Abstract
Pharmacological studies have indicated that the choline analog G25 is a potent inhibitor of Plasmodium falciparum growth in vitro and in vivo. Although choline transport has been suggested to be the target of G25, the exact mode of action of this compound is not known. Here we show that, similar to its effects on P. falciparum, G25 prevents choline entry into Saccharomyces cerevisiae cells and inhibits S. cerevisiae growth. However, we show that the uptake of this compound is not mediated by the choline carrier Hnm1. An hnm1Δ yeast mutant, which lacks the only choline transporter gene HNM1, was not altered in the transport of a labeled analog of this compound. Eleven yeast mutants lacking genes involved in different steps of phospholipid biosynthesis were analyzed for their sensitivity to G25. Four mutants affected in the de novo cytidyldiphosphate-choline-dependent phosphatidylcholine biosynthetic pathway and, surprisingly, a mutant strain lacking the phosphatidylserine decarboxylase-encoding gene PSD1 (but not PSD2) were found to be highly resistant to this compound. Based on these data for S. cerevisiae, labeling studies in P. falciparum were performed to examine the effect of G25 on the biosynthetic pathways of the major phospholipids phosphatidylcholine and phosphatidylethanolamine. Labeling studies in P. falciparum and in vitro studies with recombinant P. falciparum phosphatidylserine decarboxylase further supported the inhibition of both the de novo phosphatidylcholine metabolic pathway and the synthesis of phosphatidylethanolamine from phosphatidylserine. Together, our data indicate that G25 specifically targets the pathways for synthesis of the two major phospholipids, phosphatidylcholine and phosphatidylethanolamine, to exert its antimalarial activity.
Plasmodium falciparum, the causative agent of the most severe form of human malaria, is responsible for over 2 million deaths annually (49). The emergence of parasites resistant to the most commonly used antimalarials, such as chloroquine, mefloquine, and pyrimethamine, has hampered efforts to combat this disease, emphasizing the need to develop new compounds for malaria treatment and prophylaxis.
The rapid multiplication of P. falciparum in human erythrocytes requires active synthesis of new membranes. Therefore, developing drugs that target membrane biogenesis is an attractive strategy to fight malaria. The finding that quaternary ammonium choline analogs inhibit the synthesis of new membranes and block the growth of the parasite has stimulated efforts to develop this class of compounds for antimalarial chemotherapy (4-6, 11, 12). With a combinatorial chemistry approach to obtain compounds with greater specificity and potency against malaria, more than 420 choline analogs have been synthesized, and their structures were optimized with quantitative structural-activity criteria (11, 12, 44, 45). These compounds displayed a very close correlation between inhibition of parasite growth in vitro and specific inhibition of parasite membrane biogenesis (1, 47, 48).
One of these compounds, G25, inhibited P. falciparum growth in vitro and cleared malaria infection in monkeys infected with P. falciparum and Plasmodium cynomolgi at very low doses (48). A tritium-labeled bisquaternary ammonium salt analog of G25, VB5-T (50% inhibitory concentration [IC50], ≈18 nM), was shown to accumulate several hundredfold in trophozoite-infected compared to uninfected red blood cells (48). Accumulation of this agent within the parasite is linear with concentrations up to 1,000-fold above the IC50 and appears to be irreversible (48). The antimalarial potency of G25 is similar to that of chloroquine, which kills the parasite at low nanomolar extracellular concentrations but accumulates within the parasite food vacuole to the millimolar range (39). Although choline analogs are highly effective against malaria and are entering clinical evaluation, the difficulties in the experimental manipulation of P. falciparum has hampered efforts to understand their mode of action and identify their cellular targets.
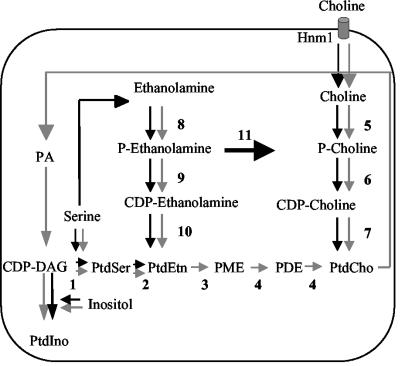
The amenability of the yeast Saccharomyces cerevisiae to genetic manipulation has made it an invaluable system to characterize the metabolic pathways involved in the synthesis of phospholipids, sterols and fatty acids. S. cerevisiae membranes consist largely of phosphatidylcholine (44%), phosphatidylethanolamine (18%), and phosphatidylinositol (19.5%) (25). These glycerolipids are thought to be essential for S. cerevisiae growth in medium that contains glucose or nonfermentable carbon sources (7, 10, 38, 41). As summarized in Fig. 1, their synthesis involves distinct but highly coregulated biosynthetic pathways: (i) the cytidyldiphosphate (CDP)-choline pathway, which uses choline as a precursor for the de novo synthesis of phosphatidylcholine (19, 21, 23, 27, 50); (ii) the CDP-ethanolamine pathway, which uses ethanolamine as a precursor for the de novo synthesis of phosphatidylethanolamine (20, 22, 26); (iii) the CDP-diacylglycerol (DAG) pathway, which utilizes serine and CDP-DAG to form phosphatidylserine, which is then decarboxylated to form phosphatidylethanolamine; and (iv) the phosphatidylinositol pathway, which synthesizes phosphatidylinositol from CDP-DAG and inositol (14, 28, 31, 34, 41-43).
FIG. 1.
Phospholipid metabolism in S. cerevisiae and P. falciparum. Pathways for the synthesis of the major phospholipids in S. cerevisiae (thin gray arrows) and P. falciparum (thick solid arrows) are shown: 1, phosphatidylserine synthase (Pss1); 2, phosphatidylserine decarboxylases (Psd1 and Psd2); 3, phosphatidylethanolamine methyltransferase (Pem1); 4, phospholipid methyltransferase (Pem2); 5, choline kinase (Cki1); 6, phosphocholine cytidylyltransferase (Pct1); 7, choline phosphotransferase (Cpt1); 8, ethanolamine kinase (Eki1); 9, phosphoethanolamine cytidylyltransferase (Ept1); 10, ethanolamine phosphotransferase (Ect1); 11, phosphoethanolamine methyltransferase (P. falciparum Pmt). PA, phosphatidic acid; CDP-DAG, cytidyldiphosphate diacylglycerol; PtdSer, phosphatidylserine; PtdEtn, phosphatidylethanolamine; PME, phosphatidylmonomethylethanolamine; PDE, phosphatidyldimethylethanolamine; PtdCho, phosphatidylcholine; PtdIno, phosphatidylinositol.
The CDP-DAG and CDP-ethanolamine pathways converge into phosphatidylethanolamine, which is subsequently methylated in a three step S-adenosyl-l-methionine-dependent methylation to form phosphatidylcholine. This reaction is catalyzed by two methyltransferases encoded by the phosphatidylethanolamine N-methyltransferase PEM1 and phospholipid N-methyltransferase PEM2 genes. The CDP-DAG pathway is the major pathway leading to the formation of phosphatidylcholine in S. cerevisiae (13). Therefore, in this organism, neither choline nor the enzymes of the CDP-choline pathway are essential for survival. The CDP-choline pathway becomes essential when the genes encoding the enzymes in the CDP-DAG pathway are altered or deleted (29).
