Abstract
For many pathogenic fungi, siderophore-mediated iron acquisition is essential for virulence. The process of siderophore production and further mechanisms to adapt to iron limitation are strictly controlled in fungi to maintain iron homeostasis. Here we demonstrate that the human pathogenic dermatophyte Arthroderma benhamiae produces the hydroxamate siderophores ferricrocin and ferrichrome C. Additionally, we show that the iron regulator HapX is crucial for the adaptation to iron starvation and iron excess, but is dispensable for virulence of A. benhamiae. Deletion of hapX caused downregulation of siderophore biosynthesis genes leading to a decreased production of siderophores during iron starvation. Furthermore, HapX was required for transcriptional repression of genes involved in iron-dependent pathways during iron-depleted conditions. Additionally, the ΔhapX mutant of A. benhamiae was sensitive to high-iron concentrations indicating that HapX also contributes to iron detoxification. In contrast to other pathogenic fungi, HapX of A. benhamiae was redundant for virulence and a ΔhapX mutant was still able to infect keratinized host tissues in vitro. Our findings underline the highly conserved role of the transcription factor HapX for maintaining iron homeostasis in ascomycetous fungi but, unlike in many other human and plant pathogenic fungi, HapX of A. benhamiae is not a virulence determinant.
Introduction
The fungal pathogen Arthroderma benhamiae belongs to a group of fungi known as dermatophytes, which exclusively infect keratinized structures such as hair, skin (stratum corneum) and nails of humans and animals [1]. In recent years, an increasing number of A. benhamiae infections have been observed worldwide [2]. Amongst others, the main reservoir of the zoophilic species A. benhamiae is the guinea pig [3] and infections of humans often occur after direct contact with an animal carrying the fungus. Usually, the infections are superficial and not life-threatening, but even in immunocompetent hosts, dermatophytosis is long-lasting and difficult to cure [4]. To date, only few putative virulence factors of dermatophytes have been identified and investigated at the molecular level yet. These are, for example, the ABC transporter TruMDR2 and pH signalling transcription factor PacC of Trichophyton rubrum as well as the keratinolytic proteases Sub3 of Microsporum canis and Sub6 of Trichophyton mentagrophytes [5–10]. Recent advances in genetic manipulation of A. benhamiae have set a basis for fundamental genetic research of dermatophytes [11]. A. benhamiae has proven to be an ideal model organism because it grows relatively fast and allows efficient targeted gene deletion as well as gene complementation [12]. Additionally, the complete genome sequence and global transcriptional profiles are available, and comprehensive in vitro and in vivo infection models have been established [13–15].
Iron is an essential trace element for almost all organisms. Its ability to exist in two redox states makes iron an important cofactor of proteins involved in a variety of cellular processes, including respiration. On the other hand, iron excess is toxic because it catalyzes the production of cell-damaging hydroxyl radicals in the presence of oxygen [16]. Thus, cellular uptake, storage and utilization of iron need to be tightly regulated to avoid the formation of reactive oxygen species. In the filamentous fungi Aspergillus nidulans and Aspergillus fumigatus, iron homeostasis is regulated by the transcription factors HapX and SreA which are interconnected by a negative regulatory feedback loop [17–20]. The Cys2-Cys2-type GATA zinc finger transcription factor SreA downregulates the expression of hapX and other genes during iron sufficiency by binding to a specific motif within the promoter region. SreA represses siderophore biosynthesis and reductive iron assimilation to avoid iron excess during iron sufficiency [21]. The basic region leucine zipper (bZIP) transcription factor HapX downregulates the expression of sreA during iron starvation by protein-protein interaction with the heterotrimeric CCAAT-binding complex (CBC) and by sequence-specific DNA binding [22, 23]. During iron-depleted conditions, the CBC-HapX complex represses iron-consuming pathways, including heme biosynthesis, tricarboxylic acid cycle and respiration to spare iron. On the other hand, it activates reductive iron assimilation, siderophore biosynthesis and siderophore uptake for iron acquisition [18, 19, 24]. Additionally, HapX is essential for iron detoxification by activating the vacuolar iron importer CccA under high-iron conditions [22, 25]. Due to its central role in iron homeostasis, the transcription factor HapX has shown to be a virulence determinant in several human fungal pathogens, such as A. fumigatus, Candida albicans, Cryptococcus neoformans as well as in the plant pathogenic fungus Fusarium oxysporum [19, 26–29]. Remarkably, until now, the role of iron during the infection of keratinized host tissues by dermatophytes has not been elucidated.
In this study, we have set out to investigate the function of the transcription factor HapX in A. benhamiae. We demonstrate that HapX function is crucial for the adaptation to iron starvation and iron excess, but is dispensable for the infection of keratinized host tissue.
Materials and Methods
Strains, media and growth conditions
The wild-type strain A. benhamiae LAU2354-2 = CBS 112371 = IHEM 20161 [30] was used for the generation of deletion mutants and reconstituted strains. For short-term storage, the wild-type A. benhamiae LAU2354-2 was cultivated on Sabouraud glucose agar [1% (w/v) peptone, 2% (w/v) glucose, 1.5% (w/v) agar] (SAB) and transformants of A. benhamiae LAU2354-2 were grown on SAB supplemented with 200 μg/ml hygromycin (ForMedium, Hunstanton, UK) or G418 (Carl Roth, Karlsruhe, Germany), according to the selectable marker used. Additionally, all fungal strains used in this study were stored as frozen glycerol stocks at -80°C (Table 1). Production of microconidia was performed on MAT agar [0.1% (w/v) peptone, 0.2% (w/v) glucose, 0.1% (w/v) MgSO4, 0.1% (w/v) KH2PO4; Carl Roth, Karlsruhe, Germany] if not otherwise stated. Further experiments were carried out at 30°C in Aspergillus minimal medium (AMM) containing 1% (w/v) glucose as the carbon source and 20 mM glutamine as the nitrogen source [31]. For solid AMM, 1.5% (w/v) agar was added. For iron-depleted conditions, iron was omitted (-Fe). Iron-replete media was supplemented with 0.03 mM FeSO4 (+Fe). For harsh iron starvation conditions, the ferrous iron chelator bathophenanthroline disulfonic acid Na2-salt (BPS) (Serva, Heidelberg, Germany) was used at a final concentration of 0.2 mM (-Fe +BPS). As xenosiderophore, the ferric iron chelator deferoxamine mesylate salt (DFOM) (Sigma-Aldrich, Taufkirchen, Germany) was added to the medium at a final concentration of 10 μM (-Fe +DFOM). For growth inhibition assays, 104 microconidia of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain were spotted on solid AMM agar supplemented with iron concentrations ranging from 1–10 mM FeSO4.
Table 1. A. benhamiae strains used in this study.
| Strain | Parent | Genotype | Reference |
|---|---|---|---|
| LAU2354-2 | wild-type strain | [30] | |
| AbenHAPXM1A | LAU2354-2 | ΔhapX::Pgpd-hph-TtrpC | This study |
| AbenHAPXM1B | LAU2354-2 | ΔhapX::Pgpd-hph-TtrpC | This study |
| AbenHAPXK1A | AbenHAPXM1A | ΔhapX::hapX-TcaACT1-PACT1-neo | This study |
| AbenHAPXK1B | AbenHAPXM1B | ΔhapX::hapX-TcaACT1-PACT1-neo | This study |
Plasmid construction
Sequence information for the gene hapX (ARB_06811) was obtained from the annotated A. benhamiae genome [13]. Plasmid construction was performed as described before [12]. For the generation of the deletion mutants, up- and downstream sequences of the hapX gene were cloned successively in the plasmid pHPH1 [12]. For deletion of the entire coding region of hapX, an ApaI-HindIII fragment containing hapX upstream sequences from positions -518 to -4 with respect to the start codon was obtained by PCR with the primers AbenHAPX-1/AbenHAPX-2. Genomic DNA from the wild-type strain A. benhamiae LAU2354-2 was used as a template. A BamHI-NotI fragment with hapX downstream sequences from positions +1417 to +1894 was amplified by PCR with the primers AbenHAPX-3/AbenHAPX-4. The hapX upstream and downstream sequences were successively cloned via the introduced restriction sites in plasmid pHPH1 to result in plasmids pAbenHAPXM1 and pAbenHAPXM2, respectively (Fig 1A). For reinsertion of the hapX gene into the knock-out mutant, the plasmid pAbenHAPXK2 was generated as follows. An ApaI-BglII DNA fragment including the hapX gene and hapX upstream sequences from positions -1008 to +1425 was amplified by PCR with the primers AbenHAPX-5/AbenHAPX-9. The BamHI-NotI fragment with hapX downstream sequences from positions +1417 to +1894 (amplified with the primers AbenHAPX-3/AbenHAPX-4) was cloned in the BamHI-NotI digested plasmid pNEO1 [12] yielding pAbenHAPXK1. The PCR product was cloned via the introduced ApaI and BglII restriction sites together with the BglII-HindIII [CaACT1T] fragment from pJetGFPACT1T1 [12] in the ApaI-HindIII digested plasmid pAbenHAPXK1 to give plasmid pAbenHAPXK2 (Fig 1B). All primers used for plasmid construction in this study are listed in S1 Table.
Fig 1. Generation of A. benhamiae ΔhapX mutants and reconstituted strains.
