Abstract
Background
Removal of the appendix might induce physiological changes in the gastrointestinal tract, and subsequently play a role in carcinogenesis. Therefore, we conducted a nationwide register-based cohort study in Sweden to investigate whether appendectomy is associated with altered risks of gastrointestinal cancers.
Methods
A population-based cohort study was conducted using the Swedish national registries, including 480,382 eligible patients followed during the period of 1970–2009 for the occurrence of site-specific gastrointestinal cancer (esophageal/gastric/colon/rectal cancer). Outcome and censoring information was collected by linkage to health and demography registers. We examined the incidence of appendectomy in Sweden using data from 1987–2009. We also calculated standardized incidence ratios (SIRs) with 95% confidence intervals (CIs) to estimate the relative gastrointestinal cancer risk through comparison to the general population.
Results
We noted an overall decrease in the age-standardized incidence of appendectomy among the entire Swedish population from 189.3 to 105.6 per 100,000 individuals between 1987 and 2009. Grouped by different discharge diagnosis, acute appendicitis, incidental appendectomy, and entirely negative appendectomy continuously decreased over the study period, while the perforation ratio (18%–23%) stayed relatively constant. Compared to the general population, no excess cancer risk was observed for gastrointestinal cancers under study with the exception of a marginally elevated risk for esophageal adenocarcinoma (SIR 1.32, 95% CI 1.09–1.58).
Conclusions
In Sweden, the incidence of appendectomy and acute appendicitis has decreased during 1987–2009. No excess gastrointestinal cancer risks were observed among these appendectomized patients, with the possible exception of esophageal adenocarcinoma.
Introduction
Appendectomy is a common procedure, which is usually performed after a clinical suspicion of acute appendicitis. In addition, it can also be conducted incidentally to other operations—that is, the appendix is removed without evidence of disease, as a preventive measure when a subject is undergoing another operation. Incidental appendectomy most commonly occurs during hysterectomy or salpingectomy, and therefore is more prevalent in females [1, 2]. Although few studies have documented this in detail, current data show a slow, long-term decline in incidence, at least in Western countries [3, 4, 5].
The main reasons for the regular use of this operation include: 1) appendicitis is a potential deadly disease; 2) historically, the vermiform appendix was defined as a vestigial organ; and 3) the loss of the appendix is thought to have few, if any, long-term effects. However, recent data indicate that the appendix might serve as a reservoir for the colonic microbiome, and therefore the excision of the appendix may affect the components of gastrointestinal microbiome, which ultimately may play a role in the development of gastrointestinal cancers [6, 7]. Although inconsistent, a few epidemiological studies have reported altered risks of cancer, especially stomach cancer, among patients with an appendectomy history [8, 9]. Moreover, a recent trial suggested that uncomplicated acute appendicitis could be treated with antibiotics rather than appendectomy, highlighting the importance of assessing the sequelae of appendectomy [10].
We revisited the hypothesis that individuals who received appendectomy would experience an increased risk of gastrointestinal cancers, possibly due to the disruption of the normal colonic microbiome, by using our nationwide register-based appendectomy cohort. In addition, we also aimed to examine the secular trend in the incidence of appendectomy among the entire Swedish population.
Methods
Databases
Based on the National Patient Registry of Sweden, a cohort consisting of patients born prior to 2003 who received appendectomy during January 1, 1970 to December 31, 2009 was established. The Swedish surgery codes for open or laparoscopic appendectomy procedure were 4510, 4511, 4517, 0058 during the time period 1970–1996, and JEA00, JEA01, JEA10 for 1997 and onwards. The National Patient Registry database was created initially in 1964/1965, and became nationwide since 1987, providing data including national registration number, which is a unique identifier for each individual, personal information (e.g. age, sex, county), dates of admission and discharge, surgical procedure codes, and medical diagnoses. External reviews demonstrated that, more than 50% of all surgeries were reported to the National Patient Registry since 1976, and the complete coverage (100%) was obtained in 1987 [11].
The dataset was then linked to the Swedish Cancer Registry, where all incident gastrointestinal cancer cases (Swedish International Classification of Diseases (ICD) (version 7) = 150 for esophageal cancer, 151 for gastric cancer, 1530-1533/ 1538/1539 for colon cancer (except appendix cancer), and 1540 for rectal cancer) [12] were ascertained. Esophageal cancer was sub-grouped as esophageal adenocarcinoma and esophageal squamous-cell carcinoma, according to the histopathological diagnoses (patho-anatomic diagnosis, PAD, ‘096’ for esophageal adenocarcinoma and ‘146’ for esophageal squamous-cell carcinoma). Gastric cancer coded as ICD-7 1511 was sub-classified as cardia cancer, while the others were counted as non-cardia gastric cancer indicating that the malignancy originated from any other subsite than the cardia. The coding for cardia cancer was introduced in 1969, and widely applied since 1970 [13]. Colon cancer was sub-divided into right-sided colon cancer (ICD7 1530–1531) and left-sided colon cancer (ICD7 1532–1533). The Swedish Cancer Register was established in 1958, with a completeness rate of 98% for gastrointestinal cancers [14]. Cross linkage with the Death Register and Emigration Register provided the necessary information for censoring follow-up.
