Abstract
Dental plaque samples from 40 children were screened for the presence of bacteria resistant to amoxicillin. Fifteen children had used amoxicillin and 25 had not used any antibiotic in the 3 months prior to sample collection. All (100%) of the children harbored amoxicillin-resistant oral bacteria. The median percentage of the total cultivable oral microbiota resistant to amoxicillin was 2.4% (range, 0.1 to 14.3%) in children without amoxicillin use and 10.9% (range, 0.8 to 97.3%) in children with amoxicillin use, with the latter value being significantly higher (P < 0.01). A total of 224 amoxicillin-resistant bacteria were isolated and comprised three main genera: Haemophilus spp., Streptococcus spp., and Veillonella spp. The biodiversity of the amoxicillin-resistant microbiota was similar among the isolates from children with and without previous antibiotic use. The amoxicillin MIC at which 90% of the isolates were inhibited for isolates from children who had used amoxicillin in the previous 3 months was higher (64 mg liter−1) than that obtained for the isolates from subjects who had not used antibiotics (16 mg liter−1). The majority of the amoxicillin-resistant isolates (65%) were also resistant to at least one of the three antibiotics tested (penicillin, erythromycin, and tetracycline), with resistance to penicillin (51% of isolates) being the most frequently encountered. However, significantly more (P < 0.05) of the amoxicillin-resistant isolates from subjects with previous amoxicillin use were also resistant to erythromycin. This study has demonstrated that a diverse collection of amoxicillin-resistant bacteria is present in the oral cavity and that the number, proportions, MICs, and resistance to erythromycin can significantly increase with amoxicillin use.
Increases in the levels of antibiotic resistance among pathogenic bacteria have led to concern in the medical, dental, and scientific communities. Antibiotic use in the general population is an important factor promoting an increase in antibiotic resistance; however, antibiotics are prescribed disproportionately, with higher rates of use in children and elderly individuals. In particular, children younger than age 5 years are the most likely age group to receive a prescription for an antibiotic, with the broad-spectrum penicillins such as amoxicillin comprising 60% of the total antibiotic prescriptions for this age group (15). Amoxicillin is not only heavily used in medicine but is also frequently prescribed in dentistry and at present is recommended for the chemoprophylaxis of infective endocarditis prior to specific dental procedures (4). The oral microbiota is an important reservoir of antibiotic-resistant bacteria, and several studies have shown that amoxicillin-resistant bacteria are carried in the oral cavity (13, 16, 19). Indeed, in a previous study (19), all of the children investigated were shown to harbor a diverse community of amoxicillin-resistant bacteria in their oral cavities. Previous studies have demonstrated that the use of amoxicillin, erythromycin, josamycin, and tetracycline can increase the number and/or the proportion of antibiotic-resistant oral bacteria (8, 18, 20, 27). However, these studies were carried out with adult populations or a model oral system and not with young children. The aims of the present investigation were to study the effects of amoxicillin use on the prevalence, proportions, and identities of amoxicillin-resistant bacteria in the oral cavities of children aged 4 to 5 years old.
MATERIALS AND METHODS
Subjects.
All children aged 4 to 5 years attending three reception classes in a United Kingdom school were included in a dental screening program. Parental consent for the collection of dental plaque samples was obtained for 48 children, and the children were recruited into the study. Six children were excluded from the study due to the use of an antibiotic other than amoxicillin in the 3 months prior to sampling, and two children were unavailable for sampling. Samples were obtained from 40 children (21 girls, 19 boys). Study group 1 consisted of 25 children who had not used any class of antibiotic in the 3 months prior to sampling, and the second study group consisted of 15 children who had used amoxicillin in the 3 months prior to sampling. The local Research and Ethics Committee approved the study.
Sample collection and processing.
