Abstract
The CRK3 cyclin-dependent kinase of Leishmania has been shown by genetic manipulation of the parasite to be essential for proliferation. We present data which demonstrate that chemical inhibition of CRK3 impairs the parasite's viability within macrophages, thus further validating CRK3 as a potential drug target. A microtiter plate-based histone H1 kinase assay was developed to screen CRK3 against a chemical library enriched for protein kinase inhibitors. Twenty-seven potent CRK3 inhibitors were discovered and screened against Leishmania donovani amastigotes in vitro. Sixteen of the CRK3 inhibitors displayed antileishmanial activity, with a 50% effective dose (ED50) of less than 10 μM. These compounds fell into four chemical classes: the 2,6,9-trisubstituted purines, including the C-2-alkynylated purines; the indirubins; the paullones; and derivatives of the nonspecific kinase inhibitor staurosporine. The paullones and staurosporine derivatives were toxic to macrophages. The 2,6,9-trisubstituted purines inhibited CRK3 in vitro, with 50% inhibitory concentrations ranging from high nanomolar to low micromolar concentrations. The most potent inhibitors of CRK3 (compounds 98/516 and 97/344) belonged to the indirubin class; the 50% inhibitory concentrations for these inhibitors were 16 and 47 nM, respectively, and the ED50s for these inhibitors were 5.8 and 7.6 μM, respectively. In culture, the indirubins caused growth arrest, a change in DNA content, and aberrant cell types, all consistent with the intracellular inhibition of a cyclin-dependent kinase and disruption of cell cycle control. Thus, use of chemical inhibitors supports genetic studies to confirm CRK3 as a validated drug target in Leishmania and provides pharmacophores for further drug development.
The leishmaniases are a group of diseases, affecting both humans and animals, caused by protozoan parasites of the genus Leishmania. The leishmaniases are endemic to the tropical and subtropical regions of 88 countries worldwide, mostly developing countries. Globally, the annual incidence is 1.5 to 2 million new cases of cutaneous leishmaniases and 500,000 new cases of visceral leishmaniasis. The overall prevalence is 12 million cases worldwide, including those with overt symptoms and asymptomatic individuals. An increasing problem emerging in southwestern Europe is coinfection with human immunodeficiency virus and leishmaniasis (1).
There are many drawbacks to current chemotherapy for leishmaniasis, including problems of low efficacy, severe toxic side effects, and emerging drug resistance (reviewed in references 2, 9, and 39). The first-line drugs for treatment of leishmaniasis (antimonials and amphotericin B) require intravenous administration over a prolonged period, which has negative implications for patient compliance. Some of the newer therapies, such as the lipid formulations of amphotericin B, are extremely expensive, which is an important factor since, in general, leishmaniasis affects those in the developing world. Thus, there is an urgent need for new antileishmanial drugs. A new treatment for leishmaniasis, miltefosine, came onto the market recently (11) and may represent a major step forward in the treatment of leishmaniasis, especially since it can be given orally. However, miltefosine is not without problems: it is teratogenic (2, 9) and has a low therapeutic index (9). In addition, it would be prudent to continue to validate new drug targets and develop new chemotherapies to combat future drug resistance (38).
Leishmania parasites possess a complex life cycle in which the parasite passes between the sandfly vector and the mammalian host, during which time the parasite oscillates between rapidly dividing and cell cycle-arrested forms. The cell cycle of Leishmania is closely regulated, as in other eukaryotes, and integrated with its differentiation between the various life cycle stages. During our investigations into the cell and life cycles of these parasites, we have isolated two cdc2-related kinase genes from Leishmania mexicana, CRK1 and CRK3 (13, 35). The encoded enzymes are homologous to the cyclin-dependent kinase (CDK) family of serine/threonine protein kinases, which are ubiquitous in eukaryotes, many of which play essential roles in the regulation and coordination of the cell cycle.
The crucial role of CDKs in the regulation of cell division and the high incidence with which their activity is abnormally regulated in human cancers suggest that CDKs would be good targets for new anticancer agents and has inspired a search for selective CDK inhibitors (reviewed in references 12 and 21). A series of chemical inhibitors, which display various degrees of CDK selectivity, have been identified, including olomoucine (17), roscovitine (29), purvalanol (14), flavopiridol (27), butyrolactone (19), indirubins (18), and paullones (42). All these inhibitors act by competing with ATP for binding at the catalytic site. The use of CDK inhibitors as cytotoxic drugs relies upon the fact that they would have more effect on tumor cells, which are rapidly dividing, than on normal cells, most of which do not divide. This same rationale can also be applied in many diseases, including those caused by parasitic protozoa. Despite the relatively high level of amino acid sequence identity between mammalian CDKs, some of the CDK-specific inhibitors display selectivity for certain subclasses of CDKs. For example, roscovitine and butyrolactone inhibit CDK1, CDK2, and CDK5 but not CDK4 or CDK6 (19, 29). Thus, it should be possible to discover, design, or develop inhibitors that are selective for parasite protein kinases of the CDK family.
Several lines of evidence indicate that CRK3 is the most likely candidate for the functional CDK1 (cdc2) homologue in Leishmania. CRK3 is active in the two proliferative life cycle stages of the parasite (the insect stage promastigote and the mammalian stage amastigote),and it binds the Schizosaccharomyces pombe cdc2 kinase subunit protein p13suc1 (13). Attempts to generate a null mutant resulted in a dramatic change in the parasite's ploidy to avoid loss of this essential gene (16); this phenotype is widely interpreted to mean that the gene is essential to the organism (3, 16). CRK3 from Leishmania major complemented an S. pombe cdc2 temperature-sensitive mutant (40). CRK3 histone H1 kinase activity was inhibited in vitro with a CDK-specific inhibitor, flavopiridol, and treatment of the parasite with flavopiridol resulted in cell cycle arrest in the G2/M phase of the cell cycle (16). CRK3 is active in the G2 phase of the cell cycle and appears to regulate progression into mitosis (16).
To investigate the potential of CRK3 as a novel antileishmanial drug target and to determine whether CRK3 inhibitors impair viability of the parasite within macrophages, we developed a microtiter plate-based histone H1 kinase assay, screened a diverse chemical library for potent inhibitors of CRK3, and then tested these inhibitors against a model of Leishmania infection in vitro. The 50% inhibitory concentrations (IC50s) of the inhibitors against the parasite kinase and against CDK1/cyclin B were determined. Several interesting groups of compounds were discovered, including the indirubins, which were investigated in more detail. These compounds caused growth arrest of the parasite in culture, and cells exposed to indirubins had aberrant morphology and an altered DNA content, consistent with disruption of the cell cycle through inhibition of a CDK.