Study design
We identified a total of 501,160 patients who underwent appendectomy in Sweden during 1970–2009. After excluding 20,778 patients with conflicting information (having emigration/death date recorded before the appendectomy date), the final cohort consisted of 480,382 eligible subjects. In each sub-cohort which was established specifically for evaluating certain gastrointestinal cancer risk (esophageal/ gastric/colon/ rectum cancer), we further excluded the patients who received a diagnosis of such cancer before the hospitalization for appendectomy. Follow-up for each participant was then started from the date of appendectomy, until certain cancer occurrence, migration out of Sweden, death, or the end of follow-up (December 31, 2009), whichever occurred first.
Moreover, since non-specific symptoms (e.g. abdominal pain) could be present due to an as-yet-undetected cancer, which may consequently increase the likelihood of having appendectomy, selection bias should be taken into consideration for assessing the post-appendectomy cancer risks. Therefore, for all further analyses, the first year of follow-up, as well as the corresponding events happening during that period, was disregarded. This study was approval by the Regional Ethical Review Board in Stockholm. All the individual records were anonymized and de-identified prior to analysis.
Appendectomy subgroups
According to the discharge diagnosis, we categorized the appendectomy patients into different subgroups: 1) perforated or abscessed appendicitis group involved all patients with the discharge diagnoses coded as (ICD-8) 54000–54003, 54302, or (ICD-9) 540A, 540B or (ICD-10) K35.0, K35.1, K38.3 in their medical records; 2) acute non-perforated appendicitis group consisted of patients having diagnosis codes of (ICD-8) 54090–54208, or (ICD-9) 540X, 541, 542, or (ICD-10) K35.9, K36, K37); 3) entirely negative appendectomy group with patients who ended up with a diagnosis of mesenterial adenititis or unspecific abdominal pain, and without any other surgical procedure appearing in the same hospitalization record; and 4) incidental appendectomy group was defined as patients without appendicitis diagnosis but having additional surgical procedure(s) performed at the same time as the appendectomy.
Statistical methods
For describing the temporal trends in the whole Sweden, we focused on the appendectomy patients recorded after 1986 (n = 269,185). We used Sweden resident population estimates for the years 1987–2009 to calculate annual incidence rates of appendectomy, standardized by world standard population (Segi 1960) and presented as appendectomy cases per 100,000 individuals per year. Trends in incidence during the period were analyzed by Poisson regression. We also studied the secular trends among different subgroups (by sex, age group, or underlying diagnoses) separately.
To estimate relative risks of each gastrointestinal cancer, we calculated standardized incidence ratios (SIRs) using the general population as reference (1970–2009). The expected numbers were estimated by multiplying the calendar period-, age-(5 year intervals) and sex-specific follow-up time in the cohort with the corresponding incidence rates derived from the entire Swedish population. We calculated 95% confidence intervals (CIs) under the assumption that the observed number of cases followed a Poisson distribution. Further analyses were stratified by underlying diagnoses, sex, age group (0–19 years, 20–39 years, 40–59 years, 60 years and above), and the duration of follow-up (1–4, 5–9, 10–14, 15–24 and 25+). We conducted trend tests by including the age, follow-up years as continuous variables in Poisson regression models, using the expected number of cases as offset. The fit of the regression model was checked using Pearson’s Chi-square statistic. All analyses were repeated using completely nationwide data only (1987–2009) as sensitivity analyses.
A P value less than 0.05 was considered to be statistically significant. All statistical analyses were performed using SAS 9.4 software (Cary, NC).
