Abstract
Purpose of review
To discuss non-daily pre-exposure prophylaxis (PrEP) modalities that may provide advantages compared with daily PrEP in cost and cumulative toxicity, but may have lower adherence forgiveness.
Recent Findings
Animal models have informed our understanding of early viral transmission events, which help guide event-driven PrEP dosing strategies. These models indicate early establishment of viral replication in rectal or cervicovaginal tissues, so event-driven PrEP should rapidly deliver high mucosal drug concentrations within hours of the potential exposure event. Macaque models have demonstrated the high biological efficacy for event-driven dosing of oral tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) against both vaginal and rectal virus transmission. In humans, the IPERGAY study demonstrated 86% efficacy for event-driven oral TDF/FTC dosing among men who have sex with men (MSM), while no similar efficacy data are available on women or heterosexual men. The HPTN 067 study showed that certain MSM populations adhere well to non-daily PrEP while other populations of women adhere more poorly to non-daily versus daily regimens. Pharmacokinetic studies following oral TDF/FTC dosing in humans, indicate that TFV-diphosphate (the active form of TFV) accumulates to higher concentrations in rectal versus cervicovaginal tissue but non-adherence in trials complicates the interpretation of differential mucosal drug concentrations.
Summary
Event-driven dosing for TFV-based PrEP has promise for HIV prevention in MSM. Future research of event-driven PrEP in women and heterosexual men should be guided by a better understanding of the importance of mucosal drug concentrations for PrEP efficacy and its sensitivity to adherence.
Keywords: HIV prevention, microbicides, tenofovir, macaque model, cellular pharmacology, adherence
Introduction
In 2012 the US Food and Drug Administration approved daily tenofovir disoproxil fumarate (TDF) in combination with oral emtricitabine (FTC) (Truvada) for pre-exposure prophylaxis (PrEP) to prevent HIV infection in men and women.(1) The decision to approve daily Truvada for PrEP was based on efficacy of 44–75% in several placebo-controlled PrEP trials.(2–4) These trials also highlighted the importance of adherence to the daily oral regimen as only ~50–80% of active arm participants had consistently quantifiable tenofovir in plasma, a biomarker of adherence. Quantifiable drug in plasma increased efficacy estimates to ~74–92%. Very low adherence (~30%) likely contributed to why two clinical trials among young African women (VOICE and FEM-PrEP) failed to show any efficacy of either daily TDF or Truvada.(5, 6) The clinical development of PrEP has benefited from animal studies that have provided proof-of-concept of efficacy of this prevention strategy and informed trial designs.
Following the demonstration of efficacy by daily TDF/FTC in macaques, attention has shifted to assessment of non-daily TDF/FTC regimens as these regimens might align better with episodic sexual practices, and result in lower drug toxicities and reduced costs compared with daily regimens. Event-driven or on-demand dosing has been the focus of microbicides development, a related PrEP strategy delivering topical antiretroviral drugs through gels or other formulations. Here, we will review the preclinical and clinical information on non-daily PrEP as a strategy for HIV prevention, with a focus on event-driven dosing.
Virologic considerations
The goal of event-driven PrEP is to deliver effective drug concentrations to HIV exposed tissues and/or systemically at the time of potential HIV exposure, and at a time when HIV is most vulnerable.(7) This will require strategic dosing relative to the HIV exposure. This differs from the goal of daily PrEP which is to sustain effective steady-state drug concentrations in HIV exposed tissues and/or systemically throughout therapy to protect the individual should an HIV exposure take place. HIV is most vulnerable during the initial hours to days following an HIV exposure.(8) The events during this vulnerable time have been elucidated for simian immunodeficiency virus (SIV) infection in macaques, as a model for HIV in humans.(7–10) After SIV inoculation in the vaginal vault, virus is rapidly diluted and cleared except for some trapping in mucus. A low number of viruses may cross the mucosal barrier (higher numbers if the mucosa is not intact) where they may encounter and infect a small number of CD4-bearing cells creating a founder population in the mucosal tissue. This process starts within hours and takes about 1–3 days to become established. It is thought that the virus is generally localized to the mucosal tissue during this time, but virus has also been observed in lymph nodes, so this may not be absolute.(10) It is during these early events that the virus is most vulnerable, so event-driven antiretroviral prophylaxis will be most effective when drug is present within the first hours of exposure to HIV.(7)
The length of time that the drug must be present is less clear for event-driven dosing. It is likely that the length of time will be dependent on whether any viral replication may have taken place initially. Theoretically, if effective drug concentrations were present early, this would prevent any viral replication compared with a situation where drug concentrations were not present or high enough such that early rounds of viral replication were to have occurred. In such a case the duration of drug action would need to be longer to adequately cover the time to prevent viral spread. This was manifested in PEP studies in macaques where short treatment of 2–3 days with either tenofovir or tenofovir/FTC combination initiated 24 hours after exposure were not protective whereas longer treatment durations up to four weeks were needed for efficacy (11, 12) This is a compelling rationale for preventing early stages of replication with effective drug concentrations.
