Abstract
The eating quality of cooked rice is important and determines its market price and consumer acceptance. To comprehensively describe the variation of eating quality in 183 rice germplasm accessions, we evaluated 33 eating-quality traits including amylose and protein contents, pasting properties of rice flour, and texture of cooked rice grains. All eating-quality traits varied widely in the germplasm accessions. Principal-components analysis (PCA) revealed that allelic differences in the Wx gene explained the largest proportion of phenotypic variation of the eating-quality traits. In 146 accessions of non-glutinous temperate japonica rice, PCA revealed that protein content and surface texture of the cooked rice grains significantly explained phenotypic variations of the eating-quality traits. An allelic difference based on simple sequence repeats, which was located near a quantitative trait locus (QTL) on the short arm of chromosome 3, was associated with differences in the eating quality of non-glutinous temperate japonica rice. These results suggest that eating quality is controlled by genetic factors, including the Wx gene and the QTL on chromosome 3, in Japanese rice accessions. These genetic factors have been consciously selected for eating quality during rice breeding programs in Japan.
Keywords: eating quality, natural variation, Oryza sativa L., temperate japonica rice accessions
Introduction
Rice (Oryza sativa L.) is the most important food crop in the world, as it is a staple food for more than half of the world’s population. Rice eating quality largely determines its market price and consumer acceptance, because consumers pay particular attention to high eating quality (Anacleto et al. 2015, Hori and Yano 2013, Juliano et al. 1964).
Grain components affect eating quality that are determined by many physicochemical properties and cooking characteristics of rice. Amylose content is widely recognized as an important determinant of eating quality, because it reflects the functionality of the rice grain’s starch (Juliano et al. 1993). The granule-bound starch synthase I enzyme (GBSSI) is required for amylose synthesis in rice, and the gene encoding GBSSI was named Waxy (Wx). Glutinous rice cultivars and waxy mutant lines carry a deletion in the Wx gene that prevents the activity of GBSSI (the wx allele), and consequently they contain no amylose. In non-glutinous rice cultivars, a single-nucleotide polymorphism (SNP) at the splice site of intron 1 of Wx defines two alleles, Wxa and Wxb, affects differences of amylose content (Cai et al. 1998, Hirano et al. 1998, Issiki et al. 1998, Wang et al. 1995, Yamanaka et al. 2004). The varietal differentiation of Wx gene, Wxa and Wxb, alleles was reported by Sano (1984). Wxa is distributed predominantly in indica accessions, and Wxb in japonica accessions (Sano et al. 1986). Protein content also plays an important role in determining eating quality, as does the grain texture and the surface hardness of cooked rice (Okadome 2005).
By focusing on physicochemical properties, such as gel consistency, gelatinization temperature, and pasting properties, several evaluation methods have been developed to estimate the eating quality of rice accessions. The Rapid Visco Analyzer (RVA) is often used to measure the gelatinization and viscosity changes that occur during heating, holding, and cooling of rice flour (Bergman et al. 2004). Because of the small sample size required for RVA evaluation, this method is suitable for screening the pasting properties of grains from the accessions produced in breeding programs. The Tensipresser is another evaluation instrument for scoring the texture of cooked rice grains (Suzuki et al. 2006). These methods have let researchers clearly differentiate among rice accessions on the basis of the physicochemical properties of their grains (Nakamura et al. 2012a, Okadome et al. 1999).
In current Japanese breeding programs, the eating quality of cooked rice is usually measured through sensory analysis by well-trained panelists of at least 20 people (Matsue 1992, Yamamoto et al. 1996). Sensory tests evaluate the glossiness, stickiness, hardness, and taste of the cooked rice grains and provide a score for overall eating quality. Sensory tests are the most effective method to determine eating quality. However, they make it difficult to evaluate many samples simultaneously and to select superior accessions in early generations such as F3 to F5 populations in breeding programs, because of the requirement for large amounts of grain (e.g., at least several hundred grams of polished rice; Takeuchi et al. 2008). To solve this problem, evaluation instruments such as the Cooked Rice Taste Analyzer and the Mido Meter have been developed to predict the eating quality that would be determined by a sensory test (Goto et al. 2014, Kwon et al. 2011). These instrumental methods showed significant correlations with the eating quality scores by sensory tests (Okadome 2005).
More than 580 QTLs for eating-quality traits have been detected on all of rice chromosomes by using segregating populations derived from crosses among rice accessions in the Q-TARO QTL database (http://qtaro.abr.affrc.go.jp/; Yonemaru et al. 2010) and the Gramene QTL database (http://archive.gramene.org/qtl/; Monaco et al. 2014). Most previous QTL studies used segregating populations derived from crosses between accessions of the indica and japonica rice variety groups, and the studies found QTLs with a large effect near the wx locus on the short arm of chromosome 6 (Hsu et al. 2014, Takeuchi et al. 2007, Tan et al. 1999). Recently, QTL studies have been performed in segregating populations derived from crosses within non-glutinous temperate japonica rice that are homozygous for the Wxb allele. These studies commonly detected eating-quality QTLs that were evaluated by sensory tests on the short arm of chromosome 3 (Hori and Yano 2013, Kobayashi and Tomita 2008, Takeuchi et al. 2008, Wada et al. 2008, 2013). However, we do not yet fully understand the genetic basis of the variation in eating quality among non-glutinous temperate japonica rice, which has been a staple food for many years in northern parts of rice cultivation areas such as Japan, Korea, and China. It is therefore necessary to comprehensively elucidate the variation of eating quality in non-glutinous temperate japonica rice accessions.
In this study, we evaluated 33 eating-quality traits and heading date in 183 Japanese rice germplasm accessions, comprising 146 non-glutinous temperate japonica rice accessions and 40 accessions that did not belong to this category (see the Methods section for details). The present study therefore represents the first study of a wide range of eating-quality traits based on a large number of Japanese rice germplasm accessions. Principal-components analysis (PCA) revealed that alleles of the Wx gene were significantly associated with a wide range of phenotypic variation in eating quality among indica, tropical japonica, and temperate japonica rice accessions. In the non-glutinous temperate japonica rice accessions, which all had the Wxb allele, a QTL on chromosome 3 was significantly associated with phenotypic variations in the eating-quality traits. These results suggest the substantial importance of the Wx gene and the other loci in future breeding programs of non-glutinous temperate japonica rice.
Materials and Methods
Plant materials
Among the genetic resources conserved in the Genebank of the National Institute of Agrobiological Sciences (NIAS), we chose 183 rice germplasm accessions (Supplemental Table 1). Landraces and improved rice accessions were selected as representative Japanese rice cultivars on the basis of their geographical origins and the results of cluster analysis by means of genome-wide DNA marker analysis (Ebana et al. 2008, Yamasaki and Ideta 2013). The rice accessions consisted of 164 temperate japonica, 14 tropical japonica, and 5 indica accessions; these comprised 160 paddy and 23 upland accessions; 164 non-glutinous and 19 glutinous accessions; and 125 improved and 58 landrace accessions. We raised 48 plants of each accession in the paddy fields at NIAS in Tsukuba, Japan (36.03°N, 140.11°E), in 2010 and 2011. Seeds were sown in April, and seedlings were transplanted into the fields in May in plots with a double row per line, with 18 cm between plants and 30 cm between rows. Cultivation management followed the standard procedures used at NIAS.
