Abstract
The activity of oral clofazimine against intracellular Mycobacterium tuberculosis was compared to that of ofloxacin in healthy volunteers by the use of whole-blood cultures. Clofazimine was inactive whether it was tested alone or combined with other drugs that are used to treat multidrug-resistant tuberculosis, despite a total dose of 2 g. Kanamycin was the most active drug tested.
Multidrug-resistant tuberculosis (MDR TB) poses a growing public health problem. Studies to assess tissue sterilization in MDR TB are particularly long and complex. Members of our laboratory recently described a method for studying the ability of administered chemotherapy to kill intracellular Mycobacterium tuberculosis cells by using whole-blood cultures (14). In patients with drug-sensitive TB, the whole-blood bactericidal activity (WBA) was correlated with two sputum markers of tissue sterilization, the 8-week sputum culture conversion and the serial sputum CFU slope (16). In the present study, the whole-blood model was used to examine the potential role of clofazimine in the treatment of MDR TB.
Our subjects consisted of 10 healthy tuberculin-nonreactive volunteers who provided written informed consent according to an Institutional Review Board-approved protocol. They were randomly assigned to receive only ofloxacin (600 mg) or clofazimine (200 mg) daily for 5 days. Pyrazinamide (25 mg/kg of body weight) was added on days 6 to 10. Ethambutol (25 mg/kg) was added on day 10. This schedule permitted the intracellular accumulation of clofazimine, ofloxacin, and pyrazinamide in phagocytic cells. Kanamycin was added to whole-blood cultures at final concentrations of 20 and 10 μg/ml in blood specimens at 2 and 6 h postdose, respectively, to mimic blood levels of 40 and 20 μg/ml, respectively. Drug addition was delayed 30 min after infection to allow for phagocytosis.
WBA was assessed as previously described (5, 14, 16). Briefly, heparinized blood was diluted 1:1 with medium and infected with M. tuberculosis H37Rv. Members of our laboratory previously documented the high efficiency of phagocytosis in whole-blood cultures (>95%) by examining CFU counts in the liquid and cellular partitions after low-speed sedimentation and by showing the lack of effect of amoxicillin-clavulanate, which does not reach adequate intracellular levels to act against M. tuberculosis (14); this eliminates the need to remove extracellular bacilli. After 72 h of incubation, the host cells were disrupted by hypotonic lysis, and bacilli were sedimented and inoculated into BACTEC medium. Bacillus killing was calculated based on days-to-positivity by using a standard curve. The total bactericidal effect was assessed as the area under the curve (AUC) (16). The results were examined by a paired or unpaired t test.
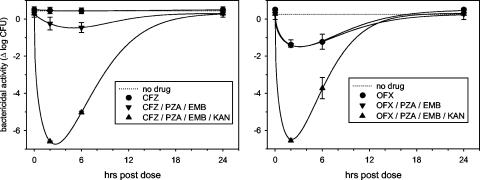
At the baseline, there was a net growth of M. tuberculosis H37Rv of 0.41 log CFU over 72 h in the subjects' blood cells. A single dose of clofazimine had no effect in blood sampled 2 h afterward (mean, 0.42 log CFU; P = 0.3), whereas the corresponding effect of ofloxacin was readily apparent (−1.21 log CFU; P < 0.001). Clofazimine remained inactive after five daily doses, as illustrated in Fig. 1 and Table 1.
FIG. 1.
WBA of MDR TB regimens based on clofazimine (CFZ) (left) and ofloxacin (OFX) (right) tested in healthy volunteers. In the left panel, the curves of clofazimine and the no-drug control are superimposed.
TABLE 1.
Total WBA of clofazimine- and ofloxacin- based regimens in 10 healthy volunteers
| Study stepa | AUC (Δlog CFU-days)b
|
P value | |
|---|---|---|---|
| Clofazimine regimen | Ofloxacin regimen | ||
| Baseline | +0.46 ± 0.2 | +0.36 ± 0.4 | 0.6 |
| Study drug (day 5) | +0.46 ± 0.2 | −0.35 ± 0.3 | <0.001 |
| Study drug plus PZA and EMB (day 10) | −0.04 ± 0.2 | −0.44 ± 0.4 | 0.067 |
| Study drug plus PZA, EMB, and KAN (day 10) | −3.18 ± 0.8 | −2.41 ± 0.9 | 0.18 |
PZA, pyrazinamide; EMB, ethambutol; KAN, kanamycin.