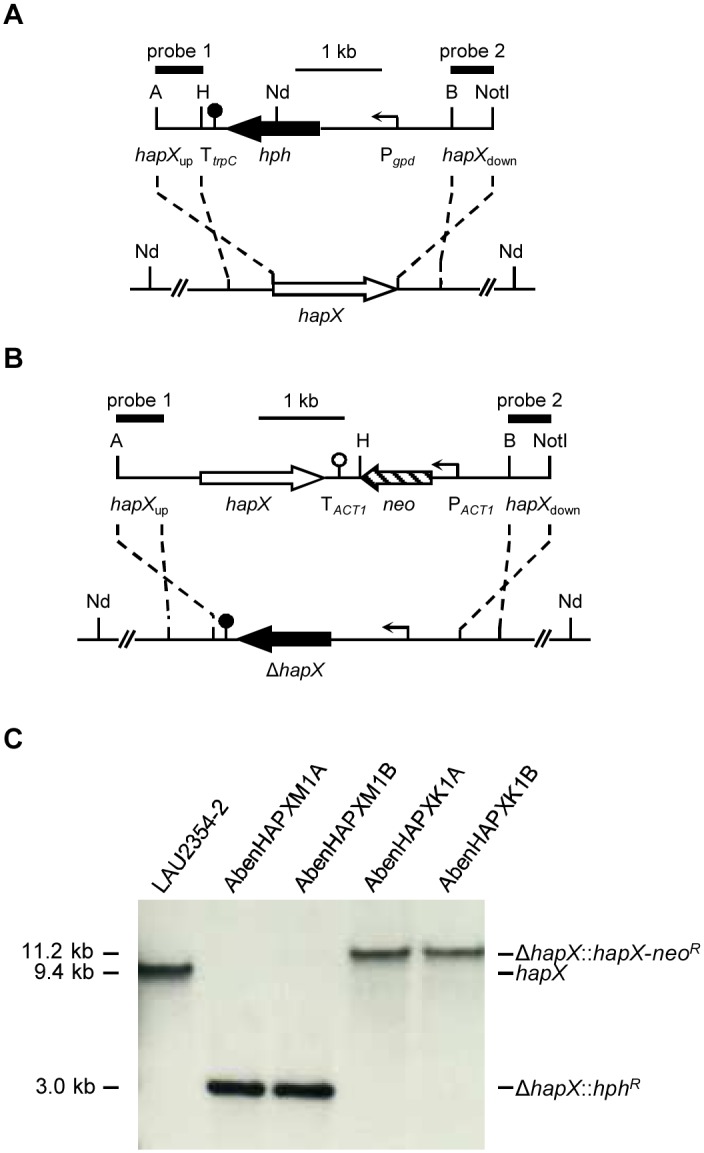
(A) For deletion of the hapX locus (white arrow) in the wild-type strain A. benhamiae LAU2354-2 (bottom) a DNA cassette, containing the hygromycin resistance gene hph (black arrow) under control of the gpd promoter (Pgpd, bent arrow) together with the termination sequence fragment TtrpC (filled circle) flanked by hapX upstream and downstream regions (hapXup and hapXdown, solid lines), was used (top). (B) For reinsertion of the hapX gene into its original locus in the ΔhapX mutants a DNA cassette, containing the coding region of hapX and the neomycin resistance gene neo (lined arrow) under control of the A. benhamiae actin promoter (PACT1, bent arrow) together with the Candida albicans actin termination sequence fragment TACT1 (blank circle) flanked by hapX upstream and downstream regions (hapXup and hapXdown, solid lines), was used. (C) Southern blot of NdeI-digested genomic DNA of the wild-type strain A. benhamiae LAU2354-2, ΔhapX mutants and hapXC reconstituted strains with hapX-specific probe 1. The probes which were used for Southern analysis of the transformants are indicated by the black bars. Only the following relevant restriction sites are given in panels a and b: A, ApaI; B, BamHI; H, HindIII; Nd, NdeI; NotI. The sizes of the hybridizing fragments are given on the left and their identities on the right.
Transformation of A. benhamiae
Transformation of A. benhamiae was carried out as previously described [12]. The ΔhapX mutant and the hapXC reconstituted strain were generated by homologous recombination. Briefly, protoplasts produced from A. benhamiae microconidia were transformed with the constructed linear DNA cassettes from plasmids pAbenHAPXM2 (ΔhapX mutant) and pAbenHAPXK2 (hapXC reconstituted strain). Hygromycin or neomycin resistant transformants were selected with either 250 μg/ml hygromycin or G418 depending on the selection marker used. For analysis of the transformants, fungal genomic DNA was isolated as stated before [12]. Targeted gene disruption or gene complementation was confirmed via Southern analyis using the Amersham ECL direct nucleic acid labeling and detection system (GE Healthcare, Little Chalfont, UK) according to the manufacturer’s instructions (Fig 1C).
Determination of cell dry weight
A volume of 100 ml AMM was used for cell dry weight determination during iron-replete conditions (+Fe), iron limitation (-Fe), harsh iron starvation (-Fe +BPS) and in the presence of DFOM (-Fe +DFOM). For high iron concentrations 50 ml AMM was supplemented with 1 mM, 3 mM, 5 mM and 7 mM FeSO4. The medium was inoculated with 106 microconidia per ml of the respective fungal strain and incubated at 30°C and 200 rpm. After 5 d of cultivation the mycelium was harvested by Miracloth (Calbiochem®, Merck Millipore, Darmstadt, Germany), thoroughly dried at 50°C and weighed.
Identification of siderophores produced by A. benhamiae
For identification of siderophores produced by A. benhamiae, fungal cultures were grown in AMM without iron for 5 d at 30°C and 200 rpm. The mycelium was harvested by Miracloth and the culture supernatant was collected. The supernatant was exhaustively extracted with ethylacetate and the resulting extract dried with Na2SO4 and concentrated under reduced pressure. For HPLC analysis the dry extract was re-dissolved in 200 μL of methanol and filtered (Ultrafree filtration system for laboratory centrifuges, Oxy-Fill Rotrac® membrane). The aqueous residue was freeze-dried and extracted with methanol. The extract was filtered using a paper filter, concentrated under reduced pressure and finally redissolved in 4 mL of 50% MeOH (H2O, v/v) for HPLC analysis. The fungal mycelium was freeze-dried, extracted with 10 mL of 50% MeOH (H2O, v/v) and filtered using a paper filter. Next, the filtrate was concentrated under reduced pressure and dissolved in 100 μL of 50% MeOH (H2O, v/v) for HPLC analysis. To convert the Fe-free derivatives into the iron complexes, all extracts were supplemented with 3 mM FeCl3 before HPLC analyses.
Analytical HPLC was performed on a Shimadzu LC-10Avp series HPLC system consisting of an autosampler, high-pressure pumps, column oven and PDA. HPLC conditions: C18 column (Eurospher 100–5, 250 x 4.6 mm) and gradient elution (MeCN/0.1% (v/v) TFA 0.5/99.5 in 30 min to MeCN/0.1% (v/v) TFA 100/0, MeCN 100% for 10 min), flow rate 1 mL min−1; injection volume: 30 μL. LC-MS measurements were performed using an Exactive Orbitrap High Performance Benchtop LC-MS with an electrospray ion source and an Accela HPLC system (Thermo Fisher Scientific, Bremen, Germany). HPLC conditions: C18 column (Betasil C18 3 μm 150 x 2.1 mm) and gradient elution (MeCN/0.1% (v/v) HCOOH (H2O) 5/95 for 1 min, going up to 98/2 in 15 min, then 98/2 for another 3 min; flow rate 0.2 mL min−1) or a Q Exactive Orbitrap High Performance Benchtop LC-MS with an electrospray ion source and an Accela HPLC system (Thermo Fisher Scientific, Bremen, Germany). HPLC conditions: C18 column (Accucore C18 3 μm 100 x 2.1 mm) and gradient elution (MeCN/0.1% (v/v) HCOOH (H2O) 5/95 for 1 min, going up to 98/2 in 15 min, then 98/2 for another 3 min; flow rate 0.2 mL min−1).
The siderophore ferricrocin (used as a standard) was isolated as the ferri-form from Aspergillus fumigatus and was kindly provided by Prof. H. Haas (Innsbruck Medical University, Austria). Ferrichrome C (used as a standard) was isolated as the ferri-form from Aspergillus ochraceous and was purchased from EMC microcollections GmbH, Tübingen, Germany.
Determination of extracellular siderophore activity
For determination of the siderophore activity in culture supernatants of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain the chrome azurol S (CAS) liquid assay was used as described [32]. A volume of 100 ml of AMM without iron or supplemented with 0.03 mM ferrous sulfate was inoculated with 108 microconidia and incubated for 3 d, 4 d and 5 d at 30°C and 200 rpm. The supernatant was collected by filtration through Miracloth and an aliquot of 100 μl culture supernatant was mixed with 100 μl CAS assay solution prepared according to Schwyn and Neilands [33]. As a reference AMM without iron was used. After incubation for 1 h at room temperature the absorbance at 630 nm was measured with a microtiter plate reader (Infinite® 200 PRO, Tecan, Switzerland). The percentage of siderophore activity was calculated by substracting the sample absorbance values from the reference according to the formula [(Ar-As)/Ar] x 100. The experiments were run in three biological replicates.
Isolation of RNA and quantitative real-time reverse transcription-PCR (qRT-PCR)
For RNA isolation fungal mycelium was harvested after cultivation in AMM during iron-replete conditions (+Fe), iron starvation (-Fe, 0.03 mM FeSO4) and high iron conditions (hFe, 3 mM FeSO4) for 5 d at 30°C and 200 rpm. For short-term iron stress the mycelium was grown for 3 d at 30°C and 200 rpm and shifted from -Fe to +Fe for 1 h (sFe). The frozen mycelium was ground with mortar and pestle and subsequently the RNeasy Plant Mini Kit (QIAGEN, Venlo, Netherlands) was used for total RNA isolation according to the manufacturer’s instructions. The quality and quantity of RNA was determined with a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, USA). For complete digestion of DNA 1000 ng RNA were treated with the TURBO DNA-free™ Kit (Ambion®, Thermo Fisher Scientific, Waltham, USA) and the purified RNA was used for first strand cDNA synthesis with oligo d(t)23 VN primer (New England Biolabs, Ipswich, USA) and RevertAid Reverse Transcriptase (Thermo Fisher Scientific, Waltham, USA) according to the manufacturer’s instructions. The qRT-PCR experiments were performed with the StepOnePlus Real-Time PCR System (Applied Biosystem, Thermo Fisher Scientific, Waltham, USA). Gene-specific primers (S2 Table) were designed with the software Primer3 [34]. The actin gene of A. benhamiae (ARB_04092) was chosen as internal control for normalization. Quantitative RT-PCR products were obtained using MyTaq HS Mix 2x (Bioline, London, UK) and EvaGreen (Biotium, Hayward, USA) as fluorescent dye. PCR conditions were 95°C for 2 min followed by 40 cycles with 15 s at 95°C, 20 s at 60°C, 15 s at 72°C and a final step at 95°C for additional 15 s. For each primer pair a standard curve with serial dilutions of genomic DNA of A. benhamiae in technical triplicates was determined and the primer efficiency (100% ±10) was used for the calculation of transcript levels by the method described by Pfaffl et al. [35]. Transcript levels are presented relative to those of A. benhamiae wild type during iron-replete conditions. The experiments were run in three biological replicates with technical duplicates.