Results
Incidence of appendectomy in Sweden, during 1987–2009
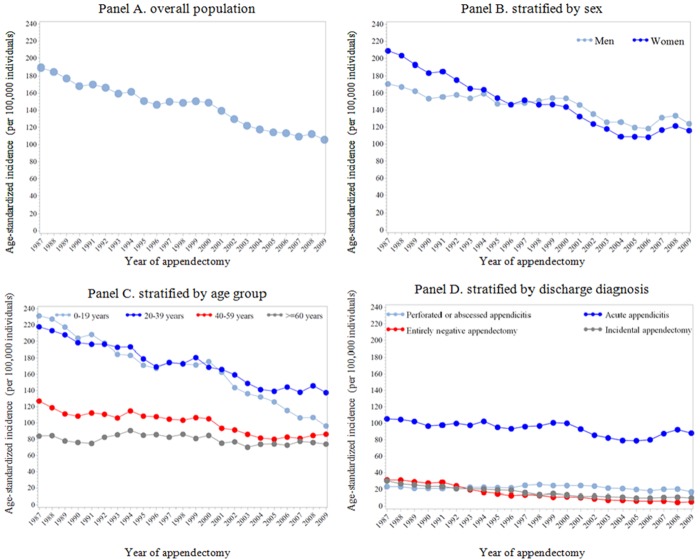
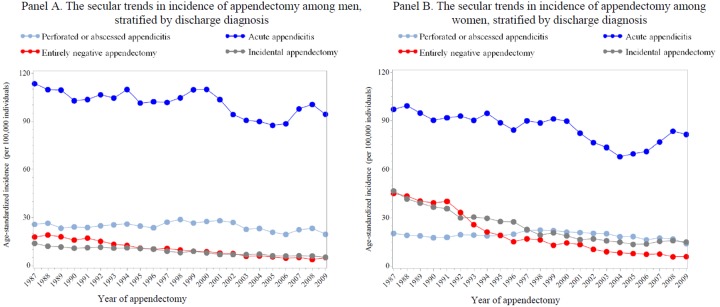
In total, 269,185 patients who underwent appendectomy during 1987–2009 were identified. Fig 1 illustrates the annual age-standardized incidence of appendectomy among the entire Swedish population over a 22-year span. An overall decreasing trend was noted (Fig 1, Panel A, from 189.3 to 105.6per 100,000 individuals between 1987 and 2009) −− the annual rate of decrease in age-standardized incidence was 2.5% (95% Cl 2.0–3.0%). Between 1987 and 1996, the age-standardized incidence rates of appendectomy for women were higher than those for men, while the opposite pattern was obtained for years after 1997 (Fig 1, Panel B). We therefore observed a more apparently decreasing trend among women, relative to men (P for difference < 0.001). The highest age-standardized incidence rate of appendectomy, together with the sharpest decline (from 236.2 to 96.4 per 100,000 between 1987 and 2009, corresponding to an annual decreasing rate of 3.5%; 95% CI 3.0–3.9%<), was observed among patients aged 0–19 years; but for individuals above 60 years old, non-significantly decreasing trends were observed (Fig 1, Panel C). Further stratified by sex, similar age group-specific temporal trends were observed. Grouped by different discharge diagnoses (Fig 1, Panel D), acute appendicitis, incidental appendectomy, and entirely negative appendectomy significantly decreased over the study period, while appendectomies attributed to perforated appendicitis remained unchanged. Notably, despite similar changing patterns regarding the incidence of acute and perforated appendicitis in both sex groups, for appendectomies due to other than appendicitis diagnosis, the annual reduction rates seemed to be higher among women, relative to men (P for difference = 0.08 for entirely negative appendectomy, and = 0.10 for incidental appendectomy) (Fig 2).
Fig 1. The age-standardized incidence of appendectomy in Sweden, during 1987–2009 (n = 269,185).
Panel A. the age-standardized incidence of appendectomy over years among overall population; Panel B. the age-standardized incidence of appendectomy over years, stratified by sex; Panel C. the age-standardized incidence of appendectomy over years, stratified by age group; Panel D. the age-standardized incidence of appendectomy over years, stratified by discharge diagnosis.
Fig 2. The secular trends in age-standardized incidence of appendectomy among men and women, stratified by discharge diagnosis.
Panel A. the secular trends in age-standardized incidence of appendectomy among men, stratified by discharge diagnosis; Panel B. the secular trends in age-standardized incidence of appendectomy among women, stratified by discharge diagnosis.
Gastrointestinal cancer risks among appendectomy patients
For cancer-risk assessment, during 1970–2009, 480,382 appendectomy patients were identified, with a mean age of 32 years (Table 1). More women were included than men (men: women = 1:1.2) in the whole cohort, which was probably driven by the obvious female-dominance in the other diagnoses subcohort. In the appendicitis cohort, however, 55% subjects were male. Regarding the underlying discharge diagnoses, acute (non-perforated) appendicitis was the most common reason for appendectomy. The entirely negative appendectomy was more frequent among young women (0–29 years), whereas incidental appendectomy tended to prevail among older females (above 40 years old).
Table 1. Description of appendectomies in Sweden during 1970–2009, grouped by underlying diagnoses.