Plaque samples were taken from the entire dentition of the supragingival region of each subject by use of a calcium alginate swab (Technical Service Consultants Ltd., Heywood, United Kingdom). The swab was immediately placed into 4 ml of prereduced Calgon-Ringer solution (Oxoid Ltd., Basingstoke, United Kingdom) in a sterile bijou containing five sterile 2-mm-diameter glass beads (BDH Chemicals, Poole, United Kingdom). The samples were mixed with a vortex mixer for a minimum of 30 s or until the calcium alginate had dissolved. A 10-fold serial dilution of the sample was prepared in prereduced tryptone soy broth (Oxoid Ltd.). The total number of cultivable bacteria in the specimen was calculated by inoculating samples onto Iso-Sensitest agar (Oxoid Ltd.) containing 5% defibrinated horse blood (TCS Microbiology, Botolphclaydon, United Kingdom). To determine the number of amoxicillin-resistant bacteria, samples were inoculated onto Iso-Sensitest agar containing 5% defibrinated horse blood and 8 mg of ampicillin liter−1 (19). The plates were incubated in an anaerobic chamber (MACS 1000; Don Whitley Scientific Ltd., Shipley, United Kingdom) at 37°C for 5 days, and a duplicate set was incubated in air supplemented with 5% carbon dioxide at 37°C for 2 days. After incubation, the amoxicillin-resistant morphotypes were enumerated separately. These were then subcultured and incubated under both aerobic and anaerobic conditions to ascertain atmospheric requirements and stored at −70°C for further identification.
Identification.
Identification of the amoxicillin-resistant bacterial species was carried out by a combination of the Gram stain reaction, enzyme and carbohydrate utilization determination (25), and partial 16S rRNA gene sequencing. The 16S rRNA gene was amplified by PCR with global primers (primers 27f and 1492r; Genosys, Sigma, Poole, United Kingdom) and by subsequent partial DNA sequencing (10, 26) with an ABI 310 genetic analyzer (PE Biosystems, Warrington, United Kingdom). The sequences were analyzed by use of the Ribosomal Database Project II database (12) and the BLAST algorithm at the National Center for Biotechnology Information (2).
Statistical analysis and biodiversity.
To calculate the percentage of the cultivable microbiota resistant to each antibiotic, the total number of resistant isolates was compared with the total number of CFU for each sample. Analysis of the total viable counts and the proportions of amoxicillin-resistant microbiota in children with and without amoxicillin use in the previous 3 months was carried out by the Mann-Whitney U test. Any differences in the proportions of amoxicillin-resistant bacteria also resistant to additional antibiotics between the two groups was assessed by the chi-square test. Data were analyzed with SPSS software (22), and the 5% level of statistical significance was used throughout these analyses. The Shannon-Weaver index of diversity (H) was used to determine the species diversity of antibiotic-resistant bacteria by using the function −Σ [Pi ln(Pi)]. Pi is equal to n/N, where n is the number of an individual species, and N is the total number of all species (21). H equals zero when only one species is present and increases with species diversity.
Antibiotic susceptibility testing.
Susceptibility testing was performed by an agar dilution method (3). Concentrations ranging from 0.06 to 512 mg of penicillin, ampicillin, erythromycin, or tetracycline liter−1 were incorporated into Iso-Sensitest agar supplemented with 5% (vol/vol) defibrinated horse blood, and incubated at 37°C for 16 to 20 h. The inoculum was standardized and applied to the agar surface with a multipoint inoculator (Mast, Merseyside, United Kingdom). The MIC was defined as the lowest concentration of antibiotic that inhibited visible growth.
RESULTS
Identification of amoxicillin-resistant bacteria.
