Abstract
The hippocampus is involved in segregating memories, an ability that utilizes the neural process of pattern separation and allows for cognitive flexibility. We evaluated a proposed role for adult hippocampal neurogenesis in cognitive flexibility using variants of the active place avoidance task and two independent methods of ablating adult-born neurons, focal X-irradiation of the hippocampus, and genetic ablation of glial fibrillary acidic protein positive neural progenitor cells, in mice. We found that ablation of adult neurogenesis did not impair the ability to learn the initial location of a shock zone. However, when conflict was introduced by switching the location of the shock zone to the opposite side of the room, irradiated and transgenic mice entered the new shock zone location significantly more than their respective controls. This impairment was associated with increased upregulation of the immediate early gene Arc in the dorsal dentate gyrus, suggesting a role for adult neurogenesis in modulating network excitability and/or synaptic plasticity. Additional experiments revealed that irradiated mice were also impaired in learning to avoid a rotating shock zone when it was added to an initially learned stationary shock zone, but were unimpaired in learning the identical simultaneous task variant if it was their initial experience with place avoidance. Impaired avoidance could not be attributed to a deficit in extinction or an inability to learn a new shock zone location in a different environment. Together these results demonstrate that adult neurogenesis contributes to cognitive flexibility when it requires changing a learned response to a stimulus-evoked memory.
Keywords: neurogenesis, hippocampus, dentate gyrus, activity-regulated cytoskeleton-associated protein (Arc), active place avoidance
INTRODUCTION
Neurons are generated throughout life in the dentate gyrus of the hippocampus (Alvarez-Buylla and Lim, 2004). In vitro, young adult-born granule cells are more excitable than mature granule cells, with a lower threshold for firing action potentials and inducing long-term potentiation (LTP) (Schmidt-Hieber et al., 2004; Ge et al., 2007). Although these young neurons develop functional synaptic inputs and outputs (van Praag et al., 2002; Toni et al., 2008), it is unclear how they affect the activity of mature granule cells once they are integrated into dentate circuitry. Furthermore, it is not known how these neurogenesis-mediated cellular changes lead to an improvement in specific cognitive functions (Shors et al., 2002; Saxe et al., 2006; Sahay et al., 2011a).
The role of adult neurogenesis in cognition has been investigated intensely, with a focus on how adult-born neurons affect the acquisition of hippocampal-dependent memories (Shors et al., 2002; Snyder et al., 2005; Saxe et al., 2006; Drew et al., 2010) or the ability to distinguish between similar memories during pattern separation (Clelland et al., 2009; Sahay et al., 2011a). It has been proposed that adult neurogenesis may also contribute to cognitive flexibility (Wiskott et al., 2006; Kempermann, 2008), which is the ability to flexibly use and ignore familiar associations when contingencies change. Evidence in support of this hypothesis comes from studies showing that suppression of adult neurogenesis leads to deficits in the flexible use of spatial information acquired in the Morris water maze (Dupret et al., 2008; Garthe et al., 2009). However, a reliable impairment has consistently been accompanied by an impairment in initial learning, calling into question the specificity of the deficit found when flexibility is required.
We evaluated the contribution of neurogenesis to cognitive flexibility using two independent and well-established methods of ablating adult-born neurons, focal X-irradiation of the hippocampus and genetic ablation of glial fibrillary acidic protein (GFAP)-positive neural progenitor cells, and the active place avoidance paradigm. Active place avoidance is a hippocampus-dependent spatial task (Cimadevilla et al., 2001; Wesierska et al., 2005) previously used to demonstrate how coordination of information in the hippocampal memory network leads to the appropriate activation of relevant information and the suppression of irrelevant information (Kubik and Fenton, 2005; Wesierska et al., 2005; Kelemen E, 2008; Fenton et al., 2010; Kelemen and Fenton, 2010). By using multiple variants of the active place avoidance task, we were able to test different types of cognitive flexibility within the same experimental framework. The cellular processes underlying neurogenesis-mediated changes in cognitive flexibility were investigated by quantifying behaviorally induced protein expression of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein).
METHODS AND MATERIALS
Animals
Procedures used to ablate adult neurogenesis were conducted at Columbia University and experiments were performed at SUNY Downstate Medical Center. Mice were housed four to five per cage and were maintained on a 12 h (06:00–18:00) light-dark schedule with free access to food and water. All experiments were conducted in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committees of Columbia University, the New York State Psychiatric Institute, and SUNY Downstate Medical Center.
Focal X-Ray Procedure
Adult male 129Sv/Ev mice were purchased from Taconic Farms (Germantown, NY) at 8 weeks of age and irradiated at 10 weeks of age. Focal X-irradiation of the hippocampus was performed as previously described (Santarelli et al., 2003; Meshi et al., 2006; Denny et al., 2011). Mice were anesthetized with ketamine (105 mg kg−1, i.p.) and xylazine (7 mg kg−1, i.p.) and then placed in a stereotaxic frame. A lead shield covered the entire body of the animal, with the exception of a small area (3.22 × 11 mm2) above the hippocampus (interaural 3.00 to 0.00). The corrected dose rate was ~1.8 Gy per min at a source to skin distance of 30 cm. During each X-ray session, mice received 5 Gy over the course of 2 min and 47 s. A total of three doses of 5 Gy were delivered, with 2–3 days between each X-ray session. Sham-treated animals did not receive radiation, but were housed, anesthetized, and transported with irradiated mice throughout the experiment. Experiments were performed 12–16 weeks after the last X-ray session, to allow for inflammation to subside (Meshi et al., 2006). As a result, testing began when mice were 23–27 weeks of age. Ablation of neurogenesis was verified with doublecortin (DCX) immunoreactivity (IR) in naïve mice perfused 12 weeks after the last X-ray session.
Genetic Ablation
Using methods previously described (Garcia et al., 2004; Saxe et al., 2006), transgenic mice (TG) expressing thymidine kinase (TK) under the regulation of the glial fibrillary acidic protein (GFAP) promoter were backcrossed onto a 129Sv/Ev background. At 6–8 weeks of age, male mice carrying the GFAP-TK transgene (GFAP-TK TG) and wild-type (WT) littermates were anesthetized with isoflurane and implanted subcutaneously with 28-day Alzet osmotic minipumps (Palo Alto, CA) filled with ganciclovir in 0.9% saline (25 mg ml−1). To provide 56 days of continuous ganciclovir treatment, transgenic mice and WT controls were given two 28-day pumps consecutively, which were rotated under the skin two to three times per week. Behavioral testing began after a 2- to 17-day ganciclovir washout period. Ablation of neurogenesis was confirmed with DCX-IR in trained mice perfused 21 days after the cessation of ganciclovir treatment.
Active Place Avoidance
A mouse was placed on a circular (40 cm diameter) platform that rotated clockwise at a speed of 1 rpm. The mouse was trained to avoid a 60° shock zone, which could be defined within a region of the room, a region of the rotating arena, or both (Fenton et al., 1998; Wesierska et al., 2005). Entrance into the shock zone resulted in a brief constant current foot-shock (500 ms, 60 Hz, 0.2 mA) that was scrambled across pairs of rods. The intershock interval was 1.5 s. The position of the mouse was tracked by PC-based software that analyzed images from an overhead camera and delivered shocks appropriately (Tracker, Bio-Signal Group Corp., Brooklyn, NY). The number of times a mouse entered the shock zone was computed by Track Analysis software (Bio-Signal Group Corp., Brooklyn, NY). Pretraining and each training trial lasted 10 min, with an intertrial interval of 50 min.
To test cognitive flexibility and alternative learning deficits within the same experimental framework, we created six variants of the active place avoidance paradigm. All mice received initial training, unless otherwise specified.
