Abstract
Although emotional functioning is impaired in children with autism, it is unclear if this impairment is due to difficulties with facial expression, autonomic responsiveness, or the verbal description of emotional states. To shed light on this issue, we examined responses to pleasant and unpleasant odors in eight children (8–14 years) with high-functioning autism and 8 age-matched typically developing controls. Despite subtle differences in the facial actions of the children with autism, children in both groups had similar facial and autonomic emotional responses to the odors. However, children with autism were less likely than controls to report an emotional reaction to the odors that matched their facial expression, suggesting difficulties in the self report of emotional states.
Keywords: Autism, Olfaction, Emotion, Facial expression, Autonomic system, Self-report
Introduction
The social-behavioral difficulties of children with autistic spectrum disorders (ASDs) have been ascribed variously to sensory or perceptive deficits (e.g. Kientz and Dunn 1997; Watling et al. 2001; Ben-Sasson et al. 2007; Tomcheck and Dunn 2007; Baker et al. 2008; Hilton et al. 2010), neurobiological deficits (e.g. Gillberg 1999; Bauman and Kemper 2005; Schroeder et al. 2010), specific deficits in the capacity to feel and integrate emotions (e.g. Capps et al. 1992; Leekam 2005), or to motor or facial expression deficits (e.g. Leary and Hill 1996; Czapinski and Bryson 2003).
Much remains to be understood about emotional functioning in persons with ASD with respect to the brain’s reception, organization, and interpretation of information from the sensory receptors, the emergence of autonomic and behavioral responses well as an inner emotional experience. According to LeDoux (1996), two different neural systems could account for the generation of a response to a given stimulus. The first is a subcortical amygdala-based system which receives sensory information from all the modalities and creates autonomous, reflex-behavioral, and hormonal reactions. Information traverses this low road rapidly, automatically, and unconsciously. The second system, the high road, processes sensory information more carefully via the prefrontal cortex, which is also able to interface with the subcortical amygdala-based system in order to amplify or attenuate the primary reaction. This high road would be mainly implicated in the elaboration of mental representations, and could allow conscious feelings, description of inner emotional experiences, identification and the naming of emotions, and attribution of sense to the sensory information. For example, the relatively crude low road may respond to a long and thin object as a dangerous snake—and trigger an immediate and primary fear response—while the slower high road then determines that the object is, in fact, a stick.
This model, based on two systems, even if still under debate, could be helpful to highlight emotional deficits encountered by children with autism. On one side, several studies have provided evidence of close-to-normal autonomic reactions during affect inductions in individuals with autism, at least if the primary physiological reaction is considered. For example, Shalom et al. (2006) found that high-functioning autistic children exposed to pleasant, unpleasant, and neutral pictures present skin conductance responses that did not differ with controls. A similar result was observed in low functioning autistic children and control children (Blair 1999). Also, heart rate patterns were found to be similar in children with autism and controls when separation from the mother occurred (Willemsen-Swinkels et al. 2000). On the other side, numerous studies have provided evidence that people with autism have difficulties in the cognitive processing of their own emotions, including difficulties in identifying and describing feelings, integrating bodily sensations of emotional arousal, and recalling previous emotions (Hill et al. 2004; Losh and Capps 2006; Rieffe et al. 2007; Capps et al. 1992, 1995). Taken together, these results raise the possibility of an impaired integration of amygdala-based information responsible of the primary reaction into cortical elaborations enabling the expression of conscious feelings.
This hypothesis has been poorly addressed until now in autism. One of the major reasons is the difficulty to conduct studies exploring in the same paradigm, autonomic, behavioral and self-reported responses during emotional processing. Odors are particularly potent in inducing emotional reactions in humans (e.g. Soudry et al. 2011). This characteristic can be explained by both anatomical and functional particularities. In the main olfactory system, axons run from the olfactory receptor neurons synapse in the olfactory bulb and then fibers project to different brain areas, including the piriform cortex, the amygdala, the entorhinal cortex and the orbitofrontal cortex. This close anatomical proximity (only two synapses) between olfactory receptors and the amygdala suggests that olfactory stimulations could be particularly efficient for activating the amygdala and for further exploring affective information processing. This supposition was confirmed by functional brain imaging studies conducted in the field of olfaction. Compared to other sensory stimuli, olfactory stimuli were even found to be the strongest predictors of amygdale activation (Costafreda et al. 2008). More precisely, fMRI studies reported that the processing of the intensity of odors is associated with activity in the amygdala and the piriform cortex (Anderson et al. 2003; Rolls et al. 2003; Jung et al. 2006), while the orbitofrontal cortex is connected to the judgment of the pleasantness of odor, odor identification, and odor memory (Zald and Pardo 1997; Royet et al. 2003; Rolls et al. 2010).
Despite the potency of this sensory modality, relatively few studies have dealt with responsiveness to odors in children with autism. Suzuki et al. (2003), for example, examined the capacity to detect odors in 12 adults with Asperger’s syndrome and 12 matched control subjects. The results revealed that the Asperger’s syndrome children were not impaired at odor detection, indicating that the olfactory perception is intact in this syndrome. The capacity to evaluate the pleasantness of odors was explored recently by Hrdlicka et al. (2011) in a group of 35 children with Asperger’s and high-functioning autism. Compared to controls, autistic subjects judged 3 of 16 odors (mainly food odors) as less pleasant, suggesting that the pleasure induced by few odors could be attenuated in the case of autism. Finally, odor identification was considered in three studies (Suzuki et al. 2003; Bennetto et al. 2007; May et al. 2010). Impaired identification of olfactory stimulation has also been observed in adults (Suzuki et al. 2003), adolescents (Bennetto et al. 2007), and children with autism spectrum disorder (May et al. 2010). With respect to behavior, Soussignan et al. (1995) examined the facial responses of children with pervasive developmental disorder (PDD) to pleasant and unpleasant odors. Children with PDD displayed distinct expressions in response to these unpleasant and pleasant odors. It is interesting to note that the design of all these studies conducted in the field of olfaction did not involve autonomic reactions, and all the conclusions were based on a single channel of expression.
