Abstract
Complex regional pain syndrome (CRPS) is a chronic condition that involves significant hyperalgesia of the affected limb, typically accompanied by localized autonomic abnormalities, and frequently motor dysfunction. Although central brain systems are thought to play a role in the development and maintenance of CRPS, these systems have not been well characterized. In this study, we used structural magnetic resonance imaging (sMRI) to characterize differences in gray matter volume between patients with right upper extremity CRPS and matched controls . Analyses were carried out using a whole brain voxel-based morphometry (VBM) approach. The CRPS group showed decreased gray matter volume in several pain-affect regions, including the dorsal insula, left orbitofrontal cortex, and several aspects of the cingulate cortex. Greater gray matter volume in CRPS patients was seen in the bilateral dorsal putamen and right hypothalamus. Correlation analyses with self-reported pain were then performed on the CRPS group. Pain duration was associated with decreased gray matter in the left dorsolateral prefrontal cortex. Pain intensity was positively correlated with volume in the left posterior hippocampus and left amygdala, and negatively correlated with the bilateral dorsolateral prefrontal cortex. Our findings demonstrate that CRPS is associated with abnormal brain system morphology, particularly pain-related sensory, affect, motor, and autonomic systems.
Keywords: Voxel-based morphometry, VBM, Complex Regional Pain Syndrome, CRPS, neuroimaging, chronic pain
1.1 Introduction
Complex Regional Pain Syndrome (CRPS) is a neurologic illness characterized by spontaneous pain that is out of proportion to the inciting event, and that extends beyond the sensory distribution of any one nerve. Swelling (edema), temperature changes, and excess sweating are commonly observed, distinguishing CRPS from other neuropathic pain disorders. Motor dysfunction is observed in 80% of patients.4,13 The majority of patients are female with upper extremity pain.4
CRPS may involve abnormalities in the central nervous system. Magntoencephalography14 and somatosensory evoked potentials 27 have shown reorganization of the contralateral primary somatosensory cortex. Functional magnetic resonance imaging (fMRI) has identified widespread somatotopic alterations during mechanical stimulation 18,19 in several areas of the brain, including the primary motor and sensory cortex, the bilateral secondary sensory cortex, cingulate cortex, and parietal association cortex. FMRI of a finger tapping task of the affected limb has also demonstrated reorganization of the motor circuits and increased activation in the bilateral primary motor cortices and the contralateral supplementary motor cortex.17
In this study, we characterized gray matter abnormalities in CRPS patients, using structural MRI (sMRI). A previous sMRI study of CRPS patients identified less gray matter in a single, large cluster encompassing the right insula, ventromedial prefrontal cortex, and nucleus accumbens.11 The previous study, however, used a heterogeneous population. We therefore recruited only females all of whom were right-handed and had right upper extremity CRPS. We hypothesized that these patients would demonstrate gray matter volume abnormalities in areas of the brain commonly seen in chronic pain patients, including the somatosensory cortex, anterior cingulate cortex, and insula.30,37 We also hypothesized that we would observe gray matter volume abnormalities in the motor system of these patients as previously non-structural imaging studies and clinical examination criteria have suggested that would help differentiate CRPS from other pain syndromes
1.2 Materials and Methods
1.2.1 Subjects
After IRB approval was received, 15 right-handed females with right upper extremity CRPS were recruited from the Stanford University Pain Clinic and surrounding community (see Supplementary Table A.1 for demographic information). Fifteen aged-matched, right-handed female controls were also recruited via community outreach and advertisements. Written consent was obtained from all study participants. The CRPS group had an age range from 20 to 68, with a mean age of 44.0. Controls were matched to within 2 years, with an age range of 20 to 68, and a mean age of 44.1. All individuals were examined and had their CRPS diagnosis confirmed by a board-certified pain specialist at Stanford using the standard IASP diagnostic criteria. All patients also met the clinical criteria as outlined by the Budapest Research Criteria. 13 Pain duration ranged from 2 to 206 months. A total of 27 patients were recruited, but 12 were excluded owing to handedness, sex, and affected limb. Subjects were not asked to change their treatment regimen during their participation in the study. The inclusionary criteria for the CRPS group were: (1) at least 18 years of age, (2) a diagnosis of CRPS with an examination by a Stanford pain specialist. The exclusionary criteria were: (1) pregnancy, (2) claustrophobia, (3) MRI incompatibility, (4) psychiatric disorders that would interfere with the participants’ ability to complete study tasks.