Pharmacologic considerations
It is important that the PrEP agents distribute and adequately accumulate in mucosal tissue (e.g. vaginal, rectal, penile) where these initial virologic events are localized.(13, 14) The possibility of virus escaping to lymph nodes at early time points suggests that systemic drug concentrations may be important as a “back-up” for this potential scenario. The relative importance of systemic versus mucosal drug concentrations has not been evaluated. It is possible that “back-up” systemic drug may contribute to PrEP efficacy. For example, PrEP trials of oral TDF-FTC dosing (eg (2, 4)) generally performed better compared with PrEP trials of topical dosing with tenofovir gel (eg (15, 16)), where mucosal drug concentrations exceed those of oral dosing by >100-fold.(17). However, it is unclear if the lower effectiveness of topical dosing reflects a lower biological efficacy or simply a generally lower adherence to the topical product. In macaques both oral TDF/FTC and tenofovir gel were found to be highly protective.(12, 18–21)
Mucosal drug distribution studies have focused on cervicovaginal and rectal tissues as these compartments are more accessible compared with penile tissue. Vaginal and rectal drug distribution has been elucidated for multiple antiretrovirals (see reviews(14, 22)) including the current oral PrEP agents, tenofovir and emtricitabine. These agents are nucleos(t)ide analogs that require phosphorylation in cells for pharmacologic activation. The resulting intracellular anabolites, tenofovir-diphosphate (TFV-DP) and emtricitabine-triphosphate (FTC-TP), are ion-trapped thereby extending their half-life at the site of action.(23) The longer half-life results in a buildup from the first dose to steady-state concentrations. This build up takes several half-lives to reach the steady state plateau. This is relevant for event-driven dosing because single (or a few) doses will result in low concentrations relative to steady-state concentrations achieved with daily dosing. These lower concentrations suggest a lower margin for error in terms of missed doses for event-driven dosing. Table 1 lists half-lives, accumulation factor, and time to reach steady-state, as well as genital and rectal distribution relative to blood for TFV-DP and FTC-TP following oral dosing.(24–27) These pharmacokinetic parameters appear to be similar in the macaque model.(12, 28)
Table 1.
| Systemic (PBMC) | Rectal | Vaginal | |||
|---|---|---|---|---|---|
| T-1/2 (days) | Tss (days) | AF (fold | Ratio with PBMC | Ratio with PBMC | |
| TFV-DP | 2 to 4 | 7 to 12 | 5 to 7 | 4 to 30 | 1 to 3 |
| FTC-TP | 1 to 2 | 3 to 7 | 2 to 3 | 1/6th | 1 to 2 |
Tss=time to 90% of steady-state
AF=fold accumulation between a single dose and steady-state
TFV-DP accumulates to several-fold higher concentrations in rectal compared to vaginal tissue and cells, suggesting preferential activity for prophylaxis of rectal HIV transmission.(22, 29, 30) Indeed, high activity has been observed for TDF-containing PrEP regimens in clinical trials among MSM,(3, 31, 32) as alluded to above and discussed further below. A potential mechanism for high TFV in the rectal tissue may be its ~30% bioavailability (33) such that 70% of the dose remains in the gastrointestinal tract providing high local concentrations. Conversely, FTC-TP accumulates quickly but relatively poorly in rectal tissue and cells (consistent with nearly complete bioavailability) (34), with better concentrations in vaginal tissue and cells, suggesting high activity for vaginal transmission. However, the Partner’s PrEP study did not find an efficacy difference for TDF alone versus TDF plus FTC among the women subgroup.(35) Penile drug distribution has not been adequately studied in humans, but limited data in macaques following oral TDF/FTC dosing show TFV-DP concentrations in urethra and foreskin within the range seen in vaginal tissues (CDC, unpublished data). Furthermore, the high PrEP efficacy observed in heterosexual men suggests sufficient drug exposure from daily oral dosing.(2, 4, 35)
While the results of clinical trials suggest high efficacy against cervicovaginal, rectal, and penile HIV exposures when adherence is high,(4, 36–39) efficacy at lower adherence levels is difficult to interpret. For instance, the placebo-controlled iPrEx trial of daily TDF-FTC in MSM found 42% efficacy (95% CI, 18% to 60%) for approximately 44% adherence as measured by quantifiable drug in blood.(36) The FEMPREP and VOICE trials in women found a lack of efficacy (95% CIs, −52% to 41% and −49% to 27%) for quantifiable drug rates of 35% and 29%, respectively.(5, 6) It is possible that differential drug penetration in mucosal tissue, as described above, could at least partly explain these findings, but the confidence intervals are wide and inconclusive .(22, 40) Nevertheless, the differences in mucosal PK may imply that non-daily dosing such as event-driven dosing may have less pharmacokinetic forgiveness for cervicovaginal or possibly penile exposures compared with rectal exposures. This should be an area of more research in terms of non-daily PrEP for vaginal and penile HIV prevention.