| All appendectomy cohort | Appendicitis subcohort | Other diagnoses subcohort | |
|---|---|---|---|
| Number | 480,382 | 327,496 | 152,886 |
| Age, mean± SD (years) | 32.5±19.6 | 30.2±18.7 | 37.4±20.6 |
| Age group, n (%) | |||
| 0–19 years | 157,024(32.7) | 120,807 (36.9) | 36,217 (23.7) |
| 20–39 years | 169823(35.3) | 120,488 (36.8) | 49,335 (32.3) |
| 40–59 years | 95527(19.9) | 53,841 (16.4) | 41,686 (27.3) |
| 60 years and above | 58008(12.1) | 32,360 (9.9) | 25,648 (16.7) |
| Sex, n (%) | |||
| Men | 217,710 (45.3) | 180,172 (55.0) | 37,538 (24.6) |
| Women | 262,672 (54.7) | 147,324 (45.0) | 115,348 (75.4) |
| Calendar year of appendectomy, n (%) | |||
| 1970–1979 | 113,499 (23.6) | 62,270 (19.0) | 51229 (33.5) |
| 1980–1989 | 139,977 (29.1) | 88,966 (27.2) | 51011 (33.4) |
| 1990–1999 | 124,677(26.0) | 92,167 (28.1) | 32510 (21.3) |
| 2000–2009 | 102,229(21.3) | 84,093 (25.7) | 18136 (11.8) |
| Duration of follow-up | |||
| mean± SD (years) | 18.6± 10.9 | 17.5± 10.6 | 20.8±11.2 |
| n (%) | |||
| 0–4 years | 66,417 (13.8) | 46,989 (14.4) | 19,428 (12.7) |
| 5–14 years | 127,550 (26.6) | 99,038 (30.2) | 28,512 (18.6) |
| 15–24 years | 134,484 (28.0) | 92,666 (28.3) | 41,818 (27.4) |
| > = 25 years | 151,931 (31.6) | 88,803 (26.1) | 63,128 (41.3) |
In Table 2, SIRs together with their 95% CIs are presented for each type of gastrointestinal cancers in all appendectomized patients, as well as stratified by discharge diagnoses (appendicitis/ other diagnoses), after excluding the first year of follow-up. We observed no association for cancers of the stomach, colon, or rectum. We found divergent results for esophageal cancers by histopathological type. Appendectomy was associated with an increased risk for esophageal adenocarcinoma (SIR 1.32, 95% CI 1.09–1.58), but a reduced risk for esophageal squamous-cell carcinoma (SIR 0.79, 95% CI 0.65–0.95). Subgroup analysis indicated this reduction to be limited to the stratum of patients with appendicitis diagnosis (SIR 0.71, 95% CI 0.54–0.91), and males (SIR 0.74, 95% CI 0.57–0.96). A sensitivity analysis restricted to complete nationwide data during 1987–2009 revealed similar results as in the primary analysis (with similar point estimates), albeit with wider CIs (S1 Table).
Table 2. Standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) for gastrointestinal cancers in appendectomy patients with different discharge diagnoses.
| All appendectomy cohort | Appendicitis | Other diagnoses | ||||
|---|---|---|---|---|---|---|
| Observed cases* | SIR (95% CI)§ | Observed cases* | SIR (95% CI)§ | Observed cases* | SIR (95% CI)§ | |
| Esophageal cancer, all | 255 | 1.00 (0.88–1.13) | 153 | 0.93 (0.79–1.09) | 102 | 1.14 (0.93–1.38) |
| Esophageal adenocarcinoma | 118 | 1.32 (1.09–1.58) | 79 | 1.25 (0.99–1.55) | 39 | 1.50 (1.07–2.05) |
| Esophageal squamous-cell carcinoma | 114 | 0.79 (0.65–0.95) | 62 | 0.71 (0.54–0.91) | 52 | 0.92 (0.69–1.21) |
| Gastric cancer, all | 831 | 1.00 (0.93–1.07) | 496 | 1.01 (0.92–1.10) | 335 | 0.98 (0.87–1.09) |
| Non-cardia gastric cancer | 685 | 0.99 (0.92–1.07) | 393 | 0.99 (0.90–1.10) | 292 | 0.99 (0.88–1.11) |
| Cardia cancer | 146 | 1.06 (0.89–1.25) | 103 | 1.11 (0.91–1.35) | 43 | 0.95 (0.69–1.28) |
| Colon cancer, all | 2473 | 1.03 (0.99–1.07) | 1373 | 1.00 (0.95–1.06) | 1100 | 0.98 (0.92–1.04) |
| Right-sided colon cancer | 1282 | 1.04 (0.99–1.10 | 693 | 1.05 (0.97–1.13) | 589 | 1.04 (0.95–1.12) |
| Left-sided colon cancer | 860 | 0.96 (0.90–1.03) | 498 | 0.99 (0.91–1.08) | 362 | 0.92 (0.83–1.02) |
| Rectal cancer | 1280 | 0.98 (0.93–1.04) | 712 | 0.94 (0.87–1.01) | 568 | 0.94 (0.87–1.01) |
*The first year of observation and corresponding events were excluded.
§ Observed to expected number of cancer cases, based on age- (5-year strata), calendar year- (5-year strata) and sex-specific incidence data in the total Swedish population. Ninety-five percent CIs of SIRs were calculated by assuming that observed cancer occurrence followed a Poisson distribution.