In total, 224 amoxicillin-resistant bacteria (MIC range, 8 to 128 mg liter−1) were isolated from the 40 children (Table 1). In addition to those shown in Table 1, three or fewer isolates of Capnocytophaga granulosa, Capnocytophaga sputigena, Fusobacterium nucleatum, Gemella haemolysans, Haemophilus haemolyticus, Haemophilus segnis, Leptotrichia buccalis, Neisseria perflava, Neisseria pharyngis, Neisseria sicca, Neisseria subflava, Staphylococcus epidermidis, Staphylococcus warneri, Rothia mucilaginosa, Streptococcus cristatus, Streptococcus intermedius, Streptococcus mutans, Streptococcus parasanguis, Streptococcus pneumoniae, and Veillonella atypica were recovered. Of these 224 isolates, 128 amoxicllin-resistant bacteria (9 genera and 25 species) were isolated from the 25 children who had not taken antibiotics and 96 amoxicillin-resistant bacteria (6 genera and 19 species) were isolated from the 15 children with previous amoxicillin use, with 5 genera and 13 species common to both. The biodiversity of the amoxicillin-resistant oral microbiota was similar in the isolates from children with (H = 2.6) and without (H = 2.8) previous amoxicillin use. Of the amoxicillin-resistant isolates recovered from children with and without amoxicillin use, the majority, 83.5 and 81.3%, respectively, comprised three main genera: Haemophilus spp., Streptococcus spp., and Veillonella spp. F. nucleatum, L. buccalis, N. pharyngis, S. cristatus, S. intermedius, and S. mutans were cultured only from children who had taken amoxicillin in the 3 months prior to sampling. However, C. granulosa, C. sputigena, G. haemolysans, H. haemolyticus, H. segnis, N. perflava, N. sicca, N. subflava, S. epidermidis, S. warneri, R. mucilaginosus, and S. pneumoniae were isolated only from subjects without recent antibiotic use.
TABLE 1.
MIC range, MIC50s, and MIC90s of amoxicillin, penicillin, erythromycin, and tetracycline for amoxicillin-resistant isolates
| Amoxicillin-resistant bacterium and antibiotic | MIC (mg liter−1)
|
Frequency of resistance (no. [%] of isolates)a | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| Haemophilus influenzae (n = 13) | ||||
| Amoxicillin | 8-132 | 16 | 16 | 13 (100) |
| Penicillin | ≤0.12-16 | 8 | 16 | —b |
| Erythromycin | 1-64 | 4 | 16 | 2 (15) |
| Tetracycline | ≤0.12-16 | 4 | 16 | 7 (54) |
| Haemophilus parainfluenzae (n = 24) | ||||
| Amoxicillin | 8-64 | 16 | 32 | 24 (100) |
| Penicillin | ≤0.12-64 | 8 | 16 | — |
| Erythromycin | 0.5-32 | 4 | 16 | 5 (21) |
| Tetracycline | ≤0.12-16 | 0.12 | 0.12 | 2 (8) |
| Haemophilus paraphrophilus (n = 25) | ||||
| Amoxicillin | 8-128 | 16 | 32 | 25 (100) |
| Penicillin | ≤0.12-32 | 8 | 32 | — |
| Erythromycin | 0.25-16 | 1 | 8 | 3 (12) |
| Tetracycline | ≤0.12-32 | 0.12 | 0.12 | 2 (8) |
| Neisseria mucosa (n = 10) | ||||
| Amoxicillin | 8-16 | 16 | 16 | 10 (100) |
| Penicillin | ≤0.12-8 | 1 | 8 | 5 (50) |
| Erythromycin | 0.5-32 | 0.5 | 32 | — |
| Tetracycline | ≤0.12 | ≤0.12 | ≤0.12 | 0 (0) |
| Prevotella melaninogenica (n = 6) | ||||
| Amoxicillin | 16-64 | 16 | 64 | 6 (100) |
| Penicillin | ≤0.12-16 | 8 | 16 | 4 (67) |
| Erythromycin | ≤0.12-16 | 0.12 | 16 | — |