Evaluation of eating quality
We evaluated 33 eating-quality traits plus the heading date of the 183 accessions (Table 1). We compared the evaluation scores of 22 accessions in 2010 and 2011 to investigate year-to-year correlations and the stability of the quality characteristics, because eating quality of cooked rice was influenced by environmental factors such as the air temperature during the ripening period. The 22 accessions showed a wide range of eating quality phenotypes, and included ancestral cultivars of Koshihikari, and cultivars derived from the same cross with Koshihikari (Supplemental Table 2). Rice seeds were harvested at the full maturity stage. Brown rice was polished to 90% of the original grain weight by a Pearlest machine (Kett Electric Laboratory, Tokyo, Japan). Polished rice grains were milled in an SRG10A mill (Satake Co. Ltd., Tokyo, Japan) to allow an evaluation of the amylose content, protein content, and pasting properties.
Table 1.
Ranges of values for the 33 eating-quality traits and for heading date in the 183 Japanese rice germplasm accessions
| Trait name | Units of measurement or score | Range of values |
|---|---|---|
| Protein content | (%) | 4.9–8.5 |
| Amylose content | (%) | 0.4–30.9 |
| Cooked Rice Taste Analyzer | ||
| Eating quality | (–) | 30.0–91.0 |
| Appearance | (–) | 0.2–9.8 |
| Hardness | (–) | 3.8–9.8 |
| Stickiness | (–) | 1.0–9.5 |
| Balance degree | (–) | 0.0–9.7 |
| Rapid Visco Analyzer | ||
| Maximum viscosity | (RVU) | 90.6–576.8 |
| Minimum viscosity | (RVU) | 31.8–264.7 |
| Breakdown | (RVU) | 58.8–391.3 |
| Final viscosity | (RVU) | 49.3–452.3 |
| Setback | (RVU) | 17.5–224.1 |
| Peak time | (min) | 3.5–7.0 |
| Gelatinization temperature | (°C) | 67.3–82.0 |
| Tensipresser | ||
| on the grain surface | ||
| Hardness H1 | (N/m2 × 102) | 33.9–136.6 |
| Stickiness S1 | (N/m2 × 102) | 0.3–21.2 |
| Adhered mass L3 | (mm) | 0.0–2.0 |
| Adhesiveness A3 | (N/m2m1 × 102) | 0.0–118.0 |
| Balance degree S1/H1 | (–) | 0.0–0.4 |
| Balance degree L3/L1 | (–) | 0.0–3.2 |
| Balance degree A3/A1 | (–) | 0.0–1.1 |
| for the whole grain | ||
| Hardness H2 | (N/m2 × 104) | 13.6–37.1 |
| Stickiness S2 | (N/m2 × 104) | 0.1–0.6 |
| Adhered mass L6 | (mm) | 0.5–4.3 |
| Adhesiveness A6 | (N/m2m1 × 104) | 3.0–27.9 |
| Balance degree S2/H2 | (–) | 0.04–0.34 |
| Balance degree L6/L4 | (–) | 0.20–2.14 |
| Balance degree A6/A4 | (–) | 0.01–0.34 |
| Sample thickness | (mm) | 2.0–3.0 |
| Tenderness | (N/m2m1 × 103) | 5.0–126.1 |
| Pliability | (–) | 0.5–1.3 |
| Toughness | (N/m2m1 × 104) | 3.1–198.4 |
| Brittleness | (–) | 1.4–7.2 |
| Heading date | (month/day) | 6/12–9/1 |
Details for individual accessions are presented in Supplemental Table 1.
The apparent amylose content was estimated according to the method reported by Ando et al. (2010). Rice flour was dissolved with 0.5 N aqueous NaOH and left overnight at room temperature. The solution was then diluted to 0.05 N with H2O, and the amylose content was determined by using an Auto Analyzer II (Bran + Luebbe Co. Ltd., Norderstedt, Germany).
The crude protein content was determined by the combustion method (American Association of Cereal Chemists International [AACCI] International Approved Method 46-30.01 (2000)). Rice flour was placed into a quartz combustion tube in an induction furnace at 900°C. Protein content was calculated from the nitrogen content using the conversion coefficient for nitrogen in protein, as N × 5.95 (Nakamura et al. 2012a).
The viscosity of the rice flour was analyzed by using a Rapid Visco Analyzer (RVA; Model No. RVA-4; Newport Scientific, Warriewood, NSW, Australia), according to the method reported by Toyoshima et al. 1997). Sample temperatures were 50°C for 1 min, followed by 93°C for 4 min, 93°C for 7 min, decreasing from 93°C to 50°C for 4 min, and 50°C for 3 min. RVA profiles were characterized by the parameters shown in Table 1.
To evaluate the characteristics of the cooked rice grains, polished rice is cooked following the method of Okadome et al. (1999). Each sample (20 g) of polished rice was placed in a pudding cup with 25 mL of water, soaked for 1 h at 20°C, and then cooked in an electric rice cooker (SR-ULH18, Panasonic, Kadoma Japan). Physical properties of the cooked rice grains were measured by using a Tensipresser MyBoy texture analyzer (Takemoto Electric, Co., Tokyo, Japan) with the high-compression/low-compression method under the conditions used by Okadome et al. (1999), and the continuous progressive compression method used by Okadome et al. (1995) and Takeyama et al. (1998). The Tensipresser physical properties in Table 1 were obtained under both the high-compression/low-compression method and the continuous progressive compression method, which are shown in Supplemental Fig. 1.
The eating quality characteristics of the rice (Table 1) were as measured by using a Cooked Rice Taste Analyzer STA1A (Satake Co. Ltd., Tokyo, Japan), according to the method reported by Mikami (2009). Eating quality was calculated by the estimation formulae based on the amount of light reflected and transmitted by the cooked rice grains.
PCA for the eating-quality traits
We used Pearson’s correlation coefficient (r) to establish relationships among all eating-quality traits. We then performed PCA using version 11 of JMP software (SAS Institute, Cary, NC, USA). PCA was based on the correlation matrix and the calculated Eigenvalues and Eigenvectors. Principal components (PCs) were extracted until they accounted for more than 80% of the cumulative contribution of the Eigenvalues.