Growth on keratin substrates
Human hair and finger nails as well as keratin powder from hooves and horns (MP Biomedicals Germany GmbH, Eschwege, Germany) were used for the analysis of fungal growth on keratin substrates. Human scalp hair from a child and finger nails from a healthy female donor were cut from the donors themselves or the next of kin in their domestic home. The provided hair and clipped finger nail samples were autoclaved. Hair, nails and keratin powder were placed on water agar plates and inoculated with 3 plugs of fresh fungal mycelium from SAB agar plates. After incubation at 22°C for 25 d (keratin powder), 30 d (nails) and 40 d (hair) in the dark, mycelia formation on the keratin substrates was documented.
Ethics Statement
The study did not include any diagnostic procedure or therapeutic method. Furthermore, the sample collection was non-invasive (the physical integrity of the donor was maintained) and did not intrude into the privacy of the donor. Based on the regulations of the ethics commission at the Jena University Hospital, Jena (Germany), an approval of this study was not necessary in this case. Only human material (scalp hair and clipped finger nails) from voluntary donors were used. Additionally, the donors or the next of kin have provided written consent for the use of the samples for research and publication. The study does not contain any patient data. Only the first author had access to any potentially identifying donor information. Identifying details are omitted in the manuscript.
Results
Identification of A. benhamiae HapX homologue
BLASTP search revealed a single HapX homologue in the genome of A. benhamiae which has significant similarity to HapX of A. nidulans (49% identity) and A. fumigatus (48% identity). A. benhamiae hapX is encoded by an open reading frame of 1425 bp with a deduced protein of 474 amino acids. Furthermore, HapX of A. benhamiae displays all the typical characteristics which are common to this class of transcription factors, including the basic region leucine zipper (bZIP) and coiled-coil domains mediating DNA-binding, an N-terminal CCAAT-binding complex (CBC) domain, which is essential for interaction with the CBC subunit HapE [18] and four conserved cysteine-rich regions (CRR) (S1 Fig). In A. fumigatus, two of the four CRR are known to be involved in detoxification of iron excess [22].
Generation of A. benhamiae ΔhapX mutants and reconstituted strains
To assess the functional role of HapX, ΔhapX mutants were generated in the wild-type strain A. benhamiae LAU2354-2 by targeted hapX gene deletion with the hph resistance cassette (Fig 1A). To ensure that the observed phenotypes were a result of the deletion of hapX, the ΔhapX mutants were complemented with a copy of the wild-type hapX gene (Fig 1B). Southern blot analysis confirmed the site-directed insertion of the linear DNA cassettes (Fig 1C). The deletion strain AbenHAPXM1A (ΔhapX) and the reconstituted strain AbenHAPXK1A (hapXC) were used for further analysis.
HapX is important for growth, conidiation and hyphal pigmentation during iron starvation
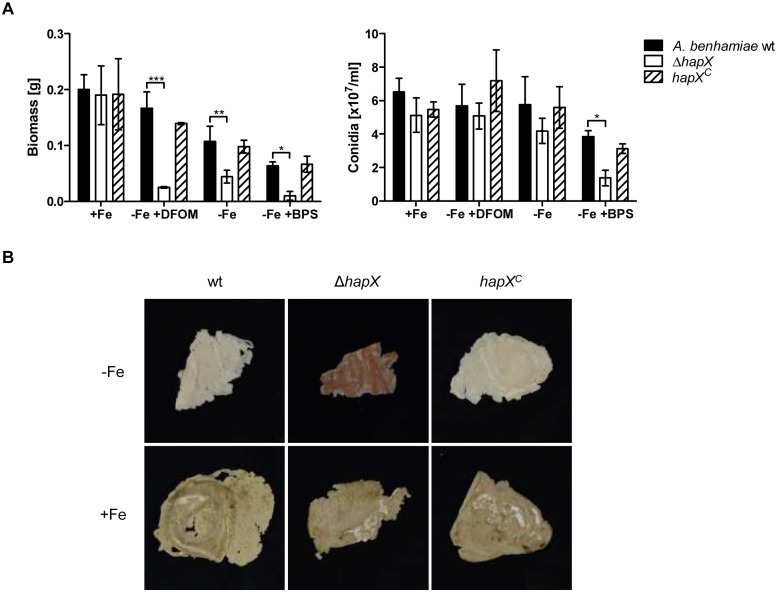
Analysis of fungal growth and conidiation revealed reduced growth and decreased conidiation of the ΔhapX mutant during iron starvation, but not during iron-replete conditions (Fig 2). The biomass production of the ΔhapX mutant in liquid culture was significantly reduced during iron starvation and in the presence of the ferrous iron chelator BPS (Fig 2A). In contrast to A. benhamiae wild type and hapXC, growth of the ΔhapX mutant was highly impaired when the medium was supplemented with the xenosiderophore DFOM (Fig 2A). Additionally, loss of HapX led to a decrease of conidiation during iron starvation (-Fe) and in the presence of the iron chelator BPS but not during iron-replete conditions or in the presence of DFOM (Fig 2A). Furthermore, the mycelium of the ΔhapX mutant showed a reddish pigmentation during iron-depleted conditions probably due to the accumulation of iron-free precursors of heme (Fig 2B). By contrast, biomass and mycelial pigmentation of the ΔhapX mutant was comparable to the wild type during iron-replete conditions. Complementation of the hapX gene, resulting in the hapXC reconstituted strain, restored the phenotype of A. benhamiae wild type in all experiments.
Fig 2. HapX of A. benhamiae is important for growth, conidiation and hyphal pigmentation during iron starvation.
(A) Cultivation of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain in AMM during iron-replete conditions (+Fe), iron limitation (-Fe), harsh iron starvation (-Fe +BPS) and in the presence of the xenosiderophore deferoxamine (-Fe + DFOM). Data represent the means and standard deviations of three biological replicates. The differences between wild type and ΔhapX mutant were statistically significant during iron starvation and in the presence of BPS and DFOM (2way ANOVA; * significant at P < 0.05, ** significant at P < 0.01, *** significant at P < 0.001). (B) Growth of the fungal strains in AMM during iron-replete conditions (+Fe) and iron starvation (-Fe) for 5 d at 30°C led to the formation of a reddish pigmented mycelium of ΔhapX mutant, particularly during iron deficiency.
HapX is necessary for the regulation of siderophore biosynthesis during iron starvation
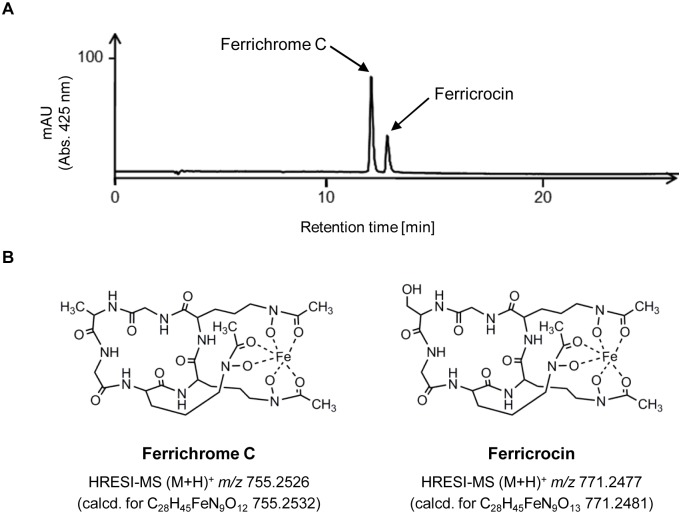
In order to identify the intra- and extracellular siderophores of A. benhamiae the supernatant and the mycelia of fungal cultures were separately extracted and analyzed by HPLC-PDA and HPLC-HRESIMS. Trace amounts of compounds with a molecular mass of m/z 753 (M-H−) and m/z 769 (M-H−) were found by MS analyses. MS/MS analyses and dereplication with commercial databases suggested a potential identity of the compounds with the hydroxamate-type siderophores ferrichrome C and ferricrocin, respectively. To unequivocally prove the structures, their UV spectra, HRESIMS-spectra, MS/MS fragmentation pattern as well as HPLC retention times were compared to authentic standards (S2–S6 Figs). As a result, ferrichrome C and ferricrocin could be identified as siderophores of A. benhamiae (Fig 3).
Fig 3. Identification of siderophores produced by A. benhamiae.
(A) HPLC chromatogram of the lyophilized culture supernatants of A. benhamiae. Ferrichrome C and ferricrocin were identified as extracellular siderophores. (B) Chemical structures and molecular masses of the siderophores ferrichrome C and ferricrocin produced by A. benhamiae.
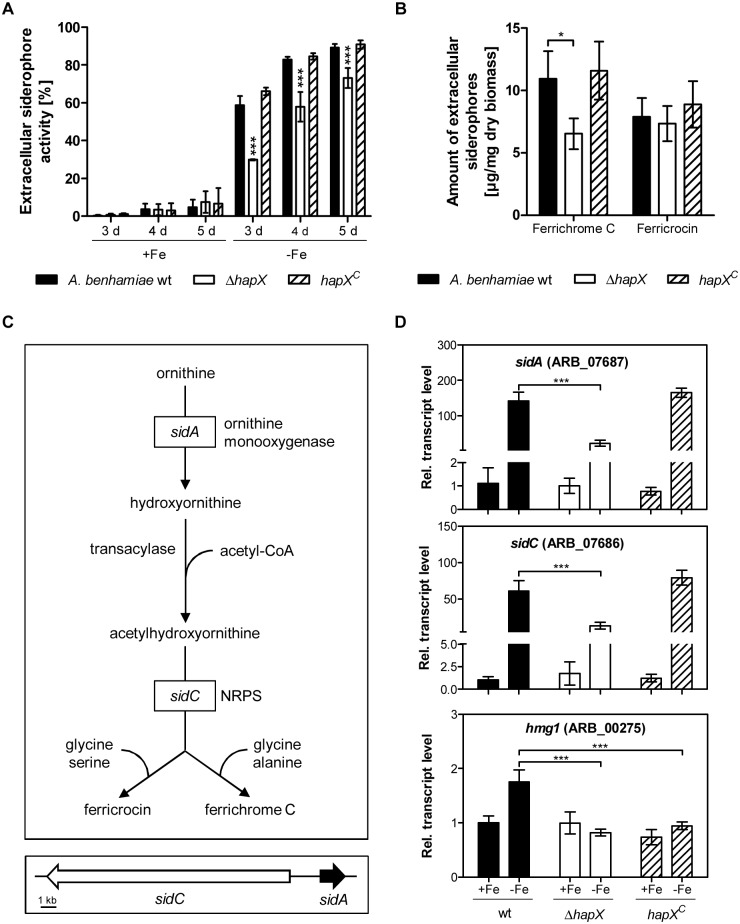
The extracellular siderophores produced by A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strains were quantified by using the CAS liquid assay (Fig 4A). During iron starvation, the extracellular siderophore production of the ΔhapX mutant was decreased in comparison to the wild type. However, all strains showed an increase of extracellular siderophore activity over time. In contrast, the siderophore production of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain was almost abolished during iron-replete conditions.