Negative values indicate killing. Blood was sampled at 0, 2, and 6 h postdose on days 5 and 10 to calculate the AUC. The concentrations of kanamycin were selected to reflect drug levels in plasma at 2 and 6 h postdose (40 and 20 μg/ml, respectively).
The treatment was then modified by the addition of pyrazinamide and ethambutol. WBA values at 2 h postdose on day 10 were −0.24 ± 0.35 and −1.40 ± 0.27 log CFU in the clofazimine and ofloxacin samples, respectively (P < 0.001). The ofloxacin regimen tended to remain superior when analyzed by the AUC (Table 1). However, both regimens were only marginally active when compared to previous studies of isoniazid and rifampin (−2 to −3 log CFU-days) (14, 16). That level of activity was not approached in the present study until kanamycin was added (mean, −2.79 log CFU-days).
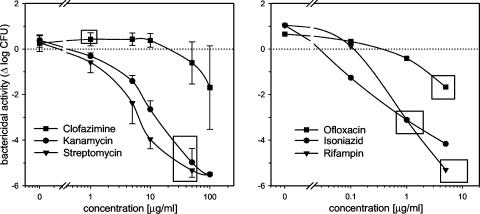
The lack of effect of clofazimine may have been due to inadequate peak drug concentrations or an insufficient total dose. Since clofazimine toxicities are related to the total dose, we could not ethically justify its prolonged administration to healthy volunteers and therefore examined the relationship between drug concentration and WBA by its direct addition to whole-blood cultures. As Fig. 2 indicates, levels of clofazimine in plasma far in excess of those achieved after oral administration are required for significant bactericidal activity against intracellular M. tuberculosis in whole-blood cultures.
FIG. 2.
Relationship between drug concentration and killing of intracellular M. tuberculosis for clofazimine, kanamycin, and streptomycin in 72-h whole-blood cultures from four healthy volunteers (left). Peak plasma drug concentrations after standard dosing are indicated by rectangles. For comparison, the results for a single volunteer for isoniazid, rifampin, and ofloxacin are shown in the right panel.
Although it is mainly used for the treatment of leprosy, clofazimine shows substantial activity against M. tuberculosis, both in vitro and in animal testing (8, 11, 13). Its MIC against M. tuberculosis H37Rv, 0.1 μg/ml, is well within the range of reported concentrations in plasma after oral administration (11). For these reasons, it is often empirically included in regimens for the treatment of highly MDR TB. Clofazimine shows substantially more intracellular bactericidal activity than kanamycin in isolated macrophages (11), yet it was inactive in the present study. In contrast, kanamycin, streptomycin, and pyrazinamide showed substantially larger effects in whole-blood cultures than in macrophages (6). The findings corresponded only for ofloxacin (14).
The basis of these divergent findings is not clear. Monocytes that are allowed to adhere to tissue culture wells show a reduced capacity to ingest and kill mycobacteria compared to cells in suspension (1, 14). Whole-blood cultures differ from those of isolated macrophages in that even in tuberculin nonreactors, cellular interactions occur between infected macrophages and other cells, such as γδ, NK, and other cells (2, 12). These interactions result in the release of cytokines and ATP, which may promote macrophage apoptosis and phagosome-lysosome fusion (9, 10). Some drugs, including aminoglycosides, penetrate poorly into resting mammalian cells (4). Little is known about the impact of cellular adherence or activation on intracellular drug accumulation and action, which may be affected by changes in the physiology of the host cell or the mycobacterium (15). Further studies of the impact of host immunity on the effectiveness of administered TB chemotherapy may help to address these questions.
Although the aminoglycosides show little, if any, early bactericidal activity, their activity on sputum CFU counts becomes apparent after 2 to 4 weeks (7). Streptomycin shares this property with rifampin and pyrazinamide, two other highly sterilizing drugs that are inactive during the first few days of TB chemotherapy (3). The sterilizing activity of the aminoglycosides has not yet been formally evaluated. Their substantial activity in the whole-blood model indicates that additional clinical studies are warranted to examine their role as sterilizing drugs for TB.