DNA marker analysis for the Wx gene and simple sequence repeats
Total DNA of each rice accession was extracted from leaves by the CTAB method reported previously (Hori et al. 2012). The alleles of the Wx gene were identified by means of derived cleaved amplified polymorphic sequences analysis following the method of Yamanaka et al. (2004). We amplified a DNA fragment containing the first exon–intron junction of the Wx gene. PCR amplification was performed using the primer set WP-B (5′-TTA ATT TCC AGC CCA ACA CC-3′) and WP-CAPS (5′-TGT TGT TCA TCA GGA AGA ACA TCT CCA AG-3′). Each PCR product was digested with EcoT14I at 37°C overnight. Digestion products were then electrophoresed in 3% agarose gels.
Simple sequence repeat (SSR) marker RM4108 on chromosome 3 was amplified by using the primer set 5′-GTC CCT CGC TTT ATA TCT AG-3′ and 5′-CAA CTC TGC TAA ACG AAT TA-3′. PCR products were fluorescently labeled and their fragment sizes were determined by using an ABI 3130xl system and version 3.6 of GeneMapper software (Applied Biosystems, Foster City, CA, USA), according to the method of Hori et al. (2012).
Results
Variation of eating-quality traits in the 183 Japanese rice accessions
All 33 eating-quality traits and heading date varied widely among the accessions (Table 1, Supplemental Fig. 2). For example, amylose content ranged from 0.4% to 30.9%, protein content ranged from 4.9% to 8.5%, the eating quality estimated by the Cooked Rice Taste Analyzer ranged from 30.0 to 91.0, maximum viscosity by the RVA ranged from 90.6 to 576.8, and stickiness on grain surface by the Tensipresser ranged from 0.3 to 21.2. The accessions with the lowest amylose content were the glutinous rice Meguromochi (0.4%), Okabo (0.5%), and Gaisenmochi (0.8%). Of the non-glutinous rice accessions, the lowest amylose content was in Sakihikari (14.2%), Shimahikari (14.4%), Nanatsuboshi (14.4%), Sorachi (14.4%), and Fukoku (146%). The accessions with low protein content were Kabashiko (4.9%), Nikomaru (5.0%), Yumetsukushi (5.0%), Aichinokaori (5.1%), and Koshihikari (5.1%). The accessions with the highest eating quality were found in the glutinous rice Hiyokumochi (91.0), Koganemochi (90.0), and Himenomochi (89.7). In the non-glutinous rice accessions, the highest eating qualities were those of Koshihikari (88.5), Itadaki (88.3), Hitomebore (88.0), Yumetsukushi (88.0), and Nikomaru (87.0). The accessions with the highest maximum viscosity were Joushuu (576.8), Kameji (548.3), Hananomai (528.7), Norin 1 (524.9), and Yamasenishiki (513.8). The accessions with the highest stickiness on the grain surface were Hinohikari (21.2), Akiho (19.9), Norin 22 (19.3), Notohikari (19.2), and Fusaotome (19.1). Year-to-year correlations between the values in 2010 and 2011 were significant for all 33 eating-quality traits except hardness of the grain surface, and the tenderness and toughness in the Tensipresser measurements (Table 2, Supplemental Table 2), indicating the consistency of these traits among years.
Table 2.
Between-year correlation coefficients and correlations with heading date for the 33 eating quality traits
| Trait name | Year-to year correlation | Correlation with heading date |
|---|---|---|
| Protein content | 0.87 *** | −0.39 *** |
| Amylose content | 0.71 *** | 0.19 ** |
| Cooked Rice Taste Analyzer | ||
| Eating quality | 0.78 *** | 0.17 * |
| Appearance | 0.80 *** | 0.15 * |
| Hardness | 0.84 *** | −0.06 |
| Stickiness | 0.82 *** | 0.23 ** |
| Balance degree | 0.76 *** | 0.17 * |
| Rapid Visco Analyzer | ||
| Maximum viscosity | 0.65 *** | −0.18 * |
| Minimum viscosity | 0.44 ** | −0.33 *** |
| Breakdown | 0.62 *** | −0.01 |
| Final viscosity | 0.69 ** | −0.19 * |
| Setback | 0.49 ** | 0.04 |
| Peak time | 0.31 * | 0.00 |
| Gelatinization temperature | −0.30 * | −0.50 *** |
| Tensipresser | ||
| on the grain surface | ||
| Hardness H1 | 0.07 | 0.00 |
| Stickiness S1 | 0.55 *** | 0.16 * |
| Adhered mass L3 | 0.32 ** | 0.04 |
| Adhesiveness A3 | 0.38 ** | 0.11 |
| Balance degree S1/H1 | 0.36 ** | 0.12 |
| Balance degree L3/L1 | 0.35 ** | 0.05 |
| Balance degree A3/A1 | 0.47 *** | 0.09 |
| for the whole grain | ||
| Hardness H2 | 0.23 * | 0.19 ** |
| Stickiness S2 | 0.38 ** | −0.26 *** |
| Adhered mass L6 | 0.58 *** | 0.00 |
| Adhesiveness A6 | 0.27 * | 0.07 |
| Balance degree S2/H2 | 0.28 * | −0.31 *** |
| Balance degree L6/L4 | 0.57 *** | 0.02 |
| Balance degree A6/A4 | 0.29 * | −0.10 |
| Sample thickness | 0.61 *** | −0.39 *** |
| Tenderness | 0.07 | 0.07 |
| Pliability | 0.22 * | −0.06 |
| Toughness | 0.09 | 0.03 |
| Brittleness | 0.23 * | 0.10 |
| Heading date | 0.98 *** | – |
Significance levels: *P < 0.05, **P < 0.01, and **P < 0.001.
The heading date also varied widely, from 12 June to 1 September (Table 1, Supplemental Fig. 2). Significant correlations with heading date were observed for 15 of the 33 eating-quality traits (Table 2). Heading date was positively correlated with amylose content; with eating quality comprosing appearance, stickiness and balance degree evaluated using the Cooked Rice Taste Analyzer; and stickiness on the grain surface and hardness of the whole grain evaluated using the Tensipresser. On the other hand, it was negatively correlated with the protein content; with the maximum viscosity, minimum viscosity, final viscosity, and gelatinization temperature evaluated using the RVA and stickiness, balance degrees, and sample thickness of the whole grain evaluated using the Tensipresser. Thus, although the correlations were generally weak to moderate (|r| ≤ 0.5), these traits were influenced to some extent by the heading date.
There were significant correlations between many pairs of the 33 eating-quality traits (Supplemental Table 3). The accessions with high eating quality showed an overall trend toward low protein content, low amylose content, high maximum viscosity, low minimum viscosity, low final viscosity, low setback, low peak time, low gelatinization temperature, low hardness, high stickiness, high adhered mass, high adhesiveness, and a high balance degree. Strong and statistically significant correlations (P < 0.001) were often observed between traits evaluated using the same instrument. The absolute values of the correlations among the five traits evaluated using the Cooked Rice Taste Analyzer were at least 0.9, so we selected eating quality as representative of all traits evaluated by the Cooked Rice Taste Analyzer, and did not subject the other four traits (appearance, hardness, stickiness and balance degree) to additional statistical analysis to avoid an excessive influence of autocorrelation on the results.