Fig 4. HapX-dependent siderophore biosynthesis of A. benhamiae during iron starvation.
(A) Quantification of extracellular siderophores produced by A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain during iron starvation (-Fe) and iron sufficiency (+Fe). Data represent the means and standard deviations of three biological replicates. The differences between wild type and ΔhapX were statistically significant during iron starvation (2way ANOVA; *** significant at P < 0.001). (B) Quantification of the extracellular siderophores ferrichrome C and ferricrocin in supernatant extracts of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain after cultivation for 5 d at 30°C during iron starvation by HPLC analysis. Data represent the means and standard deviations of three biological replicates. The differences between wild type and ΔhapX were statistically significant for ferrichrome C (2way ANOVA; * significant at P < 0.05) (C) Postulated biosynthesis pathway of the siderophores ferricrocin and ferrichrome C (based on the information from A. fumigatus) and genomic organization of the genes sidC (ARB_07686) and sidA (ARB_07687) of A. benhamiae. (D) Quantitative RT-PCR analysis of the transcript level of the genes sidA (ornithine monooxygenase), sidC (NRPS) and hmg1 (HMG-CoA reductase) of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain during iron starvation (-Fe) and iron-replete conditions (+Fe). Transcript levels are presented relative to those of A. benhamiae wild type during iron-replete conditions. Data represent the means and standard deviations of three biological replicates. The differences between wild type and ΔhapX were statistically significant during iron starvation (2way ANOVA; *** significant at P < 0.001).
Quantification of extracellular siderophores by HPLC analysis revealed a significantly reduced amount of secreted ferrichrome C in the ΔhapX mutant in comparison to the wild type (Fig 4B). By contrast, no difference between the wild type and ΔhapX mutant strain was observed for the extracellular concentration of ferricrocin (Fig 4B).
Homologues of proteins involved in the biosynthesis of the siderophores fusarinine C and TAFC were found in A. benhamiae by comparative genomic analysis with A. fumigatus (S3 Table). However, fusarinine C and TAFC were not identified in extracts of A. benhamiae culture supernatants or mycelium. Furthermore, homologous genes of the A. fumigatus TAFC esterase EstB, acetyltransferase SidG and siderophore iron transporter MirB are not clustered in A. benhamiae (S3 Table). Interestingly, the siderophore biosynthesis genes sidC (non-ribosomal peptide synthetase; ARB_07686) and sidA (ornithine monooxygenase; ARB_07687) of A. benhamiae are clustered (Fig 4C). Quantitative RT-PCR analysis of the genes sidC and sidA displayed that the transcript level of both genes is highly decreased in the ΔhapX mutant in comparison to the wild type during iron starvation (Fig 4D). In some fungi a link between siderophore biosynthesis and the isoprenoid biosynthesis pathway has been demonstrated [36, 37]. The 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase is an important enzyme for isoprenoid biosynthesis. HMG-CoA reductase is encoded by the gene hmg1 and its expression is dependent on the iron availability, but only in fungi which produce mevalonate-derived siderophores such as fusarinine C or TAFC [36]. Quantitative RT-PCR analysis of the gene hmg1 showed that the transcript level of hmg1 is slightly upregulated in A. benhamiae wild type during iron starvation. By contrast, no differences in the transcript level of hmg1 were observed during iron-replete conditions and iron starvation in the ΔhapX mutant and hapXC reconstituted strains (Fig 4D).
HapX is required for transcriptional repression of genes involved in iron-dependent pathways during iron starvation
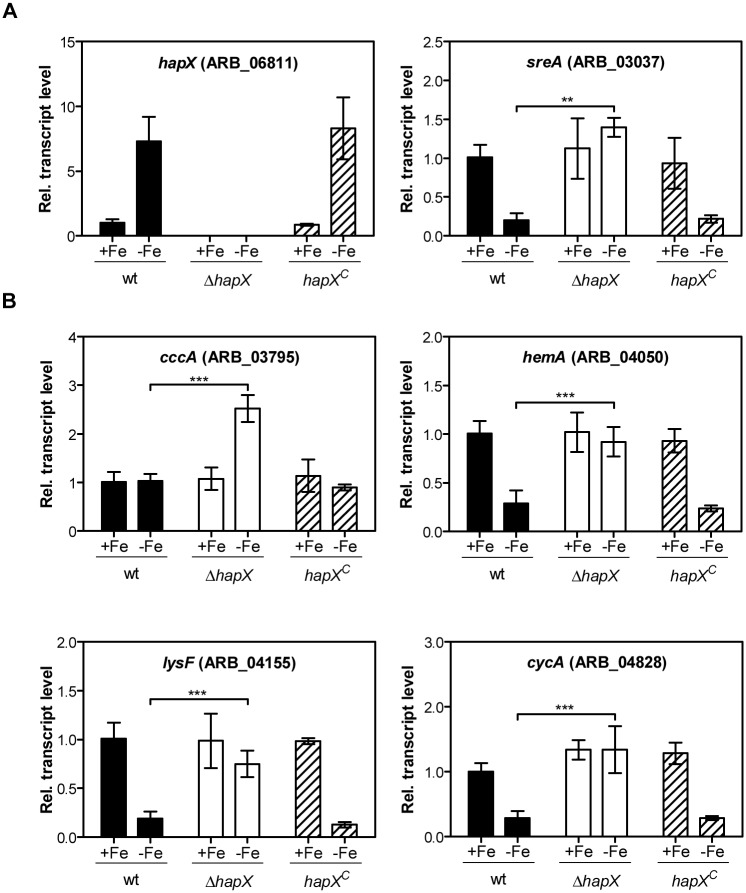
Next, we investigated the role of the transcriptional regulators HapX and SreA of A. benhamiae in the presence or absence of iron by qRT-PCR. During iron starvation, the transcript level of hapX was highly upregulated in A. benhamiae wild type in comparison to iron-replete conditions, which indicates that hapX transcription is repressed by iron (Fig 5A). By contrast, the transcript level of sreA was downregulated in A. benhamiae wild type, but significantly increased in the ΔhapX mutant during iron starvation compared to iron-replete conditions which shows that HapX represses the sreA gene during iron starvation (Fig 5A).
Fig 5. HapX of A. benhamiae is important for transcription of iron regulatory genes during iron limitation.
(A) Transcript levels of transcription factors HapX and SreA during iron starvation (-Fe) and iron-replete conditions (+Fe). (B) Transcript levels of the genes cccA, hemA, lysF and cycA during iron starvation (-Fe) and iron-replete conditions (+Fe). Transcript levels measured by quantitative RT-PCR analysis are presented relative to those of A. benhamiae wild type during iron-replete conditions. Data represent the means and standard deviations of three biological replicates. The differences between wild type and ΔhapX mutant were statistically significant during iron starvation (2way ANOVA; ** significant at P < 0.01, *** significant at P < 0.001).
Representative genes from known iron consuming pathways were chosen for further transcriptional analysis of HapX during iron starvation and iron-replete conditions. In A. fumigatus the cccA gene encodes a vacuolar iron importer which mediates the import of iron into the vacuole to avoid toxic levels of this metal in the cytosol [25]. The genes hemA (5-aminolevulinic acid synthase), cycA (cytochrome C) and lysF (homoaconitase) are involved in heme biosynthesis, respiration and lysine biosynthesis, respectively [18, 19]. The transcript levels of cccA, hemA, cycA and lysF were significantly increased in the ΔhapX mutant during iron starvation, but not during iron-replete conditions in comparison to the wild type (Fig 5B).
HapX is involved in iron detoxification
In addition to the characterization of HapX during iron starvation, the role of this transcription factor in the presence of iron excess was analyzed. Cultivation of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strains on agar plates and in liquid medium resulted in a strong growth defect of the ΔhapX mutant in the presence of 5 to 10 mM FeSO4 (Fig 6A and 6B). Quantitative RT-PCR analysis of the vacuolar iron importer encoding gene cccA showed that the transcript level of cccA was highly upregulated during a shift from iron starvation to 0.03 mM FeSO4 for 1 h (sFe) in A. benhamiae wild type but not in the ΔhapX mutant (Fig 6C). By contrast, no significant differences in the transcript level of cccA in A. benhamiae wild type and the ΔhapX mutant were observed during growth in medium supplemented with 3 mM FeSO4 (hFe) (Fig 6C).
Fig 6. HapX of A. benhamiae under high iron concentrations.
