Abstract
As epidemiologists studying foodborne illness outbreaks, we do not ask luncheon attendees to say which food caused their illnesses. Instead, we use measurement and analysis methods to estimate food-specific risk variations. Here, we adapt the foodborne outbreak approach to develop new estimates of drug use disorder risk for single-drug and polydrug users, without attributing the syndrome to a specific drug when multiple drugs have been used. We estimate drug use disorder risk for cannabis-only users as a reference value. We then derive comparative relative risk estimates for users of other drug subtypes, including polydrug combinations. Data are from the 2002 to 2003 U.S. National Comorbidity Survey Replication, a nationally representative sample of household residents (18+ years), with standardized drug use and drug dependence assessments. Multiple logistic regression provides odds ratio estimates of relative risk. With this approach, for every 1000 cannabis-only users, an estimated 17 had become cases (1.7%). By comparison, polydrug users and cocaine-only users had much greater cumulative incidence (>10%), even with adjustment for covariates and local area matching (P < 0.001). Using this approach, we find exceptionally low risk for cannabis-only users and greater risk for polydrug and cocaine-only users.
Keywords: epidemiology, drug use disorders, drug dependence, drug addiction, polydrug, multiple drug use, tobacco, alcohol, conduct problems
Introduction
Public health research challenges of polydrug use and use of multiple drugs have surfaced in periodic calls and program announcements for new research issued from the directors of the United States' National Institute on Drug Abuse (NIDA) starting in the mid-1970s.1 One rarely addressed challenge of this type involves epidemiological estimation of the risk of becoming a case of a drug use disorder when users have tried multiple subtypes of internationally regulated drugs, sometimes with co-occurring alcohol use or tobacco use as well.2,3
Making estimates of this type, epidemiologists generally have worked in a tradition that is consistent with pharmacological laboratory approaches and not always within the framework of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) from the American Psychiatric Association (APA).4 Namely, an attempt is made to investigate experience with each drug subtype, and to assign drug-specific diagnoses (e.g., cannabis use disorder), most typically via a checklist of clinical features (CFs) and standardized questions about which drug has caused each CF. In time, this epidemiological approach might be expected to change in the direction of the DSM-5 Substance Use Disorder (SUD) terminology and SUD subtypes. Meanwhile, the terms drug use disorder and drug dependence have been retained in epidemiological field studies.
In this context, when use of multiple drugs is observed, the number of drugs used as well as antecedent polydrug combinations can be studied as predictors of occurrence of each drug-specific syndrome.5–8 Before DSM-5, diagnoses for unspecified drug dependence and polysubstance dependence were allowable. Sometimes clinicians have used the 304.7[0–3]–304.8[0–3] diagnostic codes for complex polydrug-using patients using at least three different drugs indiscriminately (excluding caffeine or nicotine), showing no preference for any specific one, and showing a minimum of three drug-attributable CFs within a 12 months period. Nonetheless, epidemiological estimates for these diagnostic codes are rare.
Great humility is required about epidemiological evidence that requires the polydrug users themselves or clinician examiners to say which drug has caused each and every CF of the drug use syndromes whose epidemiology we seek to understand. We always have had to acknowledge that these causal attributions at the level of the individual case necessarily constrain validity owing to wide variations in understanding of clinical pharmacology and drug interactions, uncertain knowledge, or incorrect beliefs about the effects of various drug subtypes and their combinations. Validity constraints are especially narrow in large sample epidemiological field studies when drug users are asked to say which drug caused each symptom and CF, irrespective of whether the study design is retrospective, cross-sectional, or prospective.6,9,10 Heterogeneity across or within study samples adds more uncertainty about validity, especially in the context of multicountry surveys.11
Attempting to confront these challenges directly, we have been trying to work out a research approach for large sample epidemiological studies on these topics, with a reach toward investigations used to identify causes of food-borne illness outbreaks, that is, when the measurements ask about symptoms or other CFs of drug use disorders as might have been experienced in a syndrome cluster, and with separate questions on which drugs have been used. This measurement approach departs from the widely used drug-specific approach for research on drug use disorders, and presumes that users of multiple drugs might be uncertain about which drug has caused each symptom or clinical feature.