To address gaps in the literature, the present study explores the reaction of children with autism to pleasant and unpleasant odors while examining autonomic, behavioral, and verbal indicators. We recorded autonomic (heart rate, skin conductance), behavioral (facial), and self-reports of conscious feelings in a task in which children with high functioning autism and controls were exposed to various stimulations inducing emotional reactions. Children with high functioning autism were selected in order to obtain verbal responses as clearly as possible. Finally, everyday pleasant and unpleasant odors were used to elicit contrasting emotional states in an ecologically valid fashion.
Method
Participants
Participants included eight children with high-functioning forms of autism (HFA) recruited from an institution specializing in educating children with autism, and eight typically developed (TD) controls recruited from a standard school. In the HFA group, diagnosis of autistic disorder (F 84.0) was established by child psychologists with the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV, American Psychiatric Association 1994) and the Childhood Autism Rating Scale (CARS, Schopler et al. 1986). Only children with relatively high-functioning autism (CARS range: 32–37; scores above 30 function as a cut-off for high functioning autism). Only HFA children with relatively intact cognitive functioning as assessed with the Wechsler Intelligence Scale for Children (WISC, Wechsler 1991; Full Scale IQ range: 75–89), and with a normal grade level for age were invited to participate. Typically developing control participants had normal school performance, no known behavioral or psychological disorder, and no history of autism within their families. Children with autism and control children were matched by group based on chronological age and gender.
Odorous Stimuli
Eight odors were used in the present study: vanilla (vanillin), cheese (isovaleric acid), rose (essential oil), green grass (cis-3-hexenol), mint (menthol), chlorine, sweat (androstenon), and feces (3-methylindol). The main criterion for selecting these odors was their potency to induce pleasant or unpleasant odor perceptions. Vanilla and rose, widely used fragrances, were expected to be rated as pleasant. In the opposite, feces and cheese were predicted to be judged as unpleasant. Mint and chlorine were selected due to their property to stimulate intranasal trigeminal nerve structures (releasing sensations of freshness or irritation), and not only the main olfactory system believed to be preferentially stimulated by the other odors. Finally, the odors of green grass and sweat were added as representatives of the physical and social environment, respectively. All these odors were presented at suprathreshold concentrations. The odorous solutions and the control stimulus (distilled water) were presented in 60-ml opaque glass jars in volumes of 20 ml.
Experimental Context and Procedure
Children were tested individually at school or at a specialized institution over one session. Because children (particularly children with autism) are often disturbed when confronted with a novel situation, the experiments took place in a familiar room of the school in the presence of their usual teacher (or educator). However, the teacher stood outside the visual field of the children and did not participate in the testing procedure. The room was well ventilated before and during the test, and the temperature was maintained constant during the study (21–23 °C).
The children were then asked to take several deep nasal inspirations in order to verify their ability to regulate respiration and the absence of any nasal or respiratory problem. Children were also fitted with psycho physiological equipment to record heart rate (HR) and skin conductance response (SCR). These sensors, secured on the non-dominant hand with Velcro straps, consisted of a photopethysmograph sensor placed on the middle finger for recording heart rate, and two silver–silver chloride electrodes placed on the ring and middle fingers for recording electro dermal activity. Physiological data were collected with the Visual Energy Tester (version 6.1) by Elemaya Biofeedback System.
The testing procedure was conducted by two experimenters. Experimenter 1 was seated slightly sideways of the child and presented the glass jars containing the odorous solutions. The open jars were positioned on the child’s midline, at 2–3 cm from the nostrils, for approximately 5 s (so that it covered several inspiration-expiration cycles). The 9 stimuli were presented in random order, and prepared by Experimenter 2, so that Experimenter 1 was blind to the quality of the odor stimuli that he presented. To avoid any odor pollution, the glass jars were open just before presenting it to the subject, and immediately closed after the presentation. The onset of each stimulus trial was entered on the polygraphic recordings by Experimenter 2. The duration of stimulation and the inter-stimulus interval (minimum 1 min, in order to avoid any olfactory interactions) were controlled by Experimenter 2 through a visual signal that was only visible to Experimenter 1. Experimenter 2 sat in a separate place of the testing room outside the visual field of the subject. The odor-elicited facial responses were videotaped with a camera providing a frontal view of the subject’s face.
Approximately 15 s after each olfactory presentation, the subject was asked to rate the intensity, the familiarity, the pleasantness of the stimulus, and to identify the nature of it. Firstly, the subjects were asked to evaluate the strength of the stimulus, subjects had to select one of the 6 following terms corresponding to 6 levels of intensity: very strong, strong, normal, faint, very faint, nothing. Secondly, to judge whether the odor was familiar or unfamiliar. Thirdly, to identify the stimulus. If the answer was too general, the child was invited to specify his answer. For each stimulus, scores were assigned as follows: 0—no answer or wrong answer, 1—category descriptor (e.g. flower for rose), 2—precise name. During these evaluations, if a subject asked to smell the odor again, the request was granted. But in these cases, the next presentation was delayed in order to preserve the minimal 1-min interval between two consecutive olfactory evaluations. Finally, the experimenter asked the subject to judge the pleasantness of the odor by saying whether the odor was pleasant, unpleasant, or neither pleasant nor unpleasant. As a whole, the olfactory test lasted approximately 20 min. All subjects completed the test without any particular problems.
Data Coding
Facial responses were analyzed with the Facial Action Coding System (FACS; Ekman and Friesen 1978; Ekman et al. 2002). This anatomically based instrument is designed to measure all minimal action units (AUs) that the facial muscles can produce. Each movement of the distinct parts of the face (upper, eyes, middle, and lower) was viewed separately in slow motion and frame by frame to score onset and offset points of each AU. The precision of the duration of the measurement was thus plus or minus one video frame (±0.04 s). The intensity of each AU was rated on a 5-point scale ranging from 1 (trace of action) to 5 (maximum evidence).
