Abstract
Asymmetric ABC (ATP-binding cassette) transporters make up a significant proportion of this important superfamily of integral membrane proteins. These proteins contain one canonical (catalytic) ATP-binding site and a second atypical site with little enzymatic capability. The baker’s yeast (Saccharomyces cerevisiae) Pdr5 multidrug transporter is the founding member of the Pdr subfamily of asymmetric ABC transporters, which exist only in fungi and slime moulds. Because these organisms are of considerable medical and agricultural significance, Pdr5 has been studied extensively, as has its medically important homologue Cdr1 from Candida albicans. Genetic and biochemical analyses of Pdr5 have contributed important observations that are likely to be applicable to mammalian asymmetric ABC multidrug transporter proteins, including the basis of transporter promiscuity, the function of the non-catalytic deviant ATP-binding site, the most complete description of an in vivo transmission interface, and the recent discovery that Pdr5 is a molecular diode (one-way gate). In the present review, we discuss the observations made with Pdr5 and compare them with findings from clinically important asymmetric ABC transporters, such as CFTR (cystic fibrosis transmembrane conductance regulator), Cdr1 and Tap1/Tap2.
Keywords: asymmetric ABC transporter, molecular diode, Pdr5
INTRODUCTION: DISCOVERY OF Pdr5
The PDR5 gene was first identified as aDNA sequence that caused hyper resistance to cycloheximide and sulfometuron methyl when a high-copy-number plasmid bearing the gene was overexpressed in WT (wild-type) yeast cells [1]. Mutants that contained a disruption of the gene were hypersensitive to these drugs relative to an isogenic WT control. Two years later, Pdr5 was found to be transcriptionally regulated by Pdr1 [2], a zinc-finger transcription factor that was previously cloned and characterized [3]. The PDR5 sequence later appeared in at least three other genetic screens [4–6]. These initial studies were carried out before the era of rapid DNA sequencing, and it was not until 1994 that two laboratories published the complete sequence of Pdr5 and established that it was a member of the gigantic ABC (ATP-binding cassette) family of transporters [6,7]. At roughly the same time, Leonard, Rathod and Golin [8] used a [3H]chloramphenicol whole-cell assay to demonstrate that a null mutant of Pdr5 exhibited markedly reduced drug efflux. HPLC analysis showed that when WT Pdr5 transported the drug, it was unmodified [8]. Kolaczkowski et al. [9] observed that a deletion of Pdr5 had tremendously diminished R6G (rhodamine 6G) efflux capability. This study also demonstrated that Pdr5-deficient cells were hypersensitive to many anticancer agents, much like the mammalian drug efflux pump P-gp (P-glycoprotein)/ABCB1.
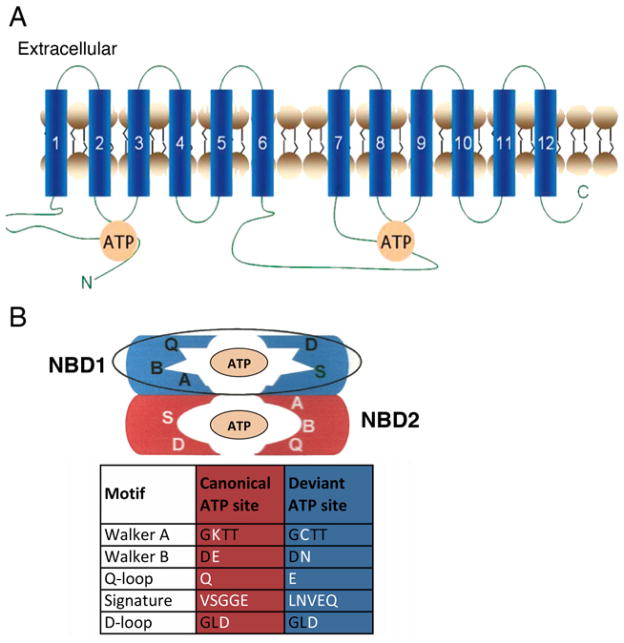
As many genomes began to be sequenced and thousands of ABC transporters were identified, it became clear that Pdr5 is a horse of a different colour. It defines its own subfamily of ABC transporters present only in fungi and slime moulds [10]. The Pdr subfamily is highly asymmetric and has a characteristic set of NBD (nucleotide-binding domain) alterations, a reverse orientation of the standard arrangement of NBDs and TMDs (transmembrane domains), and a highly unusual set of strikingly short ICLs (intracellular loops) reminiscent of some of the prokaryotic ABC importers. One Pdr5 ATP-binding site is entirely canonical and catalytic; the other is atypical and non-catalytic. A schematic diagram of the proposed secondary structure of Pdr5 and its ATP-binding sites is found in Figure 1. It is worth comparing the asymmetry of Pdr5 with non-fungal asymmetric transporters. Hohl et al. [11] offer a useful alignment of many asymmetric ABC transporters (see Figure S3), although they do not include Pdr5. These sequences follow a common pattern. In the first NBD, the Walker B catalytic glutamate residue is replaced [often by aspartate, in CFTR (cystic fibrosis transmembrane conductance regulator) by serine], and the conserved H-loop histidine residue is changed to glutamine or serine. In the second NBD, the C-loop glycine is changed to alanine, valine, glutamine or histidine. In both NBDs, the completely conserved residues of the Q-loop and Walker A motifs are unaltered. Therefore the first ATP site is atypical and the second is canonical. Given the similarity of the changes in these NBDs, it is plausible that these transporters all descend from a primordial duplication of a prokaryotic gene that was passed along through evolution. The fungal Pdr subfamily, however, has an entirely different pattern (Figure 1). Pdr5 underwent a minimum of 11 nucleotide substitutions to interchange the completely conserved residues from canonical to deviant in the same set of motifs. Remarkably, the Walker A lysine-to-cysteine alteration required three nucleotide substitutions, and an entire C-loop was interchanged (Figure 1B). Nothing like this has been observed in any other ABC transporter subfamily. The origin of the Pdr subfamily was apparently a complex independent evolutionary event.
Figure 1. Secondary structure of Pdr5 and organization of the NBDs into canonical and deviant ATP sites.
(A) Schematic diagram of the organization of Pdr5. NBD1 is followed by TMD1 composed of six α-helices followed by NBD2 and TMD2 (also made of six helices). (B) Pdr5 is an asymmetric transporter with one canonical ATP site made up of the Walker A and B motifs and Q-loop region from NBD2 and the C- and D-loop from NBD1. The second site is atypical (Walker A and B motifs and Q-loop from NBD1, C- and D-loop from NBD2) and its Walker B motif lacks a catalytic glutamate residue. This pattern of alterations is found only in the Pdr ABC subfamily of fungal transporters.
The Pdr subfamily is clinically significant. Clinical pathogens such as Candida albicans become multidrug-resistant by overexpressing Pdr5 homologues such as Cdr1 [12]. Similar multidrug resistance has been observed in Saccharomyces cerevisiae and cancer cells, which develop resistance to a variety of chemically dissimilar compounds, including anticancer drugs, primarily through the overexpression of the ABC drug transporters P-gp, ABCG2 and MRP1 (multidrug-resistance protein 1) [13,14].
Pdr5 research has made major original contributions to the study of ABC transporters, including the basis of substrate specificity [15–17], the path of the transmission interface between the ATP-and drug-binding sites [18–21], the biochemical role of deviant ATP-binding sites [22–24], and the probable gating or diode function [25]. In the present review, we focus on these important contributions. The observations made with Pdr5 are compared with other well-studied ABC transporters.
IMPORTANT FEATURES OF THE Pdr5 TRANSPORTER
Substrate size as a major basis for the tremendous promiscuity of Pdr5
Studies with Pdr5 [15–17] were among the first to systematically explore the chemical basis of multidrug transporter promiscuity. A large study indicated that Pdr5 mediates drug resistance to hundreds of different compounds [26]. Furthermore, there is some substrate overlap with the two other major Saccharomyces ABC drug transporters, Yor1 and Snq2. To explore the issue of promiscuity, our group obtained or synthesized xenobiotic compounds whose structures were systematically varied [15,16]. These included sets of trialkyltin chlorides, imidazole derivatives and tetra-alkyltins. Their chemical features were clearly different, but each series included a similar range in molecular volume. The relative Pdr5 substrate strength was determined from the ratio of the minimum inhibitory concentrations observed in the WT strain and an isogenic pdr5 deletion mutant (Δpdr5). A high ratio was indicative of a strong substrate, and a ratio of 1.0 indicated that the compound was not a substrate. These studies led to several important observations. First, tetra-alkyltin compounds were effective substrates even though they contained neither aromatic nor electron-pair donor groups. This observation suggested that substrate requirements were not the same for Pdr5 as for P-gp [27]. Secondly, we made the striking observation that, regardless of chemistry, the molecular volume rather than the hydrophobicity of a compound was the critical factor in determining substrate strength. Thus, although the compounds were generally lipophilic, two compounds with the same size but with substantial differences in log P had similar substrate strength. Strong substrates had surface volumes of 200–300 Å3 (1 Å = 0.1 nm). Above this volume, the decline in substrate capability was quite gradual. Compounds of less than 90 Å3, however, had minimum inhibitory concentration ratios of 1.0, which indicated that Pdr5 played little or no role in mediating transport. Finally, various transport and binding assays established that Pdr5 employs multiple drug-binding regions, much like P-gp [28]. A similar result was soon found for the Cdr1 transporter [29].