Initial training
Mice were habituated to handling and the training environment, during which time the shock was turned off and mice walked freely on the rotating platform (Pretraining). Then the shock was turned on and animals were trained to avoid a stationary shock zone defined by cues within the room (Fig. 1; Video 1). Afterward, separate groups of mice were tested in one of the following versions of the task.
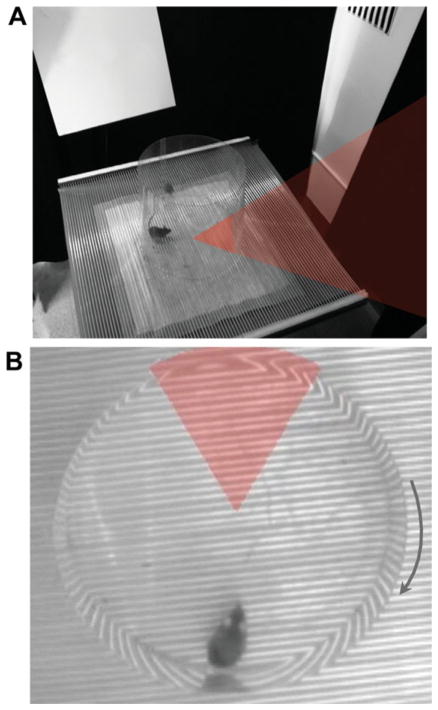
FIGURE 1.
The active place avoidance task (A) A mouse walking on a rotating circular platform in a room with multiple visual cues learns to avoid a 60° region of the room that has been designated as the shock zone. The shock zone and the corresponding section of the platform where the shocks are delivered are indicated by shading. (B) Overhead view of the mouse sitting directly opposite the stationary shock zone as the platform rotates clockwise. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Conflict
A conflict variant of the task was created to test cognitive flexibility. The location of the shock zone was moved 180° from its position during initial training, into the region where mice were primarily spending their time during the previous 2 days of training. Avoiding the new shock zone location required suppression of conditioned responses associated with avoiding its initial location. Cognitive flexibility is required to segregate the experiences associated with each shock zone and select between these two conflicting behaviors (Video 2).
Extinction
The shock was turned off and animals were free to enter the region of the room previously associated with a shock.
No conflict-initial conditions
Mice were given two additional training trials under conditions that were identical to those during initial training.
No conflict-new conditions
The location of the shock zone was moved 180° from its position during initial training and the distal room cues and local rotating platform cues were changed. This involved rearranging the curtains surrounding the rotating platform, changing the lighting, adding a peppermint scent, and reversing the direction of rotation so that the platform rotated counterclockwise. By creating a novel environment, animals have no way of identifying the initial location of the shock zone and new learning required to perform the task cannot conflict with what was previously learned.
Simultaneous two-frame avoidance
A rotating shock zone was added to the initially learned stationary shock zone. Because running through the center is required for avoiding both shock zones at the same time, the shock zones did not extend to the center of the platform. Simultaneous two-frame avoidance was tested in mice with and without initial training (Video 4).
Generation of Time-In-Location Maps
The arena was divided into 0.63 cm2 pixels and the total time spent in each pixel during the session was averaged across all the animals in a group. Time-in-location maps are presented as blue-to-red color-coded spatial histograms where blue represents the locations with the lowest dwell times and red represents the locations with maximum dwell times.
Immunohistochemistry
Histology preparation
Mice were deeply anesthetized with ketamine and perfused through the heart with cold saline followed by cold 4% paraformaledehyde in PBS. Brains were removed, postfixed overnight in 4% paraformaldehyde and then cryoprotected in 30% sucrose for at least 72 h. A cryostat (CM3050S, Leica, Germany) was used to cut 35 μm coronal sections through the entire dorso-ventral axis of the hippocampus. Sections were stored at 4°C in PBS with 0.1% NaN3. After immuno-histochemical procedures were used to stain the tissue, sections were mounted in dorsal-to-ventral order on slides (Superfrost Plus, Fisher Scientific, USA) and coverslipped with Neomount.
Doublecortin
Free-floating sections were quenched with 0.3% hydrogen peroxide in PBS:Methanol for 15 min. They were then washed with PBS containing 0.3% triton, blocked with 10% Normal Donkey Serum in PBS-0.3% triton for 2 h, and incubated overnight at 4°C with anti-DCX antibody (goat polyclonal, 1:500, Santa Cruz Biotechnology, CA) in PBS-0.3% triton. After being washed in PBS, tissue was incubated in biotinylated donkey anti-goat secondary antibody (1:500, Jackson Immuno-Research Laboratories, West Grove, PA) in PBS for 2 h, washed in PBS, and incubated with ABC reagent (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) for1 h. Tissue was visualized with diaminobenzidine (DAB Substrate kit, Vector Laboratories, Burlingame, CA).
Arc
A subset of mice tested in the “conflict” or “no conflict-initial conditions” were perfused immediately after the second trial on Day 3 of the experiment. This was 70 min after mice were put in the place avoidance apparatus, with the shock zone either in its original position or in the new position in the opposite side of the room. Naïve mice were perfused without exposure to the place avoidance task. To stain for Arc protein (Ploski et al., 2008), free floating sections were blocked with 10% Normal Donkey Serum in PBS-0.5% triton for 2 h. Then they were incubated overnight in anti-Arc antibody (mouse monoclonal, 1:1000, Santa Cruz Biotechnology, CA) in PBS-0.5% triton at 4°C. Sections were then washed in PBS and incubated with biotinylated donkey anti-mouse secondary antibody (1:500, Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS containing 10% Normal Donkey Serum for 1 h. Tissue was washed, processed with ABC solution (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) in PBS-0.5% triton for 1 h, and visualized with diaminobenzidine (DAB Substrate kit, Vector Laboratories, Burlingame, CA).
Quantification
An investigator blind to treatment status used a Zeiss (Oberkochen, Germany) Axioplan-2 upright microscope to count immunoreactive cells bilaterally in the granule cell layer (GCL) of the dentate gyrus (Santarelli et al., 2003; Meshi et al., 2006). Every sixth section throughout the entire extent of the dentate gyrus was included in the analysis. Cells were counted bilaterally using both the 20× and 40× objectives. For DCX cell counts, the total number of cells was summed across all sections for each animal and averaged for animals in each group. For Arc cell counts, sections in the dorsal half were compared to those in the ventral half of the dentate gyrus. Previous work has shown that active place avoidance relies more on the dorsal region than the ventral region (Cimadevilla et al., 2001; Pastalkova et al., 2006).
Statistical Analysis
Data were analyzed with ANOVA, Student t test for independent samples, and LSMeans Student’s t post-hoc tests, using JMP Version 5 software (SAS Institute, Cary, NC). Significance was accepted for P < 0.05.