The videotapes were coded by two certified FACS coders during the 5-s presentation of the stimulus and the 15-s post-stimulus period. Coders were blind to the odors presented and to group. The inter-coder agreement ratio regarding type, duration, and intensity of AUs was higher than 85 % in all cases. All the facial actions composing the FACS were coded. For the description of the results, the face was divided into 4 regions, each region comprising the following AUs. For the upper face: AU 1 and 2 (inner and outer eyebrow raise), AU 4 (brow lowering); for the eye region: AU5 (upper lid raise), AU6 (cheek raise), AU7 (lids tight), AU43 (eye closure), AU45 (blink), 46 (wink); for the middle face: AU9 (nose wrinkle), AU10 (upper lip raise), AU11 (nasolabial furrow deepener), AU12 (lip corner puller), AU14 (lip corner tightener), AU20 (lip stretch), AU33 (blow), AU34 (lip puff); for the lower face: AU15 (lip corner depressor), AU16 (lower lip depress), AU17 (chin raiser), AU18 (lip pucker), AU22 (lip funneler), AU23 (lip tightener), and AU24 (lip presser). On the basis of Ekman and Friesen’s work (Ekman and Friesen 1978; Ekman et al. 2002), upper lip raising (AU10) and/or nose wrinkling (AU9) was used to index disgust and negative displays; lip corner pulling (AU12) was used to index of smiling and positive displays.
For heart rate and skin conductance, difference scores were calculated by subtracting the 5-s before the stimulus release (baseline period) from the 5-s during stimulus presentation, and from the 15-s following the stimulation (post-stimulus period). In order to describe the skin conductance response, 3 parameters were used: the maximum amplitude change, the number of peaks change, and the integral (area under the curve) change. The maximum amplitude referred to the highest phasic amplitude that was recorded during each 5-s interval. Only responses equal to or greater than 0.02 microSiemens (μS) with a minimal slope of 0.01 μS/s were considered. Responses contaminated by children’s body movements or spontaneous verbal expression during stimulus presentation were eliminated from subsequent analysis. Also, aberrant data due to technical recording problems were discarded. In total, 4.4 % of data were eliminated. There was not a significant difference between the HFA (5.5 %) and TD children (3.3 %) group in the mean percentage of eliminated data, χ2 = 1.4, p = n.s.
Statistical Analysis
Nonparametric statistical comparisons were used because data were not normally distributed on all measures. The HFA and TD children were compared using the Kruskal–Wallis test. When proportions of children were compared, Chi-square tests were used. When comparing repeated measurements in a single group, the Wilcoxon signed-rank test was utilized. Finally, correlations between behavioral and self-report ratings were examined with Pearson coefficients. If p values were <.05, the results were considered significant.
Results
The results are presented in three sections: Facial expression, Autonomic reactions and Self-reported odor rating. Under facial expressions, we contrast global facial responsiveness to the control stimulus and to the odors (overall). We then compare the global facial responsiveness to odors of HFA and TD children with respect to the number, duration and intensity of facial movements in different parts of the face. Finally, we contrast the groups with respect to their facial reactions to odor using specific facial indices of affective valence. In Autonomic reactions, we compare the changes in heart rate and skin conductance during each stimulus as compared to baseline. Self-reported odor ratings are described with respect to intensity, pleasantness and familiarity in the HFA and TD children with a focus on the associations between their facial expressions and verbal responses.
Facial Expressions
Differences Between Facial Responses to the Control Stimulus and Facial Responses to Odors
The control stimulus (distilled water) induced fewer AUs than the odor stimuli both in the HFA (control stimulus Mdn = 4; olfactory stimuli Mdn = 7), Z = −2.9, p < .05, and in the TD groups (control stimulus Mdn = 3; olfactory stimuli Mdn = 9), Z = −3.2, p < .05; these effects did not differ by group, χ2 (1, N = 16) = 2.5, p = n.s. AUs during the control stimulus were also briefer than those in response to the odors for both groups (HFA children control stimulus Mdn = 1.97 s; olfactory stimuli Mdn = 2.35 s, Z = −2.9, p < .05; TD children control stimulus Mdn = 1.59 s, olfactory stimuli Mdn = 2.60 s; Z = −3.2, p < .05; group comparison χ2 (1, N = 16) = 2.7, p = n.s.) and were less intense for both group (HFA children control stimulus Mdn = 2; other stimuli Mdn = 3; Z = −2.9, p < .05; TD children control stimulus Mdn = 2; olfactory stimuli Mdn = 3; Z = −2.8, p < .05); these effects did not differ by group, χ2 (1, N = 16) = 2.3, p = n.s.). AU12 (lip corner pulling, i.e., smiling) was the most common muscular movement displayed during presentation of the control stimulus. Smiling was observed in 50 % of the children with HFA and 87.5 % of the children with TD.
Global Facial Responsiveness to Odors
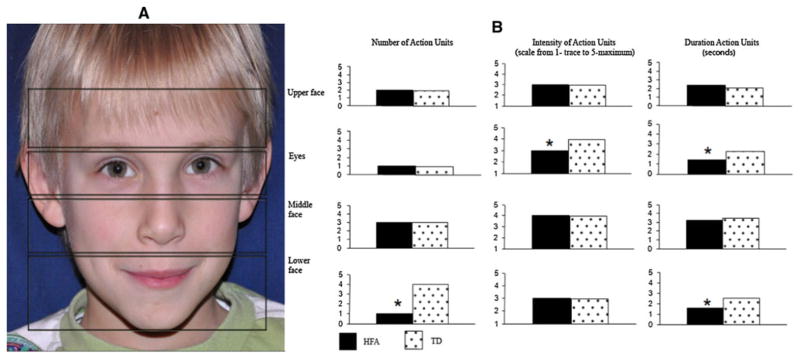
All (100 %) of the HFA and TD children reacted facially to the odors. In general, the first facial changes occurred quickly after stimulus onset (HFA children Mdn = 1.79 s; TD children Mdn = 1.43 s); these effects did not differ by group, χ2 (1, N = 16) = 2.8, p = n.s. There were subtle differences between HFA and TD children in the facial action occurring in different regions of the face. In the eye region, the AUs expressed by HFA children were of lower intensity (HFA children Mdn = 3; TD children Mdn = 4), χ2 (1, N = 16) = 4.6, p < .05, and shorter duration (HFA children Mdn = 1.45 s; TD children Mdn = 2.30 s) than those of the TD children, χ2 (1, N = 16) = 5.1, p < .05, HFA children also produced fewer AUs than TD children in the lower part of the face, in particular in the region of the mouth and the chin (HFA children Mdn = 1; TD children Mdn = 4), χ2 (1, N = 16) = 7.4, p < .05. The duration of the AUs in the lower part of the face were also briefer for the HFA children (Mdn = 1.58 s) than TD children (Mdn = 2.57 s), χ2 (1, N = 16) = 5.5, p < .05 (see Fig. 1). Individual particularities also were detected in 3 children with HFA: one had spasms in the eye region (these AUs were not introduced in the data); another one had a tendency to use a stereotyped expression in response to the stimulations; finally, one child was characterized by an asymmetry in facial expression (see Fig. 1).