| Tetracycline | ≤0.12 | 0.12 | 0.12 | 0 (0) |
| Streptococcus mitis (n = 34) | ||||
| Amoxicillin | 8-128 | 16 | 64 | 34 (100) |
| Penicillin | ≤0.12-32 | 8 | 16 | 27 (79) |
| Erythromycin | ≤0.12-32 | 4 | 16 | 18 (53) |
| Tetracycline | ≤0.12-128 | 0.12 | 64 | 11 (32) |
| Streptococcus oralis (n = 5) | ||||
| Amoxicillin | 8 | 8 | 8 | 5 (100) |
| Penicillin | 4-8 | 4 | 8 | 5 (100) |
| Erythromycin | ≤0.12-512 | 4 | 512 | 4 (80) |
| Tetracycline | ≤0.12-64 | 0.12 | 64 | 1 (20) |
| Streptococcus salivarius (n = 9) | ||||
| Amoxicillin | 8-128 | 16 | 128 | 9 (100) |
| Penicillin | ≤0.12-8 | 4 | 8 | 6 (67) |
| Erythromycin | ≤0.12-256 | 16 | 256 | 6 (67) |
| Tetracycline | ≤0.12-128 | 8 | 128 | 5 (56) |
| Streptococcus sanguis (n = 12) | ||||
| Amoxicillin | 8-128 | 8 | 64 | 12 (100) |
| Penicillin | ≤0.12-8 | 4 | 8 | 7 (58) |
| Erythromycin | ≤0.12-32 | 4 | 32 | 8 (67) |
| Tetracycline | ≤0.12-8 | 0.12 | 0.12 | 1 (8) |
| Veillonella dispar (n = 27) | ||||
| Amoxicillin | 8-128 | 16 | 32 | 27 (100) |
| Penicillin | ≤0.12-32 | 8 | 32 | 23 (85) |
| Erythromycin | ≤0.12-64 | 8 | 64 | — |
| Tetracycline | ≤0.12-32 | 0.12 | 16 | — |
| Veillonella parvula (n = 24) | ||||
| Amoxicillin | 8-64 | 16 | 16 | 24 (100) |
| Penicillin | ≤0.12-32 | 8 | 32 | 19 (79) |
| Erythromycin | ≤0.12-64 | 8 | 64 | — |
| Tetracycline | ≤0.12-32 | 0.12 | 0.12 | — |
| All isolates (n = 224) | ||||
| Amoxicillin | 8-128 | 16 | 32 | 224 (100) |
| Penicillin | ≤0.12-64 | 8 | 16 | 115 (51) |
| Erythromycin | ≤0.12-≥512 | 4 | 32 | 56 (25) |
| Tetracycline | ≤0.12-128 | 0.12 | 16 | 35 (16) |
MIC breakpoints were as follows: for Staphylococcus sp., non-S. pneumoniae Streptococcus sp., and G. haemolysans resistance, penicillin, ≥0.25 mg liter−1; erythromycin, ≥1 mg liter−1; tetracycline, ≥2 mg liter−1; for S. pneumoniae resistance, penicillin, ≥2 mg liter−1; erythromycin, ≥1 mg liter−1 tetracycline, ≥2 mg liter−1; for Neisseria sp. resistance; penicillin, ≥2 mg liter−1; tetracycline, ≥2 mg liter−1; for Haemophilus spp. resistance, erythromycin, ≥16 mg liter−1; tetracycline, ≥2 mg liter−1; for F. nucleatum, Veillonella sp., and P. melaninogenica resistance, penicillin, ≥2 mg liter−1; for Capnocytophaga spp., L. buccalis, and R. mucilaginosus, guidelines not available (3).
—, no recommendations currently available (3).
Proportion and prevalence of amoxicillin-resistant bacteria.
There was no significant difference in the total counts of the oral microbiota between the two study groups (Table 2). All 40 children harbored amoxicillin-resistant oral bacteria; consequently, there were no differences in the number of children harboring antibiotic-resistant bacteria. However, the use of amoxicillin in the 3 months prior to sampling significantly increased both the total number of amoxicillin-resistant bacteria (P < 0.01) and the proportion of the oral microbiota resistant to amoxicillin (P < 0.01). The median percentage of the amoxicillin-resistant oral microbiota increased from 2.4% in subjects who had not used antibiotics to 10.9% in subjects who had used amoxicillin in the 3 months prior to sampling, with 97% of the oral microbiota in one subject resistant to amoxicillin (Table 2).