1.2.2 Procedures
After signing informed consent, disease severity was assessed using the McGill Pain Questionnaire. 22 The average McGill questionnaire score for patients was 24.2, with the average VAS score reported for 14/15 patients at 7.25. The average McGill score for 11/15 controls was 6 and average VAS was 0.7. Due to the severity of their pain, CRPS patients were not asked to stop their medications which are listed on Supplementary Table A.1. Only 2 controls were on medication, birth control and anti-hypertensive medication. Following the pain questionnaire, participants completed the structural imaging protocol. As the protocol involved only structural imaging, participants were instructed simply to keep their head still for the duration of the scanning which totaled approximately 60 minutes
1.2.3 MR data acquisition and processing
High resolution T1-weighted anatomical images were acquired using a 3D IR-FSPGR sequence, 28 slices, and 4 mm slice thickness with1 mm skip, voxel size was 1.5 × 1.5 × 1.5 mm. Scans were conducted at Stanford University using a GE 3.0 Tesla MRI system, and a transmit-receive, end-cap, single channel head coil. All image analysis and processing was performed using SPM8 (Wellcome Trust Centre).
Anatomical images from all subjects were first segmented into gray matter, white matter, and cerebrospinal fluid images. Next, the DARTEL toolbox was used to normalize the anatomical images into a common stereotactic space. DARTEL was chosen because it has been shown to provide improved image normalization relative to many other common algorithms .15 Finally, each gray matter image was spatially smoothed with an 8-mm Gaussian kernel.
1.3 Statistical analysis
Whole brain statistics were computed using an independent Student’s t-test with healthy controls in one group and CRPS patients in the other group. Participant age and total gray matter volume were regressed out, to remove effects of non-interest. A gray matter mask was applied to eliminate voxels not containing gray matter, thus reducing the number of statistical tests being conducted. To control for the incidence of false positives, two corrections for multiple comparisons were applied. First, a false discovery rate (FDR)-controlled, voxel-level height threshold was calculated, yielding a corrected p-value of 0.0005 (t = 3.71). Second, a cluster-level extent-threshold of 30 contiguous voxels was applied, using a priori information regarding our smallest structure of interest. The cluster threshold of 30 contiguous voxels yields a volume of 45mm3 and was based on the approximate size of the caudate nucleus – as well as of the nucleus accumbens region that is commonly reported in imaging studies, and was the seed region used in the Geha 2008 paper 11
Secondary analyses were performed on the patient group only, to determine if there was relationship between gray matter volume and disease severity. Using a one-sample t-test, gray matter volume values were tested for correlations with (a) disease duration (N=15) and (b) pain severity (N=14). The p-value was set to 0.001 for these analyses to maintain a t-value threshold of 3.71 (comparable to the main analysis threshold). The 30 consecutive voxel-extent threshold was retained.
1.4 Results
Total gray matter volume (GMV) was computed for all individuals. Patients had a mean GMV volume of 0.66 (SD 0.06). The mean GMV for the control group was 0.71, SD 0.06. The T test of the GMV differences between groups was t(28)= −2.58, p= 0.015. The correlation patients with age −0.37 p=0.172, and for controls −0.76 p= 0.001 and the combined group total − 0.57 p=0.001.