Fig. 1.
a A child’s face divided into the regions used for analysis. b The median number, intensity and duration of facial action units (AU) occurring in different regions of the face in children with high functioning autism (HFA) and typically developing children (TD). * p < .05
Specific Facial Reactions to Odor Valence
Upper lip raising (AU10) and/or nose wrinkling (AU9) was used to index disgust and negative displays; lip corner pulling or smiling (AU12) was used to index of smiling and positive displays. We also analyzed the most frequent actions accompanying nose wrinkling (AU9), upper lip raising (AU10) and smiling (AU12). The most frequent actions accompanying nose wrinkling (AU9) and upper lip raising (AU10) were lip corner depressor (AU15), chin raiser (AU17), cheek raiser (AU6) and lid tightener (AU7). The most frequent actions accompanying smiling were contractions of the brow raiser (AU1 and AU2).
There was no difference in the proportion of children displaying smiling in response to the odors of vanilla (75 % of HFA children and 87.5 % of TD children), rose (75 % of HFA children and 75 % of TD children) and mint (75 % of HFA children and 87.5 % TD children). Smiling (AU12) was associated with brow raiser (AU1 and AU2), and indicator of surprise, more frequently in TD children than HFA children. Brow raiser was present with smiling for the odor of rose in 0 % of HFA children and 50 % of TD children, χ2 = 3.3, p < .05, and for the odor of vanilla in 0 % of HFA children and 62.5 % of TD children, χ2 = 4.0, p < .05.
Indices of disgust, nose wrinkling (AU9) and/or upper lip raising (AU10) were elicited in large proportions of children in reaction to the odors of feces (75 % of HFA children and 100 % of TD children), cheese (87.5 % of HFA children and 87.5 % of TD children), chlorine (75 % of HFA children and 100 % of TD children), grass (75 % of HFA children and 87.5 % of TD children), and sweat (75 % of HFA children and 87.5 % of TD children); these effect did not differ by group. Lip corner depressor (AU15) and/or chin raiser (AU17) displayed in combination with nose wrinkling (AU9) and/or upper lip raising (AU10) are a major variant of disgust (Ekman 1982). The proportion of children displaying this combination was significantly higher in TD children compared to HFA children children for feces (25 % of HFA children and 87.5 % of TD children), chlorine (25 % of HFA children and 75 % of TD children), and sweat (25 % of HFA children and 75 % of TD children), χ2 (1, N = 16) = 6.0, 4.0, and 4.0, respectively; ps < .05. In addition, TD children also exhibited more contractions of cheek raiser (AU6) and the lid tightener (AU7) in combination with nose wrinkling (AU9) and/or upper lip raising (AU10) in response to the odors of feces (37.5 % of HFA children and 87.5 % of TD children and) and chlorine (37.5 % of HFA children and 100 % of TD children), χ2 (1, N = 16) = 4.0 and 5.3, respectively; ps < .05.
Autonomic Reactions
For heart rate and skin conductance, no differences between groups were detected for comparisons involving the 15-s period following the stimulus (post-stimulus period). The presentation below focuses on comparisons involving the baseline period (5-s before the stimulus) and the period of stimulus presentation (5-s). Comparisons of these two periods resulted in a change score that was used to compare reactions to the different stimuli in the two groups.
Heart Rate (HR)
There were no differences in heart rate between baseline and the administration of the control stimulus for either group. Children of both groups displayed significantly greater HR changes with respect to baseline when they were exposed to odors as compared to when they were exposed to the control stimulus (ps < .05 in HFA and TD children).
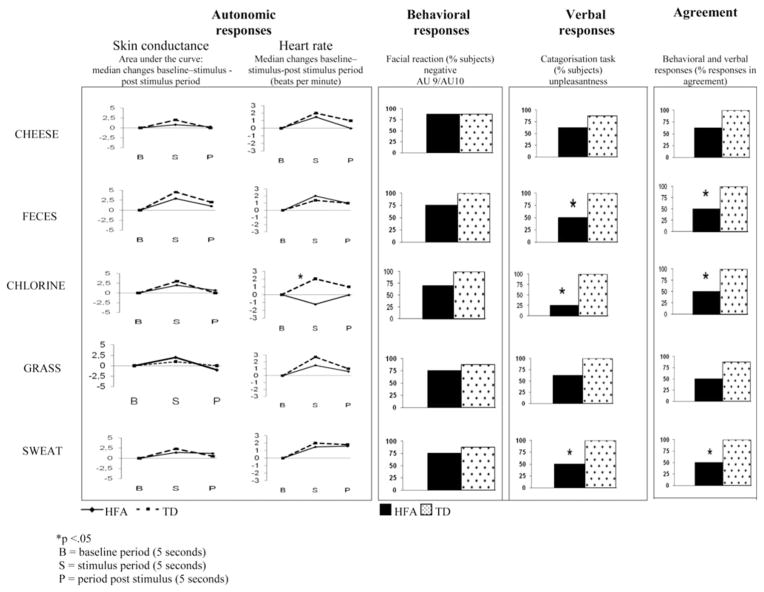
HR responsiveness to the odors did not reveal significant differences between the two groups. In both group decreased HR was observed during the pleasant odors of vanilla (TD children Mdn = −2.21 bpm, Z = 2.6, p < .05; HFA children Mdn = −1.64 bpm, Z = −1.9, p = .05) and rose compare to baseline (TD children Mdn = −1.27 bpm, Z = −1.9, p = .05; HFA children Mdn = −2.61 bpm, Z = −2.3, p < .05). In contrast, the unpleasant odors induced increased HR in children in both groups. This was particularly the case for feces (TD children Mdn = 1.53 bpm, Z = −2.1, p < .05; HFA children Mdn = 2.44 bpm, Z = −2.4, p < .05) and for sweat (TD children Mdn = 1.28 bpm, Z = −2.7, p < .05; HFA children Mdn = 1.04 bpm, Z = −1.9, p = .05). There were HR differences between groups in response to the odor of chlorine: TD children displayed an increase in HR (Mdn = 2.03 bpm), Z = −2.8, p < .05, while HFA children children reacted with a HR decrease (Mdn = −1.23 bpm), Z = −1.9, p = .05; group comparison: χ2 (1, N = 16) = 3.9, p < .05 (see Fig. 2).