In a direct adaptation of the epidemiologic research approach used in foodborne illness outbreak investigations for rapid evaluation of multiple foods that might be causal vehicles for the outbreak, this study's approach is focused on evaluation of multiple drugs as might contribute to occurrence of drug use syndromes. In brief, investigating postluncheon or banquet outbreaks, epidemiologists assemble a list of meal attendees, formulate a syndromic illness case definition, a case ascertainment plan for coverage of pertinent CFs of the syndrome, and a list of food subtypes (including beverages). Then, all attendees are contacted for interview or questionnaire assessments, without asking which food caused which CF. Estimates of food-specific syndrome attack rates are derived from the observed retrospective data; estimates of risk differences or ratios are derived from contingency table analyses (e.g., cross-product ratios from 2 × 2 tables). If required, stratified analyses or a generalized linear model is used to estimate subgroup variation in risk (e.g., food–food combinations), and to hold constant extraneous influences on illness reporting or illness experience (e.g., male–female differences). Appendix I describes an epidemiology laboratory exercise used to teach students about foodborne outbreaks, and offers online materials with additional detail.12
In this contribution, we seek to demonstrate this research approach as applied to drug use disorders in a widely known nationally representative sample of 18+ year olds that included quite a few polydrug users, namely, the National Comorbidity Survey-Replication (NCS-R) sample. In the process, we estimate suspected effects of drug subtypes used singly and in combination on risk of developing a drug dependence syndrome as a form of drug use disorder, and we study subgroup variations in risk, while holding constant potential determinants. In an extension of the typical foodborne outbreak analysis, we use life table analyses to address possible length biases due to varying retrospection intervals since onset of drug use.
In this study, the drug use disorder assessments used in the NCS-R required the presence of maladaptive drug use via a gating procedure described in three prior papers, as well as syndromic clustering of at least three symptoms or CFs associated with drug dependence. In anticipation of DSM-5, the standardized assessment items included coverage of craving, but it was not possible to anticipate that DSM-5 would shift its polythetic threshold to require just two criteria to be met, as opposed to the three required under DSM-IIIR and DSM-IV. For these reasons, we argue that the resulting diagnostic assessment resembles but is not the same as any DSM-IV or DSM-5 diagnostic assessment, as might be made when an expert clinician examines an individual patient. Instead, the construct under study is a syndromic form of a drug use disorder that we term drug dependence with maladaptive drug use (DDwMDU). This case terminology refers back to a World Health Organization Expert Committee's advocacy for use of the term drug dependence, but adds an element of maladaptive drug use that the APA's experts have integrated with the original drug dependence construct. When multiple drugs have been used and a dependence syndrome cannot be attributed to a particular drug, the construct under study most closely resembles the international and APA categories known as unspecified drug dependence or polysubstance dependence.13
A final clarification involves our use of life table conventions as illustrated in our “cannabis-only” estimates, which pertain to individuals whose cannabis use started at an onset age before or at the drug use disorder onset age. Those using both cannabis and cocaine in the same year as DDwMDU syndrome onset are counted as polydrug users with the cannabis plus cocaine combination. As in the life table approach, the experience of our cases is right-censored at the DDwMDU syndrome onset age. In this fashion, we ensured that later postdisorder drug use did not qualify as a cause of onset of the drug use syndrome under study here.
Materials and methods
Data to demonstrate our novel epidemiological approach are in public use datasets from the NCS-R,14 with English-speaking community participants, age 18 years and older, all from the 48 coterminous U.S. states, drawn via multistage area probability sampling, recruited, and assessed using an ethics committee approved protocol in 2000–2002. A participation level of 71% yielded 9282 respondents. A public use dataset version of the NCS-R data is available online,15 including the local area cluster and stratum required to perform this project's analyses.