MATERIALS AND METHODS
Parasite culture.
Leishmania mexicana mexicana (MNYC/BZ/62/M379) promastigotes were cultured in HOMEM medium supplemented with 10% heat-inactivated fetal calf serum, as described previously (35). Promastigotes were harvested during the mid-log phase of growth (0.5 × 107 to 1 × 107 cells/ml), washed twice with phosphate-buffered saline (PBS) and stored at −70°C until required.
Preparation of L. mexicana CRK3.
Transgenic L. mexicana (13) containing an episome that encodes a six-histidine-tagged copy of CRK3 (CRK3his) were harvested as described above, resuspended at 108 cells/ml in lysis buffer LSG (50 mM morpholinepropanesulfonic acid [MOPS] [pH 7.2], 100 mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM sodium orthovanadate, 10 mM NaF, 1% Triton X-100, 10% glycerol) to which a cocktail of protease inhibitors had been added (leupeptin, 0.1 mg/ml; phenylmethylsulfonyl fluoride, 0.5 mM; pepstatin A, 5 μg/ml; Pefabloc SC, 0.5 mg/ml; 1,10-phenanthroline, 1 mM), and incubated on ice for 10 min. The lysate was then centrifuged at 100,000 × g for 45 min at 4°C and the supernatant was decanted (S-100 lysate). The S-100 lysate was then filtered through a 0.2-μm-pore-size filter and applied to a Ni-chelate (BioCad) column, previously equilibrated with LSG plus 5 mM imidazole. After application of the lysate, the column was washed sequentially with LSG plus 5 mM imidazole, HSLS (50 mM MOPS [pH 7.2], 500 mM NaCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM sodium orthovanadate, 10 mM NaF, 1% Triton X-100), and LSG plus 20 mM imidazole. Then, the bound CRK3his was eluted with a linear gradient of imidazole (20 to 250 mM imidazole over 20 column volumes). Fractions were analyzed for histone H1 kinase activity, as described previously (13), and for the presence of CRK3his by Western blotting, using a CRK3-specific antibody (see below). The eluted kinase could be stored for up to 14 days at −80°C without appreciable loss of activity.
Immunoblotting.
A 20-μl aliquot of each fraction was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% acrylamide gel) and transferred onto a polyvinylidene difluoride membrane. Western blotting was performed as previously described (34) using an anti-CRK3 antiserum at 1:500 dilution. CRK3 was detected using a chemiluminescence detection system (Pierce SuperSignal West Pico). The anti-CRK3 antibody was generated against two CRK3-specific peptides (peptide 1, DAGTRDSLDRYNRLD, corresponding to amino acid positions 13 to 27 in CRK3; peptide 2, DTEGSWPGVSRLPDY, corresponding to positions 242 to 256) (Abcam, Ltd.).
Chemical library.
The library is a collection of different sublibraries each prepared by different chemists. Each compound was shown to have antimitotic activity in the starfish oocyte model (6, 30). Some of the sublibraries are extracts from plant and marine biological material. Others are sublibraries where a compound with known antimitotic activity, in most cases due to CDK-inhibitory activity, was chemically modified to prepare other compounds in the same series (14, 31, 42). Some compounds were discovered by searching the National Institutes of Health database for compounds that had the same profile of activity in their in vitro assays as the CDK inhibitor flavopiridol (42). So, taken as a whole, the library contains chemically diverse compounds. But within the library, there are also smaller subsets of compounds that were related chemically.
Microtiter plate histone H1 kinase assay.
A reaction mixture consisting of histone H1 (0.227 mg/ml; GIBCO BRL), CRK3his, and kinase assay buffer (KAB) (50 mM MOPS [pH 7.2], 20 mM MgCl2, 10 mM EGTA, 2 mM dithiothreitol) was aliquoted into the wells of a polypropylene 96-well microtiter plate (22 μl per well). Inhibitor stock solutions of various concentrations in dimethyl sulfoxide (DMSO) (or DMSO as control) were added to each well (3 μl per well). The kinase reaction was initiated by the addition of 5 μl per well of an ATP mix (16 μM ATP, [γ-32P]ATP; 1 μCi per reaction [6,000 Ci/mmol] in KAB), giving a total reaction volume of 30 μl. The microtiter plate was incubated at 30°C for 30 min, and then the reaction was stopped by trichloroacetic acid (TCA) precipitation as follows. Bovine serum albumin (120 μl; 250 μg/ml in KAB) was added to each well, and then 140 μl was removed from each well into a second microtiter plate (Millipore Multiscreen-HV) for TCA precipitation with 90 μl of ice-cold 70% TCA per well at 4°C for 10 min with shaking. The plate was then drained by pulling a vacuum through the Durapore membrane at the base of each well. Then, the TCA precipitate was washed six times with 200 μl of ice-cold 25% TCA, again pulling the wash solutions through the membranes by vacuum. The amount of radiolabeled ATP incorporated into histone H1 was assessed by scintillation counting. Values for blank wells (no-enzyme controls) were subtracted, and activities were calculated as picomoles of phosphate incorporated during the 30-min incubation and expressed as a percentage of maximal activity (no inhibitor controls).
The microtiter plate assay was also used to determine the IC50s for selected compounds. The assay was performed in triplicate (unless the compound was in limited supply), testing a range of concentrations from 0.1 nM to 0.1 mM.
Human CDK1/cyclin B was obtained from New England Biolabs. CDK1/cyclin B complex was also isolated from M-phase starfish oocytes as described in reference 29 and used to determine IC50s. The inhibitor sensitivities of the CDK1/cyclin B from starfish and humans were essentially the same.
Testing against L. donovani amastigotes in mouse PEMs.
Peritoneal exudate macrophages (PEMs) were elicited by injecting the peritoneal cavity of each BALB/c mouse with 2 ml of sterile starch solution. The PEMs were harvested 2 days later by peritoneal lavage and resuspended in RPMI 1640 plus 10% fetal calf serum (FCS) at 4 × 105/ml. The macrophages were then aliquoted into 16-chamber slides (40,000 PEMs per well) and incubated overnight at 37°C in 5% CO2 to allow them to adhere. After 24 h the PEMs were infected with L. donovani (10 parasites per PEM) and incubated for a further 24 h at 37°C. Then, the overlying medium was removed and replaced with fresh medium containing various concentrations of inhibitor (10, 3, 1, and 0.3 μM, each in quadruplicate). The infected macrophages were then incubated with the inhibitors for 3 days at 37°C. After 3 days, the medium (plus inhibitors) was changed and the infected PEMs were incubated for a further 2 days at 37°C. Then, the cells were fixed, Giemsa stained, and counted. A total of 100 macrophages from each well (4 × 100 cells counted for each drug concentration) were examined, and the percentage of cells infected was calculated for untreated controls and for each sample well. Comparison of untreated controls with samples allowed us to calculate the degree to which infection had been inhibited by the presence of the drug (percent inhibition) and to calculate the concentration that reduces the number of infected macrophages by 50% (50% effective dose [ED50]).