Animal model support
Macaque models of SIV or SHIV (a SIV/HIV chimera) transmission have been extensively used to investigate the efficacy of tenofovir and FTC in preventing HIV infection either as pre-exposure or post-exposure prophylaxis (PEP). Studies on SIV-exposed macaques receiving PEP with tenofovir showed that PEP was most effective when initiated soon after exposure and continued for 4 weeks, and helped define guidelines to manage occupational and non-occupational HIV exposures in humans.(11, 41) Indications that ARV drugs administered as PrEP could also prevent infection came from studies with subcutaneous TFV and oral SIV inoculations.(42, 43) More recent work using oral doses of FTC and TDF that recapitulate human therapeutic doses provided proof of concept of efficacy for daily PrEP with FTC/TDF.(19) In addition to using clinically relevant FTC/TDF doses, these studies used novel repeat low-dose macaque models that better mimic human transmission of HIV in many aspects including the use of a SHIV162p3 isolate containing an R5-tropic envelope similar to that of most transmitted HIV, an inoculum dose within the range of HIV-1 RNA levels in semen during acute infection, and once or twice weekly virus challenges to mimic high risk human HIV exposure.(44, 45) PrEP evaluations with the repeat low dose model generally use rhesus or pigtail macaques. While both macaque species are suitable for rectal efficacy studies, analysis of vaginal efficacy has focused on pigtail macaques because pigtails have lunar menstrual cycles and changes in hormone levels similar to humans.(46, 47) The pigtail macaque menstrual cycle averages 32.8 days and recapitulates potential fluctuations in susceptibility to HIV or SIV infection associated with the follicular and luteal phase of the menstrual cycle.(21, 47, 48) Pigtails can also be easily infected vaginally with low virus doses without the use of exogenous progestins such as depot-medroxyprogesterone acetate (DMPA).(21, 47, 49, 50) While treatment of rhesus macaques with high DMPA doses also ensures vaginal infection at lower virus doses, this DMPA dose does not fully reproduce the biological effects of DMPA seen in women.(50)
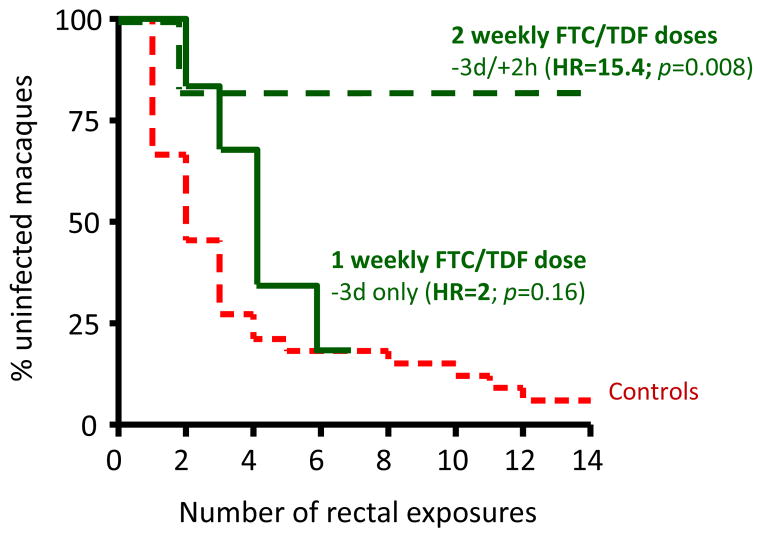
Studies with the repeat low dose model have assessed the potential for time-driven and event-driven intermittent PrEP usage as an alternative to daily PrEP.(12) Time-driven modalities were selected to model regimens containing 1 or 2 weekly pills followed by a booster post-exposure dose. For these studies, macaques received FTC/TDF orally either 3 or 7 days before SHIV challenge followed by a post-exposure dose given 2 hours later. Both scenarios were highly protective with reductions in the risk of infection of 15.4 and 9.3 (Table 2).(12) Interestingly, protection by the −3 day PrEP dose was lost when the booster dose 2 hours after challenge was not administered to the macaques (Figure 1), indicating that the duration of drug action was not long enough with the single pre-exposure dose. Although the superior efficacy in the −3d/+2h group may be explained by the high TFV-DP concentrations achieved with 2 weekly FTC/TDF doses,(28) these findings also highlighted the importance of the post-exposure dose for efficacy.
Table 2.
Efficacy of time-driven and event-driven PrEP in macaques
| Regimen (number of FTC/TDF pills)* | Macaque species | Route of virus exposure | No. animals infected/total | Reduction in risk of SHIV infection¥ |
|---|---|---|---|---|
|
| ||||
| Time-driven | ||||
| 3d before/2h after (1:1) | Rhesus | Rectal | 1/6 | 15.4; p=0.008 |
| 7d before/2h after (1:1) | Rhesus | Rectal | 2/6 | 9.3; p=0.003 |
| Event-driven | ||||
| 24h before/2h after (1:1) | Rhesus | Rectal | 1/6 | 16.7; p=0.006 |
| 2h before/24h after (1:1) | Rhesus | Rectal | 3/6 | 4.1; p=0.02 |
| 2h before/2h after (2:2) | Rhesus | Rectal | 1/6 | 15.4; p=0.008 |
relative to the time of SHIV exposure;
compared to the untreated control group
Figure 1.
Kaplan-Meier analysis and reduction in the risk of SHIV infection by FTC/TDF (−3d/+2h or -3d only)
In a different design macaques received two FTC/TDF regimens modeling event-driven PrEP usage. These regimens were initiated 24h or 2h prior to virus exposure and also contained a booster PEP dose given 24h after the initial PrEP dose. Table 2 shows that the −24h/+2h modality was highly protective with a 16.7-fold reduction in the risk of infection. Although the −2h/+24h modality also reduced the risk of infection significantly, efficacy in this scenario was substantially increased when the dose of FTC/TDF was doubled (Table 2).(12) The efficacy of this double FTC/TDF regimen has informed the design of the IPERGAY trial described below.