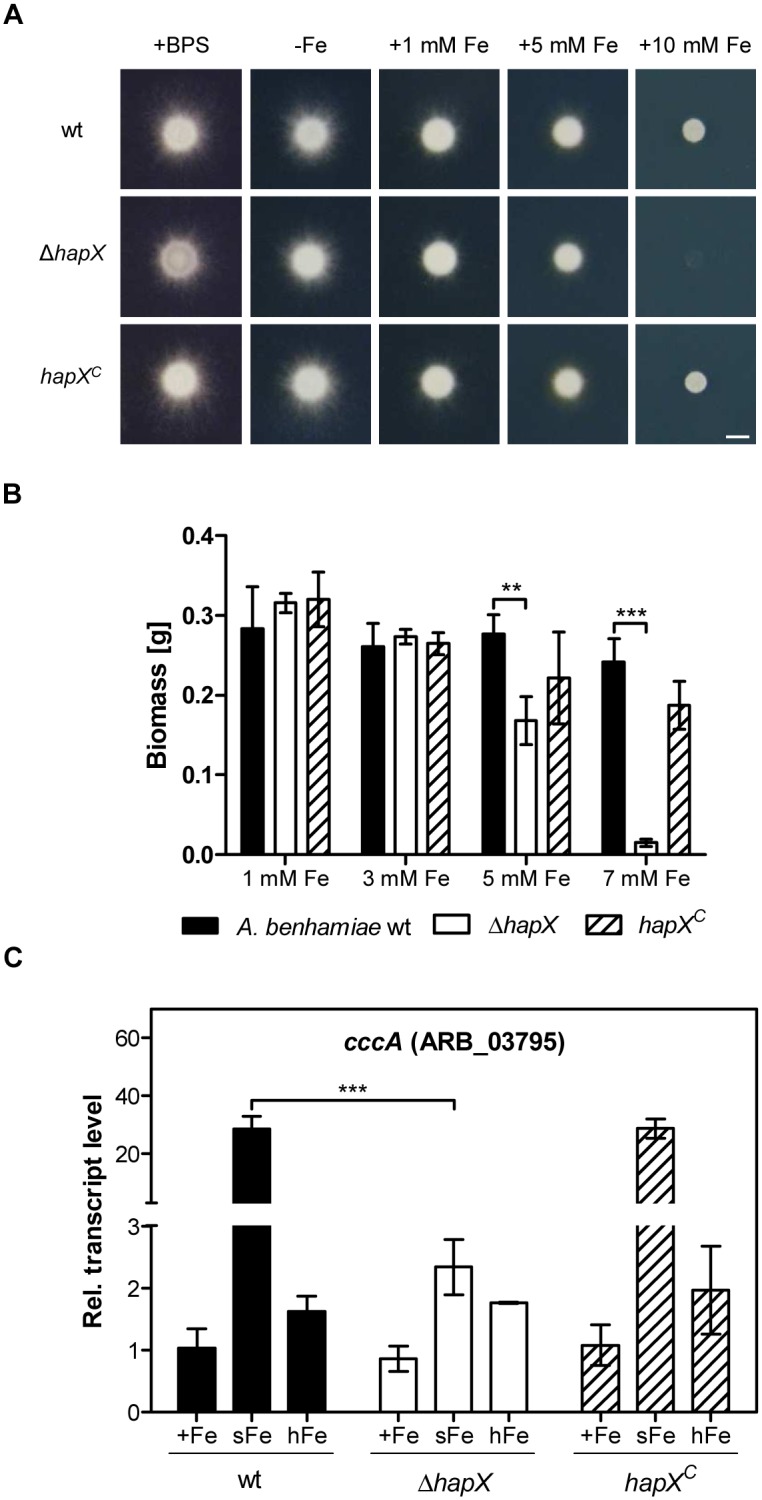
(A) Cultivation of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain during iron starvation (-Fe), high iron concentrations (1–10 mM FeSO4) and in the presence of the iron chelator BPS on solid medium for 7 d at 30°C. Scale bar represents 5 mm. (B) Cultivation of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain in AMM with high iron concentrations (1–7 mM FeSO4). Data represent the means and standard deviations of three biological replicates. The differences between wild type and ΔhapX mutant were statistically significant between 5 mM and 7 mM Fe (2way ANOVA; ** significant at P < 0.01, *** significant at P < 0.001). (C) Quantitative RT-PCR analysis of the cccA gene under different iron concentrations. The mycelium was cultivated under high iron concentrations (hFe) or shifted for 1 h from -Fe to +Fe (sFe). Data represent the means and standard deviations of three biological replicates. The differences between wild type and ΔhapX mutant were statistically significant during sFe (2way ANOVA; *** significant at P < 0.001).
HapX is dispensable for growth on keratin substrates
To date, only few models are available for testing putative virulence factors of dermatophytes [38]. Besides the application of animal models in guinea pig and mouse [39, 40], keratinized host tissues, including hair and nails, have been used for the analysis of putative pathogenicity associated factors in dermatophytes [12, 41]. To test whether the transcription factor HapX plays a role during infection, in vitro growth of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain on human hair and nails as well as on keratin powder derived from hooves and horns was analyzed. No growth differences were observed between A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain during infection of all tested keratin substrates (Fig 7).
Fig 7. HapX of A. benhamiae is dispensable for growth on keratin substrates.
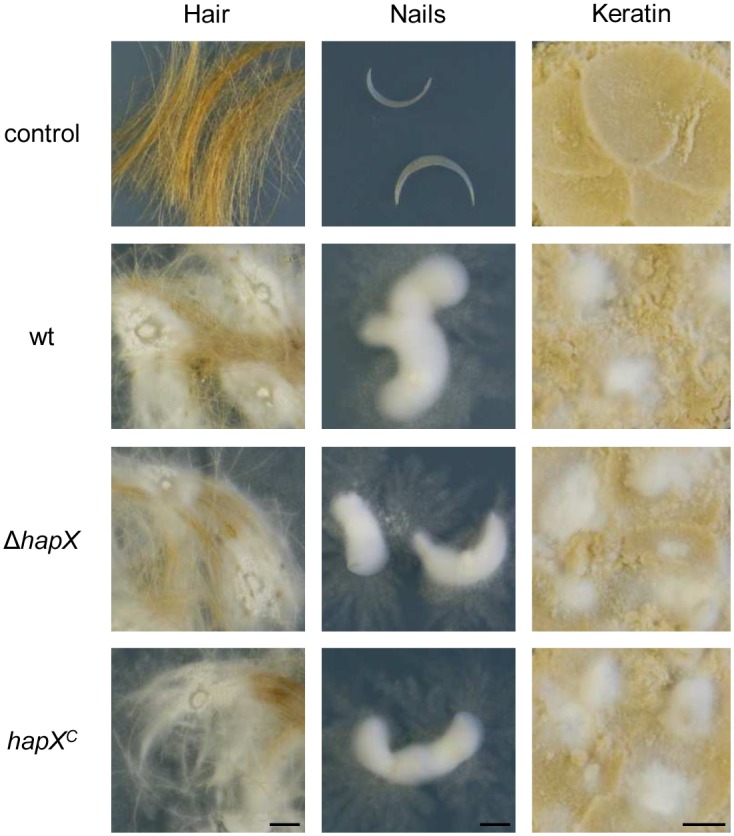
Cultivation of A. benhamiae wild type, ΔhapX mutant and hapXC reconstituted strain on human hair, finger nails and keratin powder derived from hooves and horns. Scale bar represents 5 mm.
Discussion
To overcome iron deficiency, many pathogenic fungi produce, release and take up siderophores. The chemical structure of siderophores enable them to chelate ferric iron and even extract iron from transferrin, lactoferrin or ferritin [42, 43]. The production of siderophores is usually transcriptionally regulated depending on iron availability.
As shown here, the emerging human pathogenic dermatophyte A. benhamiae produces the hydroxamate siderophores ferricrocin and ferrichrome C as intra- and extracellular siderophores. Both siderophores were isolated from the culture supernatant of the dermatophytes Microsporum gypseum, Microsporum audouinii, M. canis as well as T. rubrum [44]. Usually, filamentous fungi use ferrichrome-type siderophores for intracellular iron distribution and iron storage [45] as shown for A. nidulans, A. fumigatus, Fusarium graminearum, F. oxysporum, Neurospora crassa and Magnaporthe grisea [29, 46–50]. For iron acquisition, however, most fungi produce and secrete different hydroxamate siderophores, such as fusarinines and coprogens. The extracellular siderophores fusarinine C and triacetylfusarinine C (TAFC), for instance, are produced by A. nidulans and A. fumigatus [46, 51, 52], exclusively fusarinine C by Fusarium roseum and F. oxysporum [29, 53] and coprogens by N. crassa and M. grisea [49, 50]. In contrast to other fungi, dermatophytes appear to produce ferrichromes for both intra- and extracellular use. In this context, it is interesting to note that secretion of ferrichrome-type siderophores has been described for the yeast Schizosaccharomyces pombe [54], the basidiomycete Ustilago maydis [55] and the filamentous fungi F. roseum and F. oxysporum [29, 53]. In the presence of the bacterial siderophore deferoxamine biomass production of the A. benhamiae wild-type strain was increased under iron limitation compared to iron-depleted conditions which indicates that A. behamiae is able to use xenosiderophores as iron source. Similar results were reported for species of Paracoccidioides [56]. By contrast, growth of the ΔhapX mutant of A. benhamiae was highly negatively affected by the presence of deferoxamine. This result suggests that the ΔhapX mutant was unable to use xenosiderophore for iron acquisition, which may be caused by a defect in the siderophore uptake system in this mutant. Uptake of the ferri-form of the siderophores is mediated by siderophore-iron transporters (SITs). Although SITs are quite conserved in siderophore producing and non-producing fungal species only a few SITs have been identified and functionally characterized, so far (reviewed in [24]). In dermatophytes, SITs responsible for uptake of ferrichrome C and ferricrocin have not been identified yet.
The structure of ferricrocin differs from ferrichrome C only by a serine for alanine substitution. It has been suggested that both siderophores ferricrocin and ferrichrome C are synthesized by the ferrichrome-type NRPS SidC in F. oxysporum [29]. The genes encoding NRPS SidC and ornithine monooxygenase SidA of A. benhamiae are clustered which is typical for genes encoding components of common pathways in filamentous fungi. Similar to U. maydis, S. pombe, F. graminearum and F. oxysporum the genes sidA and sidC of A. benhamiae are probably bidirectionally transcribed from a common promoter region [29, 54, 57, 58]. In contrast, sidA and sidC of A. nidulans and A. fumigatus are located on different chromosomes [47, 59]. Conversely, the genes encoding TAFC esterase EstB, acetyltransferase SidG, siderophore iron transporter MirB and an ABC-transporter are clustered in A. fumigatus [60], but not in A. benhamiae (S3 Table). Although Arthroderma and Aspergillus both belong to the subclass Eurotiomycetidae, the organisation of their siderophore biosynthesis genes is obviously more similar to phylogenetically more distantly related phyla.
Deletion of hapX of A. benhamiae resulted in a lacking activation of the siderophore biosynthesis genes sidA and sidC during iron starvation and in a decreased production of extracellular ferrichrome C, but not ferricrocin. Similarly, the ΔhapX mutant of A. fumigatus displayed a downregulation of genes involved in siderophore biosynthesis and a decreased production of the siderophores ferricrocin and TAFC, but not fusarinine C during iron-limiting conditions [19]. Divergent from this observation, lack of HapX caused a reduced TAFC production but an increased level of ferricrocin and the sidC transcript during iron-depleted conditions in A. nidulans [18]. By contrast, inactivation of hapX in F. oxysporum led to elevated transcript levels of several siderophore genes and to an increased amount of intracellular, but not extracellular siderophores during iron starvation [29].