Stratified by time since operation (Table 3), appendectomy was associated with an increased cardia cancer risk (SIR 1.63, 95% CI 1.12–2.31) in the first 4 years of follow-up. However, this excess quickly disappeared after the initial observation period. We also observed elevated risks for esophageal adenocarcinoma (SIR 1.48, 95% CI 1.06–2.02) and right-sided colon cancer (SIR 1.12, 95% CI 1.05–1.20) between 5–14 years after appendectomy. Whereas for long-term observation (more than 15 years since appendectomy), no excess risk was noted for all of studied gastrointestinal cancers except esophageal adenocarcinoma (≥ 25 years, SIR 1.50, 95% CI 1.06–2.07).
Table 3. Standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) for gastrointestinal cancers in appendectomy patients, stratified by duration of follow-up.
| 1–4 years | 5–14 years | 15–24 years | ≥25 years | Trend test | |||||
|---|---|---|---|---|---|---|---|---|---|
| Observed cases* | SIR (95% CI)§ | Observed cases* | SIR (95% CI)§ | Observed cases* | SIR (95% CI)§ | Observed cases* | SIR (95% CI)§ | P value | |
| Esophageal cancer, all | 37 | 1.00 (0.70–1.38) | 80 | 0.92 (0.73–1.15) | 73 | 0.97 (0.76–1.22) | 65 | 1.17 (0.90–1.49) | 0.30 |
| Esophageal adenocarcinoma | 12 | 1.15 (0.59–2.01) | 40 | 1.48 (1.06–2.02) | 29 | 1.06 (0.71–1.52) | 37 | 1.50 (1.06–2.07) | 0.73 |
| Esophageal squamous-cell carcinoma | 18 | 0.79 (0.47–1.25) | 36 | 0.70 (0.49–0.96) | 38 | 0.90 (0.64–1.24) | 22 | 0.80 (0.50–1.22) | 0.60 |
| Gastric cancer, all | 171 | 1.11 (0.95–1.29) | 309 | 0.97 (0.87–1.09) | 214 | 0.95 (0.83–1.09) | 137 | 1.00 (0.84–1.18) | 0.31 |
| Non-cardia gastric cancer | 139 | 1.04 (0.87–1.23) | 265 | 0.99 (0.87–1.11) | 176 | 0.96 (0.83–1.12) | 105 | 0.99 (0.81–1.19) | 0.62 |
| Cardia cancer | 32 | 1.63 (1.12–2.31) | 44 | 0.93 (0.67–1.24) | 38 | 0.91 (0.65–1.26) | 32 | 1.10 (0.75–1.55) | 0.20 |
| Colon cancer, all | 342 | 1.05 (0.94–1.17) | 886 | 1.12 (1.05–1.20) | 711 | 0.98 (0.91–1.06) | 534 | 0.95 (0.87–1.03) | 0.01 |
| Right-sided colon cancer | 177 | 1.11 (0.95–1.28) | 451 | 1.15 (1.05–1.26) | 358 | 0.98 (0.88–1.08) | 296 | 0.96 (0.85–1.07) | 0.01 |
| Left-sided colon cancer | 108 | 0.88 (0.73–1.07) | 301 | 1.02 (0.91–1.15) | 257 | 0.97 (0.85–1.09) | 194 | 0.92 (0.79–1.06) | 0.77 |
| Rectal cancer | 186 | 1.02 (0.88–1.18) | 442 | 1.00 (0.91–1.10) | 369 | 0.94 (0.85–1.04) | 283 | 0.99 (0.88–1.11) | 0.56 |
*The first year of observation and corresponding events were excluded.
§ Observed to expected number of cancer cases, based on age- (5-year strata), calendar year- (5-year strata) and sex-specific incidence data in the total Swedish population. Ninety-fiver percent CIs of SIRs were calculated by assuming that observed cancer occurrence followed a Poisson distribution.
To evaluate the possibility of confounding due to differential distributions between the appendectomy cohort and the general population with regard to addictive substance (including tobacco and alcohol), which are thought to be important risk factors for some gastrointestinal cancers [15, 16], we also calculated SIRs for tobacco- (lung cancer) and alcohol-related (liver cancer) cancers [15, 17]. The relative risks of lung cancer among the appendectomized patients was almost equal to unity (SIR 1.03, 95% CI 0.98–1.09), whereas the corresponding figure for liver cancer risk showed a 16% reduction (SIR 0.84, 95% CI 0.70–0.98). This reduction was mainly confined to subgroups who were men (SIR 0.72, 95%CI 0.57–0.89), or with appendicitis diagnosis (SIR 0.82, 95% CI 0.68–0.98).
Discussion
Using a large, nationwide, register-based cohort study with a long follow-up duration (mean follow-up of 18.6 years), we confirmed that in Sweden, the incidence of appendectomy has decreased during last few decades. In addition, compared to the general Swedish population, a slightly increased risk of esophageal adenocarcinoma, contrasted by a reduced esophageal squamous-cell carcinoma risk, was observed among the appendectomized patients; whereas no differences were noted for gastric cancer, colon cancer, and rectal cancer.