PCA results for the 183 Japanese rice accessions
To elucidate the main components of variation in the eating-quality traits, we subjected the 29 traits (excluding appearance, hardness, stickiness, and balance degree) to PCA. The results revealed that the contributions of the first and second principal components (PC1 and PC2) were 51.2% and 18.6%, respectively (Supplemental Table 4). The sum of the contributions of the top four PCs accounted for 84.1% of the total variance. For PC1, the corresponding loadings were negative for amylose content and setback and positive for eating quality, adhered mass, adhesiveness, the three balance degrees on the grain surface, the adhered mass of the whole grain, and two of the three balance degrees of the whole grain. For PC2, the loadings were negative for protein content and positive for eating quality, maximum viscosity, minimum viscosity, breakdown, final viscosity, peak time, and stickiness on the grain surface. For PC3, the loadings were an equal mixture of positive and negative. For PC4, the loadings were almost all positive.
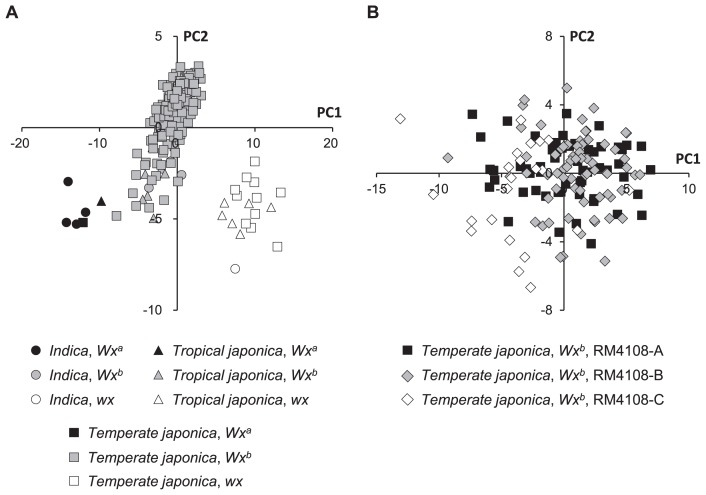
PCA for the 183 accessions revealed that PC1 clearly distinguished among alleles at wx locus (Fig. 1A). PC1 identified three groups: the Wxa, Wxb, and wx alleles were separate from each other, but members of each group were clustered together in the ordination, without differences among accessions from the indica, tropical japonica, and temperate japonica variety groups. The average PC scores for the eating-quality traits differed significantly among the three alleles at wx locus (Table 3). Compared with the rice accessions that were homozygous for the Wxa allele, rice accessions that were homozygous for the Wxb allele had a significantly lower amylose content, higher eating quality, higher maximum viscosity, and higher stickiness both on the grain surface and of the whole grain. Compared with the rice accessions that were homozygous for the Wxb allele, rice accessions that were homozygous for the wx allele had significantly lower amylose content, higher eating quality, and lower stickiness on the grain surface. These results indicate that differences in the amylose and amylopectin super long chains content, which are caused by differences in the Wx alleles, strongly affect the eating quality of the rice in the overall collection.
Fig. 1.
(A) Ordination for variations of the eating-quality traits of 183 Japanese rice accessions consisting of 5 indica, 14 tropical japonica, and 164 temperate japonica rice accessions using the first and second principal components (PC1 and PC2). (B) Ordination for variations of the eating-quality traits of the 146 non-glutinous temperate japonica rice accessions for PC1 and PC2.
Table 3.
Statistical significance of differences among the three Wx alleles (Wxa, Wxb, and wx) based on the four strongest principal components (PC1 to PC4) and six of the key eating-quality traits. The Wxa, Wxb, and wx alleles include 17, 160, and 6 rice accessions, respectively
| Trait name | Wxa allele | Wxb allele | wx allele | |
|---|---|---|---|---|
| PC1 | (–) | 9.1 ± 0.5 a | −12.5 ± 0.8 b | −0.5 ± 0.2 c |
| PC2 | (–) | −4.5 ± 0.4 a | −4.5 ± 0.7 a | 0.6 ± 0.1 b |
| PC3 | (–) | 1.3 ± 0.4 a | 4.7 ± 0.6 b | −0.3 ± 0.1 c |
| PC4 | (–) | 0.2 ± 0.3 a | −1.3 ± 0.5 b | 0.0 ± 0.1 a |
| Protein content | (%) | 6.9 ± 0.3 a | 6.0 ± 0.1 a | 6.9 ± 0.2 a |
| Amylose content | (%) | 28.7 ± 0.6 a | 16.7 ± 0.1 b | 2.2 ± 0.3 c |
| Eating quality | (–) | 33.1 ± 3.8 a | 73.9 ± 0.7 b | 84.1 ± 2.2 c |
| Maximum viscosity | (RVU) | 356.0 ± 15.3 a | 448.7 ± 2.9 b | 213.6 ± 8.8 c |
| Stickiness on the grain surface S1 | (N/m2 × 102) | 2.0 ± 1.3 a | 13.8 ± 0.3 b | 11.6 ± 0.8 c |
| Stickiness for the whole grain S2 | (N/m2 × 104) | 0.21 ± 0.02 a | 0.48 ± 0.00 b | 0.49 ± 0.01 b |
Values for a trait labeled with different letters differ significantly (P < 0.05, Tukey-Kramer HSD test).
PCA for the 146 accessions of non-glutinous temperate japonica rice that was homozygous for the same Wx allele (Wxb)
PC1 and PC2 accounted for 38.7% and 13.9%, respectively, of the cumulative variance (Supplemental Table 5). The contributions of the top six PCs accounted for 80.2% of the total variance. For PC1, the loadings were negative for protein content but positive for eating quality, stickiness, adhered mass, adhesiveness, and the three balance degrees on the grain surface. For PC2, the loadings were negative for brittleness but positive for amylose content, sample thickness, tenderness, pliability, and toughness. For PC3 to PC6, the loadings were largely associated with individual components of the RVA profile and the whole-grain Tensipresser parameters.