Transcriptional regulation of iron homeostasis and regulation of the siderophore system in A. nidulans, A. fumigatus and S. pombe is achieved by the transcription factors HapX and SreA (referred to as Php4 and Fep1 in S. pombe) which are interconnected by a negative feed-back loop [18, 19, 61, 62]. The data from our study indicate that the same model can be applied to A. benhamiae. SreA represses the expression of hapX and the siderophore system during iron sufficient conditions by an iron-sensing mechanism, while HapX represses sreA and activates the siderophore system during iron-limiting conditions resulting in efficient iron uptake and inhibition of iron-consuming pathways. As described above, deletion of hapX in A. benhamiae, A. fumigatus and A. nidulans resulted to some extent in a decreased siderophore production [18, 19]. At the same time, this result implies the existence of alternative mechanisms regulating the siderophore system. Besides HapX and SreA, the sterol regulatory element binding protein (SREBP) SrbA was shown to contribute to the activation of siderophore production in A. fumgatus [63]. Furthermore, the pH signaling transcription factor PacC, the gluconeogenesis-regulating transcription factors AcuK and AcuM and the mitogen-activated protein kinase (MAPK) MpkA have been suggested to be involved as well [64–67].
The main precursor for fungal siderophores is the non proteinogenic amino acid ornithine [45]. Additionally, the biosynthesis of fusarinine-type siderophores is linked to the isoprenoid biosynthesis pathway in A. fumigatus. The intermediate mevalonate produced by the HMG-CoA reductase serves as precursor for the biosynthesis of fusarinine C and TAFC [36]. In A. fumigatus, the transcript level of the gene encoding the HMG-CoA reductase, hmg1, is highly increased during iron starvation [36]. In contrast, the availability of iron did not significantly influence the transcript level of hmg1 in A. benhamiae. This is in line with data from the non-siderophore producing fungi S. cerevisiae, C. neoformans and C. albicans [68–70]. Similarly, the presence or absence of iron did not affect the expression of hmg1 in U. maydis [71] despite the fact that ferrichrome A biosynthesis is also linked to the isoprenoid biosynthesis pathway in U. maydis [37].
Besides altered regulation of siderophore biosynthesis, deletion of HapX in A. benhamiae resulted in decreased growth, conidiation and complete deregulation of genes from iron-dependent pathways such as vacuolar iron storage, amino acid metabolism, respiration and heme biosynthesis during iron limitation. The transcript level of cccA (vacuolar iron importer), lysF (homoaconitase), cycA (cytochrome C) and hemA (5-amino-levulinic acid synthase) was highly upregulated in the ΔhapX mutant during iron starvation. Similar results have been obtained for loss of HapX homologues in A. nidulans, A. fumigatus, F. oxysporum, C. neoformans, C. albicans and S. pombe [18, 19, 27–29, 61, 62]. Due to the activated heme biosynthesis during iron starvation, predictably the iron-free heme precursor protoporphyrin IX accumulated in the ΔhapX mutant of A. benhamiae causing a reddish pigmentation of the mycelium. The same has been previously reported from A. nidulans, A. fumigatus and F. oxysporum [18, 19, 29].
The transcription factor HapX is also essential for iron detoxification by activating the vacuolar iron importer CccA during high iron conditions [21, 25]. Consequently, deletion of hapX inhibited growth of A. fumigatus, A. nidulans, F. oxysporum and A. benhamiae in the presence of excess iron (this study, [22]). Similarly, the transcript level of cccA was highly upregulated during a shift from iron starvation to iron-replete conditions for one hour in A. benhamiae wild type, but not in the ΔhapX mutant. Interestingly, long-term periods of iron excess did not affect the transcript level of cccA in A. benhamiae which underlines the importance of the vacuolar iron importer during acute high iron stress. Distinct protein domains of HapX allow this Janus-type transcription factor to function as activator or repressor and consequently, to mediate both adaptation to iron starvation and iron resistance [22]. Additionally, it was found that the CBC-HapX complex of A. nidulans cooperatively binds to a bipartite DNA motif within the promoter of genes which are downregulated during iron limitation [23]. Characterization of this DNA motif in promoters of the genes cycA, sreA, acoA and lysF in A. nidulans resulted in the identification of the minimal motif 5’-GAT-3’, which is located at a distance of 11–12 bp downstream of the respective CCAAT box [23]. A similar motif is also evolutionary conserved in the cccA promoter of 28 fungi including species of Aspergillus and dermatophytes, e.g. A. benhamiae [22].
The critical role of iron acquistion in host-pathogen interactions has been demonstrated in various animal and plant pathogenic fungi. Siderophore-mediated iron uptake was shown to be essential for virulence of A. fumigatus, C. albicans, F. oxysporum and to a lesser extent in C. neoformans in murine infection models [19, 27–29, 47, 52, 72, 73]. Surprisingly, the ΔhapX mutant of A. benhamiae did not show a virulence defect during in vitro infection of hair and nails. A major difference between the human pathogenic fungi A. fumigatus, C. albicans, C. neoformans and A. benhamiae is the infectious life cycle. In contrast to A. fumigatus, C. albicans and C. neoformans, which are able to grow invasively in immunocompromised individuals, A. benhamiae is restricted to superficial growth on keratinized host structures such as skin (stratum corneum), hair and nails of humans and animals. We hypothesized that skin, hair and nails constitute a highly iron-restricted environment, but the ability of the ΔhapX mutant of A. benhamiae to grow on these keratinized structures might result from sufficient iron acquisition. In support of this idea, it has been described that iron is excreted by skin through desquamation of iron-loaded epithelial cells [74]. Furthermore, previous studies have shown that human epidermis, hair of children and finger nails contain variable amounts of iron [75–77]. It is possible that iron of these keratin substrates is easily accessable for dermatophytes by siderophores or alternative iron uptake mechanisms, which might explain the missing growth defect of the ΔhapX mutant of A. benhamiae on keratin. Besides siderophores, the A. benhamiae genome encodes proteins which represent homologues of iron permeases and oxidoreductases known to be involved in reductive iron assimilation (RIA) (S3 Table). These proteins may contribute to iron acquisition, too. Alternative mechanisms for iron acquisition such as low-affinity iron uptake systems have been described in S. cerevisiae, C. neoformans and A. nidulans [59, 78, 79]. Prerequisite for iron uptake by low-affinity mechanisms is the availability of ferrous iron which is the prevalent form of iron during acidic conditions. Human skin, scalp, hair shafts and nail plate have an acidic pH of 5.5 and below [80–82] which suggests that ferrous iron uptake mechanisms might play a role during superficial growth of A. benhamiae. Additionally, genes involved in iron homeostasis were not differentially regulated in the transcriptome of A. benhamiae during infection of human keratinocytes [13]. This result further supports the idea that siderophore-mediated iron uptake plays a minor role during dermatophyte infection.
Conclusions
This study underlines the highly conserved role of the fungal-specific transcription factor HapX for adaptation to iron starvation and especially, its relevance for the downregulation of iron-consuming pathways and the activation of siderophore biosynthesis during iron deficiency in ascomycetous fungi. Furthermore, HapX is a virulence factor in many plant and human pathogenic fungi in vivo, but is redundant in A. benhamiae during in vitro infection of keratinized host tissues, which might reflect the different iron supply or requirements of fungi during their respective infectious life cycle.
Supporting Information
The N-terminal CBC binding domain is indicated by bold letters, the bZIP domain is shaded in black, the coiled-coil domain is highlighted in grey and the four conserved cysteine-rich regions are indicated by black lines.
(TIF)
(A) Ferricrocin standard m/z 771. (B) Ferrichrome C standard m/z 755. (C) Mycelial extract of A. benhamiae wild type m/z 771. (D) Mycelial extract of A. benhamiae wild type m/z 755.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We are very grateful to Prof. Hubertus Haas from the Innsbruck Medical University, Austria for providing the siderophore ferricrocin. We thank Silke Steinbach for excellent technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the DFG funded excellence graduate school Jena School for Microbial Communication (JSMC; GSC 214; www.jsmc.uni-jena.de) and the Leibniz Institute for Natural Product Research and Infection Biology (www.leibniz-hki.de). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Weitzman I, Summerbell RC. The Dermatophytes. Clin Microbiol Rev. 1995;8(2):240–59. Epub 1995/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasch J, Wodarg S. Morphological and physiological features of Arthroderma benhamiae anamorphs isolated in northern Germany. Mycoses. 2015;58(2):93–8. Epub 2014/12/23. 10.1111/myc.12280 . [DOI] [PubMed] [Google Scholar]
- 3.Drouot S, Mignon B, Fratti M, Roosje P, Monod M. Pets as the main source of two zoonotic species of the Trichophyton mentagrophytes complex in Switzerland, Arthroderma vanbreuseghemii and Arthroderma benhamiae. Vet Dermatol. 2009;20(1):13–8. Epub 2008/08/14. 10.1111/j.1365-3164.2008.00691.x . [DOI] [PubMed] [Google Scholar]
- 4.Borgers M, Degreef H, Cauwenbergh G. Fungal Infections of the Skin: Infection Process and Antimycotic Therapy. Curr Drug Targets. 2005;6(8):849–62. Epub 2005/12/27. 10.2174/138945005774912726 . [DOI] [PubMed] [Google Scholar]