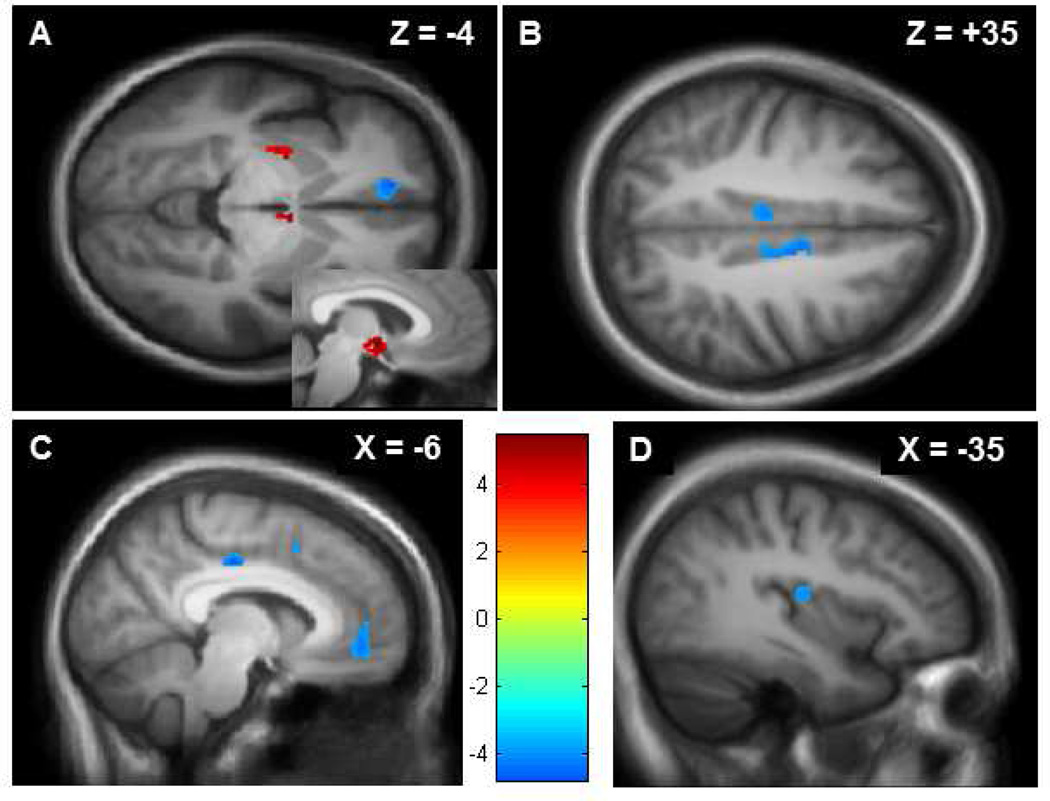
Next, region-specific differences in GMV were assessed using the whole brain analysis (see Table A.1). As compared with the healthy controls, the CRPS patients exhibited increased gray matter volume in the left dorsal putamen and the hypothalamus (Figure 1a) at p = 0.0005. Conversely, the patients had less gray matter volume than controls in the cingulate, primarily the posterior mid-cingulate cortex, but also in the bilateral anterior cingulate (Figure 1b), in the orbital frontal cortex (Figure 1a, 1c), and the left posterior insula (Figure 1d).
Table A.1.
Areas of gray matter volume differences between CRPS group and healthy control group. All regions survived a voxel-level height threshold of t = 3.71 and a cluster-extent threshold of 30 contiguous voxels.
| Region | MNI | T-score | Voxel Count (1.5 mm) |
Difference (patient vs control) |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| L orbitofrontal cortex | −10.5 | 42 | −6 | 4.69 | 276 | lesser |
| L mid-cingulate cortex | −6 | −19.5 | 33 | 4.55 | 78 | lesser |
| R mid-cingulate cortex | 9 | −4.5 | 36 | 4.55 | 150 | lesser |
| L posterior mid-cingulate cortex | 6 | −13.5 | 51 | 4.40 | 44 | lesser |
| L dorsal insula | −34.5 | −15 | 12 | 4.17 | 69 | lesser |
| L anterior mid-cingulate cortex | −4.5 | 10.5 | 40.5 | 4.00 | 32 | lesser |
| R hypothalamus | 3 | −3 | −6 | −5.42 | 168 | greater |
| L dorsal putamen | −22.5 | −4.5 | −6 | −4.67 | 86 | greater |
| L inferior temporal lobe | −46.5 | −10.5 | −27 | −4.06 | 35 | greater |
Figure A.1. Gray matter differences between CRPS patients and matched controls.