The key response variable in this study, DDwMDU, is defined as the occurrence of a syndromic clustering of at least three CFs of drug dependence over a 12-month period, with at least one manifestation of maladaptive drug use, resembling but not identical with DSM-5's “Substance Use Disorder” with respect to internationally regulated drugs. Here, MDU refers to drug use that threatens life or limb (e.g., recurrent driving under the influence) or that is socially maladaptive with failure to live up to social role expectations and obligations. The social maladaptation concept is one introduced by research groups led by Kellam,16 Rutter,17 and Rolf.18 Syndrome specifications and measurement of the DDwMDU syndrome via a “gated” Part 1 module of the UM-CIDI (Composite International Diagnostic Interview) are described in Figure 1 and Appendix II, and in other papers that provide supportive validity evidence. 19–22
Figure 1.
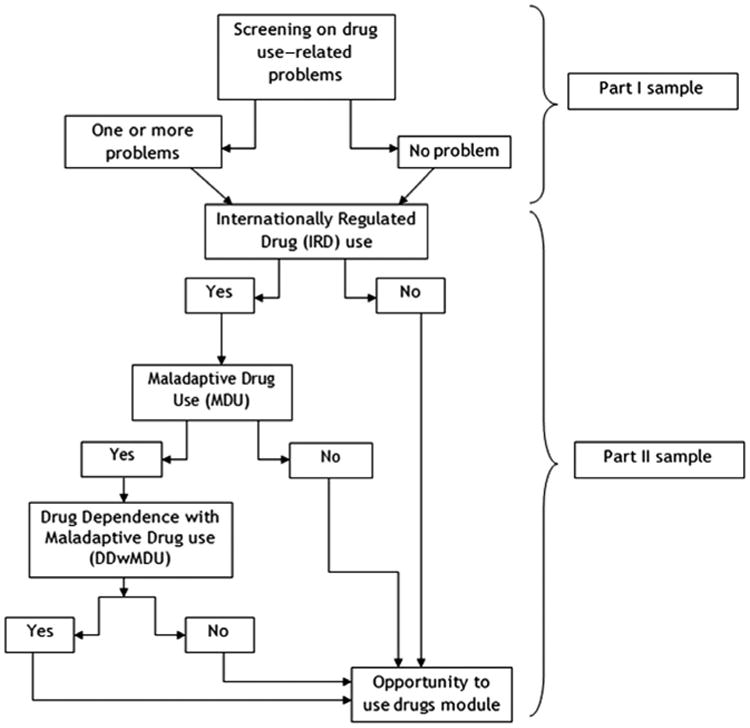
Assessment of drug dependence with maladaptive drug use (DDwMDU) in the Composite International Diagnostic Interview (CIDI).
The UM-CIDI assesses DDwMDU only when there is extramedical use of one or more subtypes of the inhalants or internationally regulated drugs (hereinafter, IRD), by which is meant using the drug to get high or otherwise beyond the boundaries of approved indications, with measurement as explained in a prior article23 and Appendix II. In the sample, among 2918 extramedical users of internationally regulated drugs (EMIRD) with complete age of onset data, 1509 had consumed just one IRD subtype before or at DDwMDU onset (e.g., 1409 “cannabis-only” users); 1409 had antecedent use of at least two IRD subtypes (Table 1). As noted in the introduction, drug-specific onset ages were used to ensure that all drug use started before or at syndrome onset age (hereinafter, IRD used extramedically before DDwMDU onset).