Significantly, work with P-gp on substrate specificity also pointed to a relationship between substrate size and transport. Loo and Clark [28] demonstrated that the ability of methanesulfonate derivatives to stimulate P-gp ATPase activity was size-dependent, with a 10–13-Å length optimum. Sauna et al. [30] looked at the effects of bivalent heterodimers of stipiamide on transport and [125I] iodoarylazidoprazosin photo affinity-labelling capability. The size of these substrates was systematically varied by adding poly(ethylene glycol) ether spacer groups. Plots of capability against molecular mass (not molecular volume) generated curves that were strikingly reminiscent of those reported for Pdr5 (although the minimum and optimum size values derived from molecular mass appear larger for P-gp).
Why does Pdr5 and P-gp substrate strength within a chemical series increase with size? One possibility is that the Pdr5 drug-binding pocket is a continuum of residues available for binding instead of a structure with discrete binding sites. Large compounds have more residues in the pocket with which to interact than do those with less surface volume. Larger compounds are therefore stronger substrates than are smaller ones. Compounds that are similar in structure share similar binding residues and can therefore overlap to the extent that each can serve as a binding antagonist for the other. Other pairs of compounds are dissimilar enough to bind to regions of the pocket that do not overlap, and the drugs in question do not behave antagonistically. In fact, recently, Chufan et al. [31] demonstrated that P-gp has multiple drug-binding sites for a single substrate.
The crystal structure of murine P-gp bound to cyclic hexapeptides lends support to this idea [32]. This structure shows a very large drug-binding cavity in which at least two substrate molecules are bound in regions that overlap. The drug-binding residues are primarily hydrophobic; however, at least seven polar residues are scattered throughout the pocket and interact directly with a cyclic hexapeptide. A similar arrangement is proposed from the more recent crystal structure of the Caenorhabditis elegans P-gp homologue [33]. The structural results showing the presence of scattered polar binding residues also explain how two substrates of similar strength can have substantially different log P values.
Pdr5 ATPase has high basal activity and exhibits trans-inhibition
Kolaczkowski et al. [9] first demonstrated that Pdr5-mediated transport is linked to ATP hydrolysis. The first report of Pdr5 ATPase activity demonstrated that this transporter had a strikingly high basal level and that it could hydrolyse other ribonucleotides. This activity, however, was not further stimulated to a significant degree by the addition of Pdr5 transport substrates [34]. High basal ATPase activity seems to be common in the Pdr fungal subfamily [35]. Although the initial biochemical characterization of Pdr5 ATPase properties was correct with respect to these important features, several kinetic parameters were in error. This was because, at the time DeCottignies et al. [34] performed the work, the other ABC transporters in the PM (plasma membrane) had not yet been identified, and they were later found to contribute to the enzymatic activity. A Δpdr5 control, for instance, retained significant ATPase activity.
More than 10 years later, two additional studies added important information about the ATPase activity [22,36]. Both studies demonstrated that some Pdr5 transport substrates allosterically inhibit enzyme activity. A similar phenomenon had already been documented with P-gp [37,38]. This inhibition is referred to as trans-inhibition. In an interesting review, Gupta et al. [39] speculated that this inhibition is used to lock the transporter in the outward-facing orientation when the transported toxic drug reaches a high extracellular concentration and could therefore return to the cell via the transporter’s binding pocket. This idea is an intriguing one, but it is important to note that many relatively strong Pdr5 substrates do not cause significant inhibition of the ATPase activity [20]. Furthermore, this mechanism alone was shown to be insufficient for preventing reflux of R6G during transport [25].
One of the studies also showed that, in contrast with the initial report, the UTPase and CTPase activities of Pdr5 were actually low and the Km values were quite high, suggesting that pyrimidine nucleotides were unlikely to serve a significant physiological role, at least with the WT enzyme [36]. The GTPase activity, however, was another matter. The Km of ~1 mM was within physiological range, and this enzyme activity, although not as robust as the ATPase activity, was clearly quite high. The GTPase activity was also notably more resistant to allosteric inhibition by clotrimazole and inhibition by vanadate and beryllium fluoride. This was a remarkable observation because it suggested that the conformation of the transporter was influenced by the type of bound nucleotide. Kolaczkowski et al. [9] had already demonstrated that R6G transport in vesicles was not mediated by Pdr5 GTPase activity. However, GTPase activity supported the transport of [3H]chloramphenicol in PM vesicles [36]. These observations suggest that the transport of some drugs is nucleotide-specific. It should be noted that GTP-mediated transport is not restricted to Pdr5. The P-gp, Bpt1 and ABCG5/ABCG8 transporters can effectively use this nucleotide [40–42].
In the second study, Ernst et al. [22] mutagenized some of the residues in both the canonical and the deviant ATP-binding sites to add additional important observations on the ATPase activity. They constructed and analysed mutations of the canonical and deviant Walker A residues (K911A and C199A), the catalytic carboxylate-containing residue of the canonical site (E1036A; as shown in Figure 1B, there is no equivalent glutamate residue in the deviant site) and the canonical Hloop (H1068A). As expected, the canonical Walker A and B mutations were completely transport-deficient. The deviant Walker A substitution C199A was phenotypically WT. These results strongly suggested that the deviant ATP site is non-catalytic, an observation clearly supported by further work with Pdr5 [23,24]. The big surprise, however, was the behaviour of the alanine substitution in the highly conserved His1068 of the H-loop. When the same substitution was made in two bacterial transporters, HlyB and MsbA, ATPase activity was almost completely eliminated [43,44]. In both of these transporters, a critical interaction between this histidine residue and the catalytic carboxylate-containing residue was proposed to create a catalytic dyad. The Pdr5 substitution, however, retained WT levels of ATPase activity, but had a striking R6G-specific transport deficiency. Clearly, then, the catalytic mechanisms of different ABC transporters are not always equivalent. The H1068A mutant also showed greatly increased resistance to trans-inhibition by R6G. In the light of these R6G-specific effects, Ernst et al. [22] proposed that His1068 plays a major role in the rate of conformational switching of the transporter from a drug-on to a drug-off conformation.
THE SIGNAL INTERFACE OF Pdr5 SHARES SOME FEATURES WITH ITS SYMMETRIC AND ASYMMETRIC COUNTERPARTS
A signal interface suggested by the atomic structure of the Sav1866 transporter
Complex polytopic proteins must have means of communication between multiple domains that perform distinct biochemical operations. ABC transporters are no exception. A transmission or signal interface must be established between the NBDs where ATP is bound and hydrolysed and the large drug-binding pocket located in the TMDs from which substrates are transported. The high-resolution atomic structure of the bacterial Sav1866 multidrug transporter suggested a possible pathway of signal transmission [45]. When ADP was bound to the homodimer, it adopted the outward-facing conformation. In particular, ICL2, which connects TMH (transmembrane helix) 2 and TMH3 made contact with the opposite NBDat two conserved sequences, the X-loop and the Q-loop, to create a trans conformation. ICL1 makes only limited contact with the cis NBD. Several seminal studies with the symmetric P-gp and asymmetric Tap1/Tap2 transporter (ABCB2/ABCB3) used cross-linking between cysteine-modified residues of ICL2 and the trans Q- or X-loops to lend strong support to the pathway suggested by the Sav1866 atomic structure [46,47]. Because P-gp and Tap1/Tap2 transporters are not especially close evolutionarily, these studies suggested that ABC transporters have a well-conserved transmission interface. It was not clear whether a transporter as deviant as Pdr5, with its extremely short ICLs, would even use a similar mode of transmission. Studies in our laboratory, however, proved that it did, with one major twist: Pdr5 employs a cis rather than a trans pathway.