A: CRPS participants showed significantly greater GMV (shown in red) in the left dorsal putamen and right hypothalamus, seen in an axial slice. The hypothalamus is also displayed in sagittal perspective (inset). Areas of statistically significant difference (threshold t = 3.71, p = 0.0005) are superimposed on an MNI-normalized average of all participants’ T1-weighted structural brain images (N = 30). B: CRPS patients showed decreased GMV (shown in blue) in the left and right posterior mid-cingulate cortex (axial perspective). C: In addition to mid-cingulate regions, CRPS patients showed significantly less GMV (shown in blue) in the left orbitalfrontal cortex (also seen in pane a). D: CRPS patients showed significantly less GMV (shown in blue) in the left posterior insula.
Finally, secondary correlation analyses were then performed on the patient group (see Table A.2). These analyses were designed to determine if any regional GMV differences were associated with disease severity. Duration was associated with decreased gray matter in the left DLPFC (Figure 2a). The VAS score correlated negatively with the right and left DLPFC (Figure 2b), and positively with the left posterior hippocampus and the left amygdala (Figure 2c and 2d), and the left post-central gyrus.
Table A.2.
Correlations between gray matter volume and symptom severity (duration and pain intensity) in patients only. All regions survived a voxel-level height threshold of t = 3.79, and 30 contiguous voxels. Negation direction means lesser gray matter volume was associated with greater illness duration or severity.
| Region | MNI | T-score | Voxel Count | Direction | Pearson | ||
|---|---|---|---|---|---|---|---|
| Patients correlated with duration: | |||||||
| L dorsolateral prefrontal cortex | −33.0 | 48 | 6 | −4.89 | 56 | negative | −0.66 |
| Patients correlated with VAS pain: | |||||||
| L posterior hippocampus | −19.5 | −33 | −4.5 | 7.01 | 34 | positive | 0.83 |
| L amygdala | −27 | −4.5 | −9 | 5.93 | 79 | positive | 0.81 |
| L postcentral gyrus | −60 | −13.5 | 18 | 5.14 | 39 | positive | 0.85 |
| L dorsolateral prefrontal cortex | −27 | 45 | 21 | −7.35 | 208 | negative | −0.61 |
| R dorsolateral prefrontal cortex | 37.5 | 48 | 12 | −5.52 | 41 | negative | −0.34 |
| R cerebellum | 21 | −61.5 | −36 | −5.41 | 38 | negative | −0.30 |
Figure A.2. Correlation analyses of CRPS patient GMV with symptom duration and pain severity.
A: Duration of CRPS symptoms was associated with decreased GMV (shown in blue) in the left dorsolateral pre-frontal cortex in the coronal view (axial view in inset) in patients only (N = 15) with a threshold of t = 3.79, p = 0.001, contiguous voxels = 30.
B: Severity of pain (0–100 visual analog scale) was associated with decreased GMV (shown in blue) in the bilateral dorsolateral prefrontal cortex seen in the coronal view.
C: Sagittal view of pain severity positively associated with increased volume (shown in red) in left posterior hippocampus and left amygdala.
D: Coronal view of increased amygdala volume (shown in red) associated with pain severity.
1.5 Discussion
1.5.1 Differences between CRPS patients and healthy controls
We identified a number of morphologic differences between CRPS patients and the matched healthy controls. Areas where patients showed lesser gray matter than controls included the posterior mid-cingulate cortex (pMCC), bilateral pregenual anterior cingulate cortex (pACC), orbitofrontal cortex (OFC), and left posterior insula. Three of these regions are included in the classic limbic system (the cingulate and the orbitofrontal cortex). We note several overlaps between our results and results seen in the literature. For example, decreased gray matter volume was observed in a more general pain population suffering from lower back pain, headache, and lower extremity joint pain30 as well as in a population of patients suffering from functional somatic syndromes such as fibromyalgia or irritable bowel syndrome. 33
Volumetric abnormalities in the limbic system suggest dysregulated emotional processing of pain information in CRPS. The pACC is chiefly involved in the components of pain having to do with unpleasantness and suffering.29 It is also involved in conditioned emotional learning and assigning emotional valence to external and internal stimuli .35 The OFC shows increased activation during certain expectation of pain and decreased activation in uncertain expectation.24 The OFC was also persistently hypoactive after long-term medication overuse headache patients discontinued chronic opiates, suggesting that the region plays a role in the persistence of chronic pain.10 Valet et al., also noted this volumetric abnormality in a group of patients with chronic pain and hypothesized that the pACC and OFC drive top-down regulation of pain, suggesting that CRPS patients may have an impaired ability to modulate pain supraspinally.33 The pMCC represents motor processing in the cingulate.34 Inputs to the pMCC include the inferior parietal lobe, motor cortex, and supplementary motor cortex.36 Dysregulation in this area may mediate motor dysfunction commonly observed in CRPS patients. The pMCC may mediate sensory, rather than affective, aspects of pain processing, given that it does not demonstrate emotional activations, but does demonstrate robust nociceptive response.35, 36 While the insula is typically thought to be part of the extended limbic system, the posterior aspect, observed in this study, is typically associated with somatosensory processing of both painful and nonpainful sensation.26 The dorsal posterior insula is thought to be activated predominantly contralaterally in response to nociceptive input.3 In addition, the dorsal posterior insula is thought to have a temperature-encoding ability8 and to be somatotopically organized.7 Using coordinates from a previous study mapping the somatotopy of the posterior insula to noxious heat stimuli,7 we found that the area of atrophy we identified corresponds well to the expected contralateral side, representing approximately the area between the face and hand.