Biochemical studies in P. falciparum and the available genome sequences have made it possible to define the pathways for synthesis of the major phospholipids (18, 46) (Fig. 1). With the exception of the choline transporter and the phospholipid methyltransferases, all the genes encoding enzymes of the CDP-choline, CDP-ethanolamine, and CDP-DAG pathways have been identified. The similarity between P. falciparum and S. cerevisiae in the biogenesis of the major phospholipids suggests that S. cerevisiae can be used as a surrogate system to characterize the function of P. falciparum phospholipid synthesizing genes and determine the mode of entry and cellular targets of antimalarial lipid inhibitors.
Here, we report that the antimalarial choline analog G25 inhibits the growth of S. cerevisiae in vitro and, in the same range of concentrations, is an effective inhibitor of choline transport in wild-type S. cerevisiae. Similar initial rate and overall uptake of a radiolabeled bisquaternary ammonium analog of G25 was measured in both wild-type and hnm1Δ cells lacking the only yeast choline transporter Hnm1. These results demonstrate that the choline carrier Hnm1 does not mediate the entry of bisquaternary ammonium compounds. Of 11 individual S. cerevisiae knockouts lacking genes involved in different steps of phosphatidylcholine biosynthesis, four mutants altered in the de novo CDP-choline pathway and one mutant lacking the phosphatidylserine decarboxylase-encoding gene PSD1 were highly resistant to G25.
Labeling studies in P. falciparum demonstrated that G25 completely and specifically inhibits the de novo CDP-choline-dependent phosphatidylcholine biosynthetic pathway. Surprisingly, higher concentrations of this compound resulted in the inhibition of synthesis of phosphatidylethanolamine from phosphatidylserine but had no effect on any other step of the CDP-DAG pathway. Interestingly, we found that G25 inhibits the phosphatidylserine decarboxylase activity of purified recombinant P. falciparum Psd1 in a way similar to the inhibition of the native enzyme. Together, our data indicate that G25 specifically targets the pathways for synthesis of the two major phospholipids, phosphatidylcholine and phosphatidylethanolamine, to exert its antimalarial activity. These novel findings constitute important information for quaternary ammonium compounds that are entering clinical studies and further support the use of S. cerevisiae as a surrogate system to identify the targets of antimalarial compounds.
MATERIALS AND METHODS
Chemicals.
G25 (1,16-hexadecamethylenebis[N-methylpyrrolidinium] dibromide) (11), T16 (1,12-dodecanemethylene bis[4-methyl-5-ethylthiazolium] diiodide), and [3H]T16 were synthesized in house (Richier et al., unpublished data).
Strains and growth conditions.
Wild-type BY4741 (MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0) and mutant (hnm1Δ, psd1Δ, cki1Δ, pem1Δ, ept1Δ, cpt1Δ, eki1Δ, psd2Δ, pct1Δ, ect1Δ, and pem2Δ) S. cerevisiae strains used in this study were purchased from Research Genetics (Invitrogen). These strains were grown on YPD (1% yeast extract, 2% dextrose, and 2% peptone) or synthetic complete medium (SD; 1.7% yeast nitrogen base, 5% ammonium sulfate, and 2% dextrose). The Nigerian strain of P. falciparum was propagated in human red blood cells at 4% hematocrit by the method of Trager and Jensen (40). Plate growth assays were performed by growing wild-type and mutant yeast strains in YPD to mid-log phase. Cultures were serially diluted 1:10 starting with a density of 3 × 107 cells/ml. The growth of cells was monitored by spotting 3 μl of each dilution onto solid medium in the absence or presence of 5 μM G25. Growth assays in liquid media were performed by inoculating wild-type and hnm1Δ cells to a density of 104 cells/ml in YPD supplemented with increasing concentrations of choline analogs. The optical density at 600 nm was measured when the control without choline analogs reached a density of 1.8 × 107 cells/ml.
Uptake assays.
S. cerevisiae strains were grown in synthetic complete medium supplemented as required to maintain cell growth to an optical density at 600 nm of 0.55 to 0.65. Cells were harvested by centrifugation at 3,200 × g for 10 min at 4°C, washed twice in cold phosphate-buffered saline and resuspended in nitrogen-free medium (SD without ammonium sulfate). Each reaction was performed in a 1-ml final volume in the presence of 12 nM [methyl-3H]choline (82 Ci/mmol; Amersham). After 3 min of incubation at 30°C with shaking, transport was immediately stopped by filtration through Whatman GF/C glass microfiber paper. The filters were washed three times with 5 ml of ice-cold phosphate-buffered saline, air dried, and analyzed in a scintillation counter.
For time course uptake, 3 × 107 to 4 × 107 cells were incubated at 30°C in 1 ml of nitrogen-free medium in the presence of 25 nM [3H]T16 (69 Ci/mmol) for 1, 2, 3, 4, 5, 7, 10, and 15 min, after which 5 ml of cold phosphate-buffered saline was added to stop the reaction. Kinetic parameters were determined after 4 min of incubation at 30°C in the presence of [3H]T16 at concentrations ranging from 25 nM to 75 μM (69 Ci to 23 mCi/mol). The samples were centrifuged at 4°C for 10 min at 1,200 × g, the supernatants were discarded, and the cells were then resuspended in 5 ml of cold phosphate-buffered saline. The reaction was terminated by filtering the cell suspension through GF/C membranes that had been treated with 15 ml of 0.05% polyethyleneimine. The filters were washed twice with 5 ml of cold phosphate-buffered saline, air dried, and analyzed in a scintillation counter. The cellular accumulation ratio was calculated as previously described for T16 and G25 (9, 48).
Labeling studies and phospholipid analysis in P. falciparum.
Nigerian strains of P. falciparum were asexually cultured in the presence of basic medium (RPMI 1640 supplemented with 25 mM HEPES, pH 7.4) and 10% AB-positive human serum (40). Parasite synchronization was obtained with three successive 5% sorbitol treatments (30). Synchronized P. falciparum-infected erythrocytes (7 to 10% parasitemia, trophozoites) were incubated for 1 h at 37°C at 4% hematocrit in 2 ml (final volume) of basic medium in the absence or the presence of different concentrations of the compound G25. The appropriate radioactive precursor of lipid metabolism was then added, followed by a further 3 h of incubation. Radioactive precursors were used as follows: 30 μM [methyl-3H]choline (334 mCi/mmol), 2 μM [3H]ethanolamine (2 Ci/mmol), and 10 μM [3-14C]serine (57 mCi/mmol).