An in vivo map of the Pdr5 transmission interface made possible by the power of yeast genetics
Yeast are amenable to several kinds of genetic analyses not readily available in mammalian cells. One of these is the ability to select suppressor mutants and thereby identify interacting residues in a biochemical pathway. This approach enabled our research group to build an in vivo map of the Pdr5 transmission interface. Fortunately, we had a loss-of-function mutant that was no longer capable of performing intradomain communication.
Ser558 lies towards the very end of TMH2 in the Pdr5 transporter. The S558Y mutant appeared in a 1995 screen of UV-mutagenized cells for survivors of this treatment that had reduced drug resistance. This mutant markedly increased hypersensitivity towards all Pdr5 transport substrates tested, including clotrimazole. Although the S558Y mutant protein was present in PM vesicles at WT levels, the mutant drug hypersensitivity phenotype closely resembled an isogenic Δpdr5 strain. Thirteen years later, further biochemical characterization was performed. The S558Y mutant protein was shown to have significant, albeit reduced, ATPase activity and WT drug-binding capability, but almost no transport function [18]. Furthermore, in contrast with the WT enzyme, the ATPase activity of the mutant was highly resistant to trans-inhibition by clotrimazole. S558Y therefore behaved as a signal-transmission-defective mutant even though it was located far away from the Q-loop and the ICLs. A plausible model proposed for S558Y is that, although Ser558 is not conserved, the large bulky tyrosine side chain in the mutant kinked TMH2 and severed the connection between ICL1 and the NBD, possibly through the Q-loop. Our research group reasoned that we could identify other residues participating in the transmission interface by plating S558Y mutant cells on medium containing a lethal concentration of clotrimazole and selecting the resulting resistant suppressor-bearing mutants.
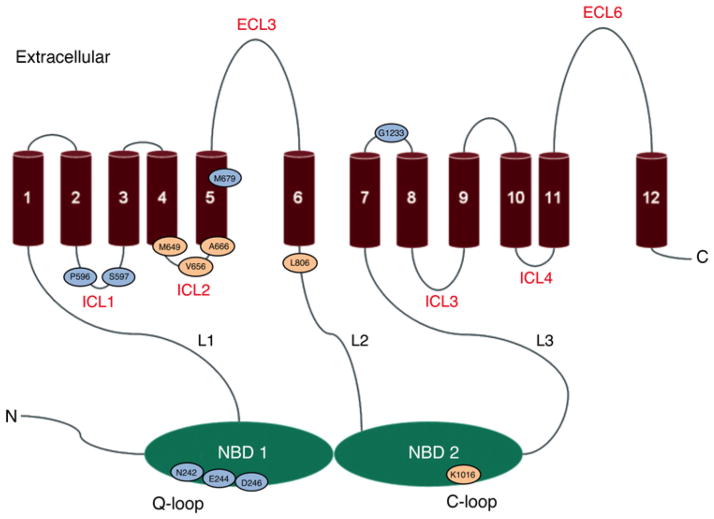
We analysed ten mutations from the suppressor screen. Their locations are shown in Figure 2. Remarkably, eight of them were in either ICL1 or the Q-loop region, including the completely conserved deviant Q-loop residue Glu244. Another, M679L, was in TMH5, which is attached to ICL2. Therefore a majority of the residues fell into regions implicated by the Sav1866 atomic structure to be involved in the transmission interface. We extensively studied several of these mutants, notably E244G and N242K [18,19]. The suppressors also re-established significant trans-inhibition.
Figure 2. Location of transmission interface residues identified using suppressor genetics.
The residues with substitution mutations that suppress the clotrimazole hypersensitivity of a S558Y strain are shown in aqua. Residues with substitutions suppressing N242K cycloheximide-hypersensitivity are shown in peach. Mutations at Asp246, Met649, Val656 and Ala666 were recovered twice and mutants at Ser597 were recovered three times. ECL, extracellular loop; ICL, intracellular loop and L1, L2 and L3 are linker regions. Figure adapted from Rutledge et al. [70]. Used by permission.
This initial collection of mutants illustrated several important principles that recurred in a subsequent screen. First, in addition to E244G, most of the other suppressor mutations occurred in highly or completely conserved residues, including Asn242, Asp246, Pro596, Met679 and Gly1233. When we uncoupled the conserved N242K and E244G mutations from S558Y and placed them in an otherwise WT background, they showed a partial loss of transport capability, even though they were present in the PM at WT levels and had significant, albeit somewhat reduced, ATPase activity [18,19]. Not surprisingly, then, these residues are essential for WT transport capability and presumably for signal transmission. Secondly, the structure of Sav1866 and the existing cross-linking studies suggested that the suppressing Q-loop residues would be in the trans canonical portion of NBD1 instead of in the deviant cis NBD2 counterpart. Thus, whereas the analysis of S558Y suppressors provided excellent in vivo evidence for the participation of the ICLs and Q-loop residues in the transmission interface, as inferred from structural studies, the cis transmission conformation was unexpected.
To try to identify additional residues in the transmission face, we took advantage of the fact that, whereas N242K increases the resistance of S558Y, it also creates a drug-hypersensitive phenotype when placed in an otherwise WT protein, much like most of the other mutants identified in this study. Thus we were looking for suppressors of a suppressing residue, a strategy we termed ping-pong genetics. To do this, we simply plated cultures of the hypersensitive N242K strain on a lethal concentration of cycloheximide and collected independent suppressors that grew up as colonies after 72–96 h [19,20].
Perhaps the most important mutant in the collection was a V656L substitution that lies in cis orientation in ICL2 with respect to the Q-loop [19,20]. Our group also constructed and tested a V656A mutant and showed that it was clearly signal-deficient. Thus two different substitutions at Val656 gave opposite phenotypes. The V656A mutant had a phenotype that was nearly identical with that of S558Y, including an ATPase that was reduced relative to WT, but markedly resistant to allosteric inhibition by clotrimazole (5-fold as resistant as the WT). These data therefore strongly suggested that, although ICL2 was a mere six or seven residues long, it still functioned as a significant part of the transmission interface, much as it does in other ABC transporters. Further evidence for the cis interface came from our observation that V656L was also a strong suppressor of the cis Q-loop drug-hypersensitive mutation E244G. We found other interesting cis-acting suppressors of N242K, notably M649I and A666G. Ala666 is a highly conserved residue in TMH5 near the boundary of ICL2. Met649 occupies a similar location in TMH4. The role of these residues in signalling is being assessed. Because ICL2 is so short, it is plausible that portions of TMH4 and TMH5 that flank it also make contact with the NBD. Significantly, work by Puri et al. [48] with the Cdr1 transporter identified alanine scan mutations in TMH5 that uncoupled ATPase activity and substrate transport.
The puzzling suppressor of N242K, however, was the K1016I substitution found towards the end of the C-loop motif of the deviant ATP site. In canonical ABC transporters, this motif is used to position the Mg-ATP complex for hydrolysis and plays no obvious signalling role in transport. Why such a substitution would appear in the N242K suppressor hunt was a mystery. However, subsequent work demonstrated that the atypical ATP site is heavily involved in signal transmission [23,24].
Our work with the collection of suppressor mutants suggested that Pdr5 employs a cis rather than a trans interface. Although no other eukaryotic efflux pumps are known to have the cis conformation, it is present in the atomic structures of several bacterial ABC importer proteins such as the Met and HI 1470/HI 1471 transporters [49,50]. The cis interface notwithstanding, the work with Pdr5 provides excellent functional evidence for residues implicated from structural studies, including those in the deviant Q-loop, ICL2 and the flanking TMHs. Our work with Pdr5 also gave rise to several criteria that seem to indicate that a residue is part of the Pdr5 signal transmission interface.
The mutants restore resistance to a known signalling-defective mutant.
A large majority of the mutants appear in places predicted by atomic structures to be part of the transmission interface.
Loss-of-function mutations have lowered, but significant, ATPase activities and often alter the allosteric inhibition to a transport substrate.
The residue is often highly conserved.
Important work has come from several other laboratories. Kueppers et al. [21] showed that atleast one transmission residue, Ser1360, also appears in the modelled drug-binding pocket. The ATPase activity of this mutant was considerably more resistant to trans-inhibition by FK506.