Fig. 2.
Example of physiological, behavioral, and verbal responses to unpleasant odors in children with high functioning autism (HFA) and typically developing children (TD)
Skin Conductance
Several skin conductance variables were used to index phasic arousal induced by odors. They were: the general profile of the curve measured by the area under the curve (integral), the number of peaks appearing on the curve, and the maximal amplitude of the curve (value of the highest peak).
The general profile of the skin conductance response was significantly modified in the presence of the odors compared to the control stimulus. The change with respect to baseline was seen in both TD (Mdn integral change for control stimulus = 110, for odors: Mdn = 244), Z = −2.8, p < .05, and HFA groups (Mdn integral change for control stimulus = 185, for odors: Mdn = 293), Z = −2.9, p < .05. We next compared the area under the curve with baseline for each odor to baseline for both groups. There was an increase with respect to baseline for each odor in both groups, with the exception of mint. In reaction to mint, the mean integral change (compared to baseline) increased in TD children (Mdn = 313), Z = −2.1, p < .05, but did not change significantly in HFA children (Mdn = −74.9), Z = −.56, p = n.s., group comparison: χ2 (1, N = 16) = 6.3, p < .05.
With respect to the number of peaks in response to odors as compared to baseline, no difference was detected between TD and HFA children, with the exception of the odors of mint and chlorine. TD children exhibited more peaks in reaction to mint than in the baseline period, but HFA children did not show this increase, χ2 (1, N = 16) = 4.3, p < .05. The same pattern was evident in response to chlorine. TD children exhibited more peaks in reaction to chlorine than in the baseline period, Z = 1.9, p < .05, but HFA children did not show this increase, Z = 0, p = n.s.; group comparison: χ2 (1, N = 16) = 4.7, p < .05.
The amplitude of the peaks was also analysed in order to quantify the level of emotional reactivity induced by the odors. There were no significant group differences between HFA and TD children.
Self-Reported Odor Ratings
After the post-stimulus period, children were asked to rate and describe the odors on four dimensions: intensity, familiarity, identification, and pleasantness (pleasant versus unpleasant). Both groups of children rated odors as relatively intense (HFA children Mdn = 4 and TD Mdn = 4 on a 0–5 scale), indicating that the odors were clearly perceived by the children. In comparison, the children of both groups rated the control stimulus at 0. The majority of the odors (vanilla, grass, rose, mint, chlorine) were judged as familiar by 50–75 % of HFA children and 50–100 % of TD children. A significant group difference was observed for the odors of feces (75 % of HFA children and 25 % of TD children) and sweat (75 % of HFA children and 12.5 % of TD children), χ2 (1, N = 16) = 4.0, and 6.3, respectively; ps < .05.
Children were also asked to identify each odor. Performance was determined by assigning a score (0—wrong answer, 1—category descriptor, 2—precise name) for each of 8 odors. Levels of identification were low overall (HFA children Mdn = 1; TD children Mdn = 1). There were not differences between groups, except for chlorine, in which TD children exhibited higher levels of identification (HFA children Mdn = 1; TD children Mdn = 2) than children with HFA children, χ2 (1, N = 16) = 5.5, p < .05.
Children were next asked to classify the odors as pleasant, unpleasant or neutral. There were no significant differences between groups for the odors of vanilla, rose, and mint. These odors were judged as pleasant by 87.5, 100 and 100 % of the TD children and by, respectively, 50, 75 and 63 % of the HFA children. Feces, cheese, grass, sweat, and chlorine were rated as unpleasant by 87.5–100 % of the TD children. However, only 25 % of HFA children rated the odor of chlorine as unpleasant, and only 50 % rated the odors of sweat and feces, and 62.5 the odors of grass and cheese as unpleasant. The tendency of TD children to perceive these odors as more unpleasant than children with HFA was significant for three odors: feces, chlorine and sweat, χ2 (1, N = 16) = 4.0, 9.6 and 4.0, respectively, ps < .05.
Agreement Between Facial and Verbal Responses
Within the HFA and TD groups, we tested for the mean proportion of correspondence between hedonic classification as revealed by facial expressions and hedonic classification as reported verbally. Each participant’s facial responsiveness was classified as positive, negative, or neutral/ambiguous. The presence of a smile indexed pleasantness; nose wrinkling and/or an upper lip raising indexed unpleasantness; other AUs lead to a classification of neutral/ambiguous. Each group was composed of 8 children who were each exposed to 8 odors, yielding 64 total responses. The responses were considered to be in agreement if, for example, a child who exhibited a positive facial expression to an odor classified that odor as pleasant in the verbal rating task. The response was not considered in agreement if, for example, the child classified a positive facial expression as unpleasant or neutral. As a whole, children with HFA children displayed a significantly lower mean proportion of responses in agreement with their facial expressions than TD children (55 % responses in children with HFA children vs. 91 % TD children), χ2 (1, N = 16) = 5.1, p < .05.