Table 1. Estimated occurrence of syndromic clustering of drug dependence with maladaptive drug use (DDwMDU) among extramedical users of internationally regulated drugs (EMIRD) in the United States; National Comorbidity Survey Replication, 2000–2002 (n = 2918).
| Weighted estimates for occurrence of DDwMDU | Unweighted numbers | P value from chi-square test | All EMIRD users (n = 2918) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| % | SE | DDwMDU case (n = 247) | Non-case controls (n = 2671) | Unweighted numbers | Weighted column % | |||
|
| ||||||||
| Column %a | SE | |||||||
| All extramedical IRD users | 6.9 | 0.05 | 247 | 2671 | 2918 | 100.0 | ||
| Cannabis users | 6.9 | 0.01 | 239 | 2569 | 0.210 | 2808 | 96.1 | 0.52 |
| Cocaine users | 12.8 | 0.01 | 153 | 888 | <0.001 | 1041 | 35.0 | 1.09 |
| Users of “other” IRD (not otherwise specified) | 17.3 | 0.01 | 144 | 597 | <0.001 | 741 | 23.3 | 1.02 |
| EM users of prescribed IRD | 15.5 | 1.61 | 132 | 644 | <0.001 | 776 | 23.8 | 1.00 |
| IRD used extramedically before DDwMDU onsetb | ||||||||
| Only cannabis | 1.7 | 0.36 | 33 | 1376 | 1409 | 50.7 | 1.21 | |
| Prescription IRD only | 1.4 | 1.36 | 1 | 50 | 51 | 1.6 | 0.30 | |
| Only cocaine | 12.3 | 6.71 | 5 | 23 | 28 | 1.3 | 0.30 | |
| Only “other” IRD (NOS) | 3.3 | 3.32 | 1 | 20 | 21 | 0.8 | 0.20 | |
| Use of IRD in 2 groups | 8.6 | 1.43 | 68 | 605 | 673 | 22.2 | 1.14 | |
| Use of IRD in 3+ groups | 16.8 | 1.45 | 139 | 597 | <0.001 | 736 | 23.5 | 1.09 |
These column estimates are based on a denominator with nonmissing values for each covariate.
There were six DDwMDU-affected EMIRD users with missing or invalid age of onset data on multiple drugs, making it impossible to confirm the sequence from onset of EMIRD use to onset of DDwMDU. Among the 673 who used IRD in 2 groups, roughly 99% of EMIRD users had used cannabis in combination with one other IRD. Among the 736 who used “IRD in 3+ groups,” 99% used cannabis in combination with two or more other IRD (e.g., cocaine + prescribed IRD).
Primary study estimates are from weighted contingency table analyses and multiple unmatched unconditional logistic regressions, as well as conditional logistic regressions with area matching. The approach is one that holds constant other UM-CIDI covariates (e.g. conduct disorders), with measurements described in Appendix II. Unmeasured macroinfluences such as local drug availability and law enforcement variations are held constant via this area matching. Uses of analysis weights and Taylor series linearization for variance estimation are noted in the tables.
Results
Overall, the DDwMDU syndrome was observed among an estimated 69 of every 1000 IRD users (i.e., ∼7%). A similar estimate was observed for those who had used cannabis, irrespective of other drugs used. A larger estimate was seen for those who had used drugs other than cannabis (128–173 for every 1000 extramedical users, or ∼12–17%). It follows that this study's DDwMDU syndrome might well be rare when use is restricted to cannabis. As shown in Table 1, for every 1000 users of “cannabis only,” an estimated 17 had developed DDwMDU by the assessment date (1.7%). By comparison, antecedent use of all other drug subtypes was followed by excess risk of developing the drug dependence syndrome under study (P < 0.05), especially for users of cocaine only and for polydrug users with extramedical use of at least 2 or 3 drug subtypes (which primarily refers to cannabis in combination with other drugs).
The subgroup of cannabis-only users can be studied as a reference category, as shown in Table 2. Table 2 estimates (with P < 0.05) disclose excess DDwMDU risk for three EMIRD subgroups, (1) cocaine-only users; (2) users of 2 subtypes; (3) users of 3+ subtypes, across a series of conditional and unconditional regression models. In the NCS-R sample, there were too few cases for statistically precise estimation of DDwMDU risk associated with the prescription IRD only and other (NOS) IRD only subgroups. Appendix III provides more detailed versions of Table 2, including relative risk (RR) estimates from conditional models that constrain alternative suspected influential antecedents (e.g., early conduct problems).