An important recent study by Kolaczkowski et al. [51] used a clever strategy that identified additional putative transmission interface residues in Cdr1. This group took a library of mutagenized Cdr1-encoding plasmids and transformed a Saccharomyces strain that lacked ABC multidrug transporters. They screened these transformants for gain-of-function mutations that conferred increased resistance to the Snq2 substrates, including resazurin and resorufin. They further characterized several mutants that did not affect the amount of Cdr1 in PM vesicles or the ATPase activity. These mutants were in the deviant H-loop (C363R), the canonical Walker A region (V885G), TMH1 (G521S/D) and TMH7 (1280V). Evidence for a signalling role was observed with C363R and G521D/S. The Cdr1 ATPase in these mutants was actually stimulated by addition of febuconazole. Therefore these mutants altered the stimulation signal, much like S558Y, V656L and V656A altered the trans-inhibition of Pdr5 ATPase by clotrimazole [18–20]. This study adds some important information (if we make the reasonable assumption that the interfaces of Pdr5 and Cdr1 are similar). The atomic structure of the Sav1866 transporter does not suggest a major role for either TMH1 or the extracellular loops. Because these locations were unanticipated and these mutants are all gain-of-function alleles, it will be important to see whether other substitutions at these residues create a loss-of-function phenotype that is consistent with a signal deficiency. This would establish beyond doubt that the residue is essential to the pathway.
The selective advantage of transmission interface manipulation
Classic multiple drug hyper resistance is often the result of genetic alterations that result in overexpression of ABC transporters [2,52]. Significantly, hyper resistant yeast strains that already overexpress Pdr5 can acquire even greater resistance to xenobiotic compounds through selection on medium containing a high concentration of cycloheximide [20]. We sequenced five such mutants. Three, V656L, P596L and A670S, were in regions making up the predicted Pdr5 signal interface (V656L and P596L were also represented in our suppressor mutant hunt).
Clues about how these alleles might manipulate the interface to create greater drug resistance came from our in-depth study of V656L [20]. This allele conferred broad increased resistance to most Pdr5 substrates tested. Western blotting established that the level of mutant protein in PM vesicles was no greater than in WT. Importantly, however, neither was the ATPase activity. Furthermore, whereas the V656L suppression of E244G restored drug-resistance to WT levels, the reduced level of ATPase activity seen in the E244G mutant remained. Taken together, the behaviour of V656L and the E244G/V656L double mutant suggested that the V656L substitution increased resistance in one of two ways. It is plausible that the V656L mutant increases the efficiency with which the energy from ATP binding and/or hydrolysis is used for transport. Alternatively, the mutant might use another nucleotide, such as GTP, more effectively than the WT. Direct tests of these hypotheses, which are not mutually exclusive, are in progress. Regardless of the exact mechanism, the implications of this finding are important. Under selective pressure, evolution allows for additional mechanisms that do not simply increase the steady-state level of the efflux pump in the membrane or the ATPase activity.
THE FUNCTION OF ATYPICAL ATP-BINDING SITES IN ASYMMETRIC ABC TRANSPORTERS
ABC transporters with atypical ATP sites are numerous and include clinically significant members such as Mrp1, CFTR, Sur1, Sur2A and Cdr1. In Saccharomyces, the three major drug efflux pumps (Pdr5, Yor1 and Snq2) all have an atypical and a canonical ATP site. The atypical ATP sites are also often referred to as degenerate, or deviant. Although the terms are often used interchangeably in scientific discourse, they could be employed to make a useful distinction. If replacing some or all of the atypical residues with canonical ones fails to greatly diminish transporter function, the ATP site is degenerate. It might have a demonstrable function apart from simply creating the NBD hybrid dimer, but such function probably can be accommodated by the canonical site or is unnecessary for significant activity. The Tap1/Tap2 transporter appears to be an example of this phenomenon [53]. Alternatively, if substitution of canonical residues for atypical ones causes significant loss of function, which is certainly the case with Pdr5 [24], the atypical ATP-binding site is actually deviant.
Structural studies and mutational analyses of asymmetric transporters, including Mrp1 [54], CFTR [55,56], Tap1/Tap2 [53,57], Sur1 [58], and most recently TM287/288 from Thermotoga maritima [11], led to several important conclusions. Some of these studies presented evidence that the dimer in the structure of the symmetric transporter Sav1866 is made in asymmetric transporters (or channels) [58] and that the atypical site is capable of binding nucleotides [53,54,57]. In the case of Tap1/Tap2, the deviant site actually binds ADP more effectively [53]. All of these studies also demonstrate that, in general, the atypical site is not a major contributor to catalysis [11,53–58]. This is because mutagenesis of the conserved motif residues present in the atypical sites of these transporters generally had mild effects, and the transporters retained significant function. Furthermore, in most instances, the atypical site lacks a catalytic glutamate residue. In contrast, mutagenesis of the conserved motifs in the canonical site causes a complete loss of transport capability.
The role of atypical ATP-binding sites in intradomain communication
Apart from their role in creating the NBD dimer, atypical sites are implicated in two other biochemical activities that are not necessarily mutually exclusive: NBD–TMD intradomain communication and interdomain (NBD–NBD) regulation of catalytic activity at the canonical site. With respect to the former, Procko et al. [59] proposed a model derived from structural and biochemical studies of Tap1. In this model, the binding of a peptide (the transport substrate) in the TMDs triggers nucleotide exchange of normally very tightly bound ADP for ATP in the atypical site, leading to a switch from an inward facing peptide-binding to an outward-facing peptide-releasing conformation. ATP hydrolysis leads to a return to the inward-facing arrangement. The lack of strong mutational effects at the atypical site in Tap1/Tap2 may be explained by the canonical site’s ability to achieve the same end, either by nucleotide exchange or perhaps through two sequential hydrolytic events. Indeed, when a Tap1/Tap2 protein is constructed with two canonical ATP-binding sites, it exhibits a nearly WT transport capability [53].
Work from two laboratories clearly established that the deviant ATP site of Pdr5 is heavily involved with intradomain signalling. These results suggest a model much like the one proposed for Tap1/Tap2. Two lines of evidence came from our laboratory. The first is that a K1016I substitution in the deviant C-loop motif suppressed the drug-hypersensitivity of the established signalling-deficient mutant N242K [19]. We also analysed a D-loop mutation, D1042N [23]. This substitution had almost no effect on the ATPase activity, but the mutant strain was almost completely devoid of transport capability and was profoundly hypersensitive to Pdr5 drug substrates. The mutant phenotype was therefore much like those of other signalling mutants, such as S558Y and V656A. Recently, Gupta et al. [24] used a somewhat different approach to study the deviant Pdr5ATP-binding site [24]. They replaced the deviant motifs, including the Walker Amotif, C-loop and Walker B motif, with canonical ones in stepwise fashion, assaying each alteration along the way. This was a substantial undertaking, because the fungal Pdr family has many atypical residues among the collection of well-studied ABC transporters. Replacing the deviant Walker A or C-loop regions with their canonical counterparts yielded an uncoupling phenotype more or less like D1042N (little or no loss in ATPase activity, significant loss of drug resistance and transport capability).
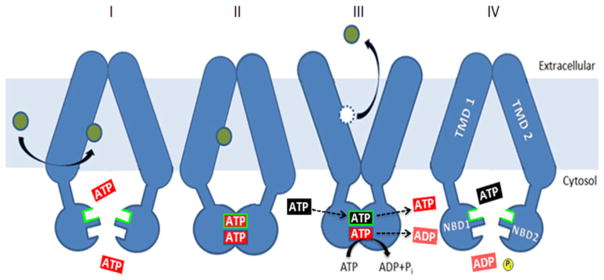
Our observations with the D1042N mutant strain and the K1016I/N242K suppressor inspired the model illustrated in Figure 3 [23]. It posits that when the deviant site exchanges nucleotide, there is intradomain communication to the TMDs at least via the deviant C-, D- and Q-loops, which causes the critical inward-facing to outward-facing shift and allows the drug substrate to be released. ATP hydrolysis at the canonical site re-establishes the drug-binding conformation. The D1042N mutation, as well as some of the replacements described by Gupta et al. [24], might inhibit the transition or perhaps adopt a novel faulty conformation that still allows canonical site ATP hydrolysis, but is ineffectual for releasing substrate. Thus this model is similar to the one proposed for Tap1/Tap2, except that Pdr5 appears to function continuously without the need for a substrate to stimulate nucleotide exchange. Because nucleotide exchange at the ATP-binding sites of Pdr5 has never been measured, this model must be regarded as speculative. There is little doubt, however, that the deviant site is at least actively involved in intradomain signalling.
Figure 3. The deviant ATP site plays a major role in intradomain signalling.