Testing compounds against L. mexicana in culture.
Cultures of promastigotes were seeded at 106 cells/ml in HOMEM plus 10% FCS in the presence or absence of a 10 μM concentration of each inhibitor in duplicate and incubated at 25°C. The cell density was determined daily for 5 days. For axenic amastigotes, cultures were seeded with stationary-phase promastigotes at 106 cells/ml in Schneider's Drosophila medium plus 20% FCS, pH 5.5, in the presence or absence of inhibitor and incubated at 32°C. Cells were allowed to differentiate for 2 days and then counted daily for 5 days.
Flow cytometry analysis.
The DNA content of L. mexicana promastigotes was determined as described previously (16). Briefly, this involved fixing the cells, staining them with propidium iodide, and determining their fluorescence, which is proportional to the DNA content. Ten thousand cells were analyzed per sample, using a Becton Dickinson FACScalibur.
DAPI.
Parasites were harvested at 1,000 × g for 10 min at 4°C, washed once in PBS, and fixed onto slides with methanol. The slides were overlaid with DAPI (4′-6-diamidino-2-phenyindole) (1 μg/ml) in PBS plus 0.5% 1,4-diazabicyclo[2,2,2]octane and analyzed immediately. Images were captured using a Zeiss Axioskop 2 microscope (at a magnification of ×1,000) and a Hamamatsu ORCA-ER digital camera and were processed using OpenLab 3.1.2.
RESULTS
Preparation of active L. mexicana CRK3.
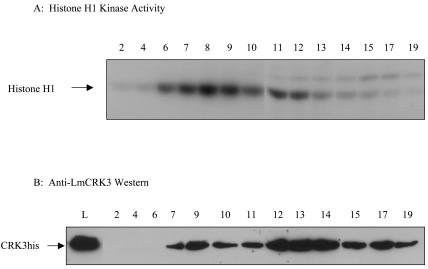
The active CRK3 protein kinase used in the chemical library screen was purified from transgenic L. mexicana cell lysates by Ni-chelate chromatography. The tagged protein (CRK3his) is fully functional, as it can be expressed in the mutant lacking both wild-type CRK3 alleles (16). Expression of recombinant CDKs in heterologous systems can lead to the production of monomer kinase that has little or no kinase activity (8, 33, 36, 37). Monomer kinase may, however, be able to bind inhibitors, thus distorting the values calculated for IC50s. The purification protocol for CRK3his was optimized to allow separation of the active kinase complexes from the inactive monomers (Fig. 1).
FIG. 1.
Ni-chelate affinity chromatography. (A) For kinase assays, 5 μl of each fraction was assayed for histone H1 kinase activity. The reaction mixtures after the reaction was stopped were subjected to SDS-PAGE and autoradiography. (B) For Western blotting, 20 μl of each fraction (or total lysate) was separated by SDS-PAGE and subjected to immunoblotting, using an antiserum specific for CRK3. Lane numbering refers to the fraction number, and L indicates the total lysate.
The total cell lysate was loaded onto the Ni-chelate column, and after substantial washing to remove contaminating proteins, the tagged protein was eluted with a linear gradient of imidazole. When the fractions were analyzed for protein kinase activity (Fig. 1A) and by Western blotting (Fig. 1B), the active fractions eluted with lower concentrations of imidazole, and the inactive monomer protein eluted in the later fractions. The active fractions were combined for use in inhibitor screening.
Primary screen.
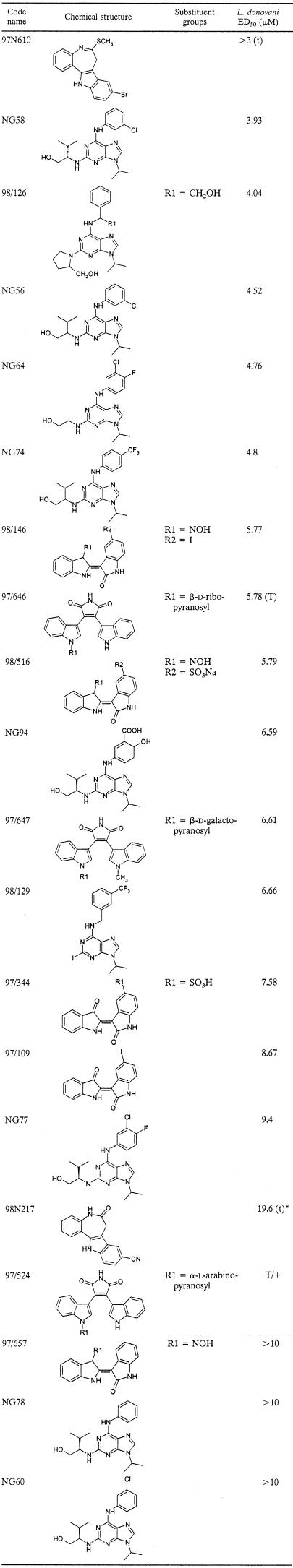
The microtiter plate assay for CRK3his was used to screen a chemically diverse library of antimitotic compounds. The library consisted of 634 compounds, divided into eight sublibraries. It included synthetic analogues of known CDK-specific inhibitors, such as olomoucine (17), natural products derived from plant sources, and some semisynthetic compounds. Each compound was provided as a stock solution in DMSO at either 1 mM or 1 mg/ml. In the initial screen, these compounds were tested against CRK3his at 100 μM or 100 μg/ml. Forty-two compounds strongly inhibited CRK3his (reducing activity by more than 90%) at the concentration tested. Four of the tested compounds (97/657, 98/516, 97/344, and NG60) (structures are shown in Table 1) inhibited the histone H1 kinase activity to background levels (100% inhibition), 12 compounds inhibited the kinase activity by between 95 and 98%, and a further 26 compounds inhibited the activity by between 90 and 95%.
TABLE 1.
Structures and activities of compounds tested against L. donovani-infected macrophagesaa
T/+, toxic to macrophages but parasites still present; (T), toxic to macrophages at 10 μM and at 3 μM; (t), some toxicity towards macrophages observed at >10 μM; *, the ED50 of 98N217 against L. mexicana-infected macrophages was 5.77 μM, although toxicity was observed at 10 μM. Compounds with an ED50 of >10 μM were 97/657, NG60, NG78, NG73, NG 71, 97N608, NG35, NG112, 98/130, and 96/422.