The aforementioned studies have provided extensive proof of concept for time-driven and event-driven PrEP regimens against rectal HIV transmission. Studies with on-demand PrEP against vaginal transmission in macaques also demonstrated high efficacy but are limited to the evaluation of a single FTC/TDF event-driven modality (Table 3). Modifications of this model to assess the impact of factors that can increase HIV susceptibility such as sexually transmitted infections (STIs) or DMPA provided important information. Prolonged administration of a physiologic dose of DMPA was found to have no impact on the efficacy of a −24h/+2h FTC/TDF regimen. .(21, 49, 50) The high protection seen with DMPA was reassuring since DMPA is the most commonly used method of contraception in sub-Saharan Africa and has been associated with higher risk of HIV infection in some studies.(51, 52) Assessing the same PrEP modaltity in macaques co-infected with Chlamydia trachomatis and Trichonomas vaginalis also found protection although infection in some animals signaled a modest loss of PrEP activity due to these two STIs.(53). No macaque data are available against penile SHIV transmission. More research is needed to develop penile PrEP models in macaques to inform clinical trials.
Table 3.
Efficacy of peri-coital FTC/TDF against vaginal SHIV infection
| Regimen (number of FTC/TDF pills)* | Model characteristics | N. of animals infected |
|---|---|---|
|
| ||
| 24h before/2h after (1:1) | Pigtails with regular menstrual cycles | 0/6, p<0.001 |
| 24h before/2h after (1:1) | Pigtails treated with a physiologic DMPA dose | 0/6, p<0.001 |
| 24h before/2h after (1:1) | Pigtails co-infected with C trachomatis and T vaginalis | 2/6, p = 0.03 |
Human experience
The HPTN 067 (ADAPT) study compared adherence and “coverage of sex acts” for three oral TDF-FTC dosing regimens; daily, twice-weekly (3 to 4 days apart) with a post-sex dose, or event-driven dosing (24 to 48 hours before and 2 hours after sex).(54–56) Coverage of sex acts was defined as at least one TDF-FTC dose within 4 days preceding sex and within 24 hours after sex. This was determined with drug concentrations and self-report. The study was conducted in South African women, Thailand MSM, and Harlem MSM. The study was not powered to compare the efficacy of the dosing regimens. The initial findings suggested that MSM may have better adherence to time-driven and event dosing compared with women as measured by coverage of sex acts. The post-sex-dose was missed most often, which has been reported in other studies.(57) As mentioned above, event-driven PrEP is unlikely to have the same level of pharmacokinetic forgiveness as daily dosing. Furthermore, event-driven dosing also assumes the individual uses advanced planning for their sexual encounters and that they can predict which encounters may result in HIV exposure. However, some studies indicate that over half of MSM do not plan sex in advance.(58) These considerations will be important as non-daily dosing studies evolve.
Molina et al. undertook the IPERGAY trial in 414 men who have sex with men (MSM) in France and Canada.(31) IPERGAY was a placebo-controlled study that evaluated a double dose of TDF-FTC two to 24 hours before sex, another tablet 24 hours later, and a fourth tablet 48 hours after the initial dose. All participants received full preventative services. The relative risk reduction for TDF-FTC compared with placebo was 86% (95% CI 40 to 99); 2 infections in the TDF-FTC arm versus 14 in the placebo arm. The two infections in the TDF-FTC arm were in participants who had stopped using the product and did not have detectable drug in their plasma at the visit that infection was diagnosed (or the visit before), suggesting that biological efficacy may be even higher than 86%. IPERGAY provides invaluable information on the duration of drug action for event-driven PrEP in MSM, as it used 2 doses following the last potential exposure.
There has been some question about whether IPERGAY adequately tested event-driven PrEP, because many participants had frequent sex such that they dosed most days of the month.(59) The median number of tablets used per month in IPERGAY was 16 (10–23), i.e. 4 tablets per week on average, as measured by pill counts. The high efficacy in IPERGAY supports pharmacology analyses from the iPrEx trial in MSM that predicted high protection for 4 or more tablets per week on average.
Of the 48 active-arm seroconverters in iPrEx, only 3/42 (7%) had quantifiable TFV-DP concentrations in PBMC at the visit with first evidence of HIV infection; concentrations were 4.2, 10.5 and 14.7 fmol/106 PBMC.(36) An inferred HIV risk-reduction model was fit to the TFV-DP and HIV acquisition data using cases and controls. A TFV-DP concentration of 9 fmol/106 PBMC (95% 1 to 16) was associated with a 75% reduction in HIV risk (EC75) relative to placebo, the EC90 was16 fmol/106 cells (95% CI 3 to 28) and the EC99 was 33 fmol/106 cells (95% CI 6 to 60).(36) A separate pharmacokinetic study called STRAND(60) used directly observed dosing in 21 participants and defined expected TFV-DP concentrations for 2, 4 and 7 tablets per week; median concentrations were 11, 32, 42 fmol/106 cells, respectively. When the iPrEx model was used to analyze STRAND concentrations, the estimated HIV risk reduction was 76% (95% CI from 56% to 96%), 96% (95% CI from 90% to >99%) and 99% (95% CI from 96% to >99%) for 2, 4 and 7 doses per week.(36)
Similar findings were reported from the iPrEx OLE study among 1603 MSM participants, of who 1225 received PrEP. Of 28 infections during PrEP, 0/28 occurred in participants who had dried blood spot (DBS) concentration of TFV-DP ≥700 fmol/punch (100% efficacy, 95% CI 86% to 100%), and 1/28 occurred in those with a DBS between 350 and 699 fmol/punch (84% efficacy, 95% CI 21% to 99%).(38) These concentrations are consistent with ≥4 pills per week and 2–3 pills per week on average based on pharmacokinetic modeling.(61)
Another analysis with the same iPrEx model may be more relevant to sporadic event-driven dosing strategies. The study used data from Cell-PrEP, which analyzed TFV-DP arising from daily TDF-FTC over the first 7 days.(27) The iPrEx model showed an inferred HIV risk reduction of 77% (95% CI, 40%–93%) after 1 dose, 89% (51%–98%) after 2 doses, 96% (95% CI, 60%–100%) after 3 doses, and 98% (67%–100%) after 4 doses. The double dose used for the first dose in IPERGAY is expected to yield a doubling of TFV-DP concentrations,(24) corresponding with inferred risk reduction of approximately 89% (51%–98%) after the first dose. Maximal efficacy was predicted to occur after a week of daily dosing in CellPrEP, which was also when TFV-DP reached the highest level in rectal tissue. Taken together, these iPrEx analyses are consistent with the IPERGAY findings, suggesting that high efficacy with 4 successive doses in MSM, even if taken for infrequent sexual encounters.