Table 2.
Estimates of the association between occurrence of syndromic clustering of drug dependence with maladaptive drug use (DDwMDU) and drugs used before DDwMDU onset among EMIRD users in the United States. National Comorbidity Survey Replication, 2000–2002 (n = 2918)
| Relative risk (RR) of occurrence of syndromic clustering of DDwMDU (all relative to DDwMDU among n = 2808 who had EM use of cannabis, Panel A, or n = 1409 who had EM use of cannabis but no other IRD use, Panel B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Estimated RR from conditional logistic regression (area-matched risk sets) | Estimated RR from conditional conditional logistic regression for discrete time data (area-matched risk sets of survival data) | Estimated RR from unconditional logistic regression with weights and Taylor series variance approach | ||||||||||
|
|
|
|
||||||||||
| RR | 95% Confidence interval (CI) | P-value | RR | 95% CI | P-value | RR | 95% CI | P-value | ||||
| Panel A | ||||||||||||
| Prescription IRD usea | 2.3 | 1.7 | 3.0 | <0.001 | 2.2 | 1.7 | 3.0 | <0.001 | 2.6 | 1.7 | 3.9 | <0.001 |
| Cocaine useb | 1.9 | 1.4 | 2.6 | <0.001 | 2.0 | 1.5 | 2.7 | <0.001 | 2.3 | 1.6 | 3.3 | <0.001 |
| “Other” IRD use (NOS)c | 2.9 | 2.2 | 3.9 | <0.001 | 2.7 | 2.0 | 3.6 | <0.001 | 3.2 | 2.3 | 4.3 | <0.001 |
| Panel B | ||||||||||||
| IRD used extramedically before DDwMDU onset | ||||||||||||
| Only cannabis (ref) | 1 | 1 | 1 | |||||||||
| Prescription IRD only | 1.0 | 0.1 | 7.7 | 0.984 | 0.5 | 0.1 | 4.3 | 0.532 | 0.9 | 0.1 | 7.3 | 0.941 |
| Only cocaine | 9.8 | 3.4 | 28.1 | <0.001 | 3.8 | 0.6 | 24.1 | 0.162 | 7.9 | 1.8 | 34.7 | 0.008 |
| Only “other” IRD (NOS) | 1.9 | 0.3 | 14.7 | 0.540 | d | – | – | 0.987 | 1.9 | 0.3 | 14.1 | 0.524 |
| Use of IRD in 2 groups | 4.6 | 3.0 | 7.1 | <0.001 | 5.4 | 3.4 | 8.8 | <0.001 | 5.1 | 3.0 | 8.6 | <0.001 |
| Use of IRD in 3+ groups | 8.7 | 5.8 | 12.9 | <0.001 | 9.9 | 6.3 | 15.5 | <0.001 | 10.8 | 6.3 | 18.6 | <0.001 |
NOTE: All models in Panel A and Panel B include covariate adjustment for sex, age, and age squared.
This model also holds constant EM use of cocaine and of drugs in the “other” subgroup.
This model also holds constant EM use of prescription IRD and of drugs in the “other” subgroup.
This model also holds constant EM use of prescription IRD and cocaine.
Too few cases for sufficiently precise estimation of RR or 95% CI, as indicated by P-value.
Another set of conditional logistic regression models was used to evaluate the association between DDwMDU onset and drugs used before DDwMDU with a sequenced exclusion of each of the 10 possible IRD combinations found among individuals who had used more than two IRD. In this fashion, via exclusion, we aimed to identify IRD combinations that might be shaping likelihood of developing DDwMDU. The analyses of age- and sex-adjusted models did not show important change in the estimates for the subgroups of individuals who used IRD in two or more groups and individuals who used three or more IRD (Appendix IV). To rule out alcohol use disorders (AUDs) as a potential confounding variable, we conducted a postestimation analysis with exclusion of all for whom AUDs preceded DDwMDU. The resulting postexclusion estimates did not differ appreciably from the estimates before exclusion (Appendix V).