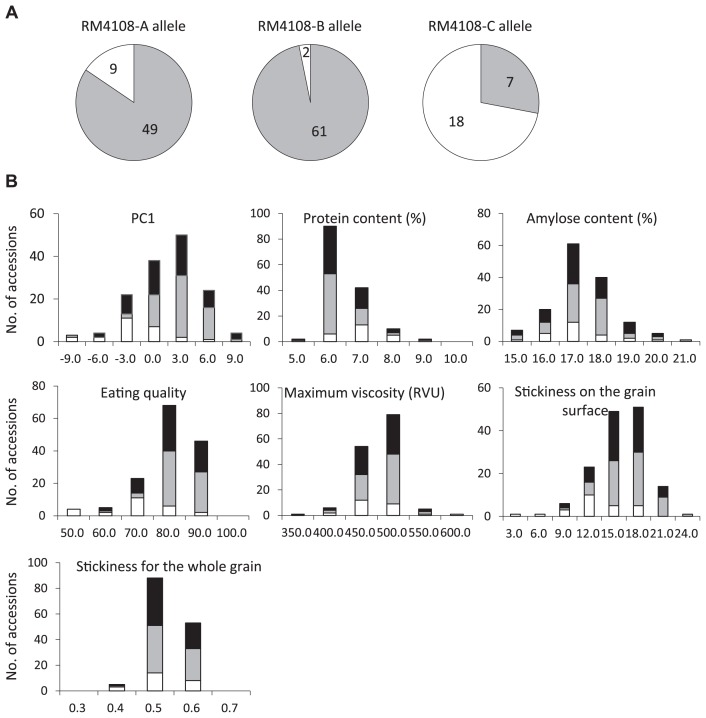
To test for the significance of genetic factors other than Wx for the eating-quality traits of the non-glutinous temperate japonica rice accessions, we focused on SSR marker RM4108, which was located near eating-quality QTLs revealed on the short arm of chromosome 3 by sensory tests. We divided the 146 accessions into three groups (A, B, and C) based on their RM4108 alleles. The group with the RM4108-C allele was somewhat distinct from the groups with the RM4108-A and RM4108-B alleles, although the distributions of the three alleles overlapped somewhat in the ordination (Fig. 1B). The RM4108-A and RM4108-B allele groups consisted mainly of improved accessions of non-glutinous temperate japonica rice, whereas the RM4108-C allele group included many landrace accessions (Fig. 2A). For example, the RM4108-A allele group included Koshihikari, Hitomebore, Aichinokaori, Nanatsuboshi, and Itadaki, whereas the RM4108-B allele group included Hinohikari, Nikomaru, Sakihikari, Fusaotome, and Notohikari (Supplemental Table 1). The mean PC1 and eating-quality values differed significantly among the three RM4108 allele groups (Fig. 2B, Table 4). Compared with the accessions with the RM4108-C allele, accessions with the RM4108-A and RM4108-B alleles showed significantly lower protein content but significantly higher eating quality and stickiness on the grain surface. There were no significant differences among the allele groups in amylose content, maximum viscosity, or stickiness of the whole grain.
Fig. 2.
(A) The proportions of landrace and improved rice accessions in the three RM4108 allele groups. Landraces are shown in white and improved rice accessions in gray. Numbers in each circle indicate the number of accessions in each group. (B) Frequency distributions for principal component 1 (PC1), protein content, amylose content, eating quality, maximum viscosity, and stickiness on the grain surface and of the whole grain for the 146 non-glutinous temperate japonica rice accessions. Rice accessions of the RM4108-A, B, and C allele groups are shown in black, gray and white, respectively.
Table 4.
Statistical significance of differences among the three RM4108 genotypes (A, B, and C) for the top four principal components (PC1 to PC4) and six key eating-quality traits. Genotypes A, B, and C include 56, 68, and 22 non-glutinous accessions of temperate japonica rice, respectively
| Trait name | RM4108-A allele | RM4108-B allele | RM4108-C allele | |
|---|---|---|---|---|
| PC1 | (–) | 0.2 ± 0.4 a | 1.0 ± 0.4 b | −3.9 ± 0.5 c |
| PC2 | (–) | 0.2 ± 0.3 a | 0.1 ± 0.3 a | −1.0 ± 0.4 b |
| PC3 | (–) | 0.1 ± 0.2 a | 0.1 ± 0.2 a | −0.5 ± 0.3 b |
| PC4 | (–) | −0.2 ± 0.2 a | 0.1 ± 0.2 a | 0.1 ± 0.3 a |
| Protein content | (%) | 5.9 ± 0.1 a | 5.8 ± 0.1 a | 6.5 ± 0.1 b |
| Amylose content | (%) | 16.8 ± 0.1 a | 16.9 ± 0.1 a | 16.6 ± 0.2 a |
| Eating quality | (–) | 75.9 ± 1.0 a | 77.4 ± 0.9 a | 65.3 ± 1.6 b |
| Maximum viscosity | (RVU) | 450.9 ± 4.5 a | 452.9 ± 4.2 a | 443.0 ± 7.4 a |
| Stickiness on the grain surface S1 | (N/m2 × 102) | 14.4 ± 0.4 a | 14.9 ± 0.4 a | 12.1 ± 0.6 b |
| Stickiness for the whole grain S2 | (N/m2 × 104) | 0.48 ± 0.01 a | 0.48 ± 0.01 a | 0.48 ± 0.01 a |
Values for a trait labeled with different letters differ significantly (P < 0.05, Tukey-Kramer HSD test).
Discussion
Researchers have recognized the wide range of eating-quality characteristics in rice germplasm accessions since the early 20th century (Ikeno 1914, Warth and Darabsett 1914). Nakamura et al. (2004, 2012b) reported varietal differences of eating quality traits in world-wide rice germplasm accessions including indica and japonica rice cultivars. Germplasm accessions provide basic knowledge that supports the discovery of genes that control eating quality and their introduction into novel rice cultivars. The present study represents a wide range of eating-quality traits using a large number of Japanese rice germplasm accessions. We evaluated 33 eating-quality traits and heading date in 183 Japanese rice germplasm accessions, and revealed that the difference of amylose content between Wxa and Wxb alleles was a primary factors involved in determining the phenotypic differences in eating-quality traits. In the 146 accessions of non-glutinous temperate japonica rice that were homozygous for Wxb allele, the protein content and surface texture of the cooked rice grains were significantly associated with phenotypic differences in eating-quality. The phenotypic variation in eating quality was controlled by one or more QTLs near SSR marker locus, RM4108, on the short arm of chromosome 3. These results suggest that genetic factors other than the Wx gene are involved in creating the natural variation of eating-quality traits in the non-glutinous temperate japonica rice accessions, and that the genetic factors have been consciously selected for eating quality during rice breeding programs in Japan.
Our study confirmed previous QTL studies that eating-quality QTLs have been commonly detected near SSR marker locus, RM4108, in non-glutinous temperate japonica rice accessions (Hori and Yano 2013, Kobayashi and Tomita 2008, Takeuchi et al. 2008, Wada et al. 2008). These previous studies used Koshihikari or one of its progeny as a crossing parent to develop mapping populations. Koshihikari has been a top cultivar in Japan since 1979, and possesses superior eating quality among the Japanese non-glutinous temperate japonica cultivars. Koshihikari and other cultivars such as Hitomebore, Itadaki and Nikomaru had high eating quality, and had the A or B alleles at SSR marker RM4108. Eating quality differed significantly among the accession groups identified on the basis of differences at marker RM4108. Therefore, the QTL or QTLs on the short arm of chromosome 3 might be associated mainly with variations of the eating-quality traits in the Japanese non-glutinous temperate japonica rice accessions. However, we found a few inconsistencies based on pedigree information of Japanese rice cultivars. For example, Koshihikari (RM4108-A allele) was derived from the cross between Norin 1 (RM4108-A allele) and Norin 22 (RM4108-A allele), but Norin 22 was derived from the cross between Norin 6 (RM41085-B allele) and Norin 8 (RM4108-C allele). These results suggested the need to add genotypes of other DNA markers located on the short arm of chromosome 3.