TABLE 2.
Total counts, prevalence, and proportions of amoxicillin-resistant bacteria in children with and without amoxicillin use in the previous 3 months
| Antibiotic use | Median (range) total count (CFU/ml) | No. (%) of children harboring ampicillin-resistant bacteria | Median (range) count (CFU/ml) of amoxicillin-resistant bacteria | Median (range) % microbiota resistant to amoxicillin |
|---|---|---|---|---|
| − | 5.5 × 106 (1.0 × 106-7.8 × 107) | 100 (25) | 1.3 × 105 (1.0 × 103-7.2 × 105) | 2.4 (0.1-14.3) |
| + | 3.2 × 106 (1.2 × 106-3.0 × 107) | 100 (15) | 6.0 × 105a (5.1 × 104-2.8 × 106) | 10.9a (0.8-97.3) |
P < 0.01 (Mann-Whitney test).
Antibiotic resistance patterns.
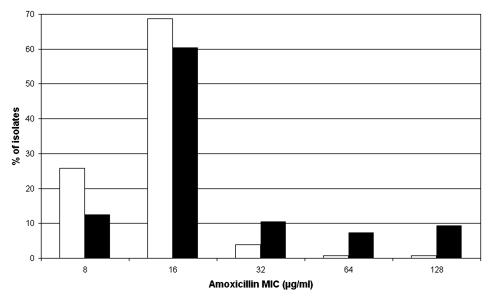
The distribution of the amoxicillin MICs for the 128 amoxicillin-resistant bacteria isolated from subjects who had not used antibiotics in the previous 3 months and the 96 amoxicillin-resistant bacteria isolated from subjects who had used amoxicillin in the previous 3 months are shown in Fig. 1. The amoxicillin MICs ranged from 8 to 128 mg liter−1 for bacteria isolated from both study groups. The MIC at which 50% of isolates are inhibited (MIC50) was 16 mg liter−1 for bacteria isolated from both study groups; however, the MIC90 for the isolates from children who had used amoxicillin in the previous 3 months was higher (64 mg liter−1) than that for the isolates from subjects who had not used antibiotics (16 mg liter−1). The majority of the amoxicillin-resistant isolates (64.1 and 65.6% of the isolates from subjects without and with amoxicillin use, respectively) were also resistant to at least one of the three other antibiotics tested: penicillin, erythromycin, and tetracycline (Table 3). Resistance to penicillin was the most frequently encountered, with 49.0 and 52.3% of the isolates from subjects with and without amoxicillin use, respectively, being resistant to this antibiotic. Only 15.6% of the isolates from both of the study groups were resistant to tetracycline. However, significantly (P = 0.03) more of the isolates (32.3%) from subjects who had used amoxicillin than isolates from subjects who had not used antibiotics (19.5%) were resistant to erythromycin. In total, 17 amoxicillin-resistant isolates were also resistant to penicillin, erythromycin, and tetracycline; and these were identified as Streptococcus mitis (8 isolates), Streptococcus salivarius (3 isolates), and single isolates each of S. mutans, Streptococcus oralis, Streptococcus sanguis, S. parasanguis, S. epidermidis, and G. haemolysans.
FIG. 1.
MIC of amoxicillin for isolates from children with and without amoxicillin use in the previous 3 months. White bars, no amoxicillin use; black bars, amoxicillin use.
TABLE 3.
