Abstract
The efficacies of colicins E1 and N against Escherichia coli strains responsible for postweaning diarrhea and edema disease, two of the most prevalent disease problems for pigs in the United States, were determined in vitro. These proteins may provide an environmentally sound means for the prevention of these infections in swine.
Postweaning diarrhea and edema disease, caused by Escherichia coli infections, are two of the most prevalent disease problems for pigs in the United States (12). More than 43% of the large swine facilities in the United States reported E. coli infections among weaned pigs in 2000, and in an attempt to prevent the spread of these infections, more than 78% of these facilities reported using prophylactic antibiotic treatments (12). The strains considered primarily responsible for these infections, F4 (K88) and F18, are not well controlled by traditional prophylactic antibiotic treatments due to the frequency and spectrum of antibiotic resistance seen in these strains (2, 8) and therefore still cause substantial losses to producers, due to both mortality and morbidity. With worldwide concern over the use of prophylactic antibiotics in animal agriculture and their contribution to the spread of antibiotic resistance (3, 4, 13), the development of alternatives to conventional antibiotics is urgently needed to protect swine from these E. coli infections.
Colicins, a class of bacteriocins produced by, and effective against, E. coli and closely related species (5), hold particular promise as alternatives to conventional antibiotics used for the control of postweaning diarrhea and edema disease in pigs. Studies examining the antibacterial activity of different colicins have typically yielded qualitative rather than quantitative determination of activity by using direct spotting or overlay techniques to determine efficacy (6, 10, 11). However, in this study, we quantitatively compared the efficacy of two purified colicins (colicins E1 [ColE1] and N [ColN]) in inhibiting the growth of the E. coli strains responsible for postweaning diarrhea and edema disease.
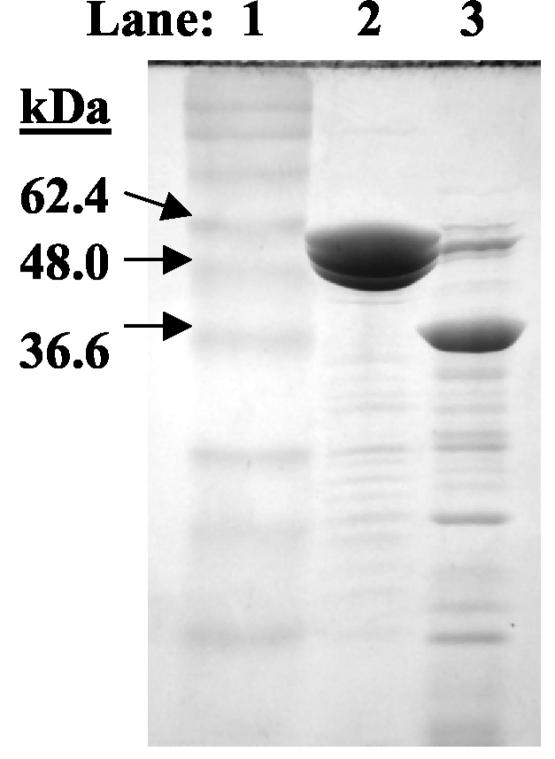
Purified colicins were obtained by inoculating colicin-producing E. coli strains (NC50132 and NC50145) obtained from the National Collection of Type Cultures (Public Health Laboratory Service, London, England) into Luria Broth (LB) to a starting optical density at 600 nm (OD600) of about 0.1 and incubating the cultures with shaking at 37°C. Colicin production was induced when cultures reached an OD600 of 0.9 by the addition of 0.2 U of mitomycin C (Sigma)/ml of culture. The cell-free supernatant was obtained by centrifugation 5.5 h later and concentrated by ultrafiltration across a regenerated cellulose membrane in a stir cell apparatus (Amicon, Millipore, Bedford, Mass.). The concentrated sample was then desalted against 10 mM Tris-Cl, pH 8, and purified by ion exchange chromatography using Q Sepharose (Amersham Biosciences, Piscataway, N.J.) equilibrated with 10 mM Tris-Cl, pH 8. The bound protein was eluted with a continuous NaCl gradient by using an AKTAprime chromatography system (Amersham Bioscience), and fractions containing the highest concentrations of colicin were pooled and concentrated by ultrafiltration. The protein concentrations of these pooled samples were determined (9), and the percentage of colicin was determined by densitometry after sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coomassie blue staining with a 16-bit megapixel charge-coupled device camera, FluorChem 8800, and FluorChem IS800 software (Alpha Innotech, San Leandro, Calif.). Yields of 1.1 mg of purified ColN/liter of culture and 7.6 mg of purified ColE1/liter of culture were obtained. The purity of the ColN and ColE1 isolates were 30 and 85%, respectively (Fig. 1).
