Summary
Idiopathic pulmonary fibrosis (IPF), a devastating progressive interstitial lung disease (ILD) with no known cause or cure, is the most common and deadly of the idiopathic interstitial pneumonias. With a median survival of 3–5 years following diagnosis, IPF is characterized by a progressive decline in lung function and quality of life in most patients. Vigilance among clinicians in recognizing IPF early in the disease course remains critical to properly caring for these patients, as this provides the widest range of management options. When IPF is suspected, a multidisciplinary evaluation (MDE) by a clinician, radiologist and pathologist with ILD expertise should occur, as this improves diagnostic agreement in both community and academic settings. When community MDE is not possible, or diagnostic doubt exists, referral to an ILD center should be considered. ILD center referral may also provide access specialized care, including clinical trials and lung transplantation, and should be considered for any patient with an established diagnosis of IPF.
Keywords: IPF, Pulmonary fibrosis, ILD, Lung transplant
Introduction
The interstitial lung diseases (ILDs) are a heterogeneous group of diffuse parenchymal lung disorders with similar clinical, radiographic, physiologic or pathologic features [1]. ILDs include disorders of known cause, and those with unknown etiology, referred to as the idiopathic interstitial pneumonias (IIPs) [2]. The most common and deadly of the IIPs is idiopathic pulmonary fibrosis (IPF) [3], defined as a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause, occurring primarily in older adults, and limited to the lungs [4]. IPF has a median survival time of 3–5 years after diagnosis, and is characterized by progressive decline in lung function and quality of life in most patients [3,5–7]. Despite an evolving understanding of the underlying disease process, the pathogenesis of IPF remains unknown.
Epidemiology
The global prevalence of IPF is difficult to establish and reflects the diagnostic complexity of the disease and the substantial resources needed to establish the diagnosis. These issues, along with heterogeneity in study design and case finding methodology, have resulted in variable epide-miologic data over the last several decades [4,8]. Two recent population studies in the US reported an IPF incidence of 6.8–8.8 and 16.3–17.4 cases per 100,000 person years using narrow and broad case definitions, respectively. These studies found the prevalence of IPF to be 14.0–27.9 (narrow case definitions) and 42.7–63.0 (broad case definitions) per 100,000 persons [9,10]. Recent epidemiologic studies from the UK demonstrate that the incidence of IPF has been rising over the last decade [11,12]. IPF has been described in all ethnic groups and in both rural and urban settings [7,13]. Affecting males predominantly, most cases are diagnosed after age 60 and incidence increases with age. [7,9–12].
Early diagnosis
Evolution of the diagnostic criteria for IPF has resulted in abandonment of the major and minor criteria set by the 2000 ATS/ERS consensus statement [14]. The latest international guidelines require exclusion of other known causes of ILD and the presence of a usual interstitial pneumonia (UIP) pattern on high-resolution computed tomography (HRCT) in patients for whom a surgical lung biopsy (SLB) has not been performed. In patients failing to demonstrate a typical UIP pattern on HRCT, IPF may still be diagnosed based on specific combinations of HRCT and SLB patterns (Table 1) [4]. This diagnostic complexity makes misdiagnosis and delayed diagnosis common [15]. A recent survey showed that a majority of patients with IPF reported seeing several physicians, and waiting over a year, before receiving the correct diagnosis [16]. Careful attention to historical clues, physical exam and other diagnostics can help establish the diagnosis early and provide those with IPF the full breadth of options available to them.
Table 1.
Combination of HRCT and surgical lung biopsy for the diagnosis of IPF4.
| HRCT patterna | Surgical lung biopsy patterna (when performed) |
Diagnosis of IPF?c |
|---|---|---|
| UIP | UIP | YES |
| Probable UIP | ||
| Possible UIP | ||
| Nonclassifiable fibrosisb | ||
| Not UIP | No | |
| Possible UIP | UIP | YES |
| Probable UIP | ||
| Possible UIP | Probabled | |
| Nonclassifiable fibrosis | ||
| Not UIP | No | |
| Inconsistent with UIP | UIP | Possibled |
| Probable UIP | No | |
| Possible UIP | ||
| Nonclassifiable fibrosis | ||
| Not UIP |
Definition of abbreviations: HRCT = high-resolution computed tomography; IPF = idiopathic pulmonary fibrosis; UIP = usual interstitial pneumonia.