For decades, the decreased incidence of appendectomy has been documented, both in Sweden [5] and other Western countries [3, 4]. This decline is most pronounced in younger age groups, and usually accompanied with a falling incidence of appendicitis. Consistent with prior reports [3, 4], both acute (non-perforated) and perforated appendicitis illustrated a male predominance. However, unlike recent US studies [18, 19] which reported an increased incidence of appendicitis, as well as a decreased odds of perforation, our data indicated that the incidence of appendectomy due to acute appendicitis kept decreasing, and the perforation ratio, nevertheless, stayed almost constant over years (about 18%-25%) among operated appendicitis patients. One possible explanation for the inconsistency could be that choice for surgery for acute appendicitis became less frequent in Sweden than before. Our data, however, did not support this statement since a relatively constant operation rate among appendicitis patients was observed during the study period. We noted a reduction in the incidence of entirely negative appendectomy. The detected pattern suggests an improved diagnostic accuracy for non-perforated appendicitis, which may be attributed to more and more frequent use of ultrasonography and computed tomography scanning. The perforation ratio, however, remained unchanged since it’s probably more related to timely surgical intervention, rather than accurate diagnosis [3, 20]. In line with the notion that women with suspected appendicitis benefit the most from pre-operative imaging tests [21, 22], our results suggested a steeper decline of negative appendectomy among females relative to males—the entirely negative appendectomy used to be 3 times more common among women than men; but the sex difference has gradually disappeared, resulting in a low incidence rate of 5 per 100,000 individuals in 2009 for both sexes. In addition, incidental appendectomy was widely performed among old females in the earliest study period because the prevailing view at that time was that it could eliminate the future risk of appendicitis in these elderly women which was associated with a considerable possibility of perforation and death, without adding any additional morbidity [23]. Moreover, gynecologists have always had difficulties with differentiating appendicitis from diseases of the right ovarium and salpinx before the era of ultrasonography which made it tempting to remove the appendix during gynecological surgeries. However, such prophylactic removals gave rise to controversy owning to conflicting results from subsequent clinical studies [24].
Few studies have assessed the association between appendectomy and the risk of subsequent gastrointestinal cancers, and the results are inconsistent. A study using Swedish Patient and Cancer registers (1965–1993) with such a focus [8] found a significantly increased risk of stomach cancer but a significantly reduced risk of colon cancer after appendectomy. However, that study was limited to subjects who had their appendix removed before 20 years of age. With the limited follow-up duration (the oldest patient was younger than 48 years old), the study was not well powered to examine individual cancer risks. A Danish study [9] also showed elevated gastric cancer risk after appendectomy, but no altered risks were noted for colon cancer. Conversely, a recent study in Taiwan [25] reported a 14% higher incidence of colorectal cancer among appendectomized patients when compared to matched controls randomly selected from inpatient claims data. The explanation for this discrepancy remains unclear. Besides the geographic differences, possible interpretations for these variations might include different study designs, inadequate statistical precision owing to limited sample size or short follow-up duration, and potential systematic errors, i.e. ascertainment or selection bias. In the current study, compared to the general Swedish population, we only observed a slightly elevated risk (32%) of esophageal adenocarcinoma among appendectomized patients, whereas the risk for esophageal squamous cell carcinoma was noted to be decreased. For all other gastrointestinal cancers, no excess risks could be noted in the study population.
To date, we found no other evidence to support the observed association between appendectomy and subsequent esophageal adenocarcinoma risk. Since in our study there was no obvious tendency of increased cancer risks with longer follow-up duration, it’s very unlikely that the observed linkage could be causative. On the other hand, with the knowledge that obesity could diminish diagnostic accuracy in the diagnosis of acute appendicitis in both children [26] and adults [27], it’s possible that obese individuals experience higher risk of appendectomies during their life time, compared to non-obese individuals. As obesity is a well-established risk factor for esophageal adenocarcinoma, the observed excess risk of esophageal adenocarcinoma among appendectomized patients could reflect the confounding effect of obesity.
The significantly reduced risk of esophageal squamous-cell carcinoma among appendectomized patients was also unexpected. Although we were not able to adjust for those potential confounding factors in the SIR calculation, our additional analysis on main tobacco-related cancer (lung cancer) and main alcohol-related cancer (liver cancer) offered a clue about the differential distribution of these factors in the study population. Despite a few previous studies reported that smoking or passive smoking in children was associated with higher possibility of having acute appendicitis [28], we found no excess risk of lung cancer among appendectomized patients. Instead, a modestly reduced risk of liver cancer was noted, and the reduction was similarly confined to the subgroups among which the reduced risk of esophageal squamous-cell carcinoma was observed. Since we have no data to assess these personal habits among our patients, these results should be interpreted cautiously.