To follow up on these group differences in levels of agreement between facial and verbal responses, we examined levels of agreement between facial and verbal responses to each odor. There were no significant differences between groups for the odors of vanilla and rose (both were 75 % in TD children and 62.5 % in children with HFA), and mint (100 % in TD children and 62.5 % in HFA). For the odor of feces, chlorine and sweat TD children’s responses were 100 % in agreement, while the response of children with HFA were 50 %. In response to the odor of grass and cheese TD children’s verbal and facial responses were 87.5 % in agreement while the responses of children with HFA were 62.5 and 50 % respectively. The significant differences were for chlorine, feces and sweat, χ2 (1, N = 16) = 4.27, respectively, p < .05.
Discussion
Individuals with autism demonstrate difficulties in emotional functioning, but it is not clear whether these difficulties are due to an alteration in the behavioral expression of emotion, difficulties in autonomic reaction, or differences in reporting subjective emotional experience. The current study examined heart rate, facial behavior and self-report in response to pleasant and unpleasant odors in HFA and TD children. Findings suggest only subtle differences between HFA and TD children with respect to their facial and autonomic reactions to odors, but difficulties in how the HFA children report their emotional reactions.
Data in this study were obtained from a relatively small group of eight HFA and eight well-matched TD children. The HFA sample was, nevertheless, relatively homogeneous with respect to IQ and all children were in an age-appropriate grade level. Global facial responses to pleasant and unpleasant stimuli were similar in HFA and TD children. In both groups of children, expressions of disgust were more frequent during unpleasant stimuli while smiles were more frequent during pleasant odors. The capacity of high functioning children with autistic spectrum disorder to facially express basic emotions in response to odors extends similar findings obtained by Soussignan et al. (1995) among low functioning children with autism.
Despite relatively intact emotional expression, there were subtle differences in facial functioning between the groups. With respect to controls, we found fewer muscle movements in the lower face in children with HFA, and a lower intensity and lower duration of muscle movements in the eye and mouth region. In conjunction with Czapinski and Bryson (2003) report of reduced and weak muscle in the eye and mouth regions in young children with high functioning autism during semi-structured play, the current results suggest subtle decrements in the movement of the facial musculature in children with autism, and the importance of continued research into the source of these differences.
Autonomic responses to the majority of odors did not yield differences between groups. The global behavioral and physiological responses do not suggest a deficit in olfactory sensitivity among HFA children. These results support and extend Suzuki et al.’s (2003) report on the intact olfactory functioning of HFA children that was based only on verbal responses to artificial stimuli (Suzuki et al. 2003). The current results indicate intact olfactory and physiological functioning in response to odors related to daily life such as grass, sweat, feces, and cheese, which lend ecological significance to the current results. Relatively normal autonomic arousability in response to odors is consonant with unremarkable autonomic responses to affect inductions using other types of stimuli such as viewing pleasant and unpleasant pictures (Shalom et al. 2006; Bölte et al. 2008), viewing pictures displaying the distress of others (Blair 1999), and experiencing a separation from the mother (Willemsen-Swinkels et al. 2000).
Despite a lack of between-group differences in response to the majority of odors, subtle differences between groups were present in response to the odors of mint and chlorine. Children with HFA exhibited lower skin conductance in response to the odor of mint and lower heart rate in response to chlorine than TD children. In comparison with the other odors utilized whose detection directly stimulates the olfactory bulb, the odors of mint and chlorine stimulate the trigeminal nerve (cranial nerve V). Recent work (Hummel 2009; Smeets and Dalton 2005) suggests that the trigeminal pathway is also stimulated by painful stimuli and may be perceived as irritating. Children with ASD are sometimes reported to be relatively insensitive to pain in intensity ratings (Kaplan et al. 1994; Kalat 1978). A lowered reactivity to odors eliciting trigeminal activation may be related to a lowered pain sensitivity.
With respect to verbal responses, both groups rated odors as relatively intense. As in previous reports, there were no group differences, suggesting that children with HFA did not suffer from a deficit in olfactory sensitivity (Bennetto et al. 2007; Suzuki et al. 2003). Turning to children’s reports of familiarity, a higher percentage of children with HFA reported the odors of sweat and feces to be familiar than did TD children. It may be that children with HFA were less embarrassed than TD children in reporting familiarity with these odors. With respect to identification, children in both groups were able to provide a category descriptor (but not a precise name) for the odors. Exceptions were mint, chlorine and rose in which TD children, but not HFA children, were able to precisely identify the odors. The results are similar to other research reporting olfactory identification deficits in people with high functioning autism (Bennetto et al. 2007; Suzuki et al. 2003; May et al. 2010).
While there were no differences between groups in judging the pleasantness of vanilla, rose, and mint, TD children were more likely to evaluate feces, cheese, grass, sweat, and chlorine odors as unpleasant than children with HFA. Moreover, while more than 90 % of TD children’s verbal reports matched their facial reactions to the odors, this was the case for only 55 % of the responses of children with HFA. Specifically, agreement between facial and verbal reactions to feces, sweat, grass and cheese was higher in TD children than children with HFA. Frequently children with HFA produced a negative facial expression in response to these odors but did not report that the odors were unpleasant.
The lack of agreement may help explain the basis for difficulties with emotion processing in individuals with HFA noted by others. Hrdlicka et al. (2011) also reported differences in evaluation of pleasantness, suggesting that pleasure induced by odors could be attenuated in the case of autism. Rieffe et al. (2007) noted deficits among children with HFA in identifying the circumstances in which they or others would experience basic negative emotions (anger, sadness and fear). Likewise, Hill et al. (2004) found that adults with HFA exhibited higher levels of depressive symptomatology than controls, but had difficulty identifying their own emotions. The currently results suggest that these problems may stem from a difficulty verbally identifying more basic emotional reactions to commonly experienced stimuli. In sum, even high-functioning individuals adults and children with HFA seem to have trouble being aware of their emotional experience and linking that with a verbal report.