Following incubation with radiolabeled precursors, cells were concentrated by centrifugation at 1,200 × g for 5 min at 4°C and washed twice, the cellular lipids were extracted by a mixture of chloroform and methanol (17), and the organic phase was evaporated under air. The dried material was dissolved in 100 μl of chloroform-methanol (9:1, vol/vol), and lipids were separated by thin-layer chromatography. Samples were applied to precoated silica gel plates (Merck, Darmstadt, Germany), which were developed in chloroform-methanol-acetic acid-0.1 M sodium borate (75:45:12:3, vol/vol/vol/vol). Phospholipids spots were revealed with iodine vapors and identified with appropriate standards. The silica gel of the lipid spots was scraped directly into scintillation vials containing 3 ml of liquid scintillation fluid and counted in a Beckman LS 5000 spectrophotometer. The amount of labeled precursors incorporated into cellular lipids was computed on the basis of radioactivity incorporated into lipids and the specific activity of the precursors in the incubation medium.
Phosphatidylserine decarboxylase assay.
Recombinant P. falciparum phosphatidylserine decarboxylase was purified as described by Baunaure and colleagues (8). The assay mixture (0.3 ml) contained 0.1 M potassium phosphate buffer (pH 6.8), 0.06% (wt/vol) Triton X-100, 200 μM l-[dipalmitoyl]phosphatidyl[3-14C]serine (1.35 mCi/mmol; Amersham), and the enzyme fraction containing recombinant protein of P. falciparum (240 μg). After incubation at 37°C for 1 h, the reaction was terminated by the addition of 400 μl of chloroform. Chloroform-soluble materials were extracted, dried, and then dissolved in chloroform-methanol (9:1, vol/vol). Phospholipids were separated by thin-layer chromatography as described above. The radioactive phospholipids were localized and identified with appropriate standards, and radioactivity was quantified with a phosphoimager analyzer (Molecular Dynamics).
RESULTS
Antimalarial drug G25 inhibits the growth of S. cerevisiae.
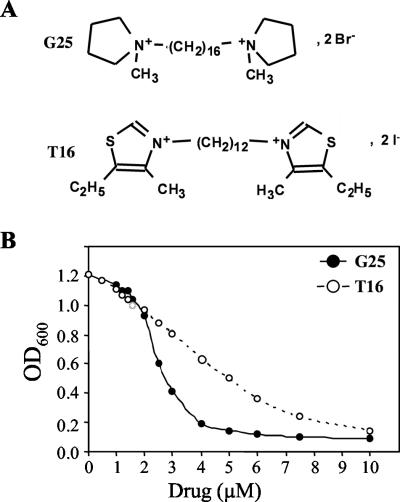
To examine the effect of the antimalarial choline analog G25(1,16-hexadecamethylenebis[N-methylpyrrolidinium] dibro-mide) (Fig. 2A) on the growth of S. cerevisiae in vitro, wild-type strain BY4741 was inoculated at 104 cells/ml in liquid medium in the presence of increasing concentrations of the compound and incubated at 30°C for 16 h. Growth inhibition was assessed by measuring the optical density at 600 nm and comparing it to that of the wild-type strain grown under the same conditions in the absence of the compound. G25 inhibited yeast growth with an IC50 of 2.5 μM (Fig. 2B). This IC50 value is in the range of the predicted intracellular concentration of G25 in P. falciparum due to the accumulative properties inside infected erythrocytes (48).
FIG. 2.
Inhibition of S. cerevisiae growth by G25 and its analog T16. (A) Chemical structure of G25 (1,16-hexadecamethylenebis[N-methylpyrrolidinium] dibromide) and T16 (1,12-dodecanemethylene bis[4-methyl-5-ethylthiazolium] diodide). (B) Liquid growth assays were performed in increasing concentrations of G25 and T16 as described in the text.
Uptake analysis and inhibition of choline transport by choline analogs in S. cerevisiae.
Bisquaternary ammonium choline analogs have been shown to inhibit choline entry into Plasmodium-infected erythrocytes (1, 3). To determine whether these compounds block choline uptake in S. cerevisiae, we examined the transport of [methyl-3H]choline in the absence and presence of various concentrations of G25 and its structural analog T16 (1,12-dodecanemethylene bis[4-methyl-5-ethylthiazolium] diiodide) (Fig. 2A), predicted by quantitative structure-activity studies and confirmed experimentally to have potent in vitro antimalarial and antifungal inhibitory activities similar to those of G25 with IC50 values of ≈16 nM (9) and ≈4 μM (Fig. 2B), respectively.
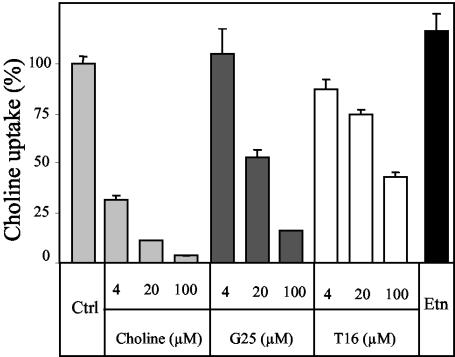
G25 inhibited choline uptake in a dose-dependent manner, with 50% inhibition of choline transport in 20-fold excess and 84% inhibition in 100-fold excess (Fig. 3). The G25 analog T16 also inhibited choline transport, albeit less efficiently than G25, with 26% inhibition of choline transport in 20-fold excess and 57% inhibition in 100-fold excess (Fig. 3). As a control, 20- and 100-fold excesses of unlabeled choline inhibited uptake of radiolabeled choline by 89 and 97%, respectively. Altogether, these data suggest that bisquaternary ammonium compounds are excellent inhibitors of choline uptake in S. cerevisiae.
FIG. 3.
Inhibition of choline uptake in S. cerevisiae by G25. Choline transport in the wild-type strain of S. cerevisiae was measured as described in Materials and Methods. The uptake of 1 μM [methyl-3H]choline in the presence of 100 μM ethanolamine (Etn) and a 4-, 20-, and 100-fold excess of unlabeled choline, G25, and T16 is shown as a percentage of the counts obtained in the control (Ctrl) without drugs, choline, or ethanolamine.
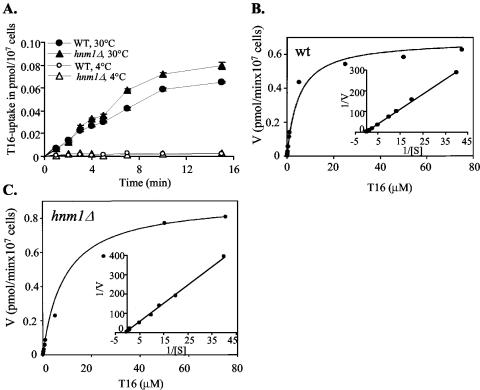
To directly examine the transport of choline analogs in S. cerevisiae, we synthesized a tritium-labeled bisquaternary ammonium salt, [3H]T16, and examined its transport properties in wild-type cells at 4 and 30°C. No significant uptake of T16 in S. cerevisiae could be measured at 4°C (Fig. 4A). In contrast, [3H]T16 uptake could be measured at 30°C and was linear during the first 12 min, after which it reached a plateau, suggesting that entry of bisquaternary ammonium compounds into yeast cells is carrier mediated (Fig. 4A). Unlike in P. falciparum-infected erythrocytes, where G25 and T16 have been shown to accumulate with cellular accumulation ratios of ≈300 and ≈500, respectively, after 3 h of incubation (9, 48), the cellular accumulation ratio of T16 in S. cerevisiae was estimated to be less than 7 (data not shown).