We found two exceptions to the general pattern of atrophy in the CRPS patients. These two regions are uncommonly seen in chronic pain and may represent a finding more unique to CRPS. Two regions, the left dorsal putamen and the hypothalamus, showed gray matter increase in these patients. The putamen is known to be involved in the processing of pain in humans.7 The putamen contains a somatotopic representation of bodily pain,2 and the posterior aspect has demonstrated gray matter volume increase in other chronic pain disorders, such as myofascial temporomandibular pain.38 The contralateral putamen has also demonstrated somatotopically arranged activation during pain. The hand representation is posterior to the foot and corresponds closely with our noted area of activity.2 Interestingly Starr et al, recently described decreased pain intensity and thermal thresholds in patients with contralateral putaminal lesions, and we might suggest the converse to be true.32 Activation of dopaminergic neurons in this region is associated with lower pain sensitivity,31 perhaps suggesting that increased gray matter volume is a compensatory action against sustained nociceptive input. In particular, the anatomical connectivity between the posterior putamen and the primary motor cortex (M1),25 which shows active reorganization during CRPS,20 suggests that M1 inputs due to CRPS pathology may be driving putamen reorganization.
The hypothalamus also showed increased gray matter in the CRPS patients. The hypothalamus has been previously shown to demonstrate abnormal processing to stimuli in CRPS patients, and altered function in that region has been posited to perhaps drive the autonomic symptoms of CRPS.16 Gray matter increase in the hypothalamus has also been shown in irritable bowel syndrome,5 another condition with supposed sympathetic and autonomic nervous system attributes. Functional activation of the hypothalamus has also been demonstrated with cluster headaches, which may also involve autonomic components.22 Martenson et al. recently put forward the hypothesis that the dorsal medial nuclei of the hypothalamus may be responsible for promoting hyperaglesia. 21
1.5.2 Correlation with pain duration and intensity
CRPS pain duration and intensity were associated with morphologic abnormalities in several limbic regions linked to the processing of affective stimuli. Interestingly, gray matter atrophy in the left DLPFC was associated both with longer duration of illness and increased pain intensity. It is possible that the decreased gray matter in that region is associated with sustained abnormal nociceptive input to the brain, rather than CRPS-specific pathology, because the same region has been found to be involved in other types of pain conditions.1 Also, whereas the left DLPFC was associated with both pain duration and intensity in patients, there was no significant difference in volume in that region between patients and matched controls. The combination of those findings suggests that left DLPFC abnormalities are not central to CRPS pathophysiology, but are instead either adaptations to increased nociceptive input, or that they represent a pre-existing vulnerability to experience greater pain. The role of this region in the processing of pain is exciting, because it is well positioned for non-invasive neuromodulatory techniques, such as transcranial magnetic stimulation (TMS). In fact, preliminary results have already demonstrated the potential analgesic benefit of left DLPFC modulation with TMS in a capsaicin model of pain.9
Greater CRPS pain was also associated with gray matter hypertrophy in the left posterior hippocampus and left amygdala. These two regions, which are classically associated with limbic reward processing and emotional intensity encoding, are also involved in the experience of pain. Both the amygdala and hippocampus have been implicated in individual differences in pain sensitivity, and they may be particularly involved in heightened pain expectancy and pain anticipation.40,28 The importance of the amygdala and hippocampus in mediating pain sensitivity has been further supported by studies of pain processing differences in war veterans with posttraumatic stress disorder.12
1.5.3 Reconciliation with previous results
We noted a general lack of agreement between our results and the results of the only other reported study of VBM and CRPS.11 In that study, only one cluster was identified, and that region encompassed the VMPFC and nucleus accumbens. Even with relaxed thresholds, we were unable to see overlap between their regions of interest and ours. However, there are several differences between the studies that make direct comparison difficult. First, Geha and colleagues recruited CRPS patients with all affected limbs (arms and legs, left and right side), while our sample was limited to right upper extremity CRPS. Second, they included left and right handed individuals, while we recruited only right handed individuals. Third, the study recruited men and women, while we recruited only women. For these reason, the present study was more homogenous, and perhaps better able to detect differences than the previous Geha study.