Bold type indicates combinations of HRCT and surgical lung biopsy patterns that correspond with a diagnosis of IPF (a YES in the far right column). The combination of UIP HRCT and probable UIP or possible UIP or Nonclassifiable fibrosis (surgical lung biopsy patterns) (for example) equals a diagnosis of IPF; the combination of UIP HRCT and Not UIP (surgical lung biopsy pattern) does not make the diagnosis of IPF.
Nonclassifiable fibrosis: Some biopsies may reveal a pattern of fibrosis that does not meet the above criteria for UIP pattern and the other idiopathic interstitial pneumonias. These biopsies may be termed “nonclassifiable fibrosis.”
The accuracy of the diagnosis of IPF increases with multidisciplinary discussion. This is particularly relevant in cases in which the radiologic and histopathologic patterns are discordant (e.g., HRCT is inconsistent with UIP and histopathology is UIP). There are data to suggest that the accuracy of diagnosis is improved with multidisciplinary discussion among interstitial lung disease experts compared to clinician-specialists in the community setting; timely referral to interstitial lung disease experts is encouraged.
Multidisciplinary discussion should include discussions of the potential for sampling error and a re-evaluation of adequacy of technique of HRCT. NOTE: In cases with an “inconsistent with UIP” HRCT pattern and a “UIP” surgical lung biopsy pattern, the possibility of a diagnosis of IPF still exists and clarification by multidisciplinary discussion among interstitial lung disease experts is indicated. Reprinted from Ref. [4] with permission of the American Thoracic Society. Copyright © 2014 American Thoracic Society.
History
Any adult over age 50 presenting with unexplained dyspnea on exertion and cough should prompt consideration of IPF [4]. Dyspnea in this disease tends to arise insidiously, often over 6 months or more, and progresses steadily [17]. Over 80% of patients report a non-productive, often intractable cough that can be debilitating and refractory to antitussive medications [18–20]. Chest pain is uncommon in IPF and because the disease is limited to the lungs, the presence of systemic inflammatory symptoms, including fever, rash, weight loss, myalgia or arthralgia, makes IPF unlikely and should prompt consideration of an alternative diagnosis.
Cigarette smoking has been strongly linked to IPF, especially in those with greater than 20 pack-year history [21–23]. Hiatal hernia and gastroesophageal reflux disease (GERD) have also been linked to IPF [24–26]. Environmental and industrial exposures associated with IPF include pesticide, metal, wood, vegetable, and animal dust exposure, as well as professions in petrochemical, farming, hairdressing and stone cutting/polishing [23,27–30]. A family history of pulmonary fibrosis or ILD should prompt consideration of familial interstitial pneumonia (FIP). Familial disease was originally thought to account for fewer than 5% of IPF cases, but recent studies suggest this estimate may be low [16,31,32].
Physical exam
On exam, patients with IPF generally exhibit inspiratory crackles, a finding reported in over 90% of patients [33,34]. These fine, Velcro-like crackles may be the earliest clinical finding in IPF and some authors suggest that auscultation of crackles is the best test for identifying early disease due to their excellent sensitivity and good specificity for IPF [35,36]. An extremity exam should be conducted in every patient with suspected IPF as 25–50% of patients will display finger clubbing [33,34,37]. Signs of connective tissue disease including joint deformity, synovitis, muscle weakness and rash make IPF unlikely and should prompt further investigation into rheumatologic disease.
Laboratory
Common laboratory assays generally provide little help in identifying patients with IPF but serologic testing should be performed to exclude underlying connective tissue disease [4]. Up to 20% of patients with IPF have a positive autoantibody but no other signs of connective tissue disease, which is of unclear clinical significance as healthy controls demonstrate a similar autoantibody frequency [38]. Several biomarkers have been linked to accelerated mortality in IPF [39,40] and substantial research is ongoing to identify unique gene signatures [41] that may serve as diagnostic and prognostic tools in the future.
Pulmonary function testing
Pulmonary function testing usually demonstrates a restrictive pulmonary defect, defined as a reduction in total lung capacity (TLC). Most patients with IPF will also exhibit a decreased forced vital capacity (FVC), normal-to-increased forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC) ratio and reduced diffusing capacity of carbon monoxide (DLCO) [7,42]. A normal FVC does not exclude IPF, as this can be near normal early in the disease course [43]. Furthermore, patients with concurrent emphysema may manifest normal lung volumes and spirometry, but will generally have a reduced DLCO [44,45]. Low baseline FVC, decline in FVC, low DLCO and decline in 6-min walk test are associated with decreased survival in IPF [46–50].