FIG. 4.
Transport kinetic of a radiolabeled G25 analog, [3H]T16, in S. cerevisiae. (A) Transport of [3H]T16 was measured as described in Materials and Methods in nitrogen-free medium. Wild-type (WT, solid and open circles) and hnm1Δ (solid and open triangles) strains were assayed for uptake of [3H]T16 over time at 30°C (solid circles and triangles) and 4°C (open circles and triangles). Each value is the mean ± standard deviation of triplicate determinations of a typical experiment. Transport of [3H]T16 as a function of the indicated concentrations of T16 was measured at 30°C for 4 min in the wild-type (B) and hnm1Δ (C) strains. The curve was fitted to the Michaelis-Menten equation (V = Vmax × S[Km + S]). The Lineweaver-Burk representation of the saturation curve is shown as an inset. For A to C, only one representative experiment performed in triplicate of two independent experiments is shown.
To determine the kinetic parameters of this transport, [3H]T16 uptake was measured after 4 min of incubation at 30°C as a function of its extracellular concentration. The Lineweaver-Burk representation of this transport resulted in an apparent Km of 5.05 ± 0.26 μM for [3H]T16 and a maximum velocity Vmax of 0.98 ± 0.48 pmol/min per 107 cells (Fig. 4B). As a control, uptake of [methyl-3H]choline in the wild-type strain was found to be carrier mediated, with a Km of 0.53 ± 0.18 μM and a Vmax of 40 pmol/min/107 cells (data not shown), as reported previously (33, 52).
To rule out the possible role of Hnm1 in the uptake of bisquaternary ammonium compounds by yeast cells, we measured the transport of [3H]T16 in an hnm1Δ mutant strain, which lacks the choline transporter gene HNM1 (Fig. 4C), and compared it to that measured in the wild-type strain (Fig. 4B). As in the wild-type strain, T16 transport in the hnm1Δ strain was found to be carrier mediated, with a Km of 7.45 ± 1.98 μM and a Vmax of 0.76 ± 0.21 pmol/min/107 cells (Fig. 4C). Thus, no differences in T16 uptake could be detected between the hnm1Δ and wild-type strains. As expected, no choline transport could be detected in the hnm1Δ mutant (data not shown). Altogether, these data suggest that yeast cells utilize other transport systems for the uptake of bisquaternary ammonium compounds.
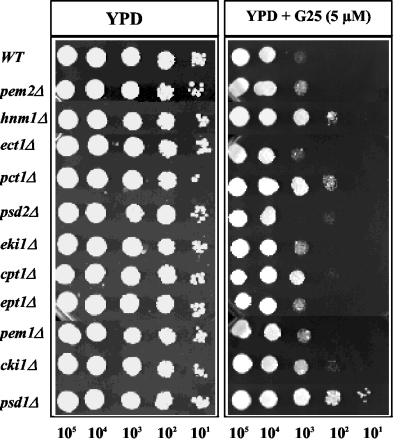
Sensitivity of mutants affected in phospholipid metabolism to choline analogs.
Choline analogs are proposed to inhibit membrane biogenesis in P. falciparum (3, 11, 45). However, the steps in the phospholipid biosynthesis pathways that are specifically targeted by these compounds are not yet known. To assess whether the mode of action of G25 is linked to disruption of phospholipid metabolism, we used S. cerevisiae as a surrogate system to compare the sensitivity to G25 of the wild-type strain and 11 individual knockouts in the CDP-choline, CDP-ethanolamine, and CDP-DAG pathways. As shown in Fig. 5, substantial resistance to G25 was conferred by loss of the choline kinase (Cki1), choline phosphotransferase (Cpt1), phosphocholine cytidylyltransferase (Pct1), and choline carrier (Hnm1) activities of the CDP-choline pathway.
FIG. 5.
Sensitivity to G25 of the wild type and mutants affected in different steps of phosphatidylcholine biosynthesis. Plate growth limiting-dilution assays were performed as described in Materials and Methods in the absence and presence of 5 μM G25. The strains used are described in Materials and Methods. The genes deleted in these strains are detailed in Fig. 1.
Surprisingly, the psd1Δ mutant strain, which lacks the PSD1 gene encoding the phosphatidylserine decarboxylase activity that converts 95% of cellular phosphatidylserine into phosphatidylethanolamine in the mitochondria (42), was also found to be highly resistant to G25 (Fig. 5). No resistance was conferred by loss of the phosphatidylethanolamine methyltransferases Pem1 and Pem2 or the enzymes of the CDP-ethanolamine pathway. Furthermore, unlike the psd1Δ mutant strain, loss of the Golgi/vacuole phosphatidylserine decarboxylase Psd2, which synthesizes only 5% of the phosphatidylethanolamine pool, had no effect on G25 sensitivity. These data indicate that the sensitivity of S. cerevisiae to G25 requires a functional de novo CDP-choline pathway for synthesis of phosphatidylcholine from choline and a functional Psd1 activity for phosphatidylethanolamine synthesis from phosphatidylserine.
G25 inhibits the CDP-choline pathway and phosphatidylethanolamine formation from phosphatidylserine in P. falciparum.
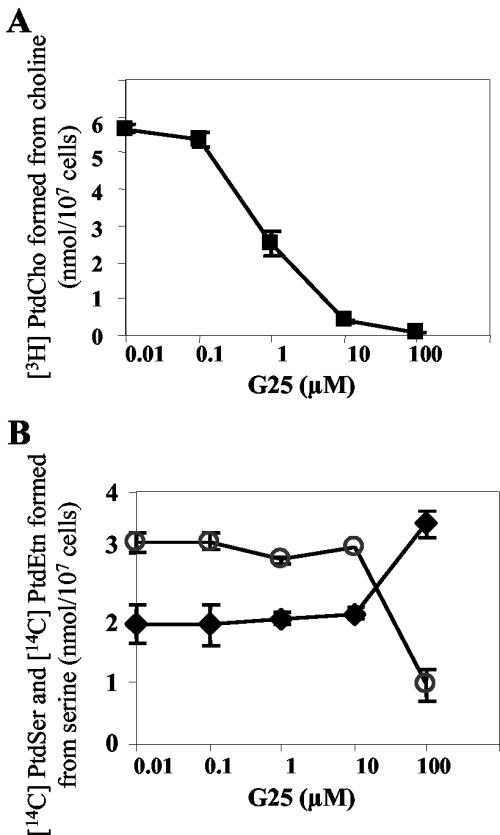
The similarity between the P. falciparum and S. cerevisiae phospholipid metabolic pathways and the finding that deletion of numerous genes of phospholipid metabolism in S. cerevisiae resulted in major resistance to G25 suggested that this compound might directly inhibit phospholipid-synthesizing enzymes in P. falciparum. To investigate the possible inhibition by G25 of the de novo CDP-choline pathway in P. falciparum, we examined the incorporation of labeled choline into phosphatidylcholine in trophozoite-infected erythrocytes in the absence and presence of increasing concentrations of G25. This assay takes into account both the inhibitory effect of G25 on choline uptake and any additional inhibition by this compound of one or multiple enzymes of the CDP-choline pathway.