1.5.4 Caveats
Our findings have the same caveats present with all other pain VBM studies. First, as a cross-sectional study, it is unknown whether the observed abnormalities represent responses to pain or causes of pain. Only longitudinal studies will be able to identify causal relationships between the brain and pain. Second, gray matter volumetric changes are difficult to interpret, given our inability to know exactly what type of gray matter is changing. While volumetric change is commonly assumed to represent neuronal growth or loss, other cell types (e.g., microglia), may also contribute to VBM changes. Current MRI methods do not allow cell types to be differentiated in this way.
Differences between subjects with CRPS and controls could be due to central changes in the face of chronic medication use. Brain system changes have been shown for antidepressants and opioids.6, 39 We cannot rule out the possibility that medication usage causes the structural changes seen in our study and in most VBM studies of chronic pain. Unfortunately, it is challenging to recruit any patients with CRPS who are not taking medications, owing to the extremely painful nature of this condition.
1.6 Conclusions
Our study has demonstrated a number of discrete volumetric abnormalities in the brains of individuals with CRPS. The pattern of volumetric abnormalities suggests widespread dysregulation of pain processing in these patients, with an emphasis on motor, sensory, affective pain, and autonomic systems. We also found a significant relationship between gray matter changes and both the duration and intensity of pain. These changes may reflect both neurobiological preconditions for central sensitization in CRPS and plastic changes as a consequence of persistent neuropathic afferent input. This study represents an advancement in our understanding of structural changes in the brain associated with CPRS. As these studies are costly and time-consuming and CRPS patients few in number, further studies should aim to add confirm and refine our studies. In addition, further focusing on areas of uniqueness not commonly noted in other pain conditions such as the hypothalamus and putamen may lead to a signature imaging finding for CRPS that clearly separates it from other pain syndromes.
Supplementary Material
Demographic information for patients and controls.
Summary.
This study examines a homogeneous CRPS population with voxel based morphometry and demonstrates volumetric changes in pain-related regions of the human brain.
Perspective.
This paper presents structural changes in the brains of patients with Complex Regional Pain Syndrome helping us differentiate CRPS from other chronic pain syndromes and furthering our understanding of this challenging disease.
ACKNOWLEDGMENTS
Supported
M.B received funding from the American Academy of Neurology Research Training Fellowship, Minneapolis, MN. S.M. reports funding from Foundation for Anesthesia Education and Research, Rochester, MN, Rocky Mountain CRPS Foundation, Denver, CO, NIH K24 DA029262, Bethesda, MD , Chris Redlich Pain Research Endowment.