- 5.Fachin AL, Ferreira-Nozawa MS, Maccheroni W Jr., Martinez-Rossi NM. Role of the ABC transporter TruMDR2 in terbinafine, 4-nitroquinoline N-oxide and ethidium bromide susceptibility in Trichophyton rubrum. J Med Microbiol. 2006;55(Pt 8):1093–9. Epub 2006/07/20. 10.1099/jmm.0.46522-0 . [DOI] [PubMed] [Google Scholar]
- 6.Maranhᾶo FC, Paiᾶo FG, Fachin AL, Martinez-Rossi NM. Membrane transporter proteins are involved in Trichophyton rubrum pathogenesis. J Med Microbiol. 2009;58(Pt 2):163–8. Epub 2009/01/15. 10.1099/jmm.0.002907-0 . [DOI] [PubMed] [Google Scholar]
- 7.Ferreira-Nozawa MS, Silveira HC, Ono CJ, Fachin AL, Rossi A, Martinez-Rossi NM. The pH signaling transcription factor PacC mediates the growth of Trichophyton rubrum on human nail in vitro. Med Mycol. 2006;44(7):641–5. Epub 2006/10/31. 10.1080/13693780600876553 . [DOI] [PubMed] [Google Scholar]
- 8.Vermout S, Tabart J, Baldo A, Monod M, Losson B, Mignon B. RNA silencing in the dermatophyte Microsporum canis. FEMS Microbiol Lett. 2007;275(1):38–45. Epub 2007/08/08. 10.1111/j.1574-6968.2007.00870.x . [DOI] [PubMed] [Google Scholar]
- 9.Baldo A, Mathy A, Tabart J, Camponova P, Vermout S, Massart L, et al. Secreted subtilisin Sub3 from Microsporum canis is required for adherence to but not for invasion of the epidermis. Br J Dermatol. 2010;162(5):990–7. Epub 2009/12/10. 10.1111/j.1365-2133.2009.09608.x . [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Niu Q, Yu X, Jia X, Wang J, Lin D, et al. Assessment of the function of SUB6 in the pathogenic dermatophyte Trichophyton mentagrophytes. Med Mycol. 2015. Epub 2015/09/01. 10.1093/mmy/myv071 [DOI] [PubMed] [Google Scholar]
- 11.Grumbt M, Monod M, Staib P. Genetic advances in dermatophytes. FEMS Microbiol Lett. 2011;320(2):79–86. Epub 2011/04/05. 10.1111/j.1574-6968.2011.02276.x . [DOI] [PubMed] [Google Scholar]
- 12.Grumbt M, Defaweux V, Mignon B, Monod M, Burmester A, Wöstemeyer J, et al. Targeted Gene Deletion and In Vivo Analysis of Putative Virulence Gene Function in the Pathogenic Dermatophyte Arthroderma benhamiae. Eukaryot Cell. 2011;10(6):842–53. Epub 2011/04/12. 10.1128/ec.00273-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burmester A, Shelest E, Glöckner G, Heddergott C, Schindler S, Staib P, et al. Comparative and functional genomics provide insights into the pathogenicity of dermatophytic fungi. Genome Biol. 2011;12(1):R7 Epub 2011/01/21. 10.1186/gb-2011-12-1-r7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaugg C, Monod M, Weber J, Harshman K, Pradervand S, Thomas J, et al. Gene Expression Profiling in the Human Pathogenic Dermatophyte Trichophyton rubrum during Growth on Proteins. Eukaryot Cell. 2009;8(2):241–50. Epub 2008/12/23. 10.1128/ec.00208-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staib P, Zaugg C, Mignon B, Weber J, Grumbt M, Pradervand S, et al. Differential gene expression in the pathogenic dermatophyte Arthroderma benhamiae in vitro versus during infection. Microbiology. 2010;156(Pt 3):884–95. Epub 2009/11/28. 10.1099/mic.0.033464-0 . [DOI] [PubMed] [Google Scholar]
- 16.Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219(1):1–14. Epub 1984/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas H, Zadra I, Stöffler G, Angermayr K. The Aspergillus nidulans GATA Factor SREA Is Involved in Regulation of Siderophore Biosynthesis and Control of Iron Uptake. J Biol Chem. 1999;274(8):4613–9. Epub 1999/02/13. 10.1074/jbc.274.8.4613 . [DOI] [PubMed] [Google Scholar]
- 18.Hortschansky P, Eisendle M, Al-Abdallah Q, Schmidt AD, Bergmann S, Thon M, et al. Interaction of HapX with the CCAAT-binding complex—a novel mechanism of gene regulation by iron. EMBO J. 2007;26(13):3157–68. Epub 2007/06/15. 10.1038/sj.emboj.7601752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrettl M, Beckmann N, Varga J, Heinekamp T, Jacobsen ID, Jöchl C, et al. HapX-Mediated Adaption to Iron Starvation Is Crucial for Virulence of Aspergillus fumigatus. PLoS Pathog. 2010;6(9):e1001124 Epub 2010/10/14. 10.1371/journal.ppat.1001124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas H. Iron—a key nexus in the virulence of Aspergillus fumigatus. Front Microbiol. 2012;3:28 Epub 2012/02/22. 10.3389/fmicb.2012.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrettl M, Kim HS, Eisendle M, Kragl C, Nierman WC, Heinekamp T, et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol Microbiol. 2008;70(1):27–43. Epub 2008/08/30. 10.1111/j.1365-2958.2008.06376.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gsaller F, Hortschansky P, Beattie SR, Klammer V, Tuppatsch K, Lechner BE, et al. The Janus transcription factor HapX controls fungal adaptation to both iron starvation and iron excess. EMBO J. 2014;33(19):2261–76. Epub 2014/08/06. 10.15252/embj.201489468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hortschansky P, Ando E, Tuppatsch K, Arikawa H, Kobayashi T, Kato M, et al. Deciphering the Combinatorial DNA-binding Code of the CCAAT-binding Complex and the Iron-regulatory Basic Region Leucine Zipper (bZIP) Transcription Factor HapX. J Biol Chem. 2015;290(10):6058–70. Epub 2015/01/16. 10.1074/jbc.M114.628677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat Prod Rep. 2014;31(10):1266–76. Epub 2014/08/21. 10.1039/c4np00071d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gsaller F, Eisendle M, Lechner BE, Schrettl M, Lindner H, Müller D, et al. The interplay between vacuolar and siderophore-mediated iron storage in Aspergillus fumigatus. Metallomics. 2012;4(12):1262–70. Epub 2012/11/16. 10.1039/c2mt20179h . [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Pande K, French SD, Tuch BB, Noble SM. An iron homeostasis regulatory circuit with reciprocal roles in Candida albicans commensalism and pathogenesis. Cell Host Microbe. 2011;10(2):118–35. Epub 2011/08/17. 10.1016/j.chom.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu PC, Yang CY, Lan CY. Candida albicans Hap43 Is a Repressor Induced under Low-Iron Conditions and Is Essential for Iron-Responsive Transcriptional Regulation and Virulence. Eukaryot Cell. 2011;10(2):207–25. Epub 2010/12/07. 10.1128/ec.00158-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung WH, Saikia S, Hu G, Wang J, Fung CK, D'Souza C, et al. HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in Cryptococcus neoformans. PLoS Pathog. 2010;6(11):e1001209 Epub 2010/12/03. 10.1371/journal.ppat.1001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López-Berges MS, Capilla J, Turrà D, Schafferer L, Matthijs S, Jöchl C, et al. HapX-Mediated Iron Homeostasis Is Essential for Rhizosphere Competence and Virulence of the Soilborne Pathogen Fusarium oxysporum. Plant Cell. 2012;24(9):3805–22. Epub 2012/09/13. 10.1105/tpc.112.098624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fumeaux J, Mock M, Ninet B, Jan I, Bontems O, Léchenne B, et al. First Report of Arthroderma benhamiae in Switzerland. Dermatology. 2004;208(3):244–50. Epub 2004/05/01. 10.1159/000077311 . [DOI] [PubMed] [Google Scholar]
- 31.Brakhage AA, Van den Brulle J. Use of reporter genes to identify recessive trans-acting mutations specifically involved in the regulation of Aspergillus nidulans penicillin biosynthesis genes. J Bacteriol. 1995;177(10):2781–8. Epub 1995/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machuca A, Milagres AM. Use of CAS-agar plate modified to study the effect of different variables on the siderophore production by Aspergillus. Lett Appl Microbiol. 2003;36(3):177–81. Epub 2003/02/13. 10.1046/j.1472-765X.2003.01290.x . [DOI] [PubMed] [Google Scholar]
- 33.Schwyn B, Neilands JB. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal Biochem. 1987;160(1):47–56. Epub 1987/01/01. 10.1016/0003-2697(87)90612-9 . [DOI] [PubMed] [Google Scholar]
- 34.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):1–12. Epub 2012/06/22. 10.1093/nar/gks596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45 Epub 2001/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yasmin S, Alcazar-Fuoli L, Grundlinger M, Puempel T, Cairns T, Blatzer M, et al. Mevalonate governs interdependency of ergosterol and siderophore biosyntheses in the fungal pathogen Aspergillus fumigatus. Proc Natl Acad Sci U S A. 2012;109(8):E497–504. Epub 2011/11/23. 10.1073/pnas.1106399108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winterberg B, Uhlmann S, Linne U, Lessing F, Marahiel MA, Eichhorn H, et al. Elucidation of the complete ferrichrome A biosynthetic pathway in Ustilago maydis. Mol Microbiol. 2010;75(5):1260–71. Epub 2010/01/15. 10.1111/j.1365-2958.2010.07048.x . [DOI] [PubMed] [Google Scholar]
- 38.Achterman RR, White TC. Dermatophyte Virulence Factors: Identifying and Analyzing Genes That May Contribute to Chronic or Acute Skin Infections. Int J Microbiol. 2012. Epub 2011/10/07. 10.1155/2012/358305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenberg JH, King RD, Krebs S, Field R. A quantitative dermatophyte infection model in the guinea pig—a parallel to the quantitated human infection model. J Invest Dermatol. 1976;67(6):704–8. Epub 1976/12/01. 10.1111/1523-1747.ep12598588 . [DOI] [PubMed] [Google Scholar]
- 40.Monod M. Development of a mouse infection model to bridge the gap between molecular biology and immunology in dermatophyte research. Br J Dermatol. 2014;170(3):491–2. Epub 2014/03/13. 10.1111/bjd.12866 . [DOI] [PubMed] [Google Scholar]
- 41.Takasuka T. Amino acid- or protein-dependent growth of Trichophyton mentagrophytes and Trichophyton rubrum. FEMS Immunol Med Microbiol. 2000;29(4):241–5. Epub 2000/12/19. [DOI] [PubMed] [Google Scholar]
- 42.Skaar EP. The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts. PLoS Pathog. 2010;6(8):e1000949 Epub 2010/08/17. 10.1371/journal.ppat.1000949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hissen AH, Chow JM, Pinto LJ, Moore MM. Survival of Aspergillus fumigatus in Serum Involves Removal of Iron from Transferrin: the Role of Siderophores. Infect Immun. 2004;72(3):1402–8. Epub 2004/02/24. 10.1128/IAI.72.3.1402-1408.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mor H, Kashman Y, Winkelmann G, Barash I. Characterization of siderophores produced by different species of the derrnatophytic fungi Microsporum and Trichophyton. BioMetals. 1992;5(4):213–6. 10.1007/BF01061220 [DOI] [Google Scholar]