As shown in Fig. 6A, G25 induced a dose-dependent inhibition of the de novo synthesis of phosphatidylcholine. At concentrations higher than 0.1 μM, G25 caused a significant decrease of phosphatidylcholine biosynthesis, with 56% inhibition at 1 μM and nearly complete inhibition at 10 μM. In contrast, under similar conditions, G25 concentrations up 100 μM had no effect on the incorporation of radiolabeled ethanolamine into phosphatidylethanolamine (not shown). These results are consistent with our data for S. cerevisiae, which showed that deletion of EK1, ECT1, and EPT1, involved in the de novo synthesis of phosphatidylethanolamine from ethanolamine, did not confer resistance to G25 (Fig. 5).
FIG. 6.
Effect of G25 on phosphatidylethanolamine and phosphatidylcholine synthesis from phosphatidylserine and choline, respectively. From 5 × 107 to 6 × 107 synchronized P. falciparum-infected erythrocytes (7% trophozoite stage) were incubated at 4% haematocrit for 1 h in RPMI-based medium containing the indicated concentration of G25 before adding (A) 30 μM [methyl-3H]choline (334 mCi/mmol) or (B) 10 μM [14C]serine (57 mCi/mmol). After incubation at 37°C for 3 h, the cellular lipids were extracted and fractionated on thin-layer chromatography plates for quantification of radioactivity in phosphatidylserine (PtdSer, solid diamonds), phosphatidylcholine (PtdCho, solid squares), and phosphatidylethanolamine (PtdEtn, open circles). Each value is the mean ± standard deviation of triplicate determinations of two independent experiments.
We also investigated the possible inhibition of phosphatidylserine decarboxylase activity in P. falciparum by G25. P. falciparum-infected erythrocytes were labeled with radiolabeled serine, which is readily incorporated into phosphatidylserine, in the presence and absence of increasing concentrations of G25, and the effect of this compound on the parasite's endogenous phosphatidylserine decarboxylase activity was measured by following the formation of phosphatidylethanolamine from phosphatidylserine (Fig. 6B). Phosphatidylethanolamine was constantly formed from phosphatidylserine in the absence or presence of low concentrations of G25. Although the total incorporation of serine was not affected by G25, high concentrations of this compound resulted in a dramatic decrease in the endogenous phosphatidylserine decarboxylase activity, with a 77% decrease in the pool of phosphatidylethanolamine formed at 100 μM G25 (Fig. 6B). Concomitantly, at this concentration of G25, phosphatidylserine was increased in the same range, indicating that the phosphatidylserine decarboxylase activity was blocked (Fig. 6B).
G25 inhibits the activity of recombinant P. falciparum phosphatidylserine decarboxylase.
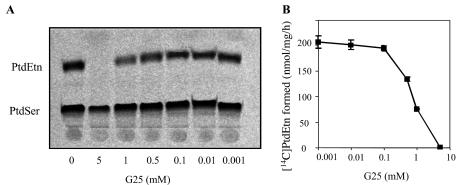
To further investigate the inhibitory effect of G25 on the formation of phosphatidylethanolamine from phosphatidylserine, we performed in vitro assays with a recombinant phosphatidylserine decarboxylase enzyme Psd1 from P. falciparum, encoded by the single-copy gene PSD1. The enzymatic activity of the recombinant P. falciparum Psd1 protein was tested under optimal conditions as described in Materials and Methods in the absence and presence of increasing concentrations of G25. Whereas in the absence of G25 recombinant P. falciparum Psd1 efficiently converted phosphatidylserine into phosphatidylethanolamine, addition of G25 resulted in a steady decrease in the activity of the enzyme as the concentration of the compound increased (Fig. 7), suggesting a direct inhibition of P. falciparum Psd1 activity by G25.
FIG. 7.
Effect of G25 on the activity of purified recombinant P. falciparum Psd1 enzyme. P. falciparum Psd1 activity was determined as described in Materials and Methods by measuring the amount of [14C]phosphatidylethanolamine (PtdEtn) formed from phosphatidylserine (PtdSer). (A) Thin-layer chromatography analysis of P. falciparum Psd1-mediated conversion of phosphatidylserine into phosphatidylethanolamine in the absence and presence of increasing concentrations of G25. (B) Quantitative analysis of the thin-layer chromatography data shown in panel A. Values are means ± standard deviation of triplicate determination of two independent experiments.
DISCUSSION
Quaternary ammonium compound analogs of choline represent a new class of drugs with a promising therapeutic future for treatment of multidrug-resistant malaria (1-3, 11, 45, 48) and possibly other parasitic infections (51). Studies in P. falciparum have suggested that choline transport might be the primary target of these compounds (1, 4). However, the role of choline influx and phosphatidylcholine biosynthesis in parasite development and survival has not been detailed. Furthermore, the difficulty in genetically manipulating P. falciparum has severely hampered efforts to understand the exact mode of action of these compounds.
Here, we provide the first evidence that the antimalarial choline analog G25 inhibits the growth of S. cerevisiae and that mutations in phospholipid metabolic genes affect the sensitivity of S. cerevisiae to this compound. The S. cerevisiae and malarial pathways of phospholipid biogenesis are similar enough that the targets of phospholipid inhibitors that we can find in S. cerevisiae are most likely to be relevant to P. falciparum. The IC50 value measured in S. cerevisiae is 2.5 μM, whereas that measured in various P. falciparum strains ranged between 1 and 5.3 nM (11). Interestingly, whereas G25 and its analog T16 accumulate in P. falciparum-infected erythrocytes with cellular accumulation ratios of ≈300 and ≈500, respectively, after 3 h of incubation (9, 48), our results indicate a cellular accumulation ratio for T16 of less than 7 in S. cerevisiae. The differences in growth inhibition assays and drug cellular accumulation could thus account for the differences in IC50s between the two organisms.
The sensitivity of S. cerevisiae to G25 and its structural analog T16 and the availability of a radioactive form of T16 led us to investigate the effect of these two compounds on the entry of choline into S. cerevisiae cells. Similar to previous studies in P. falciparum, our studies showed that G25 and T16 are very effective inhibitors of choline transport in S. cerevisiae, with 50% inhibition of choline uptake measured when G25 and T16 were present at 20- and 100-fold excess, respectively. Because choline is not essential for S. cerevisiae growth, and because the IC50 values for G25 and T16 were not affected by the presence or absence of choline in the medium (data not shown), the ability of G25 to inhibit choline transport cannot alone account for its antifungal activity.