The model shown proposes that nucleotide exchange (III) at the deviant ATP site (shown as a black ATP exchanging with a red ATP) results in a conformational switch from inward-facing drug-binding (II) to outward-facing drug-releasing. Once in the outward-facing mode, ATP (red) is hydrolysed to ADP (green)+Pi, and the transporter is restored to the drug-binding inward-facing conformation (IV). Drug molecules are indicated as green circles. Figure taken from Furman et al. [23].
Atypical ATP-binding sites as regulators of ATPase activity at the canonical site
A second model of atypical site action proposes that it is a regulator of the canonical catalytic site. Interdomain regulation could, in principle, take place in two major ways. The atypical site might exert a positive or co-operative effect to allow maximum enzymatic activity. Mutations in important atypical residues would therefore diminish ATPase activity. Alternatively, the effect could be allosteric, with some loss-of-function mutants causing an actual increase in hydrolysis. The latter is supported by a recent high-resolution structural study of the TM287/288 bacterial multidrug transporter in the apo and in the AMP-PNP- (adenosine 5′-[β,γ -imido]triphosphate, a non-hydrolysable ATP derivative) bound conformation [60]. This study made several remarkable observations, but we focus strictly on the behaviour at the atypical site. The apo and AMP-PNP-bound structures differ in the interactions seen between motif residues, including the Walker A motif and the H-, D- and C-loops. Conformational changes were also observed at the Q-loop. However, no structural changes were observed with the coupling helix itself, suggesting that the atypical ATP site does not involve itself with intradomain communication. Instead, when the investigators made a D523A substitution in the Walker B motif, ATPase activity doubled relative to the WT, suggesting that this residue (presumably as well as others) acts as an allosteric regulator of the canonical catalytic site. A comparable mutation in the Lactobacillus lactis homologue LmrCD created a modest transport deficiency.
The degenerate site of CFTR may also perform an allosteric role. Ten years ago, Vergani et al. [61] presented strong evidence that dimerization of the NBDs through the binding of ATP allows the CFTR channel to open. ATP hydrolysis then closes the channel. The open state of the channel is tremendously extended by mutations that eliminate ATPase activity at the canonical site. Subsequent work by Tsai, Li and Hwang [62] demonstrated that ATP is occluded at the degenerate site for very long periods, which are equivalent to many gating cycles. ATP hydrolysis forced only the catalytic ATP site to come apart (thus closing the channel). Therefore gating was thought to be regulated by the binding and hydrolysis of ATP at the canonical site. These observations suggested that the degenerate ATP-binding site of CFTR plays a purely structural passive role. Recently, however, Csanady et al. [63] studied the properties of the degenerate site C-loop substitution H1348A. This mutation had no effect on ATP occlusion or channel burst events, but it slowed the channel-closing rate 3-fold. The data were most consistent with an allosteric model in which hydrolytic events at the canonical site bring about essential conformational changes at the deviant one. An important caveat is that there was no direct measure of ATP hydrolysis in this mutant or the WT control. Nevertheless, the data point strongly to an active role for the CFTR deviant ATP site in channel function.
In the light of these observations, an obvious question was whether the deviant ATP site of Pdr5 regulates the canonical site ATPase activity. Gupta et al. [24] demonstrated further that replacement of multiple (Walker A, Walker B, and C-loop) deviant motifs with canonical ones in Pdr5 caused a significant loss, but not a complete elimination, of both ATPase and transport functions, rather reminiscent of the E1013A and D1042E phenotypes seen by Furman et al. [23]. These mutant phenotypes can be interpreted in several ways. Gupta et al. [24] suggested that they demonstrate a regulatory role for the deviant ATP-binding site. One strong possibility is that the site is a positive regulator of the canonical site ATPase activity rather than an allosteric one. Thus the deviant site would control both intra-(NBD–TMD) and inter- (NBD–NBD) communication. These mutants, however, do not preclude an interpretation based on intradomain regulation. Thus, for example, the cycle shown in Figure 3 could be slowed by a mutation resulting in both reduced transport and ATPase activity.
The Pdr5, Cdr1 controversy
None of the many mutations in the deviant Pdr5 ATP-binding site created by Furman et al. [23] or Gupta et al. [24] completely eliminated Pdr5ATPase activity, although some clearly reduced it. Thus it is highly unlikely that the deviant ATP site plays a major catalytic role in Pdr5. Studies have found no difference in this characteristic between Pdr5 and other asymmetric transporters. This observation is at odds with reports that the deviant site of Cdr1 plays a significant catalytic role. In a series of studies, investigators proposed a novel catalytic mechanism with Cys193 (located in the Walker A motif and the equivalent of Cys199) playing a central mechanistic role ([64–66]; see Jha et al. [65] for an illustration). The most compelling data supporting a catalytic role for the deviant ATP site of Cdr1 come from the observation that several substitution mutants of Cdr1 Asp326 (Walker B) were completely ATPase and transport deficient and that Asn327 mutants had a high Km value (ATPase).
The behaviour observed in the in vivo experiments with the Walker B Cdr1 mutants can be explained in two ways. First, the Cdr1 investigators argued that Cdr1 evolved a novel catalytic activity not present in Pdr5. The problem with this idea is that Pdr5 and Cdr1 share very high identity. Perhaps more significantly, the catalytic activity and various properties of Cdr1 (NTPase activity, lack of drug stimulation, high basal activity) appear to be nearly the same as in Pdr5, which almost certainly does not support an additional major activity. The phenotypic properties of the equivalent aspartate and asparagine mutant residues in Pdr5 were not studied. However, when the deviant Walker B region was replaced by the canonical one, the Pdr5 ATPase activity was mildly uncoupled from transport [24].
An alternative explanation for the ATPase-deficient Cdr1 mutants can be posited from the model shown in Figure 3. A critical step in the essential conformational change from drug-on to drug-off is a nucleotide-exchange event at the deviant ATP site. This switch may also be mandatory for the subsequent ATPase activity that takes place at the canonical site. Thus, without nucleotide exchange and the switch to the drug-releasing outward conformation, there may be no ATP hydrolysis. Perhaps the deviant ATP site of Cdr1 also takes part in such a mechanism. If Asp326 and Asn327 are centrally involved in the nucleotide-exchange process, mutants with no ATPase or altered ATP binding could quite possibly result.
ABC TRANSPORTERS AS ONE-WAY GATES: Pdr5 IS A MOLECULAR DIODE
Once a multidrug transporter expels a drug from a cell, reflux of the toxic substrate must be prevented. The standard model of ABC transporters, which is supported by structural and biochemical studies in bacterial and mammalian systems [67,68], invokes high-affinity substrate-binding and low-affinity substrate-releasing conformations. The latter was thought to prevent rebinding of the drugs to the binding sites. Several years ago, however, Gupta et al. [39] proposed that ABC multidrug transporters are molecular diodes or one-way gates that are remodelled after drug release to preclude re-entry. One possible scenario is that key binding residues are rotated so that the binding sites collapse. In any event, it is plausible that multiple mechanisms (loss in affinity, trans-inhibition and the presence of a molecular diode) operate to keep the excluded drug substrate from returning to the cell. Recent work in our laboratory lends credence to the diode hypothesis [25].
Strong evidence that Pdr5 is a molecular diode comes from the analysis of an alanine substitution for Ser1368, which resides in TMH11. We discovered that although the S1368A mutant strain was profoundly sensitive (5–6-fold that of WT) to a set of six drugs that are transported from multiple sites or regions, this mutant had WT levels of Pdr5 in purified PM vesicles as well as WT ATPase activity and transmembrane signalling [25]. The S1368A mutant bound drugs as well as the WT counterpart.
The results of our initial R6G whole-cell transport assay were puzzling because the effect of the S1368A substitution was so mild. We initially employed the standard R6G assay used by several laboratories [9]. Thus we pre-loaded de-energized cells with R6G for 90 min and then removed the free R6G before adding glucose for transport initiation. We measured the transport retained at various intervals in mutant and WT cells. Under these conditions, transport was clearly in the direction of the R6G concentration gradient. The difference between S1368A and the WT was surprisingly small. The mutant’s efflux rate was only modestly lower than WT: the WT rate was 2.3-fold as high as the mutant’s. It was instructive to compare the efflux difference between S1368A and D1042N, a deviant signalling-deficient mutant. This alteration created a drug-hypersensitivity phenotype very much like that of S1368A. In contrast, the D1042N mutant strain exhibited an R6G efflux rate that was only 10% as high as that of the WT.