RESULTS
Ablation of Neurogenesis
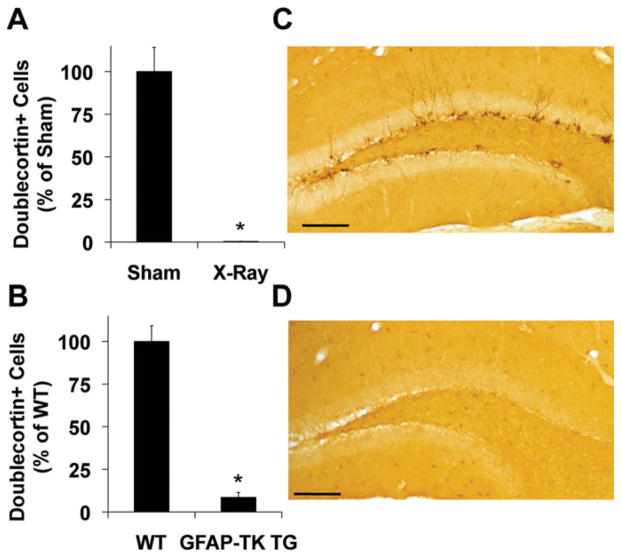
At the time of the first X-ray session, the average weight of all animals was 25.63 ± 0.67 g. At the time of the second X-ray session, the average weight of irradiated mice was 25.47 ± 0.89 g and the average weight of sham-treated mice was 25.90 ± 1.21 g. At the time of the third X-ray session, the average weight of irradiated mice was 25.19 ± 0.87 g and the average weight of sham-treated mice was 25.93 ± 1.36 g. At the time of behavioral testing, the average weight of irradiated mice was 32.88 ± 0.79 g and the average weight of sham-treated mice was 33.4 ± 1.16 g. Ablation of neurogenesis was confirmed by quantifying doublecortin (DCX)-immunoreactive (IR) cells in the dentate gyrus. Compared to sham controls (519.69 ± 73.77), the number of DCX-IR cells in irradiated mice (2.20 ± 0.82) was reduced by 99% (Fig. 2A), results that are consistent with previously published work using the same X-ray protocol (Santarelli et al., 2003; Meshi et al., 2006). Compared to WT controls (1447.32 ± 132.71), the number of DCX-IR cells in GFAP-TK TG mice (124.54 ± 42.01) was reduced by 91.4% (Figs. 2B–D).
FIGURE 2.
Ablation of adult hippocampal neurogenesis. Doublecortin immunostaining in the granule cell layer of the dentate gyrus was reduced in (A) X-ray treated mice (F1,8 = 49.21, *P < 0.01) and (B) GFAP-TK TG mice treated with ganciclovir (F1,8 = 90.30, *P < 0.01). Representative photomicrographs of doublecortin immunoreactivity in the subgranular zone of the dentate gyrus of a WT mouse (C) and a GFAP-TK TG mouse (D) are shown. Scale bars, 200 mm. Error bars represent S.E.M. X-ray-treated (N = 5); sham-irradiated (N = 5); GFAP-TK TG (N = 5); WT (N = 5). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Blocking Adult Neurogenesis Impairs Cognitive Flexibility
X-irradiated mice
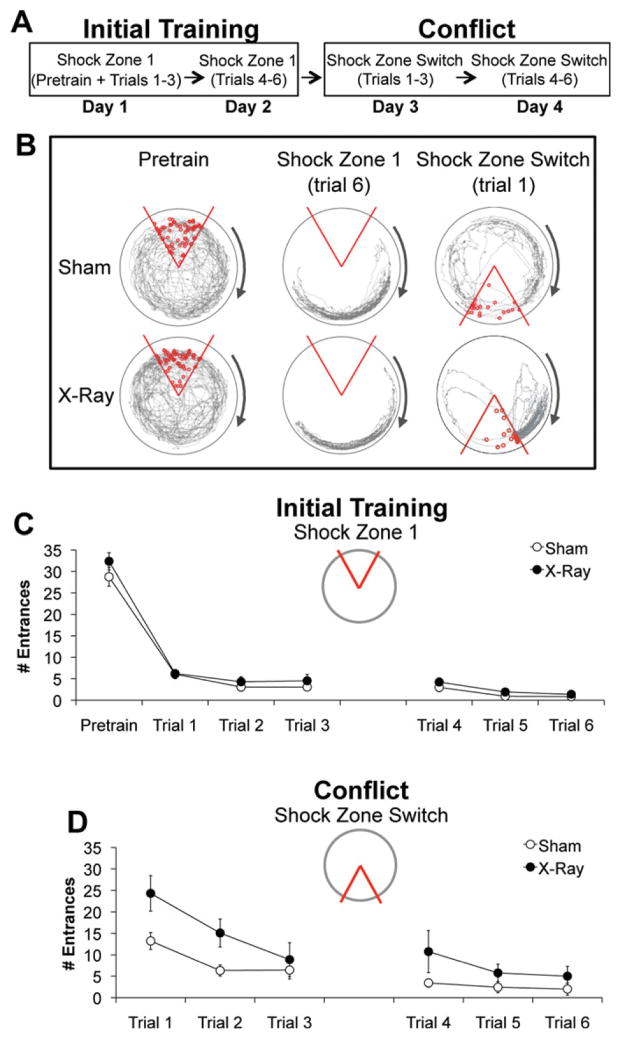
Spatial learning was evaluated in irradiated mice (Fig. 1; Video 1). Prior to training (Pretrain), irradiated mice entered the inactive shock zone (32.38 ± 2.0) as often as sham-irradiated controls (28.77 ± 2.21), (Figs. 3B, C). During initial training (Trials 1–6), all mice entered the shock zone significantly less often. Irradiated mice entered the active shock zone (3.78 ± 0.56) as infrequently as controls (2.83 ± 0.44), demonstrating unimpaired active place avoidance learning (Figs. 3B, C). The time-in-location maps confirmed there were no differences in where each group spent their time, indicating that they learned to avoid the shock zone in similar ways (Fig. 4A).
FIGURE 3.
Irradiation impaired cognitive flexibility required during conflict trials. (A) Schematic of behavioral procedures. (B) Example behavior. The path (gray) of a representative X- and sham-irradiated mouse. Open red circles indicate where the animal was shocked during each trial. The shock was off during pretraining. (C) Initial training: X- (N = 13) and sham-irradiated (N = 13) mice did not differ in the average number of times they entered the inactive shock zone during pretraining (F1,24 = 1.47, P = 0.24) or the active shock zone during six training trials (F1, 24 = 1.72, P = 0.20). The significant effect of trial confirmed that performance in both groups improved with training (F5, 20 = 10.69, P < 0.0001). (D) Conflict condition: After the location of the shock zone was switched, irradiated mice entered the new location of the shock zone significantly more than controls (F1, 101 = 14.98, P < 0.01). The significant effect of trial indicates that both groups improved with additional training (F5,101 = 30.53, P < 0.01), although the lack of an interaction suggests that performance of irradiated mice was worse than controls across all six trials (F5,101 = 2.23, P = 0.14). Error bars represent S.E.M. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
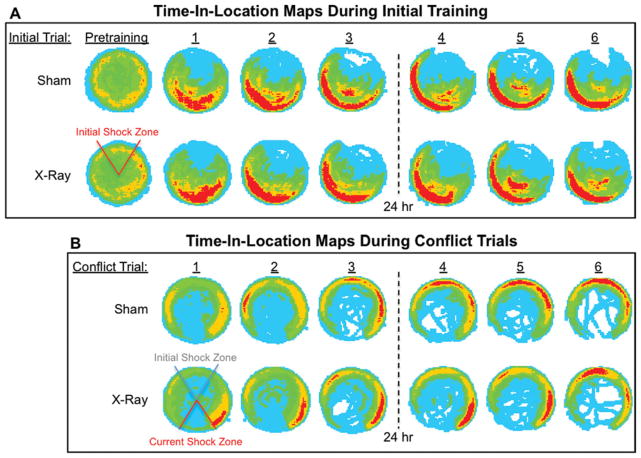
FIGURE 4.