Discussion
In a departure from prior epidemiologic research, we did not ask users or clinician examiners which drug caused each of the reported symptoms or other CFs of their drug use syndromes. Using the alternative approach demonstrated here, which substitutes data analysis in place of respondents' causal judgments, we controlled potential confounding explicitly via regression analyses, and discovered exceptionally low DDwMDU syndrome risk for cannabis-only users, with a much greater risk for polydrug users, especially when more than two drug subtypes were used (generally, cannabis in combination with other IRD). The cocaine-only subgroup also had excess risk of the DDwMDU syndrome. RR estimates for other syndrome predictors and correlates have been produced and are reported in our tables, but should be regarded as exploratory until replication firms up the evidence.
Studying DDwMDU occurrence using this alternative approach offers some methodological and practical advantages worth mentioning. First, drug use syndromes of this type can be assessed using questionnaire or interview items that focus on the syndrome and its CFs, in place of items that ask users to make causal judgments about specific effects of each of the drugs under study. The result includes reduced respondent burden and time, and there might be an improvement in the validity of the resulting estimates. Second, effect estimates can be derived in a fashion that constrains error (e.g., error when a user of cannabis and cocaine incorrectly attributes harmful effects to cocaine rather than to cannabis). Third, the resulting evidence might help guide future research for harm reduction, as when riskier combinations can be identified via data analyses of the type used to elicit more toxic food–food combinations in the illness outbreak context.
Limitations of note include our reliance on cross-sectional and retrospective self-report survey data, one of the more ubiquitous deficiencies in this type of epidemiological research, including almost all food-borne illness investigations conducted by public health departments. With respect to our RR estimates, common unmeasured genetic or other individual-level susceptibility traits might be affecting propensities to explore a variety of drug subtypes as well as the propensity to develop drug dependence (e.g., openness to experience as a trait). Furthermore, the NCS-R sample was not large enough to yield statistically precise RR estimates for some specific drug–drug combinations (e.g., cocaine + prescription pain relievers). Finally, the UM-CIDI “other drug” subtype is too heterogeneous to be recommended for use in new investigations.
Potential directions for future studies include application of this approach in prospective and longitudinal studies, including randomized prevention trials with samples large enough and measurements sufficiently refined to be informative about risk of specific forms of drug use disorders. The approach also might prove to be helpful in some clinical research contexts, as in evaluation of risks when cannabis and opioids are being used within medically prescribed boundaries, or in the clinical trials context when abbreviated drug use disorder assessments are needed.
Supplementary Material
Appendix I. Description of the foodborne approach to the study of polydrug dependence
Appendix II. Study variables selected from the Composite International Diagnostic Interview (CIDI)
Appendix III. Unadjusted and adjusted relative risk (RR) estimates of the associations between occurrence of syndromic clustering of drug dependence with maladaptive drug use (DDwMDU) and multiple covariates among EMIRD users in the United States. Results of multivariable conditional logistic regression models with area-matched risk sets. National Comorbidity Survey Replication, 2000–2002.
Appendix IV. Age- and sex-adjusted relative risk (RR) estimates of the associations between occurrence of syndromic clustering of drug dependence with maladaptive drug use (DDwMDU) and drugs used before DDwMDU onset among EMIRD users in the United States when excluding different drug combinations. Results of multivariable conditional logistic regression models with area-matched risk sets. National Comorbidity Survey Replication, 2000–2002
Appendix V. Estimates of the association between occurrence of syndromic clustering of drug dependence with maladaptive drug use (DDwMDU) and drugs used before DDwMDU onset among a sub-sample of EMIRD users who did not develop alcohol dependence before occurrence of DDwMDU in the United States. National Comorbidity Survey Replication, 2000–2002 (n = 2578)
Acknowledgments
This work was supported by the NIDA, Grants K05DA015799 (Dr. Anthony), T32DA021129 (PI: J. Anthony; Dr. Lopez-Quintero), and by Michigan State University.