FIG. 1.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified ColE1 and ColN. Lane 1, BenchMark prestained protein ladder (Invitrogen, Carlsbad, Calif.); lane 2, 5 μg of the purified ColE1 sample; lane 3, 14 μg of the purified ColN sample.
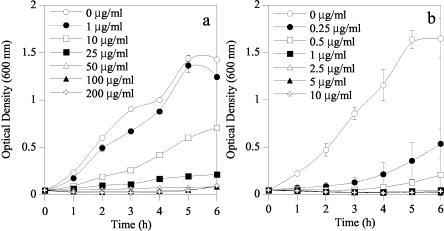
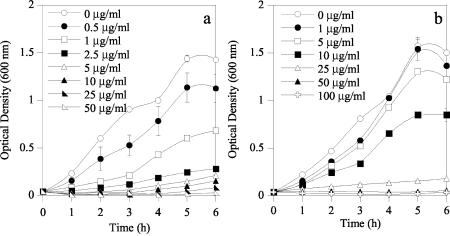
Quantitative determinations of the efficacies of these purified colicins against E. coli F4 (K88) and F18 were made by using pure cultures obtained from the culture collection at the USDA Agricultural Research Service Federal Food Safety Research Unit (College Station, Tex.). These cultures were grown overnight in LB at 37°C with shaking and then used to inoculate a flask of LB to an OD600 of about 0.05. The freshly inoculated LB was then aliquoted (5 ml) into culture tubes containing various colicin concentrations (Fig. 2 and 3). The volume of the colicin addition was made constant (175 μl) with 10 mM Tris, pH 8. These tubes were then incubated with shaking at 37°C, and their OD600 was determined hourly for 6 h. The CFU/milliliter of these E. coli strains were also determined by serial dilutions and direct plating on LB, both initially and 3 h postinoculation. These experiments were repeated in triplicate, and the values presented are means. ColN was more effective (P < 0.05) than ColE1 against E. coli F4 (K88). Tenfold more ColE1 than ColN was required to inhibit the growth of E. coli F4 (K88) (Fig. 2 and 3). After 3 h of growth in LB containing 50 μg of colicin/ml, ColN caused a 2-log reduction in CFU/milliliter, whereas ColE1 caused only a 1-log reduction (Table 1). ColE1, however, was far more effective (P < 0.05) than ColN against E. coli F18 (Fig. 2 and 3). No increase in the OD600 was seen during the 6-h incubation with 1 μg of ColE1/ml, whereas ColN concentrations greater than 25 μg/ml were needed to see this effect. Both 1 μg of ColE1 and 50 μg of ColN/ml caused a 1-log reduction in the CFU of E. coli F18/milliliter (Table 1).
FIG. 2.
Effect of ColE1 on the growth of E. coli F4 (K88) (a) and F18 (b).
FIG. 3.
Effect of ColN on the growth of E. coli F4 (K88) (a) and F18 (b).
TABLE 1.
Effect of colicins on the viability of E. coli F4 (K88) and F18 after 3 h of incubationa
| E. coli strain | ColE1
|
ColN
|
||
|---|---|---|---|---|
| Dose (μg/ml) | CFU/ml | Dose (μg/ml) | CFU/ml | |
| F4 (K88) | 0 | 3 × 109 | 0 | 3 × 109 |
| 50 | 5 × 106 | 10 | 1.1 × 107 | |
| 200 | 4 × 106 | 50 | 6 × 105 | |
| F18 | 0 | 2 × 109 | 0 | 2 × 109 |
| 1 | 1.2 × 106 | 50 | 1 × 106 | |
| 100 | 5 × 104 | 100 | 4 × 105 | |
Initial CFU/milliliter were 6 × 107 and 1 × 107 for E. coli F4 (K88) and F18, respectively.