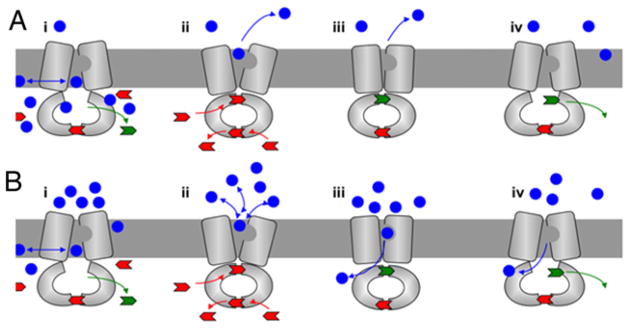
At this juncture, we began to consider the possibility that S1368A created a leak in a molecular diode. We reasoned that the R6G result could then be explained as shown in Figure 4. In the initial R6G experiment, represented in Figure 4(A), efflux along a concentration gradient means that, as the substrate exits the cell, it is immediately diluted. As a result, S1368A is more or less functional, because the effective external substrate concentration is low. However, the diode model predicted that when the external concentration was high, forcing Pdr5 to work against a gradient, the S1368A diode would leak to a much greater extent (Figure 4B).
Figure 4. Pdr5 as a molecular diode: model for the effect of the S1368A gate keeping mutant.
In the model, blue circles represent transport substrates and blue arrows show the direction of drug passage. Unhydrolysed ATP is shown in red, and ADP is shown in green. The nucleotide exchange at the deviant ATP site of Pdr5 induces a conformational switch from an inward (drug-binding) to an outward (drug-releasing) conformation. ATP hydrolysis at the canonical site allows the drug to be released and also causes remodelling of the transporter to preclude re-entry of the unwanted drug as the transporter is restored to its inward-facing conformation. When the transport is in the direction of the gradient (A) and the external concentration of the drug is relatively low, reflux because of the S1368A mutant phenotype is limited. In (B), the external drug concentration is high, transport is against a gradient and the S1368A mutant phenotype is most severe. Adapted from Mehla et al. [25].
In the next series of experiments, we simply suspended cells in a high concentration of R6G in nutrient medium and monitored the accumulation of fluorescence over time. These experiments produced a markedly different outcome. Within 30 min, we found 5-fold as much R6G in S1368A as in the WT, and by 90 min, the difference climbed to 12-fold. Therefore the outcome was consistent with a leaky diode. We needed a direct demonstration that S1368A showed significantly more drug reflux than the WT. We loaded de-energized WT and S1368A cells with unlabelled R6G for 90 min. Following this, we activated Pdr5-mediated transport by adding nutrient medium containing [3H]R6G. We determined the amount of labelled R6G present in the cells at 15 min (when there was still some unlabelled pre-loaded R6G in the cells) and 90 min. At 15 min, the S1368A mutant had accumulated 3-fold as much [3H]R6G as the WT. At 90 min, we observed no significant increase in the WT accumulation, but the S1368A cells continued to incorporate [3H]R6G, and the differential between strains rose to ~6-fold. Thus the transport experiments strongly supported the idea that Pdr5 remodels itself to preclude the re-entry of drugs that are pumped out of the cell.
Probably because of the complex phenotype that must be tested, these experiments were the first to suggest a diode mechanism in an ABC transporter. It is worth speculating whether this is a feature of at least all ABC export pumps. In the case of multidrug pumps, reflux of even very small amounts of highly toxic compounds could be lethal to a cell. Perhaps multiple mechanisms, including trans-inhibition and molecular diodes, appeared during the evolution of such pumps. However, if a transporter exports or imports substrates that are neither toxic nor rate-limiting in quantity, the simple loss of affinity in the substrate off conformation might be sufficient.
Only 2 months after the Pdr5 diode study was published, additional evidence for a molecular diode mechanism in an ABC transporter appeared in an elegant study with Tap1/Tap2 transporter [69]. These investigators presented evidence that the canonical D-loop regulates the gating of the diode in this ABC transporter.
WHAT THE FUTURE HOLDS
Many important questions remain to be addressed, including the sequence of events in the catalytic cycle, if purified Pdr5 could be successfully reconstituted with high activity. The construction of a cysteine-less Pdr5 that is active may be very difficult. Perhaps it will become feasible to identify interacting residues by using novel amino acid technology with residues capable of cross-linking. This would be quite useful, for instance, in validating or rejecting features of the recently constructed atomic model of Pdr5 [70]. This model probably already requires some revision, because the Pdr5 NBD–ICL interaction is modelled in the standard trans conformation, whereas the cis counterpart is strongly supported by suppressor genetic data. Finally, a recent study identified ~30 proteins that interact with Pdr5 [71]. Determining the role of these proteins in Pdr5 function should be extremely useful in developing a more detailed mechanistic understanding of the Pdr5-mediated transport process.
Acknowledgments
We thank Trish Weisman for her editorial assistance and George Leiman for his help with the graphics.
FUNDING
The work described in this review that was performed in the Golin laboratory was supported by National Science Foundation [grant number MCB1048838] and National Institutes of Health [grant number GM077211]. The many years of funding from these agencies is deeply appreciated. S.V.A. was supported by the Intramural Research Program of the National Institutes of Health National Cancer Institute’s Center for Cancer Research.
Abbreviations
- ABC
ATP-binding cassette
- AMP-PNP
adenosine 5′-[β,γ-imido]triphosphate
- CFTR
cystic fibrosis transmembrane conductance regulator
- ICL
intracellular loop
- NBD
nucleotide-binding domain
- P-gp
P-glycoprotein
- PM
plasma membrane
- R6G
rhodamine 6G
- TMD
transmembrane domain
- TMH
transmembrane helix
- WT
wild-type
References
- 1.Leppert G, McDevitt R, Falco SC, Van Dyk TK, Ficke MB, Golin J. Cloning by gene amplification of two loci conferring multiple drug resistance in Saccharomyces. Genetics. 1990;125:13–20. doi: 10.1093/genetics/125.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyers S, Schauer W, Balzi E, Wagner M, Goffeau A, Golin J. Interaction of the yeast pleiotropic drug resistance genes PDR1 and PDR5. Curr Genet. 1992;21:431–436. doi: 10.1007/BF00351651. [DOI] [PubMed] [Google Scholar]
- 3.Balzi E, Chen W, Ulaszewski S, Capieaux E, Goffeau A. The multidrug resistance gene PDR1 from Saccharomyces cerevisiae. J Biol Chem. 1987;262:16871–16879. [PubMed] [Google Scholar]
- 4.Kralli A, Bohen SP, Yamamoto KR. LEM1, an ATP-binding cassette transporter selectively modulates the biological potency of steroid hormones. Proc Natl Acad Sci USA. 1995;92:4701–4705. doi: 10.1073/pnas.92.10.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirata D, Yano K, Miyahara K, Miyakawa T. Saccharomyces cerevisiae YDR1 which encodes a member of the ATP-binding cassette superfamily is required for multidrug resistance. Curr Genet. 1994;26:285–294. doi: 10.1007/BF00310491. [DOI] [PubMed] [Google Scholar]
- 6.Balzi E, Wang M, Leterme S, Van Dyck L, Goffeau A. PDR5, a novel yeast multidrug resistance-conferring transporter controlled by the transcription regulator Pdr1. J Biol Chem. 1994;268:2206–2214. [PubMed] [Google Scholar]
- 7.Bissinger PH, Kuchler K. Molecular cloning and expression of the Saccharomyces cerevisiae STS1 gene product: a yeast transporter conferring mycotoxin resistance. J Biol Chem. 1994;268:4180–4186. [PubMed] [Google Scholar]
- 8.Leonard P, Rathod PK, Golin J. Loss-of-function mutation in the yeast multiple drug resistance gene PDR5 causes a reduction in chloramphenicol efflux. Antimicrob Agents Chemother. 1994;38:2492–2494. doi: 10.1128/aac.38.10.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolaczkowski M, van der Rest M, Cybularz-Kolaczkowski A, Soumillion JP, Konigs WN, Goffeau A. Anticancer drugs, ionophoric peptides, and steroids as substrates of the yeast multidrug transporter Pdr5. J Biol Chem. 1996;271:31543–31548. doi: 10.1074/jbc.271.49.31543. [DOI] [PubMed] [Google Scholar]