Testing compounds against L. donovani amastigotes within mouse peritoneal macrophages.
We tested the 27 most potent of the CRK3 inhibitors against L. donovani-infected mouse peritoneal macrophages. The results are shown in Table 1. The screen was also performed with L. mexicana-infected macrophages, and the results were broadly similar (data not shown). One of the compounds (97/524, a compound related to staurosporine [Table 1]) was toxic to the macrophages but ineffective against the parasite. Ten of the inhibitors (37%) had little or no effect on parasite growth and replication. Sixteen of the compounds (59%) suppressed the infection of macrophages by L. donovani amastigotes, 6 at an ED50 of <5 μM and 10 at an ED50 between 5 and 10 μM. Two compounds (97/646 and 98N217) also displayed toxicity toward the macrophages at higher concentrations. The 16 compounds that inhibited infection fell into four main categories: indirubins (97/109, 97/344, 98/146, and 98/516), 2,6,9-trisubstituted purines (NG56, NG58, NG64, NG74, NG77, and NG94, including a subset of C-2-alkynylated purines, 98/126 and 98/129), paullones (97N610 and 98N217), and derivatives of the nonspecific kinase inhibitor staurosporine (97/646 and 97/647).
Determination of IC50s of compounds that displayed activity toward L. donovani amastigotes.
The IC50s were determined for a subset of the inhibitors that displayed antileishmanial activity in the infection model and compared to IC50s for CDK1/cyclin B (Tables 2 and 3). The IC50s of five compounds (97/344, 98/516, NG56, NG60, and NG78) were in the nanomolar range for CRK3his. Six other compounds inhibited CRK3his when used in the low micromolar range (97/657, 98/146, 98N217, NG58, NG64, and NG74), and compound 98/126 inhibited CRK3his with a high IC50 of 69 μM. The inhibitors could be divided into two groups: those which were similarly potent toward CRK3his and CDK1/cyclin B (Table 2) and those which were more potent toward CDK1/cyclin B than toward CRK3his (Table 3).
TABLE 2.
Compounds with similar IC50 values against CRK3his and against CDK1/cyclin B
| Code name | CRK3his IC50 (nM) | CDK1/cyclin B IC50 (nM) |
|---|---|---|
| 97/344 | 47 | 51 |
| 98/516 | 16.5 | 5 |
| NG78 | 283 | 240 |
TABLE 3.
Compounds that are more potent towards CDK1/cyclin B than towards CRK3his
| Code name | CRK3his IC50 (nM) | CDK1/cyclin B IC50 (nM) | Approximate fold difference |
|---|---|---|---|
| 97/657 | 1350 | 180 | 10 |
| 98/126 | 69,000 | 80 | 1,000 |
| 98/146 | 1,200 | 25 | 50 |
| 98N217 | 2,900 | 44 | 100 |
| NG56 | 371 | 35 | 10 |
| NG58 | 2,100 | 500 | 4 |
| NG60 | 620 | 35 | 20 |
| NG64 | 4,250 | 290 | 15 |
| NG74 | 1,500 | 350 | 4 |
Testing compounds against L. mexicana in culture.
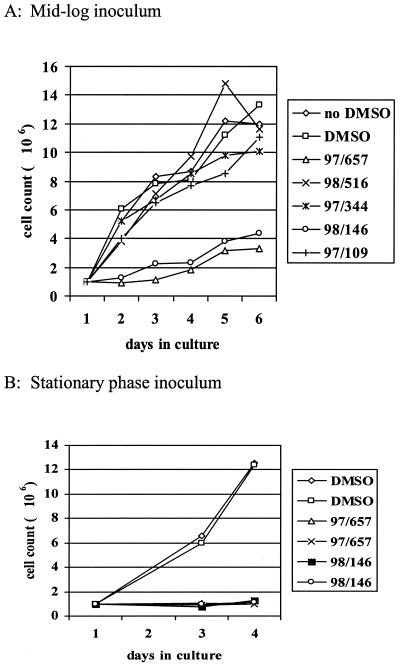
Five different members of the indirubin kinase inhibitor family (compounds 97/109, 97/344, 97/657, 98/146, and 98/516 [Table 1]), which had previously been shown to be good inhibitors of CRK3, were tested against L. mexicana promastigotes in culture (Fig. 2). Only compounds 97/657 and 98/146 had any effect on the growth of promastigotes in culture. The same results were obtained when axenic amastigotes were incubated with indirubins in culture (data not shown). Generally, growth curves were prepared using dilutions of dividing mid-log-phase cultures, but it was observed in subsequent experiments that growth inhibition was much more pronounced if the inoculating culture was in stationary phase (Fig. 2). The nature of this growth inhibition was investigated by flow cytometry analysis and DAPI staining of cells exposed to the indirubins.
FIG. 2.
Effects of indirubins on the growth of L. mexicana promastigotes in culture. Mid-log- or stationary-phase promastigote cultures were seeded at 106 cells/ml into medium. The cultures were incubated with a 10 μM concentration of one of five indirubin inhibitors at 25°C for 5 days. The cell density was determined daily.
Flow cytometry analysis of L. mexicana promastigotes exposed to indirubins.
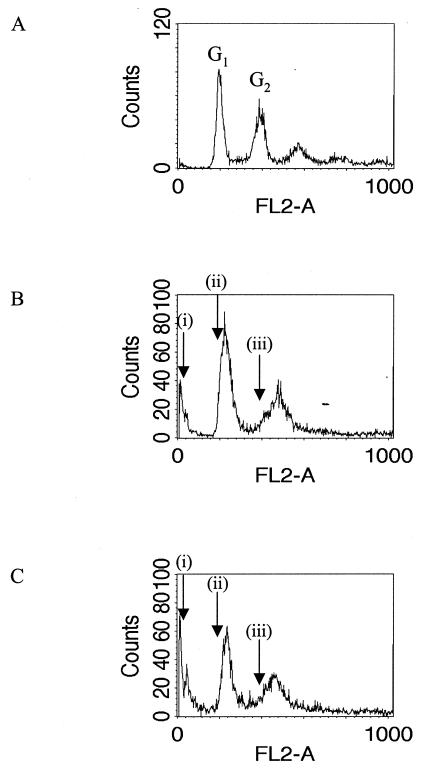
Cells were seeded at 106 cells/ml in medium and grown for 3 days at 25°C in the presence of 0.1% DMSO (Fig. 3A) (control), 10 μM 97/657 (Fig. 3B), or 10 μM 98/146 (Fig. 3C) and then subjected to flow cytometry analysis to determine the DNA content of the cells. The cell density of the control cultures had increased to >107 cells/ml, but the cultures incubated with the indirubins remained static at approximately 106 cells/ml. When control cultures were analyzed, a characteristic profile was obtained, with two peaks of fluorescence intensity corresponding to the cells being in either the G1 (DNA content of two nuclei [2N]) or G2 (4N DNA content) phase of the cell cycle (Fig. 3A). Parasites that had been incubated with the indirubins (Fig. 3B and C) displayed an elevated DNA content (an increase of ∼10%) in both the G1 and G2 peaks. In addition, a peak of very low DNA content was observed (Fig. 3B and C). This peak correlates with the increased incidence of cells with no nucleus and one kinetoplast (zoids) in the parasite population in the presence of these compounds (see below). Similar flow cytometry profiles for zoids have been demonstrated in the closely related kinetoplastid Trypanosoma brucei (15).