Multiresistance profiles as numbers and proportions of amoxicillin-resistant bacteria from subjects with and without amoxicillin use in the previous 3 months resistant to three additional antibiotics
| Antimicrobial agent | No. (%) of resistant isolates
|
|
|---|---|---|
| AMXa not used | AMX used | |
| AMX | 46 (35.9) | 33 (34.4) |
| AMX-penicillin | 52 (40.6) | 28 (29.2) |
| AMX-erythromycin | 4 (3.1) | 9 (9.4)b |
| AMX-tetracycline | 5 (3.9) | 3 (3.1) |
| AMX-penicillin-erythromycin | 6 (4.7) | 11 (11.5) |
| AMX-penicillin-tetracycline | 0 (0) | 1 (1.0) |
| AMX-erythromycin-tetracycline | 6 (4.7) | 3 (3.1) |
| AMX-penicillin-erythromycin-tetracycline | 9 (7.0) | 8 (8.3) |
| Total | 128 (100) | 96 (100) |
AMX, amoxicillin.
P < 0.05 (chi-square test).
DISCUSSION
Amoxicillin is used in dentistry for the treatment of dental alveolar abscesses, endodontic infections, advanced forms of periodontal diseases, and prophylaxis for infective endocarditis and is used in medicine for the treatment of otitis media, sinusitis, bronchitis, and community-acquired pneumonia (4). Consequently, factors that promote an increase in amoxicillin resistance in the oral microbiota are of concern to both dental and medical practitioners.
Antibiotic resistance studies have generally relied on the isolation of bacteria on antibiotic-free plates and subsequent testing of susceptibility to a range of antibiotics. This methodology selects for the detection of resistance in the predominating microbiota. In this study, the use of ampicillin-containing plates allowed the isolation of resistant bacteria. The proportion of resistant bacteria ranged from 0.1 to 97% of the total cultivable oral microbiota. Previous studies have shown that concentrations of amoxicillin in the oral cavity after administration of a single antibiotic dose range from 0.1 to 0.5 mg liter−1 in saliva (14, 23) and 1 to 14 mg liter−1 in gingival crevicular fluid (1, 24). The concentration of 8 mg of ampicillin liter−1 was chosen, as it reflects the concentration found in the oral cavity and is comparable to that used in other relevant studies (18, 19). Amoxicillin-resistant bacteria were readily isolated, with all of the 40 children harboring amoxicillin-resistant bacteria in their oral cavities, which concurs with the results obtained in a previous study (19). The use of amoxicillin significantly increased both the total number of amoxicillin-resistant bacteria and the proportions of the oral microbiota resistant to amoxicillin. Previous studies have shown that amoxicillin use can increase the number of amoxicillin-resistant oral streptococci isolated from the oral cavity (8, 27) and that the use of beta-lactam antibiotics can increase the rate of carriage of penicillin-resistant S. pneumoniae (7). Interestingly, the counts for the total microbiota did not significantly increase with amoxicillin use, which suggests that there was a loss of the amoxicillin-susceptible microbiota that was replaced by a significant increase in amoxicillin-resistant bacteria. The amoxicillin-resistant bacteria comprised 31 different species and 11 different genera. Amoxicillin use did not alter the biodiversity of the amoxicillin-resistant microbiota, which suggests that the increase in the number and proportion of the amoxicillin-resistant microbiota observed was not due to a clonal increase but, rather, to an overall increase in a diverse collection of amoxicillin-resistant bacteria.
The MIC90s of amoxicillin were increased for the isolates from children who had used amoxicillin during the 3 months prior to sampling. Previous studies have reported increased MICs after penicillin-susceptible strains of S. pneumoniae were subcultured in amoxicillin- or amoxicillin-clavulanate-containing media (5, 17), and the presence of amoxicillin in the saliva and gingival crevicular fluid during amoxicillin use may have provided the selective pressure necessary for this increase in the MIC90. The subjects in this study would have received amoxicillin as an oral suspension (125 mg/5 ml) rather than in the tablet form, which is used in adults. Therefore, in addition to the presence of amoxicillin in the saliva and gingival crevicular fluid, which would occur in all patients after amoxicillin use, it is possible that young children have an additional antibiotic challenge, as the antibiotic suspension itself may also be able to persist in the oral cavity. Hence, other studies have demonstrated that oral suspensions of carbohydrates can remain in the oral cavity for over 1 h (11) and that young children (age, 3 to 7 years old) have a slower salivary clearance than older children and adults (6).