Chest radiograph
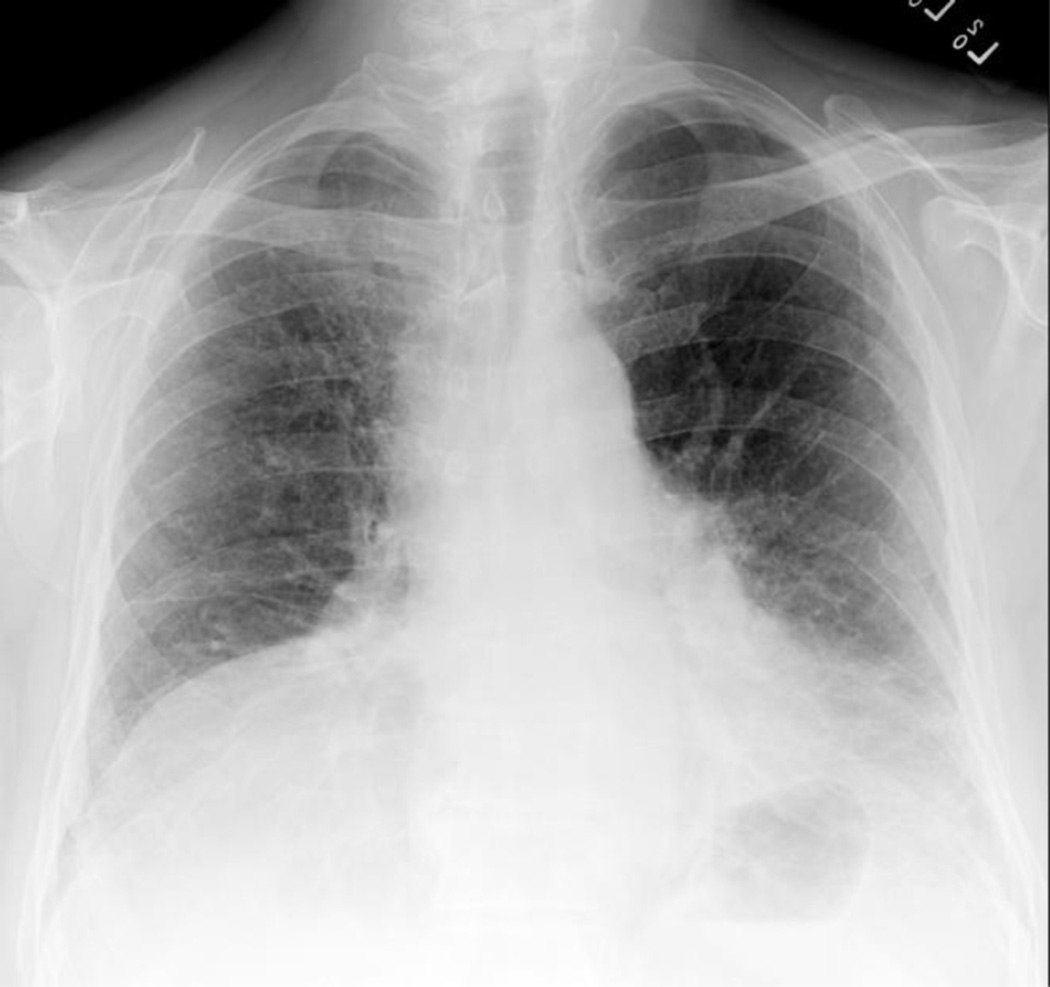
Patients with IPF tend to demonstrate an asymmetric peripheral, basilar reticular opacity with lower lobe volume loss on chest radiograph (Fig. 1) [51–53]. The low sensitivity for detecting subtle interstitial changes can result in a normal chest radiograph in some patients with IPF [37,54]. The presence of airspace filling opacities makes IPF unlikely and should prompt investigation into an alternative diagnosis. While the chest radiograph may identify IPF, it does not provide a high degree of confidence and therefore is not used in the diagnostic criteria [4,53].
Figure 1.
Chest radiograph of a patient with IPF. Findings include asymmetric peripheral and basilar reticular opacity and lower lobe volume loss.