FIG. 3.
Flow cytometry analysis of L. mexicana promastigotes exposed to indirubins. Cells were seeded at 106 cells/ml in medium and grown for 3 days at 25°C in the presence of 0.1% DMSO (A), 10 μM 97/657 (B), or 10 μM 98/146 (C). Arrows indicate the positions of the G1 and G2 peaks in the controls, labeled (ii) and (iii), respectively. Arrows labeled (i) indicate the peak of low DNA content that correlates with an increased incidence of zoids in the parasite population (see Fig. 4).
DAPI.
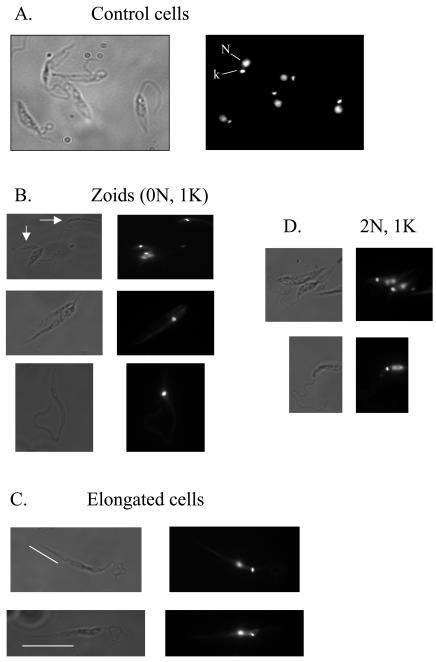
When L. mexicana mid-log promastigotes were fixed and stained with DAPI and then examined by fluorescence microscopy, two discrete stained bodies were observed, corresponding to the nucleus and the kinetoplast (Fig. 4A). When parasites incubated with indirubins were similarly treated, a high proportion of aberrant cells was observed (Table 4; Fig. 4B and C), especially zoids. There was also a significant increase in the proportion of cells with a 2N 1 kinetoplast (1K) karyotype (Fig. 4D). There was also a high proportion of aberrantly shaped cells, where the posterior part of the cell body, between the tip of the cell and the nucleus, was particularly elongated (Fig. 4C). The presence of zoids and other aberrant cells is strongly indicative of a disrupted and disorganized cell cycle in these parasites, consistent with the inhibition of a CDK.
FIG. 4.
Karyotype of L. mexicana promastigotes treated with inhibitors. L. mexicana promastigotes, grown in culture, were fixed and stained with DAPI. (A) Normal cells are predominantly 1N 1K. Parasites incubated with indirubin 3′ monoxime had an increased incidence of zoid cells (0N 1K) (B), cells with an aberrant elongated morphology (C), and 2N 1K cells (D). Bars highlight the elongated posterior portion of the cells.
TABLE 4.
Analysis of DAPI-stained L. mexicana exposed to the indirubin, 97/657
| DMSO (control) (198 cells)
|
97/657 (2 days) (289 cells)
|
97/657 (4 days) (337 cells)
|
|||
|---|---|---|---|---|---|
| Karyotype | % | Karyotype | % | Karyotype | % |
| 1N 1K | 97 | 1N 1K | 78 | 1N 1K | 76 |
| 2N 1K | 1 | 2N 1K | 11 | 2N 1K | 8 |
| 1N 0K | 1.5 | 1N 0K | 0.7 | 1N 0K | 2 |
| Zoid | 0.5 | Zoid | 8 | Zoid | 12.5 |
| Others | Others | 2 | Others | 1.2 | |
DISCUSSION
The data presented in this work show that CDK inhibitors have potent antileishmanial activity (Table 1) and that disruption of cell cycle progression has an irreversible catastrophic effect on Leishmania cell structure and morphology (Fig. 2 and 4). The rationale for carrying out an inhibitor screen against CRK3 was the finding that the gene encoding CRK3 is essential (16). CRK3 is highly homologous to the CDKs from other eukaryotes that play crucial roles in the regulation of cell division. Hence, one might expect that inhibition of CRK3 would prevent parasite replication and thus impair leishmanial infection. Indeed, CRK3 inhibitors did cause growth arrest in culture and did inhibit macrophage infection in vitro, thus providing further evidence that CRK3 is a valid drug target in Leishmania.
For activity, CDKs require association with a regulatory subunit protein, a cyclin, and must have the correct phosphorylation status (dephosphorylated in the ATP-binding pocket and phosphorylated on the T-loop residue). Thus, it is problematic to express fully active recombinant CDKs in heterologous systems. To circumvent these difficulties, we developed a transgenic L. mexicana cell line (13), in which we introduced an episome encoding a six-histidine-tagged version of CRK3. The advantages of this system are that the CRK3 is expressed in its natural host cell, which should minimize the risk of the protein misfolding frequently seen when expressing recombinant proteins in heterologous systems, for example Escherichia coli. In addition, the kinase will bind its natural cyclin partner and will be exposed to the natural processing and modifying enzymes, such as activating kinases and phosphatases. Clearly, when we wish to use the purified enzyme to screen for inhibitors as potential new drug leads, it is important that the enzyme activity is the same as that which occurs in vivo.
This novel approach has application in the expression and purification of active CDK complexes from Leishmania, as well as the isolation of other parasite proteins that are difficult to express in heterologous systems, for example, multicomponent complexes and proteins for which posttranslational modification is important for activity. Indeed, recently the use of a nonpathogenic Leishmania species has been put forward as a suitable alternative to Pichia- and baculovirus-infected insect cells for the expression of posttranslationally modified eukaryotic proteins (7).
The main aim of this work was to prove that screening a chemical library against CRK3 in vitro would identify compounds with antileishmanial activity—not just preventing parasite replication in vitro but also impairing the parasite's viability within macrophages. A large proportion (59%) of the compounds identified in the original screen as potent CRK3his inhibitors displayed antileishmanial activity in the in vitro infection model, thus substantiating our hypothesis.