We showed that entry of the G25 analog T16 into wild-type and hnm1Δ yeast strains occurs through a temperature-dependent carrier-mediated process with similar kinetic characteristics, indicating a mode of entry of bisquaternary ammonium in S. cerevisiae distinct from that of the choline carrier. Deves and Krupka have shown that the lengthening of the alkyl chain in choline analogs makes them high-affinity inhibitors of choline transport but prevents their entry via the erythrocytic choline carrier (15). A similar mechanism might account for the ability of G25 and T16 to inhibit choline transport in S. cerevisiae and P. falciparum without being transported via the endogenous choline carriers. We suggest that in S. cerevisiae and most likely in P. falciparum as well, G25 is not transported via the choline transporter Hnm1 and that once inside the cell, this compound exerts its activity by interfering with specific cellular functions. Future studies will focus on determining the primary route of entry of this compound in S. cerevisiae.
Our data showed that S. cerevisiae mutants lacking specific phospholipid-synthesizing genes display substantial resistance to G25. Interestingly, loss of every gene of the de novo CDP-choline pathway, choline transporter (HNM1), choline kinase (CKI1), choline phosphotransferase (CPT1), and phosphocholine cytidylyltransferase (PCT1) resulted in resistance to this compound. Remarkably, a psd1Δ strain, which lacks PSD1, was also found to be highly resistant to G25. In S. cerevisiae, phosphatidylserine, which is synthesized in the endoplasmic reticulum and mitochondrion-associated membrane, is first transported to the inner mitochondrial membrane and Golgi/vacuole compartments, the sites of phosphatidylserine decarboxylase 1 (Psd1p) and 2 (Psd2), respectively. It is subsequently converted to phosphatidylethanolamine (41, 42). Psd1p is the major phosphatidylserine decarboxylase, converting 95% of the cellular phosphatidylserine and producing most of the cellular phosphatidylethanolamine in the absence of an ethanolamine precursor (42). In addition to its role in S. cerevisiae membrane structure, phosphatidylethanolamine plays a central role in lysosome/vacuole autophagy by covalently conjugating to Apg8p (24) and also serves as a donor of ethanolamine phosphate to glycosylphosphatidylinositol anchors, whose synthesis is essential for yeast cell viability (16, 32). Because P. falciparum possesses homologues of the S. cerevisiae PSD1, CKI1, CPT1, and PCT1 genes, we hypothesized that G25 might exert its antimalarial activity by blocking the synthesis of phosphatidylcholine from choline, and phosphatidylethanolamine from phosphatidylserine.
Labeling studies in P. falciparum with the phospholipid precursors choline and serine demonstrated that G25 inhibited both the incorporation of choline into phosphatidylcholine and phosphatidylserine decarboxylation in a dose-dependent manner. A concentration of only 1 μM of this compound was sufficient to inhibit phosphatidylcholine synthesis from choline, and inhibition was complete at 10 μM G25. Although this inhibition could be accounted for solely by the ability of choline analogs to inhibit choline entry into Plasmodium-infected erythrocytes (1), we cannot at this stage exclude additional inhibition by this compound of one or multiple enzymes of the CDP-choline pathway. Nonetheless, G25 concentrations up to 100 μM had no effect on the de novo biosynthesis of phosphatidylethanolamine from ethanolamine in P. falciparum, suggesting that the effect of this compound on the de novo phosphatidylcholine biosynthetic pathway is very specific. Similarly, albeit at higher concentrations, G25 was able to affect the incorporation of serine into phosphatidylethanolamine via the CDP-DAG pathway by specifically inhibiting the decarboxylation step of phosphatidylserine into phosphatidylethanolamine. At a concentration of 100 μM, G25 inhibited phosphatidylethanolamine formation from phosphatidylserine by 77%. Interestingly, at this concentration, G25 had no effect on the first step of the CDP-DAG pathway catalyzed by the phosphatidylserine synthase.
Two possible hypotheses could account for the resistance of S. cerevisiae mutants to G25. First, G25 might not directly kill S. cerevisiae cells but rather be converted into toxic derivatives by Psd1 and other enzymes of the CDP-choline pathway. Deletion of the genes encoding those enzymes reduces the toxicity of the compound. Second, G25 might directly inhibit specific enzymes of the phospholipid metabolic pathways, and deletion of PSD1 or any of the four genes of the CDP-choline pathway, although not essential for survival, results in changes in the composition and/or structure of the S. cerevisiae membranes, leading to low entry and/or effect of G25. In S. cerevisiae, phosphatidylcholine can be synthesized either via the CDP-choline pathway from choline transported via the choline transporter Hnm1 or via the transmethylation of phosphatidylethanolamine by two methyltransferases encoded by the PEM1/CHO2 and PEM2/OPI3 genes (13). The genes involved in these pathways are highly regulated by the availability of the phospholipid precursors inositol and choline (13, 37).
Yeast cells utilize the CDP-DAG pathway as the primary route of synthesis of phosphatidylcholine. The CDP-choline pathway, although not essential, is also active even in the absence of choline in the medium (13, 35). This suggests that although the two pathways can compensate for each other to allow survival, the composition of phosphatidylcholine synthesized by each pathway might be different under normal conditions. Considering the mechanism of catalysis of choline kinase, phosphocholine cytidyltransferase, CDP-choline phosphotransferase, and phosphatidylserine decarboxylase, it is difficult to envisage that G25 could be a substrate for those enzymes. Furthermore, studies in P. falciparum with a radioactive analog of G25, VB5-T, have shown that this compound was not metabolized and that it acts directly as an active compound (48). Our in vitro studies with recombinant P. falciparum Psd1 showed that G25 specifically inhibited the phosphatidylserine decarboxylation reaction catalyzed by this enzyme, providing further support for the second hypothesis.
The recent discovery in P. falciparum of a plant-like pathway for phosphatidylcholine biosynthesis involving methylation of phosphoethanolamine into phosphocholine by a phosphoethanolamine methyltransferase, Pmt (36), suggests that choline uptake might not be essential for parasite survival, whereas the later steps of the CDP-choline pathway catalyzed by the phosphocholine cytidyltransferase and CDP-choline phosphotransferase enzymes might be essential. Future genetic studies to determine the importance of the CDP-choline pathway in P. falciparum and future biochemical studies with recombinant P. falciparum choline kinase, phosphocholine cytidyltransferase, or CDP-choline phosphotransferase enzymes to directly determine their sensitivity to G25 are warranted.
In conclusion, our data unraveled two new mechanisms of action of G25 in P. falciparum and S. cerevisiae. G25 specifically inhibits the de novo synthesis of phosphatidylcholine from choline and the phosphatidylserine decarboxylase-dependent formation of phosphatidylethanolamine from phosphatidylserine. These novel findings constitute important information for quaternary ammonium compounds that are entering clinical studies. These studies further support the use of S. cerevisiae as a surrogate system to identify the targets of antimalarial compounds.
Acknowledgments
This work was supported by the University of Connecticut Health Center Research Fund, the Robert Leet and Clara Guthrie Patterson Trust (to R.Z. and C.B.M.), the U.S. Army Medical Research and Material Command (to C.B.M.), the European Communities, QLK2-CT-2000-01166 (to H.V.), and the French Ministere de l'Education Nationale et Recherche Scientifique (to R.R.).