Color-coded time-in-location maps for each treatment group during active place avoidance. Group-averaged time-in-location maps for each 10-min trial were computed to visually assess the patterns of avoidance behavior, which evolved with training experience. A blue-to-red scale was used, where blue represented the locations with the lowest dwell time and red represented the locations with the maximum dwell times. (A) Pretraining and each trial of initial training are shown. The same color category assignments were used for all maps, where the minimum time for each color assignment in seconds was: 0.09 (light blue), 2.45 (green), 6.09 (orange), 10.36 (red). (B) Each conflict trial is shown. The same color category assignments were used for all maps, where the minimum time for each color assignment in seconds was: 0.25 (light blue), 1.75 (green), 6.50 (orange), 15.25 (red). X-ray-treated (N = 13); sham-treated (N = 13). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Next, we used a conflict variant of the active place avoidance task to evaluate the consequences of ablating neurogenesis on cognitive flexibility. After the shock zone had been switched, both groups entered the shock zone more than they had during initial training, indicating interference between initial training and new learning. However, irradiated mice entered the new shock zone location significantly more than controls (Fig. 3D), indicating impaired cognitive flexibility.
During the first two conflict trials, irradiated mice spent significantly more time (94.12 ± 21.30 s) in the 20° region directly counter-clockwise of the shock zone than controls (42.48 ± 7.67 s), (Fig. 3B right; Fig. 4B; Videos 2–3). Note that because the direction of the arena rotation was clockwise, this counter-clockwise region was optimal for avoiding the initial location of the shock zone but was least effective for avoiding its current location. It appeared that irradiated mice entered the new location of the shock zone, because they were avoiding its initial location. Irradiated mice persisted in this activity in subsequent trials, after they began to successfully avoid the new location of the shock zone, indicating that they learned how to avoid both shock zone locations simultaneously. In contrast, sham controls moved into the initial location of the shock zone while avoiding its new location (Fig. 4B). Together, these findings indicate that irradiation impaired the ability to suppress previously learned behavioral responses associated with avoiding the initial shock zone location, which is consistent with a deficit in segregating relevant and irrelevant memories. These data provide further evidence that ablating neurogenesis impairs cognitive flexibility.
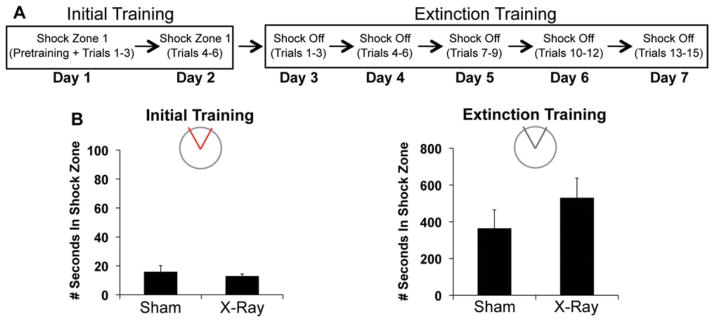
To account for whether the impairment is attributable to an impaired ability to extinguish an avoidance response (Deng et al., 2009; Ko et al., 2009), we tested extinction in a different cohort of X- and sham-irradiated mice (Fig. 5A). The amount of time irradiated mice spent in the shock zone during all six initial training trials (12.77 ± 1.62 s) was comparable to that of sham controls (15.72 ± 4.38 s), (Fig. 5B), confirming that irradiation does not impair acquisition of active place avoidance. Across 5 days of extinction training, the total number of times irradiated mice entered the inactive shock zone (111.88 ± 17.25) and the amount of time within the inactive shock zone (529.16 ± 108.61 s) during all fifteen trials was similar to that of sham-treated mice (80.13 ± 18.79; 362.94 ± 101.95 s, respectively). There were also no group differences when we limited the analysis to the first two extinction trials (Irradiated: 7.25 ± 2.38 entrances; 23.30 ± 8.22 seconds vs. Sham: 4.00 ± 1.69 entrances; 16.50 ± 6.55 s).
FIGURE 5.
Irradiated mice were not impaired in extinguishing avoidance responses. (A) Schematic of behavioral procedures. (B, left) Initial training: Irradiated (N = 8) and sham-irradiated (N = 8) mice did not differ in the total amount of time they spent in the active shock zone during six trials of initial training (F1,14 = 0.40, P = 0.54) (B, right) Extinction training: Groups did not differ in the total amount of time they spent in the inactive shock zone during 15 trials of extinction training (F1,14 = 1.25, P = 0.28). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
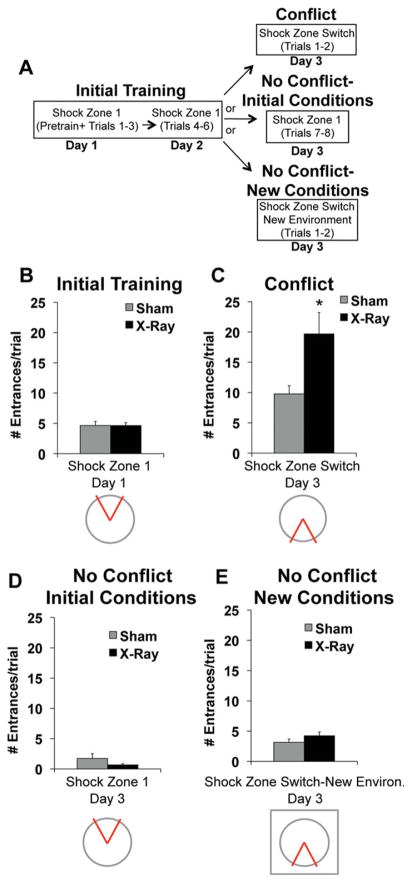
To test whether the impairment is caused by reduced memory capacity instead of cognitive flexibility, we replaced the conflict trials with trials that did not conflict with the initial place avoidance memory (Fig. 6A). Because the impairment in irradiated mice was most pronounced during the first two conflict trials, we focused all subsequent analyses on these trials.
FIGURE 6.
Irradiated mice were specifically impaired in trials involving a conflict. (A) Schematic of behavioral procedures. (B) Initial training: X- (N = 25) and sham-irradiated mice (N = 25) did not differ in the average number of times they entered the shock zone during the first two trials of initial training (F1,48 = 0.0006, P = 0.98). (C) Conflict condition: Irradiated mice (N = 13) entered the new location of the shock zone significantly more than sham-irradiated mice (N = 13) during the first two conflict trials (F1,24 = 6.85, P < 0.05). (D) No conflict-initial conditions: X- (N = 6) and sham-irradiated mice (N = 6) did not differ in the average number of times they entered the shock zone during two trials that were identical to the initial training trials (F1,10 = 1.88, P = 0.20). (E) No conflict-new conditions: X (N = 6) and sham-irradiated mice (N = 6) did not differ in the average number of times they entered a new location of the shock zone during two trials that occurred within a novel context (F1,10 = 1.74, P = 0.22). Error bars represent S.E.M. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Data acquired during initial training were collapsed for all mice subsequently allocated to the conflict/no conflict conditions. On average, irradiated mice entered the shock zone (4.64 ± 0.50) as often as sham controls (4.66 ± 0.65) during the first two trials of initial learning (Fig. 6B). The average number of times irradiated mice entered the new location of the shock zone during the first two conflict trials (conflict), (19.69 ± 3.54) was significantly higher than sham controls (9.77 ± 1.35), (Fig. 6C). In contrast, irradiated mice given two additional training trials identical to initial training (no conflict-initial conditions) entered the shock zone (0.67 ± 0.21) as infrequently as sham-treated mice (1.75 ± 0.76), (Fig. 6D). Therefore, the impairment cannot be attributed to group differences in memory retrieval that emerge as a result of being tested for an additional day. Irradiated mice also entered the new location of the shock zone (4.25 ± 0.62) as often as sham controls (3.17 ± 0.54) when they were tested in a novel environment (no conflict-new conditions), (Fig. 6E). Irradiation does not impair the ability to learn a new location of a shock zone when the new location does not conflict with what was previously learned.