The CRK3 inhibitors with antileishmanial activity fell into four chemical classes. (i) Group one consisted of the 2,6,9-trisubstituted purines, including the C-2-alkynylated purines. Many of the known CDK-specific kinase inhibitors belong to this group of chemical compounds: olomoucine (17), roscovitine (29), and purvalanol B (14). (ii) Group two consisted of the indirubins. Indirubin was originally discovered as the active component in a traditional Chinese medicine for the treatment of leukemia (18). Indirubin derivatives can inhibit CDK and GSK-3 kinases (10, 25, 28) and possess antitumor activity (28). (iii) Group three consisted of the paullones, which were originally discovered by screening the National Cancer Institute Human Tumor Cell Line Anti-Cancer Drug Screen data for compounds with patterns of activity similar to those of the known CDK inhibitor flavopiridol (42) and which are also inhibitors of CDKs and GSK-3 kinases (23, 26, 42). Our laboratory has already shown that flavopiridol inhibits CRK3 and causes cell cycle arrest at the G2/M phase of the cell cycle in L. mexicana (16). (iv) Group four consisted of the derivatives of staurosporine, the nonspecific kinase inhibitor. Both the paullones and the staurosporine derivatives displayed toxicity toward the host macrophage cells.
The most potent CRK3 inhibitors discovered in the original screen were both indirubins (compounds 97/344 and 98/516), the IC50s of which were 47 and 16.5 nM, respectively. Hence, we decided to study the indirubins in more detail. Four indirubins displayed antileishmanial activity in the infection model in vitro, and two (97/657 and 98/146) inhibited both promastigote and axenic amastigote growth in culture.
When the cellular effects of incubating L. mexicana promastigotes with these indirubins were examined, it was found that they exerted a number of effects consistent with disruption of cell cycle control. Compounds 97/657 (indirubin 3′ monoxime) and 98/146 (5-iodoindirubin 3′ monoxime) exerted a growth arrest that was more pronounced if the cells used to inoculate the culture were in stationary phase. Cultures of L. mexicana that are allowed to grow to stationary phase undergo metacyclogenesis (4) and are thought to arrest in the G1 phase of the cell cycle. Thus, parasites that have been synchronized in G1 and then exposed to indirubins do not appear to be able to overcome this cell cycle block and the growth inhibition is complete.
Two different methods were employed to study parasites exposed to indirubins: flow cytometry analysis and microscopy of DAPI-stained cells. Flow cytometry analysis indicates the changes within the population as a whole and showed two things. (i) An increase in a population of parasites with very low DNA content was observed. This peak correlates with the increased incidence of anucleate or zoid cells observed by microscopy. Similar flow cytometry profiles for zoids have been demonstrated in the closely related kinetoplastid T. brucei (15). Low DNA content peaks are also observed in cells undergoing apoptosis (41), but since there is a direct correlation between this peak and the increased incidence of cells with a low but defined (i.e., 1K) DNA content, rather than cells in which their DNA is being degraded, this would seem to be the most appropriate explanation. Anucleate or zoid cells are abnormal and must have arisen from an aberrant cell division event. (ii) An elevated DNA content in both G1 and G2 peaks compared to controls was observed. The positions of these peaks are dictated by the size of the genome: G1, corresponding to cells with one copy of each of the diploid chromosomes (a 2N DNA content), and G2, corresponding to cells in which the DNA has been replicated but not yet segregated between two daughter cells (a 4N DNA content). As can be seen from the flow cytometry spectra (Fig. 3), there is variation within the population, with a spread of DNA contents around a peak value for both G1 and G2. The movement of the peak position to a higher DNA content may indicate some genomic instability within the parasite population.
One possible explanation for this phenomenon may be that the checkpoint that ensures the genome is replicated only once per cell cycle may be impaired. In Saccharomyces cerevisiae, CDKs are required for initiation of replication but also prevent rereplication during the same cell cycle. Prereplicative complexes (PRCs) can form only at replication origins in the absence of CDKs, in late mitosis and G1, leaving the replication origin ready to initiate DNA replication when the cell enters S phase (5) The PRCs are then removed from the DNA after it has been duplicated, and this mechanism ensures that DNA is replicated only once per cell cycle (5). Normally, an essential component for the PRC, Mcm4, is found in the nucleus only in the absence of CDK activity and is exported into the cytoplasm during S and G2 phases by a CDK-dependent process (24). Inhibition of CDKs prevents Mcm4 being exported from the nucleus (24) and allows PRCs to form throughout the cell cycle. Random rereplication of chromosomes would result in genome instability and could account for the shift in the G1 and G2 DNA content peaks observed when L. mexicana promastigotes were incubated with the indirubin 97/657.
Microscopy of DAPI-stained cells also reveals that parasites exposed to compound 97/657 have aberrant morphologies, with a high proportion of cells having diffuse nuclei, an increased incidence of zoids, and abnormally elongated cells. There was also an increased incidence of cells with the karyotype 2N 1K. In Leishmania, nuclear division occurs prior to kinetoplast segregation (32), and 2N 1K cells do occur naturally in a normal parasite population. However, the increased proportion of 2N 1K cells may indicate a block or delay at this cell cycle stage. Alternatively, these cells may have arisen from an abnormal cell division in which one daughter cell receives two nuclei and one kinetoplast (2N 1K) and the other daughter cell receives one kinetoplast (0N 1K). This explanation would also account for the increased incidence of zoids within the parasite population.
Taken together with the results from the flow cytometry analysis, these results suggest that the regulatory mechanisms by which cells normally faithfully duplicate and segregate their DNA (both nuclear and kinetoplast) between daughter cells have been disrupted and that other functions of CDKs (for example, morphogenesis) are also disrupted. Thus, all the effects observed when cells are exposed to the indirubin 97/657 were consistent with the deregulation of cell cycle control and the inhibition of a CDK. A similar phenotype, with disruption of the cell cycle and an increased incidence of zoids, was observed in procyclic T. brucei, when RNA interference was used to inhibit the function the CRK3/CYC6 kinase (15). Thus, when we incubate Leishmania with indirubins, we obtain a phenotype consistent with inhibition of CRK3 and similar to that observed when the CRK3 mitotic kinase is selectively down-regulated in T. brucei (15), implying that CRK3 may well be the intracellular target for the indirubins. However, we have not formally identified the target of any of these compounds. We are pursuing several approaches, including affinity chromatography (20, 22), to determine their intracellular targets.