The efficacy of event-driven dosing with oral PrEP has not been studied adequately in women or heterosexual men at risk for HIV. However, two large placebo-controlled trials in women studied topical 1% tenofovir gel within 12 hours before sex and again within 12 hours after sex. Topical delivery results in > 100-fold higher TFV-DP in mucosal tissue compared with oral dosing, but systemic concentrations in PBMC are much lower.(17) The high local drug concentrations were expected to yield high efficacy, but this was not observed. The CAPRISA trial showed that 39% efficacy (95% CI 6% to 60%) for this topical dosing strategy. However, the efficacy estimate was increased to 54% if adherence was >80% as measured by returned gel applicators, and 65% for a TFV concentration of ≥100 ng/mL in cervicovaginal fluid.(15, 62) The FACTS 001 trial was a similarly-designed with the same dosing strategy, but this trial showed no efficacy compared with placebo (0% efficacy, 95% CI −40% to 30%).(16) This study also evaluated the effect of adherence on efficacy and reported an estimated 52% efficacy (95% CI 3% to 77%) among women who reported sex within 10 days and who had quantifiable TFV in vaginal specimens. These studies suggest a certain level of biological efficacy for topical event-driven dosing of TFV in women particularly with high adherence but also illustrate the limitations of data and complexities of inferring efficacy when a high proportion of study participants is non-adherent. These data also suggest the need to identify more desirable topical products for women such as rings, fast dissolving vaginal formulations, or similar modalities that are currently under development.
While event-driven modeling in macaques demonstrated high vaginal efficacy in cycling and DMPA-treated macaques as well as in STI-infected macaques (as described above), it is important to note that event-driven oral TDF-FTC dosing in women may be more sensitive to adherence than in men given the lower mucosal drug distribution in vaginal tissue for TFV-DP.(22, 40) This potential lack of adherence forgiveness should be a consideration in testing future PrEP modalities in women.
Conclusion
Event-driven dosing may provide an option for people unwilling to commit to chronic daily oral therapy, and it would be expected to reduce drug costs and improve long-term safety. Event-driven dosing of TDF-FTC for MSM is supported by high efficacy in well-established animal models, efficacy in a clinical trial (IPERGAY), and substantial pharmacology data including iPrEx and Cell-PrEP that predict high efficacy for less than daily dosing. It will be interesting to see the next steps for studying event-driven TDF-FTC for MSM, such as demonstration projects, and how these data might inform recommendations for event-driven PrEP for MSM.
The potential for event-driven oral dosing of TDF-FTC for HIV prevention in women and heterosexual men is not yet known but is supported for cervicovaginal transmission by evidence of efficacy in macaque models.(21) Future research of event-driven PrEP in these populations should be guided by an understanding of the importance of mucosal drug concentrations for PrEP efficacy and defining efficacy sensitivity to adherence.
Key Points.
Lower cost, less toxicity, and better adherence are potential advantages of event-driven dosing.
Animal models support event-driven dosing for rectal and cervicovaginal exposures but data for penile exposures are not available.
Pharmacology studies show preferential accumulation of tenofovir-diphosphate in rectal versus cervicovaginal tissue.
Human studies show promise for event-driven dosing in MSM, but research is needed to guide the field for women and heterosexual men.
Event-driven PrEP is likely to have less forgiveness for missed doses compared with daily dosing.
Acknowledgments
The authors thank David Glidden for helpful discussions.
Funding: This work was supported by NIH/NIAID U01 AI 106499 and intramural funds from the Centers for Disease Control and Prevention. Partial support was also provided by an inter agency agreement # Y1-AI-0681-02 between the Centers for Disease Control and Prevention and the National Institutes of Health.
Footnotes
Conflicts of interest: Dr. Anderson receives study drug and contract work from Gilead Sciences. J. Gerardo Garcia-Lerma and Walid Heneine are named on a US government patent on inhibition of HIV infection through chemoprophylaxis.
Disclaimer: The findings and conclusions of this article are those of the authors and do not necessarily represent the official position of the Centers of Disease Control and Prevention.