The previously reported association between gastric cancer risk and appendectomy [8, 9], was not replicated in our study. Mechanisms posited by others included confounding by low socioeconomic status, which is also linked to a higher risk of appendectomy [29] and Helicobacter pylori (H. pylori) infection during childhood [30]. Moreover, although no evidence suggests that H. pylori infection could directly affect the pathogenesis of appendicitis [31], the unspecific abdominal pain caused by H. pylori [32] might potentially increase the likelihood of having appendectomy. With regard to cancers of the colon or rectum, most prospective studies [9, 33], including ours, found no evidence of an association between appendectomy and colorectal cancer risk, while few others indicated a positive association [25].
To the best of our knowledge, this is the largest population-based study to date on gastrointestinal cancer risk after appendectomy—both in terms of sample size and length of follow-up. The structure of Swedish health care enabled us to include all patients with appendectomy and precise linkages to other complete and high-quality national registers permitted complete follow-up for cancer outcomes as well as for censoring events. However, this study has a few limitations. First, we did not have information on lifestyle factors, and consequently could not adjust for important confounders, such as body weight, smoking/diet habits, and other socioeconomic factors. Given the unexpected altered risks of esophageal adenocarcinoma and esophageal squamous-cell carcinoma, confounding effects of such factors might be substantial. Second, surveillance bias might be another concern. Appendectomy performed due to symptoms or diseases other than appendicitis might per se indicate a higher risk of certain cancers. In our analysis, although we have used one-year lag time for ‘wash-out’, such bias might still exist. For instance, the excess risk of cardia cancer which can only be observed in the first few years of follow-up was probably caused by such a surveillance bias.
In conclusion, we observed that, in Sweden, the incidence of appendectomy has decreased during 1987–2009. Also, except for a possible alteration in esophageal cancer risks, no association with stomach, colon, or rectum cancer risks was observed among the appendectomzied patients.
Supporting Information
(DOC)
Abbreviations
- CI
Confidence interval
- H. pylori
Helicobacter pylori
- SIR
standardized incidence ratio
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by grants from Swedish Research Council (SIMSAM Grant, grant number: 80748301) and Intramural Research Program of the National Cancer Institute, NIH; H.S. was also partly supported by a scholarship from China Scholarship Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Charfi S, Sellami A, Affes A, Yaich K, Mzali R, Boudawara TS. Histopathological findings in appendectomy specimens: a study of 24,697 cases. International journal of colorectal disease 2014;29:1009–12. 10.1007/s00384-014-1934-7 [DOI] [PubMed] [Google Scholar]
- 2.Andersson RE. Short and long-term mortality after appendectomy in Sweden 1987 to 2006. Influence of appendectomy diagnosis, sex, age, co-morbidity, surgical method, hospital volume, and time period. A national population-based cohort study. World journal of surgery 2013;37:974–81. 10.1007/s00268-012-1856-x [DOI] [PubMed] [Google Scholar]
- 3.Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. American journal of epidemiology 1990;132:910–25. [DOI] [PubMed] [Google Scholar]
- 4.Andreu-Ballester JC, Gonzalez-Sanchez A, Ballester F, Almela-Quilis A, Cano-Cano MJ, Millan-Scheiding M, et al. Epidemiology of appendectomy and appendicitis in the Valencian community (Spain), 1998–2007. Digestive surgery 2009;26:406–12. 10.1159/000235956 [DOI] [PubMed] [Google Scholar]
- 5.Blomqvist P, Ljung H, Nyren O, Ekbom A. Appendectomy in Sweden 1989–1993 assessed by the Inpatient Registry. Journal of clinical epidemiology 1998;51:859–65. [DOI] [PubMed] [Google Scholar]
- 6.Randal Bollinger R, Barbas AS, Bush EL, Lin SS, Parker W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. Journal of theoretical biology 2007;249:826–31. [DOI] [PubMed] [Google Scholar]
- 7.Guinane CM, Tadrous A, Fouhy F, Ryan CA, Dempsey EM, Murphy B, et al. Microbial composition of human appendices from patients following appendectomy. mBio 2013;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cope JU, Askling J, Gridley G, Mohr A, Ekbom A, Nyren O, et al. Appendectomy during childhood and adolescence and the subsequent risk of cancer in Sweden. Pediatrics 2003;111:1343–50. [DOI] [PubMed] [Google Scholar]
- 9.Mellemkjaer L, Johansen C, Linet MS, Gridley G, Olsen JH. Cancer risk following appendectomy for acute appendicitis (Denmark). Cancer causes & control: CCC 1998;9:183–7. [DOI] [PubMed] [Google Scholar]