None of the compounds active against the parasite displayed selectivity toward the parasite kinase. It is promising, however, that these inhibitors were still active against the parasite in vitro. Plus, the fact that there were several inhibitors whose IC50 toward CDK1/cyclin B was much lower than those of CRK3his (for example, 98/126) indicates that there are significant differences between the mammalian and parasite protein kinases which could be exploited. It could be argued that the search for a selective inhibitor should have been paramount. However, prior to this investigation, although we had demonstrated that CDK inhibitors could prevent parasite replication in vitro, we had not shown that this would translate into a deleterious effect on macrophage infection. Now that we have established that CRK3 inhibitors can indeed diminish Leishmania viability within macrophages, we can focus on the design or discovery of selective inhibitors. A combination of more-extensive screening and targeted drug design will be used in the future in the hunt for parasite-selective inhibitors. Active-site modeling or X-ray crystallography of the parasite kinase to determine the structural differences in the ATP-binding pocket, in combination with detailed structure-activity analysis, will be used to design parasite-specific kinase inhibitors.
Acknowledgments
This work was supported by the Medical Research Council and the Robertson Trust.
J.C.M. is an MRC Senior Research Fellow.
We thank Nathaniel Gray, Peter Schultz, and Michel Legraverend for providing the purine derivatives and Conrad Kunick for providing of the paullones.
REFERENCES
- 1.Alvar, J., C. Cañavate, B. Gutiérrez-Solar, M. Jiménez, F. Laguna, R. López-Vélez, R. Molina, and J. Moreno. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arana, B., N. Rizzo, and A. Diaz. 2001. Chemotherapy of cutaneous leishmaniasis: a review. Med. Microbiol. Immunol. 190:93-95. [DOI] [PubMed] [Google Scholar]
- 3.Barrett, M. P., J. C. Mottram, and G. H. Coombs. 1999. Recent advances in identifying and validating drug targets in trypanosomes and leishmanias. Trends Microbiol. 7:82-88. [DOI] [PubMed] [Google Scholar]
- 4.Bates, P. A., and L. Tetley. 1993. Leishmania mexicana: induction of metacyclogenesis by cultivation of promastigotes at acidic pH. Exp. Parasitol. 76:412-423. [DOI] [PubMed] [Google Scholar]
- 5.Blow, J. J., and T. A. Prokhorova. 1999. Saying a firm ‘no’ to DNA re-replication. Nat. Cell Biol. 1:E175-E177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgne, A., A. C. Ostvold, S. Flament, and L. Meijer. 1999. Intra-M phase-promoting factor phosphorylation of cyclin B at the prophase/metaphase transition. J. Biol. Chem. 274:11977-11986. [DOI] [PubMed] [Google Scholar]
- 7.Breitling, R., S. Klingner, N. Callewaert, R. Pietrucha, A. Geyer, G. Ehrlich, R. Hartung, A. Müller, R. Contreras, S. M. Beverley, and K. Alexandrov. 2002. Non-pathogenic trypanosomatid protozoa as a platform for protein research and production. Protein Express. Purif. 25:209-218. [DOI] [PubMed] [Google Scholar]
- 8.Brizuela, L., G. Draetta, and D. Beach. 1989. Activation of human cdc2 protein as a histone h-1 kinase is associated with complex-formation with the p62 subunit. Proc. Natl. Acad. Sci. USA 86:4362-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryceson, A. 2001. Current issues in the treatment of visceral leishmaniasis. Med. Microbiol. Immunol. 190:81-84. [DOI] [PubMed] [Google Scholar]
- 10.Damiens, E., B. Baratte, D. Marie, G. Eisenbrand, and L. Meijer. 2001. Anti-mitotic properties of indirubin-3′-monoxime, a CDK/GSK-3 inhibitor: induction of endoreplication following prophase arrest. Oncogene 20:3786-3797. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, C., A. Voss, and J. Engel. 2001. Development status of miltefosine as first oral drug in visceral and cutaneous leishmaniasis. Med. Microbiol. Immunol. 190:85-87. [DOI] [PubMed] [Google Scholar]
- 12.Fischer, L., J. Endicott, and L. Meijer. 2003. Cyclin-dependent kinase inhibitors, p. 235-248. In L. Meijer, A. Jézéquel, and M. Roberge (ed.), Cell cycle regulators as therapeutic targets. Progress in cell cycle research, vol. 5. Editions “Life in Progress,” Station Biologique de Roscoff, Roscoff, France. [PubMed] [Google Scholar]
- 13.Grant, K. M., P. Hassan, J. S. Anderson, and J. C. Mottram. 1998. The crk3 gene of Leishmania mexicana encodes a stage-regulated cdc2-related histone H1 kinase that associates with p12cks1. J. Biol. Chem. 273:10153-10159. [DOI] [PubMed] [Google Scholar]
- 14.Gray, N. S., L. Wodicka, A. H. Thunnissen, T. C. Norman, S. J. Kwon, F. H. Espinoza, D. O. Morgan, G. Barnes, S. Leclerc, L. Meijer, S. H. Kim, D. J. Lockhart, and P. G. Schultz. 1998. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281:533-538. [DOI] [PubMed] [Google Scholar]
- 15.Hammarton, T. C., J. Clark, F. Douglas, M. Boshart, and J. C. Mottram. 2003. Stage-specific differences in cell cycle control in Trypanosoma brucei revealed by RNA interference of a mitotic cyclin. J Biol. Chem. 278:22877-22886. [DOI] [PubMed] [Google Scholar]
- 16.Hassan, P., D. Fergusson, K. M. Grant, and J. C. Mottram. 2001. The CRK3 protein kinase is essential for cell cycle progression of Leishmania mexicana. Mol. Biochem. Parasitol. 113:189-198. [DOI] [PubMed] [Google Scholar]
- 17.Havlícek, L., J. Hanus, J. Vesely, S. Leclerc, L. Meijer, G. Shaw, and M. Strnad. 1997. Cytokinin-derived cyclin-dependent kinase inhibitors: synthesis and cdc2 inhibitory activity of olomoucine and related compounds. J. Med. Chem. 40:408-412. [DOI] [PubMed] [Google Scholar]