References
- 1.FDA. [Accessed 08/25/2015];FDA approves first drug for reducing the risk of sexually acquired HIV infection. 2012 Jul 16; Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm312210.htm.
- 2.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. Epub 2012/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. Epub 2010/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi: 10.1056/NEJMoa1110711. Epub 2012/07/13. [DOI] [PubMed] [Google Scholar]
- 5**.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. Epub 2015/02/05. Major clinical trial including oral and topical tenofovir-based dosing among women. Adherence greatly impacted the lack of efficacy observed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. doi: 10.1056/NEJMoa1202614. Epub 2012/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Lerma JG, Paxton L, Kilmarx PH, Heneine W. Oral pre-exposure prophylaxis for HIV prevention. Trends Pharmacol Sci. 2010;31(2):74–81. doi: 10.1016/j.tips.2009.10.009. Epub 2009/12/08. [DOI] [PubMed] [Google Scholar]
- 8.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annual review of medicine. 2011;62:127–39. doi: 10.1146/annurev-med-080709-124959. Epub 2010/11/09. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458(7241):1034–8. doi: 10.1038/nature07831. Epub 2009/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79(14):9217–27. doi: 10.1128/JVI.79.14.9217-9227.2005. Epub 2005/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai C-C, Emau P, Follis KE, Beck TW, Benveniste RE, Bischofberger N, et al. Effectiveness of Postinoculation (R)-9-(2-Phosphonylmethoxypropyl)Adenine Treatment for Prevention of Persistent Simian Immunodeficiency Virus SIVmne Infection Depends Critically on Timing of Initiation and Duration of Treatment. J Virol. 1998;72(5):4265–73. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Lerma JG, Cong ME, Mitchell J, Youngpairoj AS, Zheng Q, Masciotra S, et al. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci Transl Med. 2010;2(14):14ra4. doi: 10.1126/scitranslmed.3000391. Epub 2010/04/08. [DOI] [PubMed] [Google Scholar]
- 13.Heneine W, Kashuba A. HIV Prevention by Oral Preexposure Prophylaxis. Cold Spring Harbor perspectives in medicine. 2012;2(3):a007419. doi: 10.1101/cshperspect.a007419. Epub 2012/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson CG, Cohen MS, Kashuba AD. Antiretroviral pharmacology in mucosal tissues. J Acquir Immune Defic Syndr. 2013;63(Suppl 2):S240–7. doi: 10.1097/QAI.0b013e3182986ff8. Epub 2013/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. Epub 2010/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rees H, Delany-Moretlwe S, Lombard C, Baron D, Pandchia R, Myer L, et al. FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women. Conference on Retroviruses and Opportunistic Infections; 2015 Feb 23–26; Seattle, WA. [Google Scholar]
- 17.Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS ONE. 2013;8(1):e55013. doi: 10.1371/journal.pone.0055013. Epub 2013/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobard C, Sharma S, Martin A, Pau CP, Holder A, Kuklenyik Z, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol. 2012;86(2):718–25. doi: 10.1128/JVI.05842-11. Epub 2011/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Lerma JG, Otten RA, Qari SH, Jackson E, Cong ME, Masciotra S, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5(2):e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parikh UM, Dobard C, Sharma S, Cong M-e, Jia H, Martin A, et al. Complete Protection from Repeated Vaginal Simian-Human Immunodeficiency Virus Exposures in Macaques by a Topical Gel Containing Tenofovir Alone or with Emtricitabine. Journal of Virology. 2009;83(20):10358–65. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radzio J, Aung W, Holder A, Martin A, Sweeney E, Mitchell J, et al. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS ONE. 2012;7(12):e50632. doi: 10.1371/journal.pone.0050632. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Cottrell ML, Srinivas N, Kashuba ADM. Pharmacokinetics of antiretrovirals in mucosal tissue. Expert Opinion on Drug Metabolism & Toxicology. 2015;11(6):893–905. doi: 10.1517/17425255.2015.1027682. Comprehensive review of the biology and importance of mucosal drug disposition including a summary for various antiretroviral drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson PL, Kiser JJ, Gardner EM, Rower JE, Meditz A, Grant RM. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011;66(2):240–50. doi: 10.1093/jac/dkq447. Epub 2010/12/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hendrix CW, Andrade A, Kashuba A, Marzinke M, Anderson PL, Moore A, et al. Tenofovir-Emtricitabine Directly Observed Dosing: 100% Adherence Concentrations (HPTN 066). Conference on Retroviruses and Opportunistic Infections; 2014 Mar 3–6; Boston, MA. [Google Scholar]