The surface texture of cooked rice grains is one of important traits that determines the eating quality of non-glutinous temperate japonica rice accessions (Okabe 1979, Okadome et al. 1999, Suzuki et al. 2006). The accumulation of storage proteins in the outer layer of the rice endosperm is one factor that influences the surface texture of cooked rice grains (Okadome 2005). Our results also indicate a substantial effect of surface texture on the protein content and eating quality of rice grains in the non-glutinous temperate japonica rice accessions. In this study, accessions with high eating quality had a low protein content and strong stickiness on the surface of the cooked rice grain. These results reflect the preferences of Japanese consumers for rice grain characteristics.
Amylose content has been considered an important factor that affects eating quality in rice (Juliano et al. 1993). The 146 non-glutinous temperate japonica accessions that were homozygous for the same Wxb allele showed amylose contents ranging from 14.2% to 20.4%. Varietal difference of the amylose content might be due to alteration of heading date and ambient temperature in the grain filling period among rice cultivars. However, Biselli et al. (2014) reported 21 alleles of the Wx gene in 125 japonica rice accessions with a wide range of amylose content (from <5% to >25%). Therefore, some of the Wx gene alleles that we did not investigate in the present study are likely to be associated with differences in amylose contents among rice accessions that were homozygous for the same Wxb allele. Amylose content in the non-glutinous temperate japonica rice accessions would also be controlled by genetic factors other than the Wx gene. Recently, a QTL for amylose content has been detected and fine-mapped on chromosomes 2 in populations derived from crosses between Koshihikari and Akihikari (Kobayashi et al. 2008). Another QTL for amylose content has been detected and fine-mapped on chromosomes 9 in populations derived from crosses between Itadaki and Kuiku 162 (Ando et al. 2010, Takemoto-Kuno et al. 2015). Koshihikari and Kuiku 162 alleles at these QTLs showed a relatively small decrease (2% to 3%) in the amylose content. These QTLs may explain some of the variation of amylose content among the non-glutinous temperate japonica rice accessions in the present study.
Several previous studies evaluated the phenotypic variations of grain characteristics associated with eating quality, and elucidated genetic variation of these traits by means of PCA in several dozen non-glutinous japonica accessions. Kamara et al. (2010) evaluated the free amino acid contents in 49 accessions. Matsuzaki et al. (1992) carried out PCA for the contents of amylose, amino acids, and essential elements (N, P, K, Mg, and Ca) in 18 accessions. Kim et al. (2010) investigated the flavonoid and carotenoid contents in five accessions. Mabashi et al. (2010), Nakamura et al. (2012b) and Tsujii and Takano (2015) measured activities of endogenous enzymes of rice grains in five, 42 and nine accessions, respectively. In the present study, PCA let us summarize the relationships among the many eating-quality traits. Our results for 33 eating-quality traits in 146 accessions will complement these previous studies and enhance knowledge of the natural variation in eating-quality traits in non-glutinous japonica accessions.
We found that accessions with high eating quality tended to have high maximum viscosity but low levels of the other RVA traits. In contrast, eating quality was negatively correlated with amylose content. Previous studies reported that the amylase content had a significant negative correlation with maximum viscosity (and, thus, a significant positive correlation with eating quality) and significant positive correlations with minimum viscosity, final viscosity, and setback (Nakamura et al. 2012a, Tong et al. 2014). In the Tensipresser evaluation scores, accessions with high eating quality tended toward low hardness, high stickiness, high adhered mass and adhesiveness, and high balance degrees of both the grain surface and the whole cooked grain (Supplemental Table 3). These observations agree with those in previous studies (Kobayashi et al. 2008, Okadome 2005, Wada et al. 2013). Therefore, the RVA and Tensipresser evaluations can provide a good estimate of the eating quality of rice germplasm accessions.
QTL detection and gene cloning are necessary to dissect the genetic variation of eating-quality traits. Recent advances in molecular genomics technologies that have permitted genome-wide SNP detection and large-scale SNP genotyping allowed the detection of QTLs involved in various agronomic traits, even in genetically close groups of accessions such as non-glutinous temperate japonica rice (Hori et al. 2012). However, these QTL studies examined too few eating-quality traits. Genome-wide association studies (GWAS) make it possible to directly detect QTLs in germplasms without the need to develop genetically distinct populations, and has already been successfully applied in analyses of heading date and grain yield, two agronomically important traits in rice (Huang et al. 2012, Zhao et al. 2011). GWAS would also be a suitable way to detect QTLs for eating-quality traits in the non-glutinous temperate japonica rice accessions because of the relatively simple population structure within this group (Yamasaki and Ideta 2013, Yonemaru et al. 2014). Additional research will be needed to enhance the identification of genes that control eating-quality traits in the non-glutinous temperate japonica accessions. These future studies will support the development of novel rice cultivars with high eating quality.
Supplementary Material
Acknowledgments
We are grateful to the technical staff of the Field Management Division at the NIAS for their technical assistance and management of the rice fields. This work was supported by grants from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation QTL4010; Research Project for Genomics-based Technology for Agricultural Improvement, RBS2011).