The prophylactic use of antibiotics in animal agriculture has been greatly scrutinized in recent years due to concerns regarding its role in contributing to antibiotic resistance. This scrutiny has led to increased regulation over the use of antibiotics in animal agriculture (3, 4, 13) and will likely continue towards zero tolerance for the use of prophylactic or growth-promoting antibiotic use in animals. Therefore, it is essential for the sustainability of animal agriculture to examine alternatives to conventional antibiotics to improve animal health and production efficiency. Because of their in vitro efficacy against E. coli F4 (K88) and F18, the strains responsible for causing postweaning diarrhea and edema disease in pigs, ColE1 and ColN should be examined as alternatives to conventional antibiotics in swine production. Since the modes of cell recognition and transport through the periplasm are dramatically different between ColE1 and ColN (1, 7), a combination of these colicins may be more effective than either colicin alone for their use in animal agriculture.
REFERENCES
- 1.Cao, Z., and P. E. Klebba. 2002. Mechanism of colicin binding and transport through outer membrane porins. Biochimie 84:399-412. [DOI] [PubMed] [Google Scholar]
- 2.Choi, C., H. J. Ham, D. Kwon, J. Kim, D. S. Cheon, K. Min, W. S. Cho, H. K. Chung, T. Jung, K. Jung, and C. Chae. 2002. Antimicrobial susceptibility of pathogenic Escherichia coli isolated from pigs in Korea. J. Vet. Med. Sci. 64:71-73. [DOI] [PubMed] [Google Scholar]
- 3.Federal Register. 1999. Consideration of the human health impact of the microbial effects of antimicrobial new animal drugs intended for use in food producing animals. Food and Drug Administration guidance no. 78. Fed. Regist. 64:70715. [Google Scholar]
- 4.Federal Register. 2002. Evaluating the safety of antimicrobial new animal drugs with regard to their microbiological effects on bacteria of human health concern. Food and Drug Administration draft guidance no. 152. Fed. Regist. 67:58058. [Google Scholar]
- 5.Fredericq, P. 1957. Colicins. Annu. Rev. Microbiol. 11:7-22. [DOI] [PubMed] [Google Scholar]
- 6.Jordi, B. J. A. M., K. Boutaga, C. M. E. van Heeswijk, F. van Knapen, and L. J. A. Lipman. 2001. Sensitivity of Shiga toxin-producing Escherichia coli (STEC) strains for colicins under different experimental conditions. FEMS Microbiol. Lett. 204:329-334. [DOI] [PubMed] [Google Scholar]
- 7.Lakey, J. H., and S. L. Slatin. 2001. Pore-forming colicins and their relatives. Curr. Top. Microbiol. Immunol. 257:131-161. [DOI] [PubMed] [Google Scholar]
- 8.Lanz, R., P. Kuhnert, and P. Boerlin. 2003. Antimicrobial resistance and resistance gene determinants in clinical Escherichia coli from different animal species in Switzerland. Vet. Microbiol. 91:73-84. [DOI] [PubMed] [Google Scholar]
- 9.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 10.Murinda, S. E., R. F. Roberts, and R. A. Wilson. 1996. Evaluation of colicins for inhibitory activity against diarrheagenic Escherichia coli strains, including serotype O157:H7. Appl. Environ. Microbiol. 62:3196-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schamberger, G. P., and F. Diez-Gonzalez. 2002. Selection of recently isolated colicinogenic Escherichia coli strains inhibitory to Escherichia coli O157:H7. J. Food Prot. 65:1381-1387. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Department of Agriculture. 2001. Reference of swine health and management in the United States, 2002. N338.0801. National Animal Health Monitoring System, Fort Collins, Colo.
- 13.World Health Organization. 2000. Global principles for the containment of antimicrobial resistance in animals intended for food. [Online.] http://www.who.int/emc-documents/zoonoses/docs/whocdscsraph20004.pdf.