In conclusion, the current investigation suggests relatively intact facial and autonomic responsitivity along with an autism-related deficit in reporting one’s own emotional responses. Together with related findings, this potential deficit might suggest an impairment in the connectivity between the amygdala and functionally associated cortical areas (Phan et al. 2002; Sabbagh et al. 2004; Bachevalier and Loveland 2006; Carper and Courchesne 2005). This interpretation is supported by recent findings of abnormal brain connectivity in the autism spectrum disorders such as local over-connectivity and long-distance under-connectivity (Wass 2011). Specifically, disruptions in later-developing cortical regions such as prefrontal cortex may be implicated in the disjunction between autonomic and facial responsivity to odors and verbal report of emotional response.
Acknowledgments
The authors wish to thank the children and parents who accepted to participate to this study. This research was supported by the Regione Friuli Venezia Giulia (Italy), the Centre National de la Recherche Scientifique (France) and the French Ministry of Health (PHRC program to Luc Marlier); Daniel Messinger’s involvement in this project was supported by grants from Autism Speaks, the National Science Foundation (1052736 & 0808767), and the National Institutes of Health (HD047417 & HD057284). We gratefully thank: Manuel Devetak for informatic support, William Geroldini, Thierry Pebayle, and Arnaud Eschenlauer for their technical support, Franco De Marchi, Clara Sforzina and Giovanni Grube for their organisation in the recruitment of subjects.
Contributor Information
Jasna Legiša, Email: jlegisa@units.it, Università degli Studi di Trieste, Friuli Venezia Giulia, Italy. Centro Ricerca FACS, Friuli Venezia Giulia, Italy. Centre National de la Recherche Scientifique, Alsace, France. Université de Strasbourg, Alsace, France. Centro Ricerca FACS, Via Artemidoro 11, Trieste, Italy.
Daniel S. Messinger, University of Miami, Coral Gables, FL, USA
Enzo Kermol, Università degli Studi di Trieste, Friuli Venezia Giulia, Italy. Centro Ricerca FACS, Friuli Venezia Giulia, Italy.
Luc Marlier, Centre National de la Recherche Scientifique, Alsace, France. Université de Strasbourg, Alsace, France.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. The American Psychiatric Association; Washington D.C: 1994. DSM-IV. Traslation it. (1995). Manuale Diagnostico e Statistico dei Disturbi mentali. Masson, Milano. [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland K. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neuroscience and Biobehavioral Reviews. 2006;30:97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Baker AEZ, Lane A, Angley MT, Young RL. The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. Journal Autism and Developmental Disorders. 2008;38:867–875. doi: 10.1007/s10803-007-0459-0. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Neuroanatomic observations of the brain in autism: A review and future directions. International Journal of Developmental Neuroscience. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bennetto L, Kurschner ES, Hyman SL. Olfaction and taste processing in autism. Biological Psychiatry. 2007;62:1015–1021. doi: 10.1016/j.biopsych.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Carter, et al. Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. The American Journal of Occupational Therapy. 2007;61(5):584–592. doi: 10.5014/ajot.61.5.584. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Psychophysiological responsiveness to the distress of others in children with autism. Personality and Individual Differences. 1999;26:477–485. [Google Scholar]
- Bölte S, Feineis-Matthews S, Poustka F. Brief report: Emotional processing in high-functioning autism—physiological reactivity and affective report. Journal of Autism and Developmental Disorders. 2008;38(4):776–781. doi: 10.1007/s10803-007-0443-8. [DOI] [PubMed] [Google Scholar]
- Capps L, Yirmiya N, Sigman M. Understanding of simple and complex emotions in non-retarded children with autism. Journal of Child Psychology and Psychiatry. 1992;33(7):1169–1182. doi: 10.1111/j.1469-7610.1992.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Capps L, Sigman M, Yirmiya N. Self-competence and emotional understanding in high-functioning children with autism. Development and Psychopathology. 1995;7:137–149. [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biological Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Czapinski P, Bryson SE. Reduced facial muscle movements in autism: Evidence for dysfunction in the neuromuscular pathway. Brain and Cognition. 2003;51:177–179. [Google Scholar]
- Ekman P. Emotion in the human face. Cambridge University Press; 1982. [Google Scholar]
- Ekman P, Friesen WV. The facial action coding system: A technique for the measurement of facial movement. Palo Alto: Consulting Psychologist Press; 1978. [Google Scholar]
- Ekman P, Friesen WV, Hager JC. Facial action coding system. 2. Salt Lake City, UT: Research Nexus ebook; 2002. [Google Scholar]
- Gillberg Neurodevelopmental processes and psychological functioning in autism. Development and Psychopathology. 1999;11(3):567–587. doi: 10.1017/s0954579499002217. [DOI] [PubMed] [Google Scholar]
- Hill E, Berthoz S, Frith U. Brief report: Cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. Journal of Autism and Developmental Disorders. 2004;34:229–235. doi: 10.1023/b:jadd.0000022613.41399.14. [DOI] [PubMed] [Google Scholar]
- Hilton CL, Harper JD, Kueker RH, Lang AR, Abbacchi AM, Todorov A, et al. Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40(8):937–945. doi: 10.1007/s10803-010-0944-8. [DOI] [PubMed] [Google Scholar]
- Hrdlicka M, Vodicka J, Havlovicova M, Urbanek T, Blatny M, Dudova I. Brief report: Significant differences in perceived odor pleasantness found in children with ASD. Journal of Autism and Developmental Disorders. 2011;41(4):524–527. doi: 10.1007/s10803-010-1084-x. [DOI] [PubMed] [Google Scholar]
