-negative-pathogen septic shock rat model. Antimicrob. Agents Chemother. 46:101-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devine, D. A., and R. E. Hancock. 2002. Cationic peptides: distribution and mechanisms of resistance. Curr. Pharm. Des. 8:703-714. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein, B. P., J. Wei, K. Greenberg, and R. Novick. 1998. Activity of nisin against Streptococcus pneumoniae, in vitro, and in a mouse infection model. J. Antimicrob. Chemother. 42:277-278. [PubMed] [Google Scholar]
- 8.Gunseren, F., L. Mamikoglu, S. Ozturk, M. Yucesoy, K. Biberoglu, N. Yulug, M. Doganay, B. Sumerkan, S. Kocagoz, S. Unal, S. Cetin, S. Calangu, I. Koksal, H. Leblebicioglu, and M. Gunaydin. 1999. A surveillance study of antimicrobial resistance of gram-negative bacteria isolated from intensive care units in eight hospitals in Turkey. J. Antimicrob. Chemother. 43:373-378. [DOI] [PubMed] [Google Scholar]
- 9.Hancock, R. E., and G. Diamond. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402-410. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, R. E., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingham, A., M. Ford, R. J. Moore, and M. Tizard. 2003. The bacteriocin piscicolin 126 retains antilisterial activity in vivo. J. Antimicrob. Chemother. 51:1365-1371. [DOI] [PubMed] [Google Scholar]
- 12.Joly-Guillou, M. L., M. Wolff, J. J. Pocidalo, F. Walker, and C. Carbon. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 41:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehrer, R. I., and T. Ganz. 1999. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 11:23-27. [DOI] [PubMed] [Google Scholar]
- 14.McPhee, J. B., S. Lewenza, and R. E. Hancock. 2003. Cationic antimicrobial peptides activate a two-component regulatory system, PmrA-PmrB, that regulates resistance to polymyxin B and cationic antimicrobial peptides in Pseudomonas aeruginosa. Mol. Microbiol. 50:205-217. [DOI] [PubMed] [Google Scholar]
- 15.Navon-Venezia, S., R. Feder, L. Gaidukov, Y. Carmeli, and A. Mor. 2002. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46:689-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolas, P., and A. Mor. 1995. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 49:277-304. [DOI] [PubMed] [Google Scholar]
- 17.Oren, Z., J. Hong, and Y. Shai. 1997. A repertoire of novel antibacterial diastereomeric peptides with selective cytolytic activity. J. Biol. Chem. 272:14643-14649. [DOI] [PubMed] [Google Scholar]
- 18.Pacor, S., A. Giangaspero, M. Bacac, G. Sava, and A. Tossi. 2002. Analysis of the cytotoxicity of synthetic antimicrobial peptides on mouse leucocytes: implications for systemic use. J. Antimicrob. Chemother. 50:339-348. [DOI] [PubMed] [Google Scholar]
- 19.Pag, U., M. Oedenkoven, N. Papo, Z. Oren, Y. Shai, and H. G. Sahl. 2004. In vitro activity and mode of action of diastereomeric antimicrobial peptides against bacterial clinical isolates. J. Antimicrob. Chemother. 53:230-239. [DOI] [PubMed] [Google Scholar]
- 20.Papo, N., Z. Oren, U. Pag, H. G. Sahl, and Y. Shai. 2002. The consequence of sequence alteration of an amphipathic alpha-helical antimicrobial peptide and its diastereomers. J. Biol. Chem. 277:33913-33921. [DOI] [PubMed] [Google Scholar]
- 21.Pouny, Y., D. Rapaport, A. Mor, P. Nicolas, and Y. Shai. 1992. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues with phospholipid membranes. Biochemistry 31:12416-12423. [DOI] [PubMed] [Google Scholar]
- 22.Russell, P. E., R. J. Milling, and K. Wright. 1995. The need for new antibiotics: possible ways forward, p. 67-85. In P. A. Hunter, G. K. Darby, and N. J. Russell (ed.), Fifty years of antimicrobials: past perspectives and future trends. Cambridge University Press, Cambridge, United Kingdom.
- 23.Scott, M. G., and R. E. Hancock. 2000. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit. Rev. Immunol. 20:407-431. [PubMed] [Google Scholar]
- 24.Scott, M. G., H. Yan, and R. E. Hancock. 1999. Biological properties of structurally related alpha-helical cationic antimicrobial peptides. Infect. Immun. 67:2005-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shai, Y. 2002. From innate immunity to de-novo designed antimicrobial peptides. Curr. Pharm. Des. 8:715-725. [DOI] [PubMed] [Google Scholar]
- 26.Shai, Y. 1999. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1462:55-70. [DOI] [PubMed] [Google Scholar]
- 27.Shai, Y. 2002. Mode of action of membrane active antimicrobial peptides. Biopolymers 66:236-248. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg, D. A., M. A. Hurst, C. A. Fujii, A. H. Kung, J. F. Ho, F. C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 30.Wade, D., A. Boman, B. Wahlin, C. M. Drain, D. Andreu, H. G. Boman, and R. B. Merrifield. 1990. All-d amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. USA 87:4761-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weidenmaier, C., S. A. Kristian, and A. Peschel. 2003. Bacterial resistance to antimicrobial host defenses—an emerging target for novel antiinfective strategies? Curr. Drug Targets 4:643-649. [DOI] [PubMed] [Google Scholar]
- 32.Wu, M., E. Maier, R. Benz, and R. E. Hancock. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235-7242. [DOI] [PubMed] [Google Scholar]
- 33.Zasloff, M. 2002. Antimicrobial peptides in health and disease. N. Engl. J. Med. 347:1199-1200. [DOI] [PubMed] [Google Scholar]