The authors thank Kim M. Mauer, MD, Assistant Professor in Anesthesia and Perioperative Medicine, Oregon Health & Science University, Portland, OR for her assistance in behavioral data collection, Gary H. Glover, PhD , Professor of Radiology at Stanford and Director of the Lucas Center for Neuroimaging, Palo Alto, CA. for assistance and advice in imaging, and Rebecca McCue, BA, Pain Division Research Manager, Stanford, Palo Alto, CA, for editing and administrative assistance. This study was made possible through funding by the American Academy of Neurology Research Training Fellowship, Foundation for Anesthesia Education and Research, Rocky Mountain CRPS Foundation, NIH K24 DA029262, Chris Redlich Pain Research Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
N.C., T.U., J.Y. have no disclosures
Contributor Information
Meredith J Barad, Clinical Assistant Professor Anesthesia (Pain Management) and Neurology & Neurological Sciences. Stanford University School of Medicine, Stanford University, Palo Alto, CA.
Takefumi Ueno, Research Associate Professor, Department of Neuropsychiatry, Kyushu University Graduate School of Medical Sciences, Japan.
Jarred Younger, Assistant Professor (Research) of Anesthesia, Stanford University School of Medicine, Stanford University, Palo Alto, CA.
Neil Chatterjee, Medical Student, Feinberg School of Medicine, Northwestern University, Chicago, IL.
Sean Mackey, Professor of Anesthesia, Stanford University School of Medicine, Stanford University, Palo Alto, CA.
References
- 1.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bingel U, Gläscher J, Weiller C, Büchel C. Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cereb. Cortex. 2004;14:1340–1345. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]
- 3.Bingel U, Quante M, Knab R, Bromm B, Weiller C, Büchel C. Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. Neuroimage. 2003;18:740–748. doi: 10.1016/s1053-8119(02)00033-2. [DOI] [PubMed] [Google Scholar]
- 4.Birklein F, Riedl B, Sieweke N, Weber M, Neundörfer B. Neurological findings in complex regional pain syndromes--analysis of 145 cases. Acta Neurol. Scand. 2000;101:262–269. doi: 10.1034/j.1600-0404.2000.101004262x./. [DOI] [PubMed] [Google Scholar]
- 5.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 6.Bremner JD, Vermetten E. Neuroanatomical changes associated with pharmacotherapy in posttraumatic stress disorder. Ann NY. Acad. Sci. 2004;1032:154–157. doi: 10.1196/annals.1314.012. [DOI] [PubMed] [Google Scholar]
- 7.Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 8.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat. Neurosci. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- 9.Fierro B, De Tommaso M, Giglia F, Giglia G, Palermo A, Brighina F. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res. 2010;203:31–38. doi: 10.1007/s00221-010-2206-6. [DOI] [PubMed] [Google Scholar]
- 10.Fumal A, Laureys S, Di Clemente L, Boly M, Bohotin V, Vandenheede M, Coppola G, Salmon E, Kupers R, Schoenen J. Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain. 2006;129:543–550. doi: 10.1093/brain/awh691. [DOI] [PubMed] [Google Scholar]
- 11.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geuze E, Westenberg HG, Jochims A, de Kloet CS, Bohus M, Vermetten E, Schmahl C. Altered pain processing in veterans with posttraumatic stress disorder. Arch. Gen. Psychiatry. 2007;64:76–85. doi: 10.1001/archpsyc.64.1.76. [DOI] [PubMed] [Google Scholar]
- 13.Harden RN, Bruehl S, Perez RS, Birklein F, Marinus J, Maihofner C, Lubenow T, Buvanendran A, Mackey S, Graciosa J, Mogilevski M. Christoph Ramsden: Validation of proposed diagnostic criteria (the "Budapest Criteria") for Complex Regional Pain Syndrome. Pain. 2010;150:268–274. doi: 10.1016/j.pain.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juottonen K, Gockel M, Silén T, Hurri H, Hari R, Forss N. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain. 2002;98:315–323. doi: 10.1016/S0304-3959(02)00119-7. [DOI] [PubMed] [Google Scholar]
- 15.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroi mage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebel A, Becerra L, Wallin D, Moulton EA, Morris S, Pendse G, Jasciewicz J, Stein M, Aiello-Lammens M, Grant E, Berde C, Borsook D. fMRI reveals distinct CNS processing during symptomatic and recovered complex regional pain syndrome in children. Brain. 2008;131:1854–1879. doi: 10.1093/brain/awn123. [DOI] [PubMed] [Google Scholar]