Multiantibiotic-resistant bacteria were frequently seen in the samples taken from the children. Indeed, we observed that the use of amoxicillin significantly increased the number of amoxicillin-resistant isolates which were additionally resistant to erythromycin. We have previously shown in vitro that the use of tetracycline selected for bacteria that were resistant to several antibiotics, leading to an increase in the overall level of resistance in the population (18). Multiple prescriptions of antibiotics are common throughout childhood (13), and thus, their use could result in the repeated selection of an antibiotic-resistant oral microbiota. This is of concern, as patients who become infected with antibiotic-resistant bacteria in the community have been shown to have a higher incidence of hospitalization, longer durations of stay in hospital, and increased mortality compared to patients infected with antibiotic-sensitive strains (9).
This study has demonstrated that amoxicillin-resistant bacteria comprising a wide variety of species are present in the oral cavity and that the number, proportions, MICs, and resistance to erythromycin can significantly increase with amoxicillin use.
Acknowledgments
We thank Julia Roche for collecting the plaque samples and Adam P. Roberts and Lindsay Sharp for help with 16S rRNA gene sequencing.
This work was supported by the Charles Wolfson Charitable Trust.
REFERENCES
- 1.Akimoto, Y., H. Nishimura, M. Komiya, T. Shibata, K. Kaneko, A. Fujii, and T. Tamura. 1985. Ampicillin concentrations in human serum, gingiva, the mandibular bone, and dental follicle following a single oral administration. Gen. Pharmacol. 16:125-128. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, J. M., for the BSAC Working Party on Susceptibility Testing. 2001. BSAC standardized disc susceptibility testing method. J. Antimicrob. Chemother. 48(Suppl. 1):43-57. [DOI] [PubMed] [Google Scholar]
- 4.British National Formulary. 2003. Drugs used in the treatment of infections. British National Formulary, British Medical Association and the Royal Pharmaceutical Society of Great Britain, London, United Kingdom.
- 5.Carsenti-Etesse, H., J. Durant, F. De Salvador, M. Bensoussan, F. Bensoussan, C. Pradier, A. Thabaut, and P. Dellamonica. 1995. In vitro development of resistance of Streptococcus pneumoniae to beta-lactam antibiotics. Microb. Drug Resist. 1:85-89. [DOI] [PubMed] [Google Scholar]
- 6.Crossner, C. G., J. C. Hase, and D. Birkhed. 1991. Oral sugar clearance in children compared with adults. Caries Res. 25:201-206. [DOI] [PubMed] [Google Scholar]
- 7.Guillemot, D., C. Carbon, B. Balkau, P. Geslin, H. Lecoeur, F. Vauzelle-Kervroedan, G. Bouvenot, and E. Eschwege. 1998. Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA 279:365-370. [DOI] [PubMed] [Google Scholar]
- 8.Harrison, G. A., M. P. Rubin, R. M. Davies, and D. C. Speller. 1985. Resistance in oral streptococci after repetition of a single-dose amoxycillin prophylactic regimen. J. Antimicrob. Chemother. 15:501-503. [DOI] [PubMed] [Google Scholar]
- 9.Holmberg, S. D., S. L. Solomon, and P. A. Blake. 1987. Health and economic impacts of antimicrobial resistance. Rev. Infect. Dis. 9:1065-1078. [DOI] [PubMed] [Google Scholar]
- 10.Lane, D. J. 1996. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons Ltd., Chichester, United Kingdom.