- 10.Salminen P, Paajanen H, Rautio T, Nordstrom P, Aarnio M, Rantanen T, et al. Antibiotic Therapy vs Appendectomy for Treatment of Uncomplicated Acute Appendicitis: The APPAC Randomized Clinical Trial. Jama 2015;313:2340–8. 10.1001/jama.2015.6154 [DOI] [PubMed] [Google Scholar]
- 11.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC public health 2011;11:450 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cancer Incidence in Sweden 2011. Stockholm:The National Board of Health and Welfare, 2013. [Google Scholar]
- 13.Ye W, Nyren O. Risk of cancers of the oesophagus and stomach by histology or subsite in patients hospitalised for pernicious anaemia. Gut 2003;52:938–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekstrom AM, Signorello LB, Hansson LE, Bergstrom R, Lindgren A, Nyren O. Evaluating gastric cancer misclassification: a potential explanation for the rise in cardia cancer incidence. Journal of the National Cancer Institute 1999;91:786–90. [DOI] [PubMed] [Google Scholar]
- 15.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M, Comparative Risk Assessment collaborating g. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet 2005;366:1784–93. [DOI] [PubMed] [Google Scholar]
- 16.Nordenvall C, Nilsson PJ, Ye WM, Nyren O. Smoking, snus use and risk of right- and left-sided colon, rectal and anal cancer: a 37-year follow-up study. Int J Cancer 2011;128:157–65. 10.1002/ijc.25305 [DOI] [PubMed] [Google Scholar]
- 17.WHO. The world health report. Reducing Risks, Promoting Healthy Life, 2002. [DOI] [PubMed]
- 18.Buckius MT, McGrath B, Monk J, Grim R, Bell T, Ahuja V. Changing epidemiology of acute appendicitis in the United States: study period 1993–2008. The Journal of surgical research 2012;175:185–90. 10.1016/j.jss.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 19.Anderson JE, Bickler SW, Chang DC, Talamini MA. Examining a common disease with unknown etiology: trends in epidemiology and surgical management of appendicitis in California, 1995–2009. World journal of surgery 2012;36:2787–94. 10.1007/s00268-012-1749-z [DOI] [PubMed] [Google Scholar]
- 20.Malt RA. The Perforated Appendix. New Engl J Med 1986;315:1546–7. [DOI] [PubMed] [Google Scholar]
- 21.Bendeck SE, Nino-Murcia M, Berry GJ, Jeffrey RB Jr. Imaging for suspected appendicitis: negative appendectomy and perforation rates. Radiology 2002;225:131–6. [DOI] [PubMed] [Google Scholar]
- 22.Webb EM, Nguyen A, Wang ZJ, Stengel JW, Westphalen AC, Coakley FV. The negative appendectomy rate: who benefits from preoperative CT? AJR American journal of roentgenology 2011;197:861–6. 10.2214/AJR.10.5369 [DOI] [PubMed] [Google Scholar]
- 23.Salom EM, Schey D, Penalver M, Gomez-Marin O, Lambrou N, Almeida Z, et al. The safety of incidental appendectomy at the time of abdominal hysterectomy. Am J Obstet Gynecol 2003;189:1563–7. [DOI] [PubMed] [Google Scholar]
- 24.Voitk AJ, Lowry JB. Is Incidental Appendectomy a Safe Practice. Can J Surg 1988;31:448–51. [PubMed] [Google Scholar]
- 25.Wu SC, Chen WT, Muo CH, Ke TW, Fang CW, Sung FC. Association between Appendectomy and Subsequent Colorectal Cancer Development: An Asian Population Study. PLoS One 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito JM, Yan Y, Evashwick TW, Warner BW, Tarr PI. Use and Accuracy of Diagnostic Imaging by Hospital Type in Pediatric Appendicitis. Pediatrics 2013;131:E37–E44. 10.1542/peds.2012-1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto F, Pinto A, Russo A, Coppolino F, Bracale R, Fonio P, et al. Accuracy of ultrasonography in the diagnosis of acute appendicitis in adult patients: review of the literature. Crit Ultrasound J 2013;5 Suppl 1:S2 10.1186/2036-7902-5-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery SM, Pounder RE, Wakefield AJ. Smoking in adults and passive smoking in children are associated with acute appendicitis. Lancet 1999;353:379. [DOI] [PubMed] [Google Scholar]
- 29.Ekbom A. Appendicectomy and childhood hygiene: different sides of the same coin? Gut 1998;43:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malaty HM, Graham DY. Importance of childhood socioeconomic status on the current prevalence of Helicobacter pylori infection. Gut 1994;35:742–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kell MR, Winter DC, Ryan D, Lynch M, Brew B, Rajpal P, et al. Nitric oxide synthetase and Helicobacter pylori in patients undergoing appendicectomy. The British journal of surgery 1999;86:1538–42. [DOI] [PubMed] [Google Scholar]
- 32.Heldenberg D, Wagner Y, Heldenberg E, Keren S, Auslaender L, Kaufshtein M, et al. The role of Helicobacter pylori in children with recurrent abdominal pain. The American journal of gastroenterology 1995;90:906–9. [PubMed] [Google Scholar]
- 33.Friedman GD, Fireman BH. Appendectomy, appendicitis, and large bowel cancer. Cancer research 1990;50:7549–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.