- 25.Louissaint NA, Cao YJ, Skipper PL, Liberman RG, Tannenbaum SR, Nimmagadda S, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013;29(11):1443–50. doi: 10.1089/aid.2013.0044. Epub 2013/04/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seifert SM, Meditz A, Castillo-Mancilla J, Gardner E, Klein B, Kerr B, et al. Steady-state TDF/FTC in Genital, Rectal, and Blood Compartments in Males vs Females [abstract 525]. Conference on Retroviruses and Opportunistic Infections; 2015 Feb 23–26; Seattle, WA, USA. [Google Scholar]
- 27*.Seifert SM, Glidden DV, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, et al. Dose Response for Starting and Stopping HIV Preexposure Prophylaxis for Men Who Have Sex With Men. Clin Infect Dis. 2015;60(5):804–10. doi: 10.1093/cid/ciu916. Epub 2014/11/20. Model that predicts the dose response for PrEP in MSM including the onset and duration of PrEP action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson PL, Glidden DV, Bushman LR, Heneine W, Garcia-Lerma JG. Tenofovir diphosphate concentrations and prophylactic effect in a macaque model of rectal simian HIV transmission. J Antimicrob Chemother. 2014;69(9):2470–6. doi: 10.1093/jac/dku162. Epub 2014/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendrix CW. Exploring Concentration Response in HIV Pre-Exposure Prophylaxis to Optimize Clinical Care and Trial Design. Cell. 2013;155(3):515–8. doi: 10.1016/j.cell.2013.09.030. Epub 2013/11/19. [DOI] [PubMed] [Google Scholar]
- 30.Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):112re4. doi: 10.1126/scitranslmed.3003174. Epub 2011/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molina JM, Capitant C, Spire B, Pialoux G, Chidiac C, Charreau I, et al. On Demand PrEP With Oral TDF-FTC in MSM: Results of the ANRS Ipergay Trial [abstract 23LB]. Conference on Retroviruses and Opportunistic Infections; 2015 Feb 23–26; Seattle, WA, USA. [Google Scholar]
- 32.McCormack S, Dunn D. Pragmatic Open-Label Randomised Trial of Preexposure Prophylaxis: The PROUD Study [abstract 22LB]. Conference on Retroviruses and Opportunistic Infections; 2015 Feb 23–26; Seattle, WA, USA. [Google Scholar]
- 33.Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother. 2001;45(10):2733–9. doi: 10.1128/AAC.45.10.2733-2739.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truvada® (tenofovir disoproxil fumarate and emtricitabine) Foster City, CA: Gilead Sciences; Oct, 2013. Product information. [Google Scholar]
- 35**.Baeten JM, Donnell D, Mugo NR, Ndase P, Thomas KK, Campbell JD, et al. Single-agent tenofovir versus combination emtricitabine plus tenofovir for pre-exposure prophylaxis for HIV-1 acquisition: an update of data from a randomised, double-blind, phase 3 trial. The Lancet Infectious Diseases. 2014;14(11):1055–64. doi: 10.1016/S1473-3099(14)70937-5. Efficacy comparison of TDF alone versus TDF plus FTC in serodiscordant couples including subpopulations among the cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4(151):151ra25. doi: 10.1126/scitranslmed.3004006. Epub 2012/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37*.Donnell D, Baeten JM, Bumpus NN, Brantley J, Bangsberg DR, Haberer JE, et al. HIV Protective Efficacy and Correlates of Tenofovir Blood Concentrations in a Clinical Trial of PrEP for HIV Prevention. J Acquir Immune Defic Syndr. 2014 doi: 10.1097/QAI.0000000000000172. Epub 2014/05/03 Assessed the adherence-efficacy relationship in serodiscordant couples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9. doi: 10.1016/S1473-3099(14)70847-3. Epub 2014/07/30. Assessed the adherence-efficacy relationship in an open label PrEP trial among MSM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haberer JE, Baeten JM, Campbell J, Wangisi J, Katabira E, Ronald A, et al. Adherence to Antiretroviral Prophylaxis for HIV Prevention: A Substudy Cohort within a Clinical Trial of Serodiscordant Couples in East Africa. PLoS Medicine. 2013;10(9):e1001511. doi: 10.1371/journal.pmed.1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cottrell ML, Yang KH, Prince HA, Sykes C, White N, Malone S, et al. Predicting effective Truvada(®) PrEP dosing strategies with a novel PK-PD model incorporating tissue active metabolites and endogenous nucleotides (EN) AIDS Res Hum Retroviruses. 2014;30(Suppl 1):A60. [Google Scholar]
- 41.Tsai CC, Follis KE, Sabo A, Beck TW, Grant RF, Bischofberger N, et al. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270(5239):1197–9. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 42.Van Rompay KK, Berardi CJ, Aguirre NL, Bischofberger N, Lietman PS, Pedersen NC, et al. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. Aids. 1998;12(9):F79–83. doi: 10.1097/00002030-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Van Rompay KK, Kearney BP, Sexton JJ, Colon R, Lawson JR, Blackwood EJ, et al. Evaluation of oral tenofovir disoproxil fumarate and topical tenofovir GS-7340 to protect infant macaques against repeated oral challenges with virulent simian immunodeficiency virus. J Acquir Immune Defic Syndr. 2006;43(1):6–14. doi: 10.1097/01.qai.0000224972.60339.7c. [DOI] [PubMed] [Google Scholar]
- 44.Kim CN, Adams DR, Bashirian S, Butera S, Folks TM, Otten RA. Repetitive exposures with simian/human immunodeficiency viruses: strategy to study HIV pre-clinical interventions in non-human primates. J Med Primatol. 2006;35(4–5):210–6. doi: 10.1111/j.1600-0684.2006.00169.x. Epub 2006/07/29. [DOI] [PubMed] [Google Scholar]