J.C. Anthony was responsible for study conceptualization and design; C. Lopez-Quintero managed the literature searches, conducted the statistical analyses, and created the tables. Both authors contributed to writing up the evidence, and have approved the final manuscript.
The NCS-R was supported by the National Institute of Mental Health (NIMH; U01-MH60220) with supplemental support from the NIDA, the Substance Abuse and Mental Health Services Administration, the Robert Wood Johnson Foundation (Grant 044708), and the John W. Alden Trust. Collaborating investigators included Ronald C. Kessler (Principal Investigator, Harvard Medical School), Kathleen Merikangas (Co-principal Investigator, NIMH), James Anthony (Michigan State University), William Eaton (the Johns Hopkins University), Meyer Glantz (NIDA), Doreen Koretz (Harvard University), Jane McLeod (Indiana University), Mark Olfson (Columbia University College of Physicians and Surgeons), Harold Pincus (University of Pittsburgh), Greg Simon (Group Health Cooperative), Michael Von Korff (Group Health Cooperative), Philip Wang (Harvard Medical School), Kenneth Wells (UCLA), Elaine Wethington (Cornell University), and Hans-Ulrich Wittchen (Institute of Clinical Psychology, Technical University Dresden and Max Planck Institute of Psychiatry). A complete list of NCS publications and the full text of all NCS-R instruments can be found at http://www.hcp.med.harvard.edu/ncs.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Supporting Information: Additional supporting information may be found in the online version of this article.
References
- 1.Dupont RL. Polydrug abuse and the maturing national drug abuse data base. Ann N Y Acad Sci. 1976;281:311–320. doi: 10.1111/j.1749-6632.1976.tb27941.x. [DOI] [PubMed] [Google Scholar]
- 2.Grant BF, Harford TC. Concurrent and simultaneous use of alcohol with cocaine: results of a nation survey. Drug Alcohol Depend. 1990;25:97–104. doi: 10.1016/0376-8716(90)90147-7. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal RN, Levounis P. Polysubstance use, abuse, and dependence. In: Frances R, Miller S, Mack A, editors. Clinical Textbook of Addictive Disorders. New York/London: The Guilford Press; 2011. pp. 245–270. [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5) Washington (DC): American Psychiatric Association; 2013. [Google Scholar]
- 5.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- 6.Anthony JC, Helzer J. Syndromes of drug abuse and dependence. In: Regier D, Robins L, editors. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. New York: Free Press; 1991. pp. 116–154. [Google Scholar]
- 7.O'Brien MS, Anthony JC. Risk of becoming cocaine dependent: epidemiological estimates for the United States, 2000–2001. Neuropsychopharmacology. 2005;30:1006–1018. doi: 10.1038/sj.npp.1300681. [DOI] [PubMed] [Google Scholar]
- 8.Perkonigg A, Goodwin RD, Fiedler A, et al. The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction. 2008;103:439–449. doi: 10.1111/j.1360-0443.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- 9.Anthony JC, Folstein M, Romanoski AJ, et al. Comparison of the lay diagnostic interview schedule and a standardized psychiatric diagnosis: experience in eastern Baltimore. Arch Gen Psychiatry. 1985;42:667–675. doi: 10.1001/archpsyc.1985.01790300029004. [DOI] [PubMed] [Google Scholar]
- 10.Anthony JC, Echeagaray-Wagner F. Epidemiologic analysis of alcohol and tobacco use. Alcohol Res Health. 2000;24:201–208. [PMC free article] [PubMed] [Google Scholar]
- 11.Anthony JC. Novel phenotype issues raised in cross-national epidemiological research on drug dependence. Ann N Y Acad Sci. 2010;1187:353–369. doi: 10.1111/j.1749-6632.2009.05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Center for Disease Control and Prevention. [Accessed February 12, 2014];Steps of an outbreak investigation. 2013 http://www.cdc.gov/foodsafety/outbreaks/investigating-outbreaks/investigations/index.html.