HRCT
A high-resolution computed tomography (HRCT) of the chest should be performed in any patient with an abnormal chest radiograph and clinical findings suggestive of IPF or another ILD.
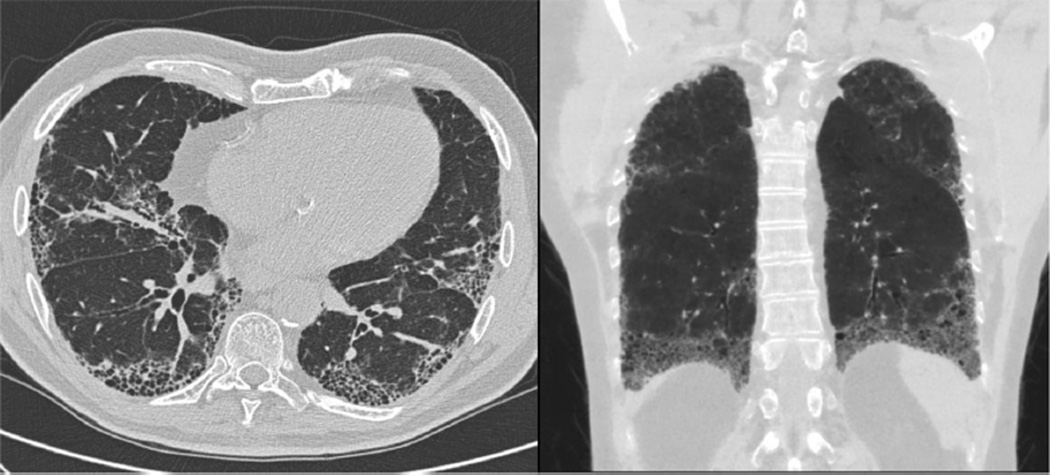
HRCT, which provides excellent specificity for IPF in the appropriate clinical setting, can be used to diagnose IPF in the majority of cases [55–59]. Patients with IPF generally demonstrate a pattern of usual interstitial pneumonia (UIP) on HRCT, which is characterized by a subpleural, basilar predominant reticular abnormality with honeycombing and often traction bronchiectasis (Fig. 2). Features of UIP, possible UIP and those inconsistent with UIP are summarized in Table 2A. Due to institutional variability in ordering and performing HRCT, pre-test discussion with a radiologist should occur to ensure the appropriate ILD protocol is followed. The necessary components of HRCT, as recommended by the American College of Radiology [60], are summarized in Table 2B. Adhering to these guidelines helps avoid repeat imaging and radiation exposure.
Figure 2.
HRCT of the chest demonstrating classic UIP pattern. Findings include peripheral and basilar predominant reticulation with traction bronchiectasis and honeycombing.
Table 2.
HRCT specifications and findings in IPF.
| A) | |
|---|---|
| Specifications for Performing HRCTa | |
|
|
| B) | |
|---|---|
| HRCT findings in IPFb | |
Usual Interstitial Pneumonia (UIP)
|
Inconsistent with UIP
|
Bronchoscopy
While bronchoalveolar lavage (BAL) may assist in diagnosing inflammatory disorders such as hypersensitivity pneumonitis or eosinophilic pneumonia, it provides little help in identifying patients with IPF [7,61]. The same is true of transbronchial biopsy. Raghu and colleagues reported that 85% of patients undergoing surgical lung biopsy (SLB) prior to referral for ILD evaluation had undergone a non-diagnostic transbronchial biopsy [57]. Accordingly, the latest international guidelines for diagnosis and management of IPF recommend against performing a BAL or trans-bronchial biopsy in the majority of patients in whom IPF is suspected [4].
Surgical lung biopsy
The decision to pursue a SLB requires substantial risk/ benefit consideration and should occur only after a comprehensive ILD evaluation. This highly morbid procedure has several known complications, including persistent air leak, prolonged mechanical ventilation, pneumonia and acute exacerbation of IPF. A mortality rate of 4–16% was reported in two case series of patients with ILD undergoing SLB; however, a substantial proportion of these were open lung biopsies and more recent data suggest video-assisted thorascopic surgery (VATS) biopsy may be safer [62–64]. In these studies, male gender and low DLCO were risk factors for SLB-associated complications, while low DLCO and acute exacerbation at the time of biopsy were independently associated with increased 30-day mortality [62,63].