We thank Patrick Eldin and Francoise Baunaure for help with the phosphatidylserine decarboxylase assay. We are grateful to Stephen Wikel and Justin Radolf for advice and critical reading of the manuscript.
REFERENCES
- 1.Ancelin, M. L., M. Calas, J. Bompart, G. Cordina, D. Martin, M. Ben Bari, T. Jei, P. Druilhe, and H. J. Vial. 1998. Antimalarial activity of 77 phospholipid polar head analogs: close correlation between inhibition of phospholipid metabolism and in vitro Plasmodium falciparum growth. Blood 91:1426-1437. [PubMed] [Google Scholar]
- 2.Ancelin, M. L., M. Calas, A. Bonhoure, S. Herbute, and H. J. Vial. 2003. In vivo antimalarial activities of mono- and bisquaternary ammonium salts interfering with Plasmodium phospholipid metabolism. Antimicrob. Agents Chemother. 47:2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancelin, M. L., M. Calas, V. Vidal-Sailhan, S. Herbute, P. Ringwald, and H. J. Vial. 2003. Potent inhibitors of Plasmodium phospholipid metabolism with a broad spectrum of in vitro antimalarial activities. Antimicrob. Agents Chemother. 47:2590-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ancelin, M. L., and H. J. Vial. 1986. Quaternary ammonium compounds efficiently inhibit Plasmodium falciparum growth in vitro by impairment of choline transport. Antimicrob. Agents Chemother. 29:814-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancelin, M. L., H. J. Vial, M. Calas, L. Giral, G. Piquet, E. Rubi, A. Thomas, W. Peters, C. Slomianny, S. Herrera, and et al. 1994. Present development concerning antimalarial activity of phospholipid metabolism inhibitors with special reference to in vivo activity. Mem Inst Oswaldo Cruz 89 Suppl. 2:85-90. [DOI] [PubMed] [Google Scholar]
- 6.Ancelin, M. L., H. J. Vial, and J. R. Philippot. 1985. Inhibitors of choline transport into Plasmodium-infected erythrocytes are effective antiplasmodial compounds in vitro. Biochem. Pharmacol. 34:4068-4071. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson, K. D., B. Jensen, A. I. Kolat, E. M. Storm, S. A. Henry, and S. Fogel. 1980. Yeast mutants auxotrophic for choline or ethanolamine. J. Bacteriol. 141:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baunaure, F., P. Eldin, A. M. Cathiard, and H. Vial. 2004. Characterization of a non-mitochondrial type I phosphatidylserine decarboxylase in Plasmodium falciparum. Mol. Microbiol. 51:33-46. [DOI] [PubMed] [Google Scholar]
- 9.Biagini, G. A., E. Richier, P. G. Bray, M. Calas, H. Vial, and S. A. Ward. 2003. Heme binding contributes to antimalarial activity of bis-quaternary ammoniums. Antimicrob. Agents Chemother. 47:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birner, R., M. Burgermeister, R. Schneiter, and G. Daum. 2001. Roles of phosphatidylethanolamine and of its several biosynthetic pathways in Saccharomyces cerevisiae. Mol. Biol. Cell 12:997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calas, M., M. L. Ancelin, G. Cordina, P. Portefaix, G. Piquet, V. Vidal-Sailhan, and H. Vial. 2000. Antimalarial activity of compounds interfering with Plasmodium falciparum phospholipid metabolism: comparison between mono- and bisquaternary ammonium salts. J. Med. Chem. 43:505-516. [DOI] [PubMed] [Google Scholar]
- 12.Calas, M., G. Cordina, J. Bompart, M. Ben Bari, T. Jei, M. L. Ancelin, and H. Vial. 1997. Antimalarial activity of molecules interfering with Plasmodium falciparum phospholipid metabolism. Structure-activity relationship analysis. J. Med. Chem. 40:3557-3566. [DOI] [PubMed] [Google Scholar]
- 13.Carman, G. M., and S. A. Henry. 1999. Phospholipid biosynthesis in the yeast Saccharomyces cerevisiae and interrelationship with other metabolic processes. Prog. Lipid Res. 38:361-399. [DOI] [PubMed] [Google Scholar]
- 14.Clancey, C. J., S. C. Chang, and W. Dowhan. 1993. Cloning of a gene (PSD1) encoding phosphatidylserine decarboxylase from Saccharomyces cerevisiae by complementation of an Escherichia coli mutant. J. Biol. Chem. 268:24580-24590. [PubMed] [Google Scholar]
- 15.Deves, R., and R. M. Krupka. 1979. The binding and translocation steps in transport as related to substrate structure. A study of the choline carrier of erythrocytes. Biochim. Biophys. Acta 557:469-485. [DOI] [PubMed] [Google Scholar]
- 16.Englund, P. T. 1993. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu. Rev. Biochem. 62:121-138. [DOI] [PubMed] [Google Scholar]
- 17.Folch, J., M. Lees, and G. H. S. Stanley. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497-509. [PubMed] [Google Scholar]
- 18.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419:498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hjelmstad, R. H., and R. M. Bell. 1987. Mutants of Saccharomyces cerevisiae defective in sn-1,2-diacylglycerol cholinephosphotransferase. Isolation, characterization, and cloning of the CPT1 gene. J. Biol. Chem. 262:3909-3917. [PubMed] [Google Scholar]
- 20.Hjelmstad, R. H., and R. M. Bell. 1991. sn-1,2-diacylglycerol choline- and ethanolaminephosphotransferases in Saccharomyces cerevisiae. Nucleotide sequence of the EPT1 gene and comparison of the CPT1 and EPT1 gene products. J. Biol. Chem. 266:5094-5103. [PubMed] [Google Scholar]
- 21.Hjelmstad, R. H., and R. M. Bell. 1990. The sn-1,2-diacylglycerol cholinephosphotransferase of Saccharomyces cerevisiae. Nucleotide sequence, transcriptional mapping, and gene product analysis of the CPT1 gene. J. Biol. Chem. 265:1755-1764. [PubMed] [Google Scholar]
- 22.Hjelmstad, R. H., and R. M. Bell. 1988. The sn-1,2-diacylglycerol ethanolaminephosphotransferase activity of Saccharomyces cerevisiae. Isolation of mutants and cloning of the EPT1 gene. J. Biol. Chem. 263:19748-19757. [PubMed] [Google Scholar]
- 23.Hosaka, K., T. Kodaki, and S. Yamashita. 1989. Cloning and characterization of the yeast CKI gene encoding choline kinase and its expression in Escherichia coli. J. Biol. Chem. 264:2053-2059. [PubMed] [Google Scholar]
- 24.Ichimura, Y., T. Kirisako, T. Takao, Y. Satomi, Y. Shimonishi, N. Ishihara, N. Mizushima, I. Tanida, E. Kominami, M. Ohsumi, T. Noda, and Y. Ohsumi. 2000. A ubiquitin-like system mediates protein lipidation. Nature 408:488-492. [DOI] [PubMed] [Google Scholar]