- 11.Luke, G. A., H. Gough, J. A. Beeley, and D. A. Geddes. 1999. Human salivary sugar clearance after sugar rinses and intake of foodstuffs. Caries Res. 33:123-129. [DOI] [PubMed] [Google Scholar]
- 12.Maidak, B. L., J. R. Cole, T. G. Lilburn, C. T. Parker, Jr., P. R. Saxman, J. M. Stredwick, G. M. Garrity, B. Li, G. J. Olsen, S. Pramanik, T. M. Schmidt, and J. M. Tiedje. 2000. The RDP (Ribosomal Database Project) continues. Nucleic Acids Res. 28:173-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millar, M. R., T. R. Walsh, C. J. Linton, S. Zhang, J. P. Leeming, and P. M. Bennett. 2001. Carriage of antibiotic-resistant by healthy children. J. Antimicrob. Chemother. 47:605-610. [DOI] [PubMed] [Google Scholar]
- 14.Nelson, J. D., C. M. Ginsburg, O. Mcleland, J. Clahsen, M. C. Culbertson, Jr., and H. Carder. 1981. Concentrations of antimicrobial agents in middle ear fluid, saliva and tears. Int. J. Pediatr. Otorhinolaryngol. 3:327-334. [DOI] [PubMed] [Google Scholar]
- 15.Office for National Statistics. 2003. Antibiotic prescription items per 1000 patient years at risk by BNF sub-section, age, sex and calendar year: 1994-98. [Online.] http://www.statistics.gov.uk/.
- 16.Packer, S., N. Woodley, M. Wilson, and P. Mullany. 1999. Prevalence and persistence of amoxycillin-resistant bacteria in the dental plaques of adults. Microbios 100:135-144. [PubMed] [Google Scholar]
- 17.Pankuch, G. A., S. A. Jueneman, T. A. Davies, M. R. Jacobs, and P. C. Appelbaum. 1998. In vitro selection of resistance to four beta-lactams and azithromycin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2914-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ready, D., A. P. Roberts, J. Pratten, D. A. Spratt, M. Wilson, and P. Mullany. 2002. Composition and antibiotic resistance profile of microcosm dental plaques before and after exposure to tetracycline. J. Antimicrob. Chemother. 49:769-775. [DOI] [PubMed] [Google Scholar]
- 19.Ready, D., R. Bedi, D. A. Spratt, P. Mullany, and M. Wilson. 2003. Prevalence, proportions, and identities of antibiotic-resistant bacteria in the oral microflora of healthy children. Microb. Drug Resist. 9:367-372. [DOI] [PubMed] [Google Scholar]
- 20.Sefton, A. M. 1999. Macrolides and changes in the oral flora. Int. J. Antimicrob. Agents 11(Suppl. 1):S23-S29. [DOI] [PubMed] [Google Scholar]
- 21.Shannon, C. E., and W. Weaver. 1963. The mathematical theory of communication. University of Illinois Press, Urbana.
- 22.SPSS Inc. 2000. SPSS 10.0 syntax reference guide. SPSS Inc., Chicago, Ill.
- 23.Stewart S. M., M. Fisher, J. E. Young, and W. Lutz. 1970. Ampicillin levels in sputum, serum, and saliva. Thorax 25:304-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenenbaum, H., F. Jehl C. Gallion, and M. Dahan. 1997. Amoxicillin and clavulanic acid concentrations in gingival crevicular fluid. J. Clin. Periodontol. 24:804-807. [DOI] [PubMed] [Google Scholar]
- 25.Whiley, R. A., and D. Beighton. 1998. Current classification of the oral streptococci. Oral Microbiol. Immunol. 13:195-216. [DOI] [PubMed] [Google Scholar]
- 26.Wilson, M. J., A. J. Weightman, and W. G. Wade. 1997. Applications of molecular ecology in the characterisation of uncultured microorganisms associated with human disease. Rev. Med. Microbiol. 8:91-101. [Google Scholar]
- 27.Woodman, A. J., J. Vidic, H. N. Newman, and P. D. Marsh. 1985. Effect of repeated high dose prophylaxis with amoxycillin on the resident oral flora of adult volunteers. J. Med. Microbiol. 19:15-23. [DOI] [PubMed] [Google Scholar]