- 13.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th. Washington DC.; American Psychiatric Association: 1994. [Google Scholar]
- 14.Kessler RC, Merikangas KR. The national comorbidity survey replication (NCS-R): background and aims. Int J Methods Psychiatr Res. 2004;13:60–68. doi: 10.1002/mpr.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States National Institute of Mental Health Collaborative Psychiatric Epidemiology Surveys (CPES) [Accessed October 30, 2014]; http://www.icpsr.umich.edu/icpsrweb/CPES/index.jsp.
- 16.Kellam SG. Mental Health and Going to School: The Woodlawn Program of Assessment, Early Intervention, and Evaluation. Chicago: University of Chicago Press; 1975. [Google Scholar]
- 17.Quinton D, Pickles A, Maughan B, Rutter M. Partners, peers and pathways: assortative pairing and continuities in conduct disorder. Dev Psychopathol. 1994;5:763–783. [Google Scholar]
- 18.Rolf J, Masten AS, Cicchetti D, et al. Risk and Protective Factors in the Development of Psychopathology. New York: Cambridge University Press; 1990. [Google Scholar]
- 19.Degenhardt L, Chiu WT, Conkay K, et al. Does the ‘gateway’ matter? Associations between the order of drug use initiation and the development of drug dependence in the National Comorbidity Study Replication. Psychol Med. 2009;39:157–167. doi: 10.1017/S0033291708003425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swendsen J, Anthony JC, Conway KP, et al. Improving targets for the prevention of drug use disorders: sociodemographic predictors of transitions across drug use stages in the national comorbidity survey replication. Prev Med. 2008;47:629–634. doi: 10.1016/j.ypmed.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glantz MD, Anthony JC, Berglund PA, et al. Mental disorders as risk factors for later substance dependence: estimates of optimal prevention and treatment benefits. Psychol Med. 2009;39:1365–1377. doi: 10.1017/S0033291708004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haro JM, Arbabzadeh-Bouchez S, Brugha TS, et al. Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the World Health Oorganization, World Mental Health surveys. Int J Methods Psychiatr Res. 2006;15:167–180. doi: 10.1002/mpr.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degenhardt L, Chiu WT, Sapson N, et al. Epidemiological patterns of extra-medical drug use in the United States: evidence from the National Comorbidity Survey Replication, 2001–2003. Drug Alcohol Depend. 2007;90:210–223. doi: 10.1016/j.drugalcdep.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix I. Description of the foodborne approach to the study of polydrug dependence
Appendix II. Study variables selected from the Composite International Diagnostic Interview (CIDI)
Appendix III. Unadjusted and adjusted relative risk (RR) estimates of the associations between occurrence of syndromic clustering of drug dependence with maladaptive drug use (DDwMDU) and multiple covariates among EMIRD users in the United States. Results of multivariable conditional logistic regression models with area-matched risk sets. National Comorbidity Survey Replication, 2000–2002.
Appendix IV. Age- and sex-adjusted relative risk (RR) estimates of the associations between occurrence of syndromic clustering of drug dependence with maladaptive drug use (DDwMDU) and drugs used before DDwMDU onset among EMIRD users in the United States when excluding different drug combinations. Results of multivariable conditional logistic regression models with area-matched risk sets. National Comorbidity Survey Replication, 2000–2002
Appendix V. Estimates of the association between occurrence of syndromic clustering of drug dependence with maladaptive drug use (DDwMDU) and drugs used before DDwMDU onset among a sub-sample of EMIRD users who did not develop alcohol dependence before occurrence of DDwMDU in the United States. National Comorbidity Survey Replication, 2000–2002 (n = 2578)