- Hummel T. Central processing of trigeminal activation in humans. Annals of the New York Academy Of Sciences, Interantional symposium on Olfaction and Taste. 2009;1170:190–195. doi: 10.1111/j.1749-6632.2009.03910.x. [DOI] [PubMed] [Google Scholar]
- Jung J, Hudry J, Ryvlin P, Royet JP, Bertrand O, Lachaux JP. Functional Significance of olfactory-induced oscillations in the human amygdala. Cerebral Cortex. 2006;16:1–8. doi: 10.1093/cercor/bhi090. [DOI] [PubMed] [Google Scholar]
- Kalat JW. Speculations on similarities between autism and opiate addiction. Journal of Autism and Childhood Schizophrenia. 1978;8:477–479. doi: 10.1007/BF01538051. [DOI] [PubMed] [Google Scholar]
- Kaplan HI, Sadock BJ, Grebb JA. Kaplan and Sadock’s synopsis of psychiatry: Behavioral sciences, clinical psychiatry. 7. Baltimore: Williams and Wilkins; 1994. [Google Scholar]
- Kientz MA, Dunn W. A comparison of the performance of children with and without autism on the sensory profile. The American Journal of Occupational Therapy. 1997;51(7):530–537. doi: 10.5014/ajot.51.7.530. [DOI] [PubMed] [Google Scholar]
- Leary M, Hill D. Moving on: Autism and movement disturbance. Mental Retardation. 1996;34(1):39–53. [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain. New York: Simon & Schuster; 1996. [Google Scholar]
- Leekam S. Why do children with autism have a joint attention impairment? In: Eilan N, Hoerl C, McCormack T, Roessler J, editors. Joint attention: Communication and other minds. Oxford: Oxford University Press; 2005. [Google Scholar]
- Losh M, Capps L. Understanding of emotional experience in autism: Insights from the personal accounts of high-functioning children with autism. Developmental Psychology. 2006;42(5):809–818. doi: 10.1037/0012-1649.42.5.809. [DOI] [PubMed] [Google Scholar]
- May T, Brewer TW, Rinehart NJ, Enticott PG, Brereton AV, Bruce J, et al. Differential olfactory identification in children with autism and Asperger’s disorder: A comparative and longitudinal study. Journal of Autism and Developmental Disorders. 2010;41(7):837–847. doi: 10.1007/s10803-010-1101-0. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Rieffe C, Terwogt MM, Kotronopoulou K. Awareness of single and multiple emotions in high-functioning children with autism. Journal of Autism and Developmental Disorders. 2007;37(3):455–465. doi: 10.1007/s10803-006-0171-5. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, De Araujo IET. Different representations of pleasant and unpleasant odours in the human brain. European Journal of Neuroscience. 2003;18(3):695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA. Neural systems underlying decisions about affective odors. Journal of Cognitive Neuroscience. 2010;22(5):1069–1082. doi: 10.1162/jocn.2009.21231. [DOI] [PubMed] [Google Scholar]
- Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. NeuroImage. 2003;20:713–728. doi: 10.1016/S1053-8119(03)00388-4. [DOI] [PubMed] [Google Scholar]
- Sabbagh MA, Moulson M, Harkness KL. Neural correlates of mental state recognition in human adults: An ERP study. Journal of Cognitive Neuroscience. 2004;16:415–426. doi: 10.1162/089892904322926755. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, Rochen Renner B. The childhood autism rating scale (CARS) for diagnostic screening and classification of autism. New York: Irvington; 1986. [Google Scholar]
- Schroeder JH, Desrocher M, Bebko JM, Cappadocia MC. The neurobiology of autism: Theoretical applications. Research in Autism Spectrum Disorders. 2010;4:555–564. [Google Scholar]
- Shalom BD, Mostofsky SH, Hazlett RL, Goldberg MC, Landa RL, Faran Y, et al. Normal physiological emotions but differences in expression of conscious feelings in children with high-functioning autism. Journal of Autism and Developmental Disorders. 2006;36(3):395–400. doi: 10.1007/s10803-006-0077-2. [DOI] [PubMed] [Google Scholar]
- Smeets MA, Dalton P. Evaluating the human response to chemicals: Odor, irritation and non-sensory factors. Environmental Toxicology and Pharmacology. 2005;19(3):581–588. doi: 10.1016/j.etap.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Soudry Y, Lemogne C, Malinvaud C, Consoli SM, Bonfils P. Olfactory system and emotion: Common substrates. European Annals of Otorhinolaryngology Head and Neck Diseases. 2011;128(1):18–23. doi: 10.1016/j.anorl.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Soussignan R, Schaal B, Schmit G, Nadel J. Facial responsiveness to odours in normal and pervasively developmentally disordered children. Chemical Senses. 1995;20:47–59. doi: 10.1093/chemse/20.1.47. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Critchley HD, Rowe A, Howlin P, McMurphy DGM. Impaired olfactory identification in Asperger’s syndrome. Journal of Neuropsychiatry and Clinical Neuroscience. 2003;15:105–107. doi: 10.1176/jnp.15.1.105. [DOI] [PubMed] [Google Scholar]
- Tomcheck SD, Dunn W. Sensory processing in children with and without autism: A comparative study using the short sensory profile. American Journal of Occupational Therapy. 2007;61(2):190–200. doi: 10.5014/ajot.61.2.190. [DOI] [PubMed] [Google Scholar]
- Wass S. Distortions and disconnections: Disrupted brain connectivity in autism. Brain and Cognition. 2011;75(1):18–28. doi: 10.1016/j.bandc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Watling RI, Deitz J, White O. Comparison of sensory profile scores of young children with and without autism spectrum disorders. American Journal of Occupational Therapy. 2001;55:416–423. doi: 10.5014/ajot.55.4.416. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler intelligence scale for children. 3. San Antonio, XT: The Psychological Corporation; 1991. [Google Scholar]
- Willemsen-Swinkels SHN, Bakermans-Kranenburg MJ, Buitelaar JK, van Ijzendoorn MH, Van Engeland H. Insecure and disorganized attachment in children with autistic behavior: Relationship with social interaction and heart rate. Journal of Child Psychology and Psychiatry. 2000;41:759–767. [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction and the amygdala: Amygdala activation during aversive olfaction in humans. Proceedings of the National Academy of Sciences. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]