- 18.Hoessel, R., S. Leclerc, J. A. Endicott, M. E. M. Nobel, A. Lawrie, P. Tunnah, M. Leost, E. Damiens, D. Marie, D. Marko, E. Niederberger, W. C. Tang, G. Eisenbrand, and L. Meijer. 1999. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1:60-67. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa, M., T. Okabe, H. Ogino, H. Matsumoto, I. Suzuki-Takahashi, T. Kokubo, H. Higashi, S. Saitoh, Y. Taya, H. Yasuda, Y. Ohba, S. Nishimura, N. Tanaka, and A. Okuyama. 1993. Butyrolactone I, a selective inhibitor of cdk2 and cdc2 kinase. Oncogene 8:2425-2432. [PubMed] [Google Scholar]
- 20.Knockaert, M., N. Gray, E. Damiens, Y.-T. Chang, P. Grellier, K. M. Grant, D. Fergusson, J. C. Mottram, M. Soete, K. Le Roch, C. Doerig, P. G. Schultz, and L. Meijer. 2000. Intracellular targets of cyclin-dependent kinase inhibitors: identification by affinity chromatography using immobilised inhibitors. Chem. Biol. 7:411-422. [DOI] [PubMed] [Google Scholar]
- 21.Knockaert, M., P. Greengard, and L. Meijer. 2002. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol. Sci. 23:417-425. [DOI] [PubMed] [Google Scholar]
- 22.Knockaert, M., K. Wieking, S. Schmitt, M. Leost, K. M. Grant, J. C. Mottram, C. Kunick, and L. Meijer. 2002. Intracellular targets of paullones: Identification following affinity purification on immobilized inhibitor. J. Biol. Chem. 277:25493-25501. [DOI] [PubMed] [Google Scholar]
- 23.Kunick, C., C. Schultz, T. Lemcke, D. W. Zaharevitz, R. Gussio, R. K. Jalluri, E. A. Sausville, M. Leost, and L. Meijer. 2000. 2-Substituted paullones: CDK1/cyclin B-inhibiting property and in vitro antiproliferative activity. Bioorg. Med. Chem. Lett. 10:567-569. [DOI] [PubMed] [Google Scholar]
- 24.Labib, K., J. F. Diffley, and S. E. Kearsey. 1999. G1-phase and B-type cyclins exclude the DNA-replication factor Mcm4 from the nucleus. Nat. Cell Biol. 1:415-422. [DOI] [PubMed] [Google Scholar]
- 25.Leclerc, S., M. Garnier, R. Hoessel, D. Marko, J. A. Bibb, G. L. Snyder, P. Greengard, J. Biernat, Y. Z. Wu, E. M. Mandelkow, G. Eisenbrand, and L. Meijer. 2001. Indirubins inhibit glycogen synthase kinase-3β and CDK5/P25, two protein kinases involved in abnormal tau phosphorylation in Alzheimer's disease: a property common to most cycline-dependent kinase inhibitors? J. Biol. Chem. 276:251-260. [DOI] [PubMed] [Google Scholar]
- 26.Leost, M., C. Schultz, A. Link, Y. Z. Wu, J. Biernat, E. M. Mandelkow, J. A. Bibb, G. L. Snyder, P. Greengard, D. W. Zaharevitz, R. Gussio, A. M. Senderowicz, E. A. Sausville, C. Kunick, and L. Meijer. 2000. Paullones are potent inhibitors of glycogen synthase kinase-3β and cyclin-dependent kinase 5/p25. Eur. J. Biochem. 267:5983-5994. [DOI] [PubMed] [Google Scholar]
- 27.Losiewicz, M. D., B. A. Carlson, G. Kaur, E. A. Sausville, and P. J. Worland. 1994. Potent inhibition of CDC2 kinase activity by the flavonoid L86-8275. Biochem. Biophys. Res. Commun. 201:589-595. [DOI] [PubMed] [Google Scholar]
- 28.Marko, D., S. Schätzle, A. Friedel, A. Genzlinger, H. Zankl, L. Meijer, and G. Eisenbrand. 2001. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br. J. Cancer 84:283-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijer, L., A. Borgne, O. Mulner, J. P. J. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 30.Meijer, L., and E. Raymond. 2003. Roscovitine and other purines as kinase inhibitors. From starfish oocytes to clinical trials. Acc. Chem. Res. 36:417-425. [DOI] [PubMed] [Google Scholar]
- 31.Mettey, Y., M. Gompel, V. Thomas, M. Garnier, M. Leost, I. Ceballos-Picot, M. Noble, J. Endicott, J. M. Vierfond, and L. Meijer. 2003. Aloisines, a new family of CDK/GSK-3 inhibitors. SAR study, crystal structure in complex with CDK2, enzyme selectivity, and cellular effects. J. Med. Chem. 46:222-236. [DOI] [PubMed] [Google Scholar]
- 32.Milligan, K. 1996. Cell cycle, growth and differentiation in Trypanosoma brucei and Leishmania species. Ph.D. thesis. University of Glasgow, Glasgow, United Kingdom.
- 33.Moreno, S., J. Hayles, and P. Nurse. 1989. Regulation of p34cdc2 protein kinase during mitosis. Cell 58:361-372. [DOI] [PubMed] [Google Scholar]
- 34.Mottram, J. C., and K. M. Grant. 1996. Leishmania mexicana p12cks1, a functional homologue of fission yeast p13suc1, associates with a stage-regulated histone H1 kinase. Biochem. J. 316:833-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mottram, J. C., J. Kinnaird, B. R. Shiels, A. Tait, and J. D. Barry. 1993. A novel CDC2-related protein kinase from Leishmania mexicana, lmmCRK1, is post-translationally regulated during the life-cycle. J. Biol. Chem. 268:21044-21051. [PubMed] [Google Scholar]
- 36.Pan, Z.-Q., and J. Hurwitz. 1993. Reconstitution of cyclin-dependent cdc2 and cdk2 kinase activities in vitro. J. Biol. Chem. 268:20433-20442. [PubMed] [Google Scholar]
- 37.Pavletich, N. P. 1999. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J. Mol. Biol. 287:821-828. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Victoria, J. M., F. J. Perez-Victoria, A. Parodi-Talice, I. A. Jimenez, A. G. Ravelo, S. Castanys, and F. Gamarro. 2001. Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob. Agents Chem. 45:2468-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundar, S. 2001. Treatment of visceral leishmaniasis. Med. Microbiol. Immunol. 190:89-92. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Y. X., K. Dimitrov, L. K. Garrity, S. Sazer, and S. M. Beverley. 1998. Stage-specific activity of the Leishmania major CRK3 kinase and functional rescue of a Schizosaccharomyces pombe cdc2 mutant. Mol. Biochem. Parasitol. 96:139-150. [DOI] [PubMed] [Google Scholar]
- 41.Yang, Y., S. Zhao, and J. Song. 2004. Caspase-dependent apoptosis and -independent poly(ADP-ribose) polymerase cleavage induced by transforming growth factor beta1. Int. J. Biochem. Cell Biol. 36:223-234. [DOI] [PubMed] [Google Scholar]
- 42.Zaharevitz, D. W., R. Gussio, M. Leost, A. M. Senderowicz, T. Lahusen, C. Kunick, L. Meijer, and E. A. Sausville. 1999. Discovery and initial characterization of the paullones, a novel class of small-molecule inhibitors of cyclin-dependent kinases. Cancer Res. 59:2566-2569. [PubMed] [Google Scholar]