Literature Cited
- American Association of Cereal Chemists Approved Methods Committee (2000) Approved methods of the American Association of Cereal Chemists, 10th edn AACC, St. Paul, pp. 1–2. [Google Scholar]
- Anacleto, R., Cuevas, R.P., Jimenez, R., Llorente, C., Nissila, E., Henry, R. and Sreenivasulu, N. (2015) Prospects of breeding high-quality rice using post-genomic tools. Theor. Appl. Genet. 128: 1449–1466. [DOI] [PubMed] [Google Scholar]
- Ando, I., Sato, H., Aoki, N., Suzuki, Y., Hirabayashi, H., Kuroki, M., Shimizu, H., Ando, T. and Takeuchi, Y. (2010) Genetic analysis of the low-amylose characteristics of rice cultivars Oborozuki and Hokkai-PL9. Breed. Sci. 60: 187–194. [Google Scholar]
- Bergman, C.J., Bhattacharya, K.R. and Ohtsubo, K. (2004) Rice end-use quality analysis. In: Champagne, E.T. (ed.) Rice: Chemistry and Technology, 3rd edn., AACCI, MN, USA, pp. 415–472. [Google Scholar]
- Biselli, C., Cavalluzzo, D., Perrini, R., Gianinetti, A., Bagnaresi, P., Urso, S., Orasen, G., Desiderio, F., Lupotto, E., Cattivelli, L.et al. (2014) Improvement of marker-based predictability of Apparent Amylose Content in japonica rice through GBSSI allele mining. Rice 7: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, X.L., Wang, Z.Y., Xing, Y.Y., Zhang, J.L. and Hong, M.M. (1998) Aberrant splicing of intron 1 leads to the heterogeneous 5′ UTR and decreased expression of waxy gene in rice cultivars of intermediate amylose content. Plant J. 14: 459–465. [DOI] [PubMed] [Google Scholar]
- Ebana, K., Kojima, Y., Fukuoka, S., Nagamine, T. and Kawase, M. (2008) Development of mini core collection of Japanese rice landrace. Breed. Sci. 58: 281–291. [Google Scholar]
- Goto, H., Asanome, N., Suzuki, K., Sano, T., Saito, H., Abe, Y., Chuba, M. and Nishio, T. (2014) Objective evaluation of whiteness of cooked rice and rice cakes using a portable spectrophotometer. Breed. Sci. 63: 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano, H.Y., Eiguchi, M. and Sano, Y. (1998) A single base change altered the regulation of the Waxy gene at the posttranscriptional level during the domestication of rice. Mol. Biol. Evol. 15: 978–987. [DOI] [PubMed] [Google Scholar]
- Hori, K., Kataoka, T., Miura, K., Yamaguchi, M., Saka, N., Nakahara, T., Sunohara, Y., Ebana, K. and Yano, M. (2012) Variation in heading date conceals quantitative trait loci for other traits of importance in breeding selection of rice. Breed. Sci. 62: 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, K. and Yano, M. (2013) Genetic improvement of grain quality in Japonica rice. In: Varshney, R.K. and Yuberosa R. (eds.) Translational Genomics for Crop Breeding Vol. II Abiotic Stress, Yield and Quality, Wiley Blackwell, Iowa, USA, pp. 143–160. [Google Scholar]
- Hsu, Y.C., Tseng, M.C., Wu, Y.P., Lin, M.Y., Wei, F.J., Hwu, K.K., Hsing, Y.I. and Lin, Y.R. (2014) Genetic factors responsible for eating and cooking qualities of rice grains in a recombinant inbred population of an inter-subspecific cross. Mol. Breed. 34: 655–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X., Zhao, Y., Wei, X., Li, C., Wang, A., Zhao, Q., Li, W., Guo, Y., Deng, L., Zhu, C.et al. (2012) Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat. Genet. 44: 32–39. [DOI] [PubMed] [Google Scholar]
- Ikeno, S. (1914) Ueber die Bestaubung und die Bastardierung von Reis. Zeit. f. Pflanzenzucht. 2: 495–503. [Google Scholar]
- Issiki, M., Morino, K., Nakajima, M., Okagaki, R.J., Wessler, S.R., Izawa, T. and Shimamoto, K. (1998) A naturally occurring functional allele of the rice waxy locus has a GT to TT mutation at the 5′ splice site of the first intron. Plant J. 15: 133–138. [DOI] [PubMed] [Google Scholar]
- Juliano, B.O., Bautista, G.M., Lugay, J.C. and Reyes, A.C. (1964) Rice quality, studies on physicochemical properties of rice. J. Agr. Food Chem. 12: 131–138. [Google Scholar]
- Juliano, B.O., Perez, C.M. and Cuevas-Perez, F. (1993) Screening for stable high head rice yields in rough rice. Cereal Chem. J. 70: 650–655. [Google Scholar]
- Kamara, J.S., Konishi, S., Sasanuma, T. and Abe, T. (2010) Variation in free amino acid profile among some rice (Oryza sativa L.) cultivars. Breed. Sci. 60: 46–54. [Google Scholar]
- Kim, J.K., Lee, S.Y., Chu, S.M., Lim, S.H., Suh, S.C., Lee, Y.T., Cho, H.S. and Ha, S.H. (2010) Variation and correlation analysis of flavonoids and carotenoids in Korean pigmented rice (Oryza sativa L.) cultivars. J. Agr. Food Chem. 58: 12804–12809. [DOI] [PubMed] [Google Scholar]
- Kobayashi, A. and Tomita, K. (2008) QTL detection for stickiness of cooked rice using recombinant inbred lines derived from crosses between japonica rice cultivars. Breed. Sci. 58: 419–426. [Google Scholar]
- Kobayashi, A., Tomita, K., Yu, F., Takeuchi, Y. and Yano, M. (2008) Verification of quantitative trait locus for stickiness of cooked rice and amylose content by developing near-isogenic lines. Breed. Sci. 58: 235–242. [Google Scholar]
- Kwon, S.W., Cho, Y.C., Lee, J.H., Suh, J.P., Kim, J.J., Kim, M.K., Choi, I.S., Hwang, H.G., Koh, H.J. and Kim, Y.G. (2011) Identification of quantitative trait loci associated with rice eating-quality traits using a population of recombinant inbred lines derived from a cross between two temperate japonica cultivars. Mol. Cells 31: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabashi, Y., Miwa, Y., Ookura, T. and Kasai, M. (2010) Role of endogenous enzymes in milled rice of various cultivars in the accumulation of chemical components in rice grain during cooking. J. Cookery Sci. Jpn. 4: 228–236. [Google Scholar]
- Matsue, Y. (1992) On a sensory test of cooked rice in which 10 cultivars were evaluated by 13 panel members. J. Home Econ. Jpn. 43: 1027–1032. [Google Scholar]
- Matsuzaki, A., Takano, T., Sakamoto, S. and Kuboyama, T. (1992) Relation between eating quality and chemical components in milled rice and amino acid contents in cooked rice. Jpn. J. Crop Sci. 61: 561–567. [Google Scholar]
- Mikami, T. (2009) Development of evaluation systems for rice taste quality. Jpn. J. Food Eng. 4: 191–197. [Google Scholar]