- 45.Otten RA, Adams DR, Kim CN, Jackson E, Pullium JK, Lee K, et al. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis. 2005;191(2):164–73. doi: 10.1086/426452. Epub 2004/12/21. [DOI] [PubMed] [Google Scholar]
- 46.Steiner RA, Schiller HS, Illner P, Blandau R, Gale CC. Sex hormones correlated with sex skin swelling and rectal temperature during the menstrual cycle of the pigtail macaque (Macaca nemestrina) Laboratory animal science. 1977;27(2):217–21. Epub 1977/04/01. [PubMed] [Google Scholar]
- 47.Vishwanathan SA, Guenthner PC, Lin CY, Dobard C, Sharma S, Adams DR, et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011;57(4):261–4. doi: 10.1097/QAI.0b013e318220ebd3. Epub 2011/05/07. [DOI] [PubMed] [Google Scholar]
- 48.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. Aids. 2008;22(15):1909–17. doi: 10.1097/QAD.0b013e3283060ea4. Epub 2008/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Radzio J, Hanley K, Mitchell J, Ellis S, Deyounks F, Jenkins L, et al. Depot-medroxyprogesterone acetate does not reduce the prophylactic efficacy of emtricitabine and tenofovir disoproxil fumarate in macaques. J Acquir Immune Defic Syndr. 2014;67(4):365–9. doi: 10.1097/QAI.0000000000000340. Epub 2014/09/10. Macaque model for cervicovaginal viral exposures that included depot-medroxyprogesterone therapy, which is important for resource limited settings that use this contraceptive strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Radzio J, Hanley K, Mitchell J, Ellis S, Deyounks F, Jenkins LT, et al. Physiologic doses of depot-medroxyprogesterone acetate do not increase acute plasma simian HIV viremia or mucosal virus shedding in pigtail macaques. Aids. 2014;28(10):1431–9. doi: 10.1097/QAD.0000000000000294. Epub 2014/04/25. [DOI] [PubMed] [Google Scholar]
- 51.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13(9):797–808. doi: 10.1016/S1473-3099(13)70155-5. Epub 2013/07/23. [DOI] [PubMed] [Google Scholar]
- 52.Polis CB, Phillips SJ, Curtis KM. Hormonal contraceptive use and female-to-male HIV transmission: a systematic review of the epidemiologic evidence. Aids. 2013;27(4):493–505. doi: 10.1097/QAD.0b013e32835ad539. Epub 2012/10/20. [DOI] [PubMed] [Google Scholar]
- 53.Radzio J, Henning T, Mitchell J, Holder A, Hanson D, McNicholl J, et al. FTC/TDF Prevents SHIV Infection in C trachomatis and T vaginalis-Infected Macaques. Conference on Retroviruses and Opportunistic Infections; 2015 Feb 23–26; Seattle WA. [Google Scholar]
- 54.Bekker LG, Hughes J, Amico KR, Roux S, Hendrix C, Anderson PL, et al. HPTN 067/ADAPT Background and Methods and Cape Town Results. IAS 2015; July 19–22, 2015; Vancouver CA. [Google Scholar]
- 55.Mannheimer SB, Hirsch-Moverman Y, Loquere A, Franks J, Hughes J, Ou SS, et al. Feasibility of Intermittent PrEP Among US MSM: Data from the Harlem Site. IAS 2015; July 19–22, 2015; Vancouver, CA. [Google Scholar]
- 56.Holtz TH, Chitwarakorn A, Curlin ME, Hughes J, Amico KR, Hendrix C, et al. A Comparison of Daily and Non-daily Pre-exposure Prophylaxis Dosing in Thai Men Who Have Sex With Men, Bangkok. IAS 2015; July 19–22, 2015; Vancouver, CA. [Google Scholar]
- 57.Kibengo FM, Ruzagira E, Katende D, Bwanika AN, Bahemuka U, Haberer JE, et al. Safety, Adherence and Acceptability of Intermittent Tenofovir/Emtricitabine as HIV Pre-Exposure Prophylaxis (PrEP) among HIV-Uninfected Ugandan Volunteers Living in HIV-Serodiscordant Relationships: A Randomized, Clinical Trial. PLoS ONE. 2013;8(9):e74314. doi: 10.1371/journal.pone.0074314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volk JE, Liu A, Vittinghoff E, Irvin R, Kroboth E, Krakower D, et al. Sexual Frequency and Planning Among At-Risk Men Who Have Sex With Men in the United States: Implications for Event-Based Intermittent Pre-Exposure Prophylaxis. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2012;61(1):112–5. doi: 10.1097/QAI.0b013e31825bd87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buchbinder SP, Liu AY. CROI 2015: Advances in HIV Testing and Prevention Strategies. Top Antivir Med. 2015 Mar-Apr;23(1):8–27. [PMC free article] [PubMed] [Google Scholar]
- 60.Liu AY, Yang Q, Huang Y, Bacchetti P, Anderson PL, Jin C, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a potential adherence measure for pre-exposure prophylaxis (PrEP) PLoS ONE. 2014;9(1):e83736. doi: 10.1371/journal.pone.0083736. Epub 2014/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–90. doi: 10.1089/aid.2012.0089. Epub 2012/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62*.Kashuba AD, Gengiah TN, Werner L, Yang KH, White NR, Karim QA, et al. Genital Tenofovir Concentrations Correlate With Protection Against HIV Infection in the CAPRISA 004 Trial: Importance of Adherence for Microbicide Effectiveness. J Acquir Immune Defic Syndr. 2015;69(3):264–9. doi: 10.1097/QAI.0000000000000607. Epub 2015/07/17. Evauated pharmacologic predictors of efficacy in the CAPRISA 004 study and identified drug concentration thresholds for topical TFV delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]