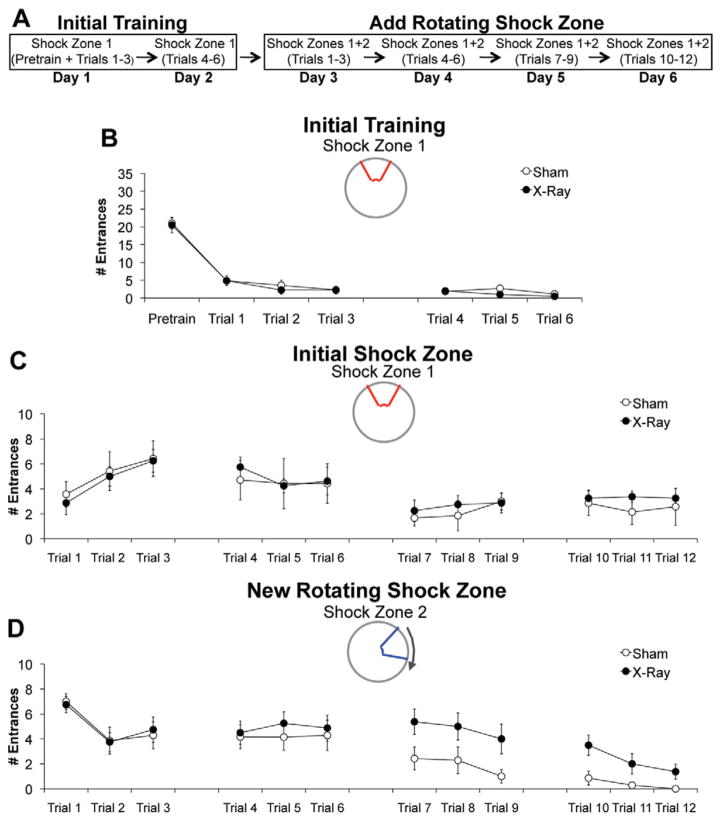
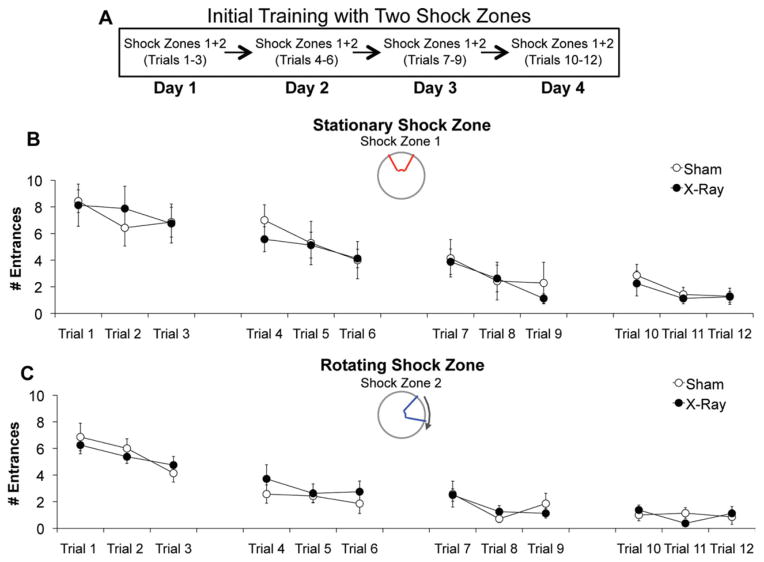
The deficit detected during conflict trials is consistent with a large body of literature demonstrating a role for hippocampus in reversal learning (Kimble and Kimble, 1965; Zeng et al., 2001). We tested the ability of irradiated mice to adapt to changing contingencies when reversal learning is not involved. After 2 days of initial training, a rotating shock zone was added to the initially learned stationary shock zone, creating a simultaneous two-frame avoidance task variant (Fig. 7A). During initial training, irradiated mice entered the stationary shock zone (2.15 ± 0.54) as often as sham controls (2.74 ± 0.46), (Fig. 7B). During the simultaneous task variant, irradiated mice entered the initially learned stationary shock zone (3.88 ± 0.46) as often as sham controls (3.59 ± 1.04) across all 4 additional days of training (Fig. 7C), but entered the new rotating shock zone (3.54 ± 0.88) significantly more than controls (1.14 ± 0.44) during the last 2 days of training, which is when control mice began to avoid this region (Fig. 7D). In contrast, when irradiated mice without any initial experience in place avoidance were tested in the identical simultaneous task variant (Fig. 8A), they entered the stationary (4.15 ± 0.53) and rotating shock zone (2.77 ± 0.20) as often as sham controls (stationary: 4.37 ± 0.53; rotating: 2.67 ± 0.38), (Figs. 8B, C). Therefore, ablating neurogenesis does not impair learning to avoid two shock zones simultaneously, but does impair cognitive flexibility in the absence of reversal learning.
FIGURE 7.
Irradiation impaired cognitive flexibility required with the addition of a shock zone. (A) Schematic of behavioral procedures. (B) Initial training: X- (N = 8) and sham-irradiated (N = 7) mice did not differ in the average number of times they entered the stationary shock zone during six initial training trials (F1,13 = 0.67, P = 0.43). (C, D). After the conditions of the task were changed, there was a significant effect of treatment (sham vs. irradiation) (F1,312 = 5.88, P < 0.05) and a significant treatment ×shock zone (initial vs. new) interaction (F1,312 = 4.70, P < 0.05). Post-hoc tests: Average entrances of irradiated mice into initial stationary shock zone = average entrances of sham mice into initial stationary shock zone. Average entrances of irradiated mice into new rotating shock zone > average entrances of sham mice into new rotating shock zone. Error bars represent S.E.M. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 8.
Irradiation did not impair initial learning of the simultaneous two-frame avoidance task variant. (A) Schematic of behavioral procedures. (B, C) Compared to sham controls (N = 7), irradiated mice (N = 8) were not impaired in learning to avoid the stationary shock zone or the rotating shock zone across all 12 trials. The effect of treatment (sham vs. irradiation), (F1,312 = 0.05, P = 0.82) and the treatment × shock zone (stationary vs. rotating) interaction (F1, 312 = 0.38, P = 0.54) were not significant. Error bars represent S.E.M. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
GFAP-TK TG mice
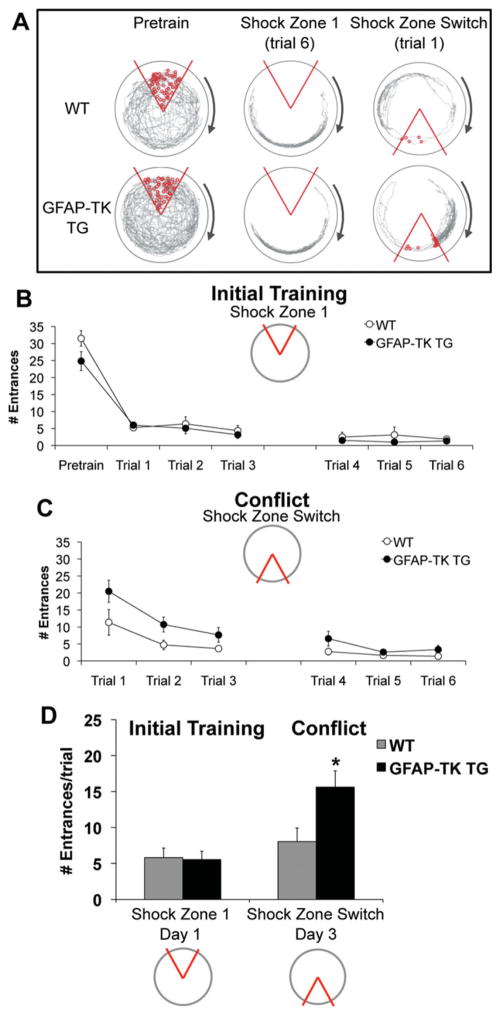
Active place avoidance was also tested following genetic ablation of adult neurogenesis. On average, GFAP-TK TG mice entered the initial shock zone (3.01 ± 0.55) as often as WT controls (3.92 ± 0.92), (Fig. 9A, middle; Fig. 9B), across all six trials and during the first two trials (GFAP-TK TG = 5.54 ± 1.16; WT = 5.81 ± 1.32), (Fig. 9D).