- Monaco, M.K., Stein, J., Naithani, S., Wei, S., Dharmawardhana, P., Kumari, S., Amarasinghe, V., Youens-Clark, K., Thomason, J., Preece, J.et al. (2014) Gramene 2013: comparative plant genomics resources. Nucleic Acids Res. 42: D1193–D1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, S., Okadome, H., Yoza, K., Haraguchi, K., Okunishi, T., Suzuki, K., Sato, H. and Ohtsubo, K. (2004) Differentiation and search for palatability-factors of world-wide rice grains by PCR method. J. Jpn. Soc. Food Sci. 78: 764–779. [Google Scholar]
- Nakamura, S., Suzuki, D., Kitadume, R. and Ohtsubo, K. (2012a) Quality evaluation of rice crackers based on physicochemical measurements. Biosci. Biotechnol. Biochem. 76: 794–804. [DOI] [PubMed] [Google Scholar]
- Nakamura, S., Machida, K. and Ohtsubo, K. (2012b) Search for cell-wall-degrading enzymes of world-wide rice grains by PCR and their effects on the palatability of rice. Biosci. Biotechnol. Biochem. 76: 1645–1654. [DOI] [PubMed] [Google Scholar]
- Okabe, M. (1979) Texture measurement of cooked rice and its relationship to the eating quality. J. Texture Stud. 10: 131–152. [Google Scholar]
- Okadome, H., Toyoshima, H. and Ohtsubo, K. (1995) Many-sided evaluation of physical properties of cooked rice grains with a single apparatus. J. Jpn. Soc. Food Sci. 43: 1004–1011. [Google Scholar]
- Okadome, H., Toyoshima, H. and Ohtsubo, K. (1999) Multiple measurements of physical properties of individual cooked rice grains with a single apparatus. Cereal Chem. 76: 855–860. [Google Scholar]
- Okadome, H. (2005) Application of instrument-based multiple texture measurement of cooked milled-rice grains to rice quality evaluation. JARQ 39: 261–268. [Google Scholar]
- Sano, Y. (1984) Differential regulation of waxy gene expression in rice endosperm. Theor. Appl. Genet. 68: 467–473. [DOI] [PubMed] [Google Scholar]
- Sano, Y., Katsumata, M. and Okuno, K. (1986) Genetic studies of speciation in cultivated rice. 5. Inter- and intraspecific differentiation in the waxy gene expression of rice. Euphytica 35: 1–9. [Google Scholar]
- Suzuki, K., Okadome, H., Nakamura, S. and Ohtsubo, K. (2006) Quality evaluation of various “new characteristic rice” varieties based on physicochemical measurements. J. Jpn. Soc. Food Sci. 53: 287–295. [Google Scholar]
- Takemoto-Kuno, Y., Mitsueda, H., Suzuki, K., Hirabayashi, H., Ideta, O., Aoki, N., Umemoto, T., Ishii, T., Ando, I., Kato, H.et al. (2015) qAC2, a novel QTL that interacts with Wx and controls the low amylose content in rice (Oryza sativa L.). Theor. Appl. Genet. 128: 563–573. [DOI] [PubMed] [Google Scholar]
- Takeuchi, Y., Nonoue, Y., Ebitani, T., Suzuki, K., Aoki, N., Sato, H., Ideta, O., Hirabayashi, H., Hirayama, M., Ohta, H.et al. (2007) QTL detection for eating quality including glossiness, stickiness, taste and hardness of cooked rice. Breed. Sci. 57: 231–242. [Google Scholar]
- Takeuchi, Y., Hori, K., Suzuki, K., Nonoue, Y., Takemoto-Kuno, Y., Maeda, H., Sato, H., Hirabayashi, H., Ohta, H., Ishii, T.et al. (2008) Major QTLs for eating quality of an elite Japanese rice cultivar, Koshihikari, on the short arm of chromosome 3. Breed. Sci. 58: 437–445. [Google Scholar]
- Takeyama, S., Toyama, R., Fujiwara, Y. and Arakawa, Y. (1998) Physical properties of steamed cakes made of miscellaneous cereals. Res. Bull. Iwate Ind. Res. Inst. 5: 28. [Google Scholar]
- Tan, Y.F., Li, J.X., Yu, S.B., Xing, Y.Z., Xu, C.G. and Zhang, Q. (1999) The three important traits for cooking and eating quality of rice grains are controlled by a single locus in an elite rice hybrid, Shanyou 63. Theor. Appl. Genet. 99: 642–648. [DOI] [PubMed] [Google Scholar]
- Tong, C., Chen, Y., Tang, F., Xu, F., Huang, Y., Chen, H. and Bao, J. (2014) Genetic diversity of amylose content and RVA pasting parameters in 20 rice accessions grown in Hainan, China. Food Chem. 161: 239–245. [DOI] [PubMed] [Google Scholar]
- Toyoshima, H., Okadome, H., Ohtsubo, K., Suto, M., Horisue, N., Inatsu, O., Narizuka, A., Aizaki, M., Okawa, T., Inouchi, N.et al. (1997) Cooperative test on the small-scale rapid method for the gelatinization properties test of rice flours with a rapid-visco-analyser (RVA). J. Jpn. Soc. Food Sci. 44: 579–584. [Google Scholar]
- Tsujii, Y. and Takano, K. (2015) Effects of cultivar and cultivation region on endosperm enzyme activity involved in the palatability of cooked rice. J. Jpn. Soc. Food Sci. 62: 34–40. [Google Scholar]
- Wada, T., Ogata, T., Tsubone, M., Uchimura, Y. and Matsue, Y. (2008) Mapping of QTLs for eating quality and physicochemical properties of the japonica rice ‘Koshihikari’. Breed. Sci. 58: 427–435. [Google Scholar]
- Wada, T., Yasui, H., Inoue, T., Tsubone, M., Ogata, T., Doi, K., Yoshimura, A. and Matsue, Y. (2013) Validation of QTLs for eating quality of japonica rice ‘Koshihikari’ using backcross inbred lines. Plant Prod. Sci. 16: 131–140. [Google Scholar]
- Wang, Z.Y., Zheng, F.Q., Shen, G.Z., Gao, J.P., Snustad, D.P., Li, M.G., Zhang, J.L. and Hong, M.M. (1995) The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. Plant J. 7: 613–622. [DOI] [PubMed] [Google Scholar]
- Warth, F.J. and Darabsett, D.B. (1914) Disintegration of rice grains by means of alkali. Bull. Agr. Res. Inst. 38: 1–9. [Google Scholar]
- Yamamoto, T., Horisue, N. and Ikeda, R. (1996) Rice breeding manual. Yokendo Ltd., Tokyo, pp. 74–124. [Google Scholar]
- Yamanaka, S., Nakamura, I., Watanabe, K.N. and Sato, Y.I. (2004) Identification of SNPs in the waxy gene among glutinous rice cultivars and their evolutionary significance during the domestication process of rice. Theor. Appl. Genet. 108: 1200–1204. [DOI] [PubMed] [Google Scholar]
- Yamasaki, M. and Ideta, O. (2013) Population structure in Japanese rice population. Breed. Sci. 63: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemaru, J., Yamamoto, T., Fukuoka, S., Uga, Y., Hori, K. and Yano, M. (2010) Q-TARO: QTL Annotation Rice Online Database. Rice 3: 194–203. [Google Scholar]
- Yonemaru, J., Mizobuchi, R., Kato, H., Yamamoto, T., Yamamoto, E., Matsubara, K., Hirabayashi, H., Takeuchi, Y., Tsunematsu, H., Ishii, T.et al. (2014) Genomic regions involved in yield potential detected by genome-wide association analysis in Japanese high-yielding rice cultivars. BMC Genomics 15: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, K., Tung, C.W., Eizenga, G.C., Wright, M.H., Ali, M.L., Price, A.H., Norton, G.J., Islam, M.R., Reynolds, A., Mezey, J.et al. (2011) Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat. Commun. 2: 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.