- 10.Taglicht D, Michaelis M. Saccharomyces cerevisiae ABC proteins and their relevance to human health and disease. Methods Enzymol. 1998;292:130–162. doi: 10.1016/s0076-6879(98)92012-2. [DOI] [PubMed] [Google Scholar]
- 11.Hohl M, Briand C, Grütter MG, Seeger MA. Crystal structure of a heterodimeric ABC transporter in its inward-facing conformation. Nat Struct Mol Biol. 2012;19:395–402. doi: 10.1038/nsmb.2267. [DOI] [PubMed] [Google Scholar]
- 12.Konotoyiannis D, Lewis RE. Antifungal drug resistance of pathogenic fungi. Lancet. 2002;355:1135–1144. doi: 10.1016/S0140-6736(02)08162-X. [DOI] [PubMed] [Google Scholar]
- 13.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 14.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 15.Golin J, Barkatt A, Cronin S, Eng G, May L. Chemical specificity of the PDR5 multidrug resistance gene product of Saccharomyces based on studies with tri-n-alkyltin chlorides. Antimicrob Agents Chemother. 2000;44:134–138. doi: 10.1128/aac.44.1.134-138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golin J, Ambudkar SV, Gottesman MM, Habib AD, Sczepanski J, Ziccardi W, May L. Studies with novel Pdr5p substrates demonstrate a strong size dependence for xenobiotic efflux. J Biol Chem. 2003;278:5963–5969. doi: 10.1074/jbc.M210908200. [DOI] [PubMed] [Google Scholar]
- 17.Hanson L, May L, Tuma P, Keeven J, Mehl P, Ferens M, Ambudkar SV, Golin J. The role of hydrogen bond acceptor groups in the interaction of substrates with Pdr5p, a major yeast drug transporter. Biochemistry. 2005;44:9703–9713. doi: 10.1021/bi0502994. [DOI] [PubMed] [Google Scholar]
- 18.Sauna Z, Bohn SS, Rutledge RM, Dougherty MP, Cronin S, May L, Xia D, Ambudkar SV, Golin J. Mutations define crosstalk between the N-terminal nucleotide-binding domain and transmembrane helix-2 of the yeast multidrug transporter Pdr5: possible conservation of a signaling interface for coupling ATP hydrolysis to drug transport. J Biol Chem. 2008;283:35010–35022. doi: 10.1074/jbc.M806446200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ananthaswamy N, Rutledge RM, Sauna ZE, Ambudkar SV, Dine E, Nelson E, Xia D, Golin J. The signaling interface of the yeast multidrug transporter Pdr5 adopts a cis-conformation and there are functional overlap and equivalence of the deviant and canonical Q-loop residues. Biochemistry. 2010;49:4440–4449. doi: 10.1021/bi100394j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downes M, Mehla J, Ananthaswamy N, Wakschlag A, LaMonde M, Dine E, Ambudkar SV, Golin J. The transmission interface of the Saccharomyces cerevisiae multidrug transporter Pdr5: Val-656 located in intracellular loop 2 plays a major role in drug resistance. Antimicrob Agents Chemother. 2013;57:1025–1034. doi: 10.1128/AAC.02133-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kueppers P, Gupta RP, Stindt J, Smits SHJ, Schmitt L. Functional impact of a single mutant within the transmembrane domain of the multidrug transporter Pdr5. Biochemistry. 2013;52:2184–2195. doi: 10.1021/bi3015778. [DOI] [PubMed] [Google Scholar]
- 22.Ernst R, Kueppers P, Klein CH, Schwarzmueller T, Kuchler K, Schmitt L. A mutation of the H-loop selectively affects rhodamine transport by the yeast ABC transporter Pdr5. Proc Natl Acad Sci USA. 2008;105:5069–5074. doi: 10.1073/pnas.0800191105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furman C, Mehla J, Ananthaswamy N, Arya N, Kulesh B, Kovach I, Golin J. The deviant ATP-binding site of the multidrug efflux pump Pdr5 plays an active role in the transport cycle. J Biol Chem. 2013;288:30420–30431. doi: 10.1074/jbc.M113.494682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta RP, Kueppers P, Hanekop N, Schmitt L. Generating symmetry in the asymmetric ATP-binding cassette (ABC) transporter Pdr5 from Saccharomyces cerevisiae. J Biol Chem. 2014;289:15272–15279. doi: 10.1074/jbc.M114.553065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehla J, Ernst R, Moore R, Wakschlag A, Marquis MK, Ambudkar SV, Golin J. Evidence for a molecular diode-based mechanism in a multispecific ATP-binding cassette (ABC) exporter: Ser1368 as a gatekeeping residue in the yeast multidrug transporter Pdr5. J Biol Chem. 2014;289:26597–26606. doi: 10.1074/jbc.M114.586032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogers B, Decottignies A, Kolaczkowski M, Caravajal E, Balzi E, Goffeau A. The pleiotropic drug ABC transporters from Saccharomyces cerevisiae. J Mol Microbiol Biotechnol. 2001;3:207–214. [PubMed] [Google Scholar]
- 27.Chufan EE, Sim HM, Ambudkar SV. Molecular basis of the poly specificity of P-glycoprotein (ABCB1): recent biochemical and structural studies. Adv Cancer Res. 2015;125:71–96. doi: 10.1016/bs.acr.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loo TW, Clark DM. Defining the drug-binding sites in multidrug resistance P-glycoprotein using a methanethiosulfonate analog of verapamil, MTS-verapamil. J Biol Chem. 2001;276:14972–14979. doi: 10.1074/jbc.M100407200. [DOI] [PubMed] [Google Scholar]
- 29.Shukla S, Sani P, Jha S, Ambudkar SV, Prasad R. Functional characterization of the Candida albicans ABC transporter Cdr1. Eukaryot Cell. 2003;2:1361–1375. doi: 10.1128/EC.2.6.1361-1375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sauna ZE, Andrus MB, Tainer TM, Ambudkar Biochemical basis of polyvalency as a strategy for enhancing the efficacy of P-glycoprotein (ABCB1) modulators: stipamide homodimers separated with defined-length spacers reverse drug efflux with great efficacy. Biochemistry. 2004;43:2262–2271. doi: 10.1021/bi035965k. [DOI] [PubMed] [Google Scholar]
- 31.Chufan EE, Kapoor K, Sim HM, Singh S, Talele TT, Durell SR, Ambudkar SV. Multiple transport-active binding sites are available for a single substrate on human P-glycoprotein. PLoS ONE. 2013;8:e82463. doi: 10.1371/journal.pone.0082463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for polyspecific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin MS, Oldham ML, Zhang Q, Chen J. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature. 2012;490:566–570. doi: 10.1038/nature11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeCottignies A, Kolaczkowski M, Balzi E, Goffeau A. Solubilization and characterization of the over expressed Pdr5 multidrug resistance nucleotide triphosphatase of yeast. J Biol Chem. 1994;269:12797–12803. [PubMed] [Google Scholar]
- 35.Prasad RP, Goffeau A. ATP-binding cassette transporters conferring multidrug resistance. Annu Rev Microbiol. 2012;66:39–63. doi: 10.1146/annurev-micro-092611-150111. [DOI] [PubMed] [Google Scholar]
- 36.Golin J, Kon ZN, Wu CP, Martello J, Hanson L, Supernavage S, Ambudkar SV, Sauna ZE. Complete inhibition of the Pdr5 multidrug efflux pump ATPase activity by its transport substrate clotrimazole suggests GTP as well as ATP may be used as an energy source. Biochemistry. 2007;46:13109–13119. doi: 10.1021/bi701414f. [DOI] [PubMed] [Google Scholar]
- 37.Litman T, Zeuthan T, Skovsgaard T, Stein WD. Non-competitive and competitive interactions between the substrates of P-glycoprotein as measured by its ATPase activity. Biochim Biophys Acta. 1997;1361:169–176. doi: 10.1016/s0925-4439(97)00027-6. [DOI] [PubMed] [Google Scholar]
- 38.Litman T, Zeuthan T, Skovsgaard T, Stein WD. Structure-activity relationships of P-glycoprotein interacting drugs; kinetic characterization of their effects on ATPase activity. Biochim Biophys Acta. 1997;1361:159–168. doi: 10.1016/s0925-4439(97)00026-4. [DOI] [PubMed] [Google Scholar]
- 39.Gupta RP, Kueppers P, Schmitt L, Ernst R. The multidrug transporter Pdr5: a molecular diode? Biol Chem. 2011;392:53–60. doi: 10.1515/BC.2011.011. [DOI] [PubMed] [Google Scholar]
- 40.Lelong IH, Padmanabhan R, Lovelace E, Pastan I, Gottesman MM. ATP and GTP as alternative energy sources for vinblastine transport by P-170 in KB-V1 plasma membrane vesicles. FEBS Lett. 1992;304:256–260. doi: 10.1016/0014-5793(92)80632-q. [DOI] [PubMed] [Google Scholar]