FIGURE 9.
Genetic ablation of adult neurogenesis impaired cognitive flexibility required during conflict trials. (A) Example behavior. The path (gray) of a representative WT and GFAP-TK TG mouse. (B) Initial training: GFAP-TK TG (N = 12) and WT (N = 8) mice did not differ in the average number of times they entered the inactive shock zone during pretraining (F1,18 = 2.93, P = 0.10) or the active shock zone during six initial training trials (F1,18 = 0.80, P = 0.38). Avoidance behavior improved across trials in both groups (F5, 14 = 6.42, P < 0.01). (C) Conflict condition: After the location of the shock zone was switched, GFAP-TK TG mice entered the new shock zone location significantly more than WT mice (F1, 18 = 4.66, P < 0.05). There was a significant effect of trial (F5, 14 = 7.60, P < 0.01) and no significant interaction (F5, 14 = 1.19, P = 0.36). (D) Average number of times each group entered the shock zone during the first two initial training and conflict trials. There were significant effects of shock zone location (F1, 36 = 11.37, P <0.01) and genotype × shock zone location interaction (F1, 36 = 4.62, P < 0.05). Post-hoc tests confirmed that GFPAP-TK TG mice entered the switched shock zone more than WT controls (*P < 0.05). Error bars represent S.E.M. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
During the conflict variant of the task, GFAP-TK TG mice entered the new shock zone location (8.57 ± 1.56) more than WT mice (4.24 ± 0.68) across all six conflict trials (Fig. 9A, right; Fig. 9C) and during the first two conflict trials (GFAP-TK TG = 15.63 ± 2.27; WT = 8.04 ± 1.87) (Fig. 9D). GFAP-TK TG mice also spent more time (73.42 ± 11.26 s) in the 20° region directly counter-clockwise of the shock zone than WT controls (37.33 ± 13.02 s; F1,18 = 4.29, P = 0.05) during the first two conflict trials. These results confirm that blocking adult neurogenesis impairs cognitive flexibility. Because neurogenesis in the subventricular zone (SVZ) was ablated in these animals (Saxe et al., 2006) but not in irradiated animals (Santarelli et al., 2003), our results indicate that ablation of SVZ neurogenesis does not exacerbate this deficit.
Impaired Cognitive Flexibility is Associated With Increased Arc Expression
We used expression of the immediate early gene Arc (Steward et al., 1998; Guzowski et al., 2000) to investigate hippocampal neural correlates of the deficit in cognitive flexibility. Arc was quantified after exposure to the following behavioral conditions: naïve, no conflict-initial conditions, conflict.
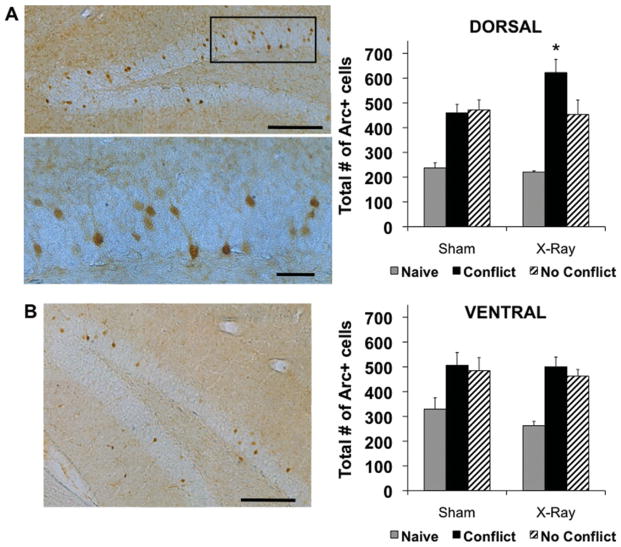
Consistent with previous reports (Chawla et al., 2005), we found the pattern of Arc+ cells to be sparse, with more labeled cells in the upper blade than the lower blade of the GCL (Fig. 10, left). Cell counts in the dorsal half of the GCL revealed that Naïve mice had at least 50% fewer Arc+ cells than trained animals, indicating that performance in the active place avoidance task induced significant upregulation of Arc protein in both X- and sham-irradiated mice. Irradiated mice in the Conflict condition had significantly more Arc+ cells (622.30 ± 52.83) than sham mice in the Conflict condition (459.50 ± 34.56), sham mice in the no conflict condition (471.10 ± 41.12), and irradiated mice in the no conflict condition (452.80 ± 58.86), none of which were significantly different from each other (Fig. 10A, right). The number of Arc+ cells in Naïve irradiated (220.70 ± 4.63) and Naïve sham mice (237.10 ± 20.74) were also not significantly different from each other. The lack of a difference in Arc protein between irradiated and sham-treated mice in the naïve and no conflict conditions indicates that upregulation of Arc in the Conflict condition is not the result of X-ray-induced morphological changes, which is consistent with previous work (Santarelli et al., 2003).
FIGURE 10.
Impaired cognitive flexibility was associated with upregulation of Arc protein in the dentate gyrus. (A, left) Representative ×10 (top) and × 20 (bottom) photomicrographs of Arc+ cells in the dorsal GCL of a sham mouse tested in the No conflict condition. Scale bar, 200 mm (top) and 50 mm (bottom). Multiple photographs were taken at × 20 (top) and × 40 (bottom) and reconstructed. (A, right) Quantification of Arc+ cells in the dorsal half of the GCL. There were significant effects of behavioral condition (F2,25 = 33.20, P < 0.01) and treatment × behavioral condition interaction (F2,25 = 3.51, P < 0.05). Post-hoc tests: irradiated mice in the conflict condition (N = 5) > sham mice in the conflict condition (N = 6) = sham mice in the no conflict condition (N = 5) = irradiated mice in the no conflict condition (N = 5) >Naïve irradiated mice (N = 5) = Naive sham mice (N = 5), (B, left) Representative ×10 photomicrograph of Arc+ cells in the ventral GCL of a sham-irradiated mouse in the no conflict condition. Scale bar, 200 mm. Multiple photographs were taken at ×20 and reconstructed. (B, right) Quantification of Arc+ cells in the ventral half of the GCL. There was a significant effect of behavioral condition (F2, 25 = 14.07, P < 0.01) but not treatment (F1,25 = 0.85, P = 0.37) or treatment ×behavioral condition interaction (F2, 25 = 0.28, P = 0.76). Post-hoc tests: Mice in the no conflict condition = mice in the conflict condition > naïve mice. Error bars represent S.E.M. *P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Cell counts in the ventral half of the GCL revealed that Naïve mice had at least 32% fewer Arc+ cells than trained animals. However, the number of Arc+ cells in irradiated mice in the Conflict condition (500.20 ± 39.19), sham mice in the Conflict condition (506.28 ± 51.34), irradiated mice in the No Conflict condition (462.30 ± 26.85), and sham mice in the no conflict condition (484.60 ± 52.68) were not significantly different from each other (Fig. 10B, right). The deficit in cognitive flexibility following removal of adult-born cells correlates with an increase in Arc+ cells, specifically in the dorsal half of the dentate gyrus.
DISCUSSION
Main Findings
Using variants of the active place avoidance task and two independent methods of ablating adult neurogenesis, we identified and characterized the contribution of adult-born neurons to cognitive flexibility. Ablation of adult neurogenesis using either focal hippocampal X-irradiation or a genetic technique did not impair learning the initial location of a shock zone, but did impair the ability to avoid a new shock zone location if it conflicted with the initial location of shock. This impairment was associated with increased upregulation of Arc in the dorsal dentate gyrus, suggesting a role for adult neurogenesis in modulating dentate network excitability and/or synaptic plasticity. Ablating neurogenesis also impaired cognitive flexibility when it did not involve reversal learning, but did not impair extinction or the ability to learn a new shock zone location per se. Together, these results demonstrate that mice without neurogenesis were specifically impaired in their ability to flexibly adapt to a change in contingencies when it required modification of a learned response to a reinforcing stimulus.
A Role for Adult Neurogenesis in a Specific Type of Cognitive Flexibility
A role for adult-born cells in contextual learning has been debated, with some studies demonstrating that ablation of neurogenesis impairs contextual fear conditioning (Saxe et al., 2006; Winocur et al., 2006; Imayoshi et al., 2008; Warner-Schmidt et al., 2008; Wojtowicz et al., 2008; Hernandez-Rabaza et al., 2009; Ko et al., 2009), and others reporting no impairment (Shors et al., 2002; Clark et al., 2008; Dupret et al., 2008; Pollak et al., 2008; Zhang et al., 2008). A recent study accounts for some of the discrepancies in the literature by showing that adult hippocampal neurogenesis is only required for contextual fear conditioning in mice when they have not been preexposed to the training environment or when training involves a single shock (Drew et al., 2010). Using the active place avoidance paradigm, a spatial task that is sensitive to minor hippocampus dysfunction (Cimadevilla et al., 2001; Wesierska et al., 2005), we found that ablation of adult-born neurons did not impair learning the location of shock during initial training or when all spatial cues were changed during the “no conflict-new conditions” version of the task. Because animals were preexposed to the training environment during a pretraining session and received more than one shock when initially trained in each variant of the task, our findings provide additional evidence that adult hippocampal neurogenesis does not contribute to contextual learning when rapid acquisition is not required.
We tested animals with ablated neurogenesis in three variants of the active place avoidance task that challenged the ability to adapt a learned behavioral response to new contingencies. In the conflict variant, irradiated animals ran opposite the initial location of the shock zone, directly into its new location, demonstrating an impaired ability to suppress a learned response. When tested in the simultaneous two-frame avoidance task variant, irradiated mice continued to avoid the initially learned shock zone as well as controls, but were specifically impaired in avoiding the new shock zone. In both conditions, it was as if the shock elicited retrieval of the initial memory, causing impaired acquisition of the new memory. Consistent with previous reports (Ko et al., 2009), animals without neurogenesis were not impaired in extinguishing the avoidance response, which is when the shock was turned off. Together, these results indicate that adult neurogenesis plays a role in a particular type of cognitive flexibility involving the ability to change a learned response, specifically to a memory evoked by a stimulus.
Cognitive flexibility was only impaired under conditions that required animals to differentiate between highly similar experiences and generate distinct responses to each, implicating a role for pattern separation. Pattern separation is a process that involves transforming similar and potentially interfering inputs into nonoverlapping representations (Marr, 1971) and is modulated by adult neurogenesis (Clelland et al., 2009; Sahay et al., 2011a). Theoretically, pattern separation was required to segregate similar perceptual input during initial learning of the simultaneous two-frame avoidance task variant and similar memories during extinction. However, the impairment reported here indicates that a functional consequence of neurogenesis-mediated improvements in pattern separation is specifically to minimize interference between similar stimulus-evoked memories.
Our findings support hypotheses proposing a role for adult neurogenesis in the flexible use of information (Kempermann, 2008) and in minimizing interference between similar memories (Becker, 2005; Wiskott et al., 2006; Winocur et al., 2011). In contrast to work presented here, previous efforts to test these hypotheses have relied on the water maze, with results that either demonstrated an impairment in initial water maze learning (Dupret et al., 2008; Garthe et al., 2009) or no effect of blocking neurogenesis after moving the platform to the opposite quadrant (Wojtowicz et al., 2008). Active place avoidance and the water maze are both tasks that require spatial navigation. Although place responses in the active place avoidance task are not as precise as those in the water maze, place avoidance is nonetheless more sensitive than the water maze to learning impairments (Fenton and Bures, 1993; Cimadevilla et al., 2001; Kubik and Fenton, 2005) and to the maintenance of a learned place response by LTP in hippocampus (Serrano et al., 2008), which in the case of active place avoidance lasts at least 30 days (Pastalkova et al., 2006). An additional benefit of using active place avoidance is that it can be configured so that more than one spatial location is learned concurrently, which allowed us to characterize impairments in cognitive flexibility beyond a deficit in reversal learning. Perhaps these differences between the two paradigms and the use of other methods of ablation account for why prior findings using the water maze have not been as conclusive as what we observed here. Whereas we used two independent methods of ablation and consistently found no effect of ablating neurogenesis on initial learning, this has not been clear in previous studies using the water maze (Shors et al., 2002; Madsen et al., 2003; Snyder et al., 2005; Saxe et al., 2006; Dupret et al., 2008; Wojtowicz et al., 2008; Garthe et al., 2009).
Recent studies reveal a role for adult hippocampal neurogenesis in regulating the hypothalamo-pituatary-adrenal axis response. Specifically, removal of adult-born cells leads to hypersecretion of corticosterone (CORT) in response to stressful experiences (Schloesser et al., 2009; Snyder et al., 2011). Although it is unclear how stressful active place avoidance is compared to other behavioral paradigms (Friedman and Ader, 1965; Harrison et al., 2009; Shen et al., 2010), it is still possible that our behavioral tests elicited different CORT responses in animals with and without neurogenesis. Despite the association between stress, CORT, and memory (Sauro et al., 2003), our data indicate that potential differences in CORT did not affect the ability of mice to acquire active place avoidance or to extinguish an avoidance response. While an enhanced CORT response may have contributed to deficits in cognitive flexibility, our behavioral data are not consistent with this interpretation. Irradiated and GFAP-TK mice were shocked the most during the first conflict trial, during which the release of CORT is assumed to be maximal. A CORT-mediated deficit in learning would therefore be expected to worsen with additional training, yet we found that avoidance behavior improved with additional training. Together, our data indicate that the cognitive functions we measured with active place avoidance are not sensitive to changes in CORT that result from ablation of neurogenesis.
The Effect of Adult Neurogenesis on a Neural Correlate of Cognitive Flexibility
Adult-born neurons are more excitable than mature granule cells with a reduced threshold for LTP induction (Schmidt-Hieber et al., 2004; Ge et al., 2007; Mongiat et al., 2009), prompting the hypothesis that these cells encode and represent memories (Aimone et al., 2006, 2011; Deng et al., 2010). Consequently, the majority of prior neurogenesis studies using neuronal activity markers have focused specifically on immediate-early gene expression in adult-born cells (Jessberger and Kempermann, 2003; Ramirez-Amaya et al., 2006; Kee et al., 2007; Tashiro et al., 2007; Trouche et al., 2009). Here, we focus on expression of the immediate-early gene Arc in mature granule cells after ablation of adult-born cells to investigate how young neurons affect the dentate gyrus network in the awake behaving animal.
Even though adult-born cells are characterized by increased excitability, we found that their removal upregulated Arc expression, specifically when cognitive flexibility was tested. Arc is a neuronal activity marker (Guzowski et al., 1999; Guzowski et al., 2001) that is also required for synaptic plasticity and long-term memory (Guzowski et al., 2000; Plath et al., 2006). Upregulation of Arc in irradiated animals may therefore reflect greater network activation and/or increased synaptic plasticity in the remaining mature granule cells. An association between greater network activation and a deficit in cognitive flexibility is consistent with computational models of dentate gyrus function. The dentate gyrus normally uses a sparse coding scheme (Jung and McNaughton, 1993), which is thought to be conducive to pattern separation (Marr, 1971; Treves et al., 2008). Recruitment of additional cells would therefore be expected to correlate with an impaired ability to segregate and select between similar conflicting memories.
Previous studies have shown that reducing hippocampal neurogenesis increases the magnitude of spontaneous gamma bursts in the dentate gyrus (Lacefield et al., 2010) and prolongs LTP (Kitamura et al., 2009). Conversely, increasing neurogenesis through enrichment (Kempermann et al., 1997) accelerates LTP decay in the dentate gyrus (Abraham et al., 2002). Such findings have prompted the hypothesis that adult-born neurons decrease excitation of mature granule cells through mechanisms such as feedback inhibition (Lacefield et al., 2010; Sahay et al., 2011b). Our finding that removal of adult-born cells increased behaviorally induced immediate early gene expression is in line with this hypothesis. Because upregulation only occurred when cognitive flexibility was required, neurogenesis-dependent changes in inhibition may not be detectable in vivo under all conditions that evoke dentate activity. Alternatively, it is possible that even initial learning differentially affected synaptic plasticity in the dentate gyrus of mice with and without neurogenesis in a manner that was not detectable behaviorally or with Arc expression. Future studies aimed at understanding the electrophysiological changes that accompany neurogenesis-dependent learning will provide insight into how adult-born cells modulate mature granule cells in the awake behaving animal.
SUMMARY
In summary, we used variants of the active place avoidance task and two independent ablation techniques to examine the contribution of adult-born neurons to cognitive flexibility. We found that ablating neurogenesis impaired a specific type of cognitive flexibility, which was accompanied by an increase in Arc protein expression. Our results suggest that adult-born neurons in the hippocampus may contribute to cognitive processes by modulating excitability and/or synaptic plasticity in the dentate gyrus network.
Acknowledgments
Grant sponsor: NIH; Grant numbers: MH068542, MH068542-07S1, MH015174, MH084038-01; Grant sponsor: Korea Research Foundation (MOHERD); Grant number: KRF-2007-357-H0005; Grant sponsor: NAR-SAD and New York Stem Cell Initiative (NYSTEM)
Thank you to Michael Drew and Indira David for technical assistance with the GFAP-TK TG mice, Michael Saxe for initiating the collaboration, and Gijs Joost Brouwer for place avoidance photography.
References
- Abraham WC, Logan B, Greenwood JM, Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;15:722–738. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Cimadevilla JM, Wesierska M, Fenton AA, Bures J. Inactivating one hippocampus impairs avoidance of a stable room-defined place during dissociation of arena cues from room cues by rotation of the arena. Proc Natl Acad Sci USA. 2001;98:3531–3536. doi: 10.1073/pnas.051628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–1058. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning. Hippocampus. 2011 doi: 10.1002/hipo.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MR, Denny CA, Hen R. Arrest of adult hippocampal neurogenesis in mice impairs single- but not multiple-trial contextual fear conditioning. Behav Neurosci. 2010;124:446–454. doi: 10.1037/a0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Bures J. Place navigation in rats with unilateral tetrodotoxin inactivation of the dorsal hippocampus: Place but not procedural learning can be lateralized to one hippocampus. Behav Neurosci. 1993;107:552–564. doi: 10.1037//0735-7044.107.4.552. [DOI] [PubMed] [Google Scholar]
- Fenton AA, Lytton WW, Barry JM, Lenck-Santini PP, Zinyuk LE, Kubik S, Bures J, Poucet B, Muller RU, Olypher AV. Attention-like modulation of hippocampus place cell discharge. J Neurosci. 2010;30:4613–4625. doi: 10.1523/JNEUROSCI.5576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton AA, Wesierska M, Kaminsky Y, Bures J. Both here and there: Simultaneous expression of autonomous spatial memories in rats. Proc Natl Acad Sci USA. 1998;95:11493–11498. doi: 10.1073/pnas.95.19.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SB, Ader R. Parameters relevant to the experimental production of “stress” in the mouse. Psychosom Med. 1965;27:27–30. doi: 10.1097/00006842-196501000-00004. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Llorens-Martin M, Velazquez-Sanchez C, Ferragud A, Arcusa A, Gumus HG, Gomez-Pinedo U, Perez-Villalba A, Rosello J, Trejo JL, et al. Inhibition of adult hippocampal neurogenesis disrupts contextual learning but spares spatial working memory, long-term conditional rule retention and spatial reversal. Neuroscience. 2009;159:59–68. doi: 10.1016/j.neuroscience.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18:2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3:165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10:355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- Kelemen EFA. What do place cells tell us about learning to associate and learning to segregate? In: Mizumori SJ, editor. Hippocampal Place Fields, Relevance to Learning and Memory. New York: Oxford University Press; 2008. pp. 127–137. [Google Scholar]
- Kelemen E, Fenton AA. Dynamic grouping of hippocampal neural activity during cognitive control of two spatial frames. PLoS Biol. 2010;8:e1000403. doi: 10.1371/journal.pbio.1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. The neurogenic reserve hypothesis: What is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kimble DP, Kimble RJ. Hippocampectomy and response perseveration in the rat. J Comp Physiol Psychol. 1965;60:474–476. doi: 10.1037/h0022550. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Ko HG, Jang DJ, Son J, Kwak C, Choi JH, Ji YH, Lee YS, Son H, Kaang BK. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubik S, Fenton AA. Behavioral evidence that segregation and representation are dissociable hippocampal functions. J Neurosci. 2005;25:9205–9212. doi: 10.1523/JNEUROSCI.1707-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2010;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PE, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: A theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Mongiat LA, Esposito MS, Lombardi G, Schinder AF. Reliable activation of immature neurons in the adult hippocampus. PLoS One. 2009;4:e5320. doi: 10.1371/journal.pone.0005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak DD, Monje FJ, Zuckerman L, Denny CA, Drew MR, Kandel ER. An animal model of a behavioral intervention for depression. Neuron. 2008;60:149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011a;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: A common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011b;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sauro MD, Jorgensen RS, Pedlow CT. Stress, glucocorticoids, and memory: A meta-analytic review. Stress. 2003;6:235–245. doi: 10.1080/10253890310001616482. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Manji HK, Martinowich K. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport. 2009;20:553–557. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, et al. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for alpha4betadelta GABAA receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves A, Tashiro A, Witter ME, Moser EI. What is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci USA. 2009;106:5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Madsen TM, Duman RS. Electroconvulsive seizure restores neurogenesis and hippocampus-dependent fear memory after disruption by irradiation. Eur J Neurosci. 2008;27:1485–1493. doi: 10.1111/j.1460-9568.2008.06118.x. [DOI] [PubMed] [Google Scholar]
- Wesierska M, Dockery C, Fenton AA. Beyond memory, navigation, and inhibition: Behavioral evidence for hippocampus-dependent cognitive coordination in the rat. J Neurosci. 2005;25:2413–2419. doi: 10.1523/JNEUROSCI.3962-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Becker S, Luu P, Rosenzweig S, Wojtowicz JM. Adult hippocampal neurogenesis and memory interference. Behav Brain Res. 2011;227:464–469. doi: 10.1016/j.bbr.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: Avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- Wojtowicz JM, Askew ML, Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur J Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]