- 17.Maihöfner C, Baron R, DeCol R, Binder A, Birklein F, Deuschl G, Handwerker HO, Schattschneider J. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130:2671–2687. doi: 10.1093/brain/awm131. [DOI] [PubMed] [Google Scholar]
- 18.Maihöfner C, Forster C, Birklein F, Neundörfer B, Handwerker HO. Brain processing during mechanical hyperalgesia in complex regional pain syndrome: a functional MRI study. Pain. 2005;114:93–103. doi: 10.1016/j.pain.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Maihöfner C, Handwerker HO, Birklein F. Functional imaging of allodynia in complex regional pain syndrome. Neurology. 2006;66:711–717. doi: 10.1212/01.wnl.0000200961.49114.39. [DOI] [PubMed] [Google Scholar]
- 20.Maihöfner C, Handwerker HO, Neundörfer B, Birklein F. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;63:693–701. doi: 10.1212/01.wnl.0000134661.46658.b0. [DOI] [PubMed] [Google Scholar]
- 21.Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142:236–224. doi: 10.1016/j.pain.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May A, Bahra A, Büchel C, Frackowiak RS, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352:275–278. doi: 10.1016/S0140-6736(98)02470-2. [DOI] [PubMed] [Google Scholar]
- 23.Melzack R, Torgerson WS. On the language of pain. Anesthesiology. 1971;34:50–59. doi: 10.1097/00000542-197101000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Mohr C, Binkofski F, Erdmann C, Büchel C, Helmchen C. The anterior cingulate cortex contains distinct areas dissociating external from self-administered painful stimulation: a parametric fMRI study. Pain. 2005;114:347–337. doi: 10.1016/j.pain.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J. Neurol. 2000;247(Suppl 5):1–15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- 26.Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguière F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb. Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- 27.Pleger B, Tegenthoff M, Schwenkreis P, Janssen F, Ragert P, Dinse HR, Völker B, Zenz M, Maier C. Mean sustained pain levels are linked to hemispherical side-to-side differences of primary somatosensory cortex in the complex regional pain syndrome. I Exp Brain Res. 2004;155:115–119. doi: 10.1007/s00221-003-1738-4. [DOI] [PubMed] [Google Scholar]
- 28.Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ploner M, Gross J, Timmermann L, Schnitzler A. Cortical representation of first and second pain sensation in humans. Proc. Natl. Acad. Sci U.S.A. 2002;99:12444–12448. doi: 10.1073/pnas.182272899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruscheweyh R, Deppe M, Lohmann H, Stehling C, Flöel A, Ringelstein EB, Knecht S. Pain is associated with regional grey matter reduction in the general population. Pain. 2011;152:904–911. doi: 10.1016/j.pain.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J. Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, McHaffie JG, Coghill RC. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134:1987–2004. doi: 10.1093/brain/awr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valet M, Gündel H, Sprenger T, Sorg C, Mühlau M, Zimmer C, Henningsen P, Tölle TR. Patients with pain disorder show gray-matter loss in pain-processing structures: a voxel-based morphometric study. Psychosom Med. 2009;71:49–56. doi: 10.1097/PSY.0b013e31818d1e02. [DOI] [PubMed] [Google Scholar]
- 34.Vogt BA. Cingulate Neurobiology and Disease. New York: Oxford University Press; 2009. Cingulate Nociceptive Circuitry and Roles in Pain Processing-The Cingulate Premotor Pain Model; pp. 311–344. [Google Scholar]
- 35.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur. J. Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood PB. Variations in brain gray matter associated with chronic pain. Curr Rheumatol Rep. 2010;12:462–469. doi: 10.1007/s11926-010-0129-7. [DOI] [PubMed] [Google Scholar]
- 38.Younger JW, Shen YF, Goddard G, Mackey SC. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149:222–228. doi: 10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Younger JW, Chu LF, D'Arcy NT, Trott KE, Jastrzab LE, Mackey SC. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152:1803–1810. doi: 10.1016/j.pain.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziv M, Tomer R, Defrin R, Hendler T. Individual sensitivity to pain expectancy is related to differential activation of the hippocampus and amygdala. Hum Brain Mapp. 2010;31:326–338. doi: 10.1002/hbm.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic information for patients and controls.