- 45.Haas H, Eisendle M, Turgeon BG. Siderophores in Fungal Physiology and Virulence. Annu Rev Phytopathol. 2008;46:149–87. Epub 2008/08/06. 10.1146/annurev.phyto.45.062806.094338 . [DOI] [PubMed] [Google Scholar]
- 46.Charlang G, Ng B, Horowitz NH, Horowitz RM. Cellular and Extracellular Siderophores of Aspergillus nidulans and Penicillium chrysogenum. Mol Cell Biol. 1981;1(2):94–100. Epub 1981/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, et al. Distinct Roles for Intra- and Extracellular Siderophores during Aspergillus fumigatus Infection. PLoS Pathog. 2007;3(9):1195–207. Epub 2007/09/12. 10.1371/journal.ppat.0030128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oide S, Moeder W, Krasnoff S, Gibson D, Haas H, Yoshioka K, et al. NPS6, Encoding a Nonribosomal Peptide Synthetase Involved in Siderophore-Mediated Iron Metabolism, Is a Conserved Virulence Determinant of Plant Pathogenic Ascomycetes. Plant Cell. 2006;18(10):2836–53. Epub 2006/10/24. 10.1105/tpc.106.045633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horowitz NH, Charlang G, Horn G, Williams NP. Isolation and Identification of the Conidial Germination Factor of Neurospora crassa. J Bacteriol. 1976;127(1):135–40. Epub 1976/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antelo L, Hof C, Welzel K, Eisfeld K, Sterner O, Anke H. Siderophores Produced by Magnaporthe grisea in the Presence and Absence of Iron. Z Naturforsch C. 2006;61(5–6):461–4. Epub 2006/07/28. 10.1515/znc-2006-5-626 . [DOI] [PubMed] [Google Scholar]
- 51.Charlang G, Horowitz RM, Lowy PH, Ng B, Poling SM, Horowitz NH. Extracellular siderophores of rapidly growing Aspergillus nidulans and Penicillium chrysogenum. J Bacteriol. 1982;150(2):785–7. Epub 1982/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, Arst HN Jr., et al. Siderophore Biosynthesis But Not Reductive Iron Assimilation Is Essential for Aspergillus fumigatus Virulence. J Exp Med. 2004;200(9):1213–9. Epub 2004/10/27. 10.1084/jem.20041242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Emery T. Malonichrome, a new iron chelate from Fusarium roseum. Biochim Biophys Acta. 1980;629(2):382–90. Epub 1980/05/07. 10.1016/0304-4165(80)90110-5 . [DOI] [PubMed] [Google Scholar]
- 54.Schrettl M, Winkelmann G, Haas H. Ferrichrome in Schizosaccharomyces pombe—an iron transport and iron storage compound. Biometals. 2004;17(6):647–54. Epub 2005/02/04. 10.1007/s10534-004-1230-z . [DOI] [PubMed] [Google Scholar]
- 55.Budde AD, Leong SA. Characterization of siderophores from Ustilago maydis. Mycopathologia. 1989;108(2):125–33. Epub 1989/11/01. [DOI] [PubMed] [Google Scholar]
- 56.Silva-Bailᾶo MG, Bailᾶo EF, Lechner BE, Gauthier GM, Lindner H, Bailᾶo AM, et al. Hydroxamate production as a high affinity iron acquisition mechanism in Paracoccidioides Spp. PLoS One. 2014;9(8):e105805 Epub 2014/08/27. 10.1371/journal.pone.0105805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan WM, Gentil GD, Budde AD, Leong SA. Characterization of the Ustilago maydis sid2 Gene, Encoding a Multidomain Peptide Synthetase in the Ferrichrome Biosynthetic Gene Cluster. J Bacteriol. 2001;183(13):4040–51. Epub 2001/06/08. 10.1128/jb.183.13.4040-4051.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tobiasen C, Aahman J, Ravnholt KS, Bjerrum MJ, Grell MN, Giese H. Nonribosomal peptide synthetase (NPS) genes in Fusarium graminearum, F. culmorum and F. pseudograminearium and identification of NPS2 as the producer of ferricrocin. Curr Genet. 2007;51(1):43–58. Epub 2006/10/18. 10.1007/s00294-006-0103-0 . [DOI] [PubMed] [Google Scholar]
- 59.Eisendle M, Oberegger H, Zadra I, Haas H. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol Microbiol. 2003;49(2):359–75. Epub 2003/06/28. 10.1046/j.1365-2958.2003.03586.x . [DOI] [PubMed] [Google Scholar]
- 60.Kragl C, Schrettl M, Abt B, Sarg B, Lindner HH, Haas H. EstB-Mediated Hydrolysis of the Siderophore Triacetylfusarinine C Optimizes Iron Uptake of Aspergillus fumigatus. Eukaryot Cell. 2007;6(8):1278–85. Epub 2007/06/26. 10.1128/ec.00066-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mercier A, Watt S, Bähler J, Labbé S. Key Function for the CCAAT-Binding Factor Php4 To Regulate Gene Expression in Response to Iron Deficiency in Fission Yeast. Eukaryot Cell. 2008;7(3):493–508. Epub 2008/01/29. 10.1128/ec.00446-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mercier A, Pelletier B, Labbé S. A Transcription Factor Cascade Involving Fep1 and the CCAAT-Binding Factor Php4 Regulates Gene Expression in Response to Iron Deficiency in the Fission Yeast Schizosaccharomyces pombe. Eukaryot Cell. 2006;5(11):1866–81. Epub 2006/09/12. 10.1128/ec.00199-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blatzer M, Barker BM, Willger SD, Beckmann N, Blosser SJ, Cornish EJ, et al. SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus. PLoS Genet. 2011;7(12):e1002374 Epub 2011/12/07. 10.1371/journal.pgen.1002374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eisendle M, Oberegger H, Buttinger R, Illmer P, Haas H. Biosynthesis and uptake of siderophores is controlled by the PacC-mediated ambient-pH Regulatory system in Aspergillus nidulans. Eukaryot Cell. 2004;3(2):561–3. Epub 2004/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pongpom M, Liu H, Xu W, Snarr BD, Sheppard DC, Mitchell AP, et al. Divergent targets of Aspergillus fumigatus AcuK and AcuM transcription factors during growth in vitro versus invasive disease. Infect Immun. 2015;83(3):923–33. Epub 2014/12/24. 10.1128/iai.02685-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu H, Gravelat FN, Chiang LY, Chen D, Vanier G, Ejzykowicz DE, et al. Aspergillus fumigatus AcuM regulates both iron acquisition and gluconeogenesis. Mol Microbiol. 2010;78(4):1038–54. Epub 2010/11/11. 10.1111/j.1365-2958.2010.07389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain R, Valiante V, Remme N, Docimo T, Heinekamp T, Hertweck C, et al. The MAP kinase MpkA controls cell wall integrity, oxidative stress response, gliotoxin production and iron adaptation in Aspergillus fumigatus. Mol Microbiol. 2011;82(1):39–53. Epub 2011/09/03. 10.1111/j.1365-2958.2011.07778.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shakoury-Elizeh M, Tiedeman J, Rashford J, Ferea T, Demeter J, Garcia E, et al. Transcriptional remodeling in response to iron deprivation in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15(3):1233–43. Epub 2003/12/12. 10.1091/mbc.E03-09-0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lian T, Simmer MI, D'Souza CA, Steen BR, Zuyderduyn SD, Jones SJ, et al. Iron-regulated transcription and capsule formation in the fungal pathogen Cryptococcus neoformans. Mol Microbiol. 2005;55(5):1452–72. Epub 2005/02/22. 10.1111/j.1365-2958.2004.04474.x . [DOI] [PubMed] [Google Scholar]
- 70.Lan CY, Rodarte G, Murillo LA, Jones T, Davis RW, Dungan J, et al. Regulatory networks affected by iron availability in Candida albicans. Mol Microbiol. 2004;53(5):1451–69. Epub 2004/09/25. 10.1111/j.1365-2958.2004.04214.x . [DOI] [PubMed] [Google Scholar]
- 71.Eichhorn H, Lessing F, Winterberg B, Schirawski J, Kamper J, Muller P, et al. A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis. Plant Cell. 2006;18(11):3332–45. Epub 2006/12/02. 10.1105/tpc.106.043588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hissen AH, Wan AN, Warwas ML, Pinto LJ, Moore MM. The Aspergillus fumigatus Siderophore Biosynthetic Gene sidA, Encoding L-Ornithine N5-Oxygenase, Is Required for Virulence. Infect Immun. 2005;73(9):5493–503. Epub 2005/08/23. 10.1128/iai.73.9.5493-5503.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schrettl M, Ibrahim-Granet O, Droin S, Huerre M, Latgé JP, Haas H. The crucial role of the Aspergillus fumigatus siderophore system in interaction with alveolar macrophages. Microbes Infect. 2010;12(12–13):1035–41. Epub 2010/07/28. 10.1016/j.micinf.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weintraub LR, Demis DJ, Conrad ME, Crosby WH. Iron Excretion by the Skin. Selective Localization of Iron59 in Epithelial Cells. Am J Pathol. 1965;46:121–7. Epub 1965/01/01. [PMC free article] [PubMed] [Google Scholar]
- 75.Bissett DL, McBride JF. Iron content of human epidermis from sun-exposed and non-exposed body sites. J Cosmet Sci. 1992;43(4):215–7. [Google Scholar]
- 76.Jacobs A, Jenkins DJ. The iron content of finger nails. Br J Dermatol. 1960;72:145–8. Epub 1960/04/01. 10.1111/j.1365-2133.1960.tb13863.x . [DOI] [PubMed] [Google Scholar]
- 77.Lovric VA, Pepper R. Iron Content of Hair in Children in Various States of Iron Balance. Pathology. 1971;3(4):251–6. [Google Scholar]
- 78.Jacobson ES, Goodner AP, Nyhus KJ. Ferrous Iron Uptake in Cryptococcus neoformans. Infect Immun. 1998;66(9):4169–75. Epub 1998/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaplan CD, Kaplan J. Iron Acquisition and Transcriptional Regulation. Chem Rev. 2009;109(10):4536–52. Epub 2009/08/27. 10.1021/cr9001676 . [DOI] [PubMed] [Google Scholar]
- 80.Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28(5):359–70. Epub 2008/05/21. 10.1111/j.1467-2494.2006.00344.x . [DOI] [PubMed] [Google Scholar]
- 81.Murdan S, Milcovich G, Goriparthi GS. An Assessment of the Human Nail Plate pH. Skin Pharmacol Physiol. 2011;24(4):175–81. Epub 2011/02/18. 10.1159/000324055 . [DOI] [PubMed] [Google Scholar]
- 82.Gavazzoni Dias MFR, de Almeida AM, Cecato PM, Adriano AR, Pichler J. The Shampoo pH can Affect the Hair: Myth or Reality? Int J Trichology. 2014;6(3):95–9. Epub 2014/09/12. 10.4103/0974-7753.139078 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The N-terminal CBC binding domain is indicated by bold letters, the bZIP domain is shaded in black, the coiled-coil domain is highlighted in grey and the four conserved cysteine-rich regions are indicated by black lines.
(TIF)
(A) Ferricrocin standard m/z 771. (B) Ferrichrome C standard m/z 755. (C) Mycelial extract of A. benhamiae wild type m/z 771. (D) Mycelial extract of A. benhamiae wild type m/z 755.
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.