- 41.Klein M, Mamnun YM, Eggmann T, Schuller C, Wolfger H, Martinola E, Kuchler K. The ATP-binding cassette (ABC) transporter Bpt1 mediates vacuolar sequestration of glutathione conjugates in yeast. FEBS Lett. 2002;520:63–67. doi: 10.1016/s0014-5793(02)02767-9. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Sun F, Zhang D, Ma VM, Xu F, Belani JD, Cohen JC, Hobbs HH, Xie XS. Sterol transfer by ABCG5 and ABCG8: in vitro assay and reconstitution. J Biol Chem. 2006;281:27894–27904. doi: 10.1074/jbc.M605603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaitseva J, Jenewein S, Jumpertz T, Holland BI, Schmitt L. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J. 2005;24:1901–1910. doi: 10.1038/sj.emboj.7600657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchaklian AH, Klug CS. Characterization of LSGGQ and H motifs from the Escherichia coli lipid transporter MsbA. Biochemistry. 2006;45:12359–12546. doi: 10.1021/bi060830a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson RJ, Locher KP. Structure of a bacterial multidrug transporter. Nature. 2006;443:180–185. doi: 10.1038/nature05155. [DOI] [PubMed] [Google Scholar]
- 46.Zolnerciks JK, Wooding C, Linton KJ. Evidence for a Sav1866-like architecture for the human multidrug transporter P-glycoprotein. FASEB J. 2007;21:3937–3948. doi: 10.1096/fj.07-8610com. [DOI] [PubMed] [Google Scholar]
- 47.Onancea G, O’Mara ML, Drew-Bennett WF, Tielman P, Abeles R, Tampe R. Structural arrangement of the transmission interface in the antigen ABC transporter TAP. Proc Natl Acad Sci USA. 2009;106:5551–5556. doi: 10.1073/pnas.0811260106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puri N, Gaur M, Sharma M, Shukla S, Ambudkar SV, Prasad R. The amino acid residues of transmembrane helix-5 of the multidrug resistance protein CaCdr1p of Candida albicans are involved in substrate specificity and drug transport. Biochim Biophys Acta. 2009;1788:1752–1761. doi: 10.1016/j.bbamem.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinkett HW, Lee AT, Lum P, Locher KP, Rees DC. An inward-facing conformation of a putative metal-chelate-type ABC transporter. Science. 2007;315:373–377. doi: 10.1126/science.1133488. [DOI] [PubMed] [Google Scholar]
- 50.Kabada NS, Kaiser JT, Johnson E, Lee A, Rees DC. The high affinity E. coli methionine ABC transporter: structure and allosteric regulation. Science. 2008;321:250–253. doi: 10.1126/science.1157987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolaczkowski M, Sroda-Pomianek K, Kolaczkowski A, Michalak K. A conserved interdomain communication pathway of pseudo symmetrically distributed residues affects the substrate specificity of the fungal multidrug transporter Cdr1p. Biochim Biophys Acta. 2012;1828:479–490. doi: 10.1016/j.bbamem.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 52.Roninson IB, Chin JE, Choi KG, Gros P, Houseman DE, Fojo A, Shen DW, Gottesman MM, Pastan I. Isolation of human mdr sequences amplified in multidrug resistant KB carcinoma cells. Proc Natl Acad Sci USA. 1986;83:4538–4542. doi: 10.1073/pnas.83.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Procko E, O’Mara ML, Bennett WFD, Tieleman DP, Gaudet R. The mechanism of ABC transporters: general lessons from structural and functional studies of an antigenic peptide transporter. FASEB J. 2009;23:1287–1302. doi: 10.1096/fj.08-121855. [DOI] [PubMed] [Google Scholar]
- 54.Payen L, Gao M, Westlake C, Theis A, Cole SPC, Deeley R. Functional interactions between nucleotide-binding domains and leukotriene C4 binding sites of multidrug resistance protein 1 (ABCC1) Mol Pharmacol. 2005;67:1944–1953. doi: 10.1124/mol.104.007708. [DOI] [PubMed] [Google Scholar]
- 55.Tsai MF, Jih KY, Shimizu H, Li M, Hwang TC. Optimization of the degenerated interfacial ATP binding site improves the function of disease-related mutant cystic fibrosis transmembrane conductance (CFTR) channels. J Biol Chem. 2010;285:37663–37671. doi: 10.1074/jbc.M110.172817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gregory RJ, Rich DP, Cheng SH, Souza DW, Paul S, Manavalan P, Anderson MP, Welsh MJ, Smith AC. Maturation and function of cystic fibrosis transmembrane conductance regulator variants bearing mutations in putative nucleotide-binding domains 1 and 2. Mol Cell Biol. 1991;11:3886–3893. doi: 10.1128/mcb.11.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen M, Abele R, Tampe R. Functional non-equivalence of ATP-binding cassette signature motifs in the transporter associated with antigen processing (TAP) J Biol Chem. 2004;279:46073–46081. doi: 10.1074/jbc.M404042200. [DOI] [PubMed] [Google Scholar]
- 58.Masia R, Nichols CG. Functional clustering of mutations in the dimer interface of the nucleotide-binding folds of the sulfonylurea receptor. J Biol Chem. 2008;283:30322–30329. doi: 10.1074/jbc.M804318200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Procko E, Ferrin-O’Connell I, Ng SL, Gaudet R. Distinct structural and functional properties of the ATPase sites in an asymmetric ABC transporter. Mol Cell. 2006;24:51–62. doi: 10.1016/j.molcel.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 60.Hohl M, Hürlimann LE, Böhm S, Schöppe J, Grütter MG, Bordignon E, Seeger MA. Structural basis for allosteric crosstalk between the asymmetric nucleotide-binding sites of a heterodimeric ABC exporter. Proc Natl Acad Sci USA. 2014;111:11025–11030. doi: 10.1073/pnas.1400485111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–880. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai MF, Li M, Hwang TC. Stable ATP binding mediated by a partial NBD dimer of the CFTR chloride channel. J Gen Physiol. 2010;135:399–414. doi: 10.1085/jgp.201010399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Csanady L, Mihalyi C, Szollosi A, Torocsik B, Vergani P. Conformational changes in the catalytically inactive nucleotide-binding site of CFTR. J Gen Physiol. 2013;142:61–73. doi: 10.1085/jgp.201210954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jha S, Karnani N, Dhar SK, Mukhopadhaugh G, Prasad R. Characterization of the N-terminal nucleotide-binding domain of an ABC transporter: uncommon cysteine 193 of Walker A is critical for ATP hydrolysis. Biochemistry. 2005;42:10822–10832. doi: 10.1021/bi0345900. [DOI] [PubMed] [Google Scholar]
- 65.Jha S, Dabas N, Karnani N, Saini P, Prasad R. ABC multidrug transporter Cdr1p of Candida albicans has divergent nucleotide-binding domains which display functional asymmetry. FEMS Yeast Res. 2006;5:63–72. doi: 10.1016/j.femsyr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Rai V, Shukla S, Jha S, Koranth SS, Prasad R. Functional characterization of the N-terminal nucleotide-binding domain (NBD-1) of a major ABC drug transporter Cdr1p of C. albicans has a novel role in ATP hydrolysis. Biochemistry. 2005;45:14726–14739. [Google Scholar]
- 67.van Veen HW, Margolles A, Muller M, Higgins CF, Konings WN. The heterodimeric ATP-binding cassette transporter LmrA mediates transport by an alternating (two-cylinder engine) mechanism. EMBO J. 2000;19:2503–2514. doi: 10.1093/emboj/19.11.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sauna ZE, Ambudkar SV. Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc Natl Acad Sci USA. 2000;97:2515–2520. doi: 10.1073/pnas.97.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grossmann N, Vakkasoglu AS, Hulpke S, Abele R, Gaudet R, Tampe R. Mechanistic determinants of the directionality and energetics of active transport by a heterodimeric ABC transporter. Nat Commun. 2014;5:5419. doi: 10.1038/ncomms6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rutledge RM, Esser L, Ma J, Xia D. Toward understanding the mechanism of action of the yeast multidrug resistance transporter Pdr5: a molecular modeling study. J Struct Biol. 2011;173:333–344. doi: 10.1016/j.jsb.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Snider J, Hanif A, Lee ME, Jin K, Yu AR, Graaham C, Chuk MM, Damjaanovic D, Wierzbicka M, Tang P, et al. Mapping the functional yeast ABC transporter interactome. Nat Chem Biol. 2013;9:565–572. doi: 10.1038/nchembio.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]