- 25.Jakovcic, S., G. S. Getz, M. Rabinowitz, H. Jakob, and H. Swift. 1971. Cardiolipin content of wild type and mutant yeasts in relation to mitochondrial function and development. J. Cell Biol. 48:490-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, K., K. H. Kim, M. K. Storey, D. R. Voelker, and G. M. Carman. 1999. Isolation and characterization of the Saccharomyces cerevisiae EKI1 gene encoding ethanolamine kinase. J. Biol. Chem. 274:14857-14866. [DOI] [PubMed] [Google Scholar]
- 27.Kim, K. H., D. R. Voelker, M. T. Flocco, and G. M. Carman. 1998. Expression, purification, and characterization of choline kinase, product of the CKI gene from Saccharomyces cerevisiae. J. Biol. Chem. 273:6844-6852. [DOI] [PubMed] [Google Scholar]
- 28.Kiyono, K., K. Miura, Y. Kushima, T. Hikiji, M. Fukushima, I. Shibuya, and A. Ohta. 1987. Primary structure and product characterization of the Saccharomyces cerevisiae CHO1 gene that encodes phosphatidylserine synthase. J. Biochem. (Tokyo) 102:1089-1100. [DOI] [PubMed] [Google Scholar]
- 29.Kodaki, T., and S. Yamashita. 1989. Characterization of the methyltransferases in the yeast phosphatidylethanolamine methylation pathway by selective gene disruption. Eur. J. Biochem. 185:243-251. [DOI] [PubMed] [Google Scholar]
- 30.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65:418-420. [PubMed] [Google Scholar]
- 31.Letts, V. A., L. S. Klig, M. Bae-Lee, G. M. Carman, and S. A. Henry. 1983. Isolation of the yeast structural gene for the membrane-associated enzyme phosphatidylserine synthase. Proc. Natl. Acad. Sci. USA 80:7279-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menon, A. K., and V. L. Stevens. 1992. Phosphatidylethanolamine is the donor of the ethanolamine residue linking a glycosylphosphatidylinositol anchor to protein. J. Biol. Chem. 267:15277-15280. [PubMed] [Google Scholar]
- 33.Nikawa, J., K. Hosaka, Y. Tsukagoshi, and S. Yamashita. 1990. Primary structure of the yeast choline transport gene and regulation of its expression. J. Biol. Chem. 265:15996-16003. [PubMed] [Google Scholar]
- 34.Nikawa, J., Y. Tsukagoshi, T. Kodaki, and S. Yamashita. 1987. Nucleotide sequence and characterization of the yeast PSS gene encoding phosphatidylserine synthase. Eur. J. Biochem. 167:7-12. [DOI] [PubMed] [Google Scholar]
- 35.Patton-Vogt, J. L., P. Griac, A. Sreenivas, V. Bruno, S. Dowd, M. J. Swede, and S. A. Henry. 1997. Role of the yeast phosphatidylinositol/phosphatidylcholine transfer protein (Sec14p) in phosphatidylcholine turnover and INO1 regulation. J. Biol. Chem. 272:20873-20883. [DOI] [PubMed] [Google Scholar]
- 36.Pessi, G., G. Kociubinski, and C. B. Mamoun. 2004. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc. Natl. Acad. Sci. USA 101:6206-6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santiago, T. C., and C. B. Mamoun. 2003. Genome expression analysis in yeast reveals novel transcriptional regulation by inositol and choline and new regulatory functions for Opi1p, Ino2p, and Ino4p. J. Biol. Chem. 278:38723-38730. [DOI] [PubMed] [Google Scholar]
- 38.Storey, M. K., K. L. Clay, T. Kutateladze, R. C. Murphy, M. Overduin, and D. R. Voelker. 2001. Phosphatidylethanolamine has an essential role in Saccharomyces cerevisiae that is independent of its ability to form hexagonal phase structures. J. Biol. Chem. 276:48539-48548. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan, D. J., Jr., I. Y. Gluzman, D. G. Russell, and D. E. Goldberg. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA 93:11865-11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 41.Trotter, P. J., J. Pedretti, and D. R. Voelker. 1993. Phosphatidylserine decarboxylase from Saccharomyces cerevisiae. Isolation of mutants, cloning of the gene, and creation of a null allele. J. Biol. Chem. 268:21416-21424. [PubMed] [Google Scholar]
- 42.Trotter, P. J., J. Pedretti, R. Yates, and D. R. Voelker. 1995. Phosphatidylserine decarboxylase 2 of Saccharomyces cerevisiae. Cloning and mapping of the gene, heterologous expression, and creation of the null allele. J. Biol. Chem. 270:6071-6080. [DOI] [PubMed] [Google Scholar]
- 43.Trotter, P. J., and D. R. Voelker. 1995. Identification of a non-mitochondrial phosphatidylserine decarboxylase activity (PSD2) in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 270:6062-6070. [DOI] [PubMed] [Google Scholar]
- 44.Vial, H. 1996. Recent developments and rationale towards new strategies for malarial chemotherapy. Parasite 3:3-23. [DOI] [PubMed] [Google Scholar]
- 45.Vial, H., and M. Calas. 2001. Inhibitors of phospholipid metabolism, p. 347-365. Humana Press Inc., Totowa, N.J.
- 46.Vial, H. J., and M. L. Ancelin. 1998. Malaria lipids, p. 159-175. In I. W. Sherman (ed.), Malaria: parasite biology, pathogenesis, and protection. American Society for Microbiology, Washington, D.C.
- 47.Vial, H. J., and M. Calas. 2001. Inhibitors of phospholipid metabolism, p. 347-365. In P. Rosenthal (ed.), Antimalarial Chemotherapy, mechanisms of action, modes of resistance, and new directions in drug development. Humana Press, Totowa, NJ.
- 48.Wengelnik, K., V. Vidal, M. L. Ancelin, A. M. Cathiard, J. L. Morgat, C. H. Kocken, M. Calas, S. Herrera, A. W. Thomas, and H. J. Vial. 2002. A class of potent antimalarials and their specific accumulation in infected erythrocytes. Science 295:1311-1314. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. 2000. World Health Organization Expert Committee on Malaria. World Health Org. Tech. Rep. Ser. 892:i-v, 1-74. [Google Scholar]
- 50.Yamashita, S., and K. Hosaka. 1997. Choline kinase from yeast. Biochim. Biophys. Acta 1348:63-69. [DOI] [PubMed] [Google Scholar]
- 51.Zufferey, R., and C. B. Mamoun. 2002. Choline transport in Leishmania major promastigotes and its inhibition by choline and phosphocholine analogs. Mol. Biochem. Parasitol. 125:127-134. [DOI] [PubMed] [Google Scholar]
- 52.Zufferey, R., T. C. Santiago, V. Brachet, and C. Ben Mamoun. 2004. Reexamining the role of choline transporter-like (CTL) proteins in choline transport. Neurochem. Res. 29:461-467. [DOI] [PubMed] [Google Scholar]