Acknowledgments
This work was supported by the Fogarty International Central/Eastern European HIV Research Program grant 3 D43 TW00233 (to Jack DeHovitz, primary investigator) and by grant 063410/A/00/Z of the Wellcome Trust (to Jerrold Ellner, primary investigator).
REFERENCES
- 1.Barker, K., H. Fan, C. Carroll, G. Kaplan, J. Barker, W. Hellmann, and Z. A. Cohn. 1996. Nonadherent cultures of human monocytes kill Mycobacterium smegmatis, but adherent cultures do not. Infect. Immun. 64:428-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brill, K. J., Q. Li, R. Larkin, D. H. Canaday, D. R. Kaplan, W. H. Boom, and R. F. Silver. 2001. Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms. Infect. Immun. 69:1755-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brindle, R., J. Odhiambo, and D. A. Mitchison. 2001. Serial counts of Mycobacterium tuberculosis in sputum as surrogate markers of the sterilising activity of rifampicin and pyrazinamide in treating pulmonary tuberculosis. BMC Pulm. Med. 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, K. N., and A. Percival. 1978. Penetration of antimicrobials into tissue culture cells and leucocytes. Scand. J. Infect. Dis. 1978(Suppl.):251-260. [PubMed] [Google Scholar]
- 5.Cheon, S. H., B. Kampmann, A. G. Hise, M. Phillips, H. Y. Song, K. Landen, Q. Li, R. Larkin, J. J. Ellner, R. F. Silver, D. F. Hoft, and R. S. Wallis. 2002. Bactericidal activity in whole blood as a potential surrogate marker of immunity after vaccination against tuberculosis. Clin. Diagn. Lab. Immunol. 9:901-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heifets, L., M. Higgins, and B. Simon. 2000. Pyrazinamide is not active against Mycobacterium tuberculosis residing in cultured human monocyte-derived macrophages. Int. J. Tuberc. Lung Dis. 4:491-495. [PubMed] [Google Scholar]
- 7.Jindani, A., C. J. Dore, and D. A. Mitchison. 2003. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am. J. Respir. Crit. Care Med. 167:1348-1354. [DOI] [PubMed] [Google Scholar]
- 8.Klemens, S. P., M. S. DeStefano, and M. H. Cynamon. 1993. Therapy of multidrug-resistant tuberculosis: lessons from studies with mice. Antimicrob. Agents Chemother. 37:2344-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusner, D. J., and J. Adams. 2000. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J. Immunol. 164:379-388. [DOI] [PubMed] [Google Scholar]
- 10.Kusner, D. J., and J. A. Barton. 2001. ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium-dependent phagosome-lysosome fusion. J. Immunol. 167:3308-3315. [DOI] [PubMed] [Google Scholar]
- 11.Rastogi, N., V. Labrousse, and K. S. Goh. 1996. In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr. Microbiol. 33:167-175. [DOI] [PubMed] [Google Scholar]
- 12.Silver, R. F., Q. Li, W. H. Boom, and J. J. Ellner. 1998. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative, subjects. J. Immunol. 160:2408-2417. [PubMed] [Google Scholar]
- 13.Wallace, R. J., D. R. Nash, L. C. Steele, and V. Steingrube. 1986. Susceptibility testing of slowly growing mycobacteria by a microdilution MIC method with 7H9 broth. J. Clin. Microbiol. 24:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallis, R. S., M. Palaci, S. Vinhas, A. G. Hise, F. C. Ribeiro, K. Landen, S. H. Cheon, H. Y. Song, M. Phillips, R. Dietze, and J. J. Ellner. 2001. A whole blood bactericidal assay for tuberculosis. J. Infect. Dis. 183:1300-1303. [DOI] [PubMed] [Google Scholar]
- 15.Wallis, R. S., H. Y. Song, C. Whalen, and A. Okwera. 2004. TB chemotherapy: antagonism between immunity and sterilization. Am. J. Respir. Crit. Care Med. 169:771-772. [DOI] [PubMed] [Google Scholar]
- 16.Wallis, R. S., S. A. Vinhas, J. L. Johnson, F. C. Ribeiro, M. Palaci, R. L. Peres, R. T. Sa, R. Dietze, A. Chiunda, K. Eisenach, and J. J. Ellner. 2003. Whole blood bactericidal activity during treatment of pulmonary tuberculosis. J. Infect. Dis. 187:270-278. [DOI] [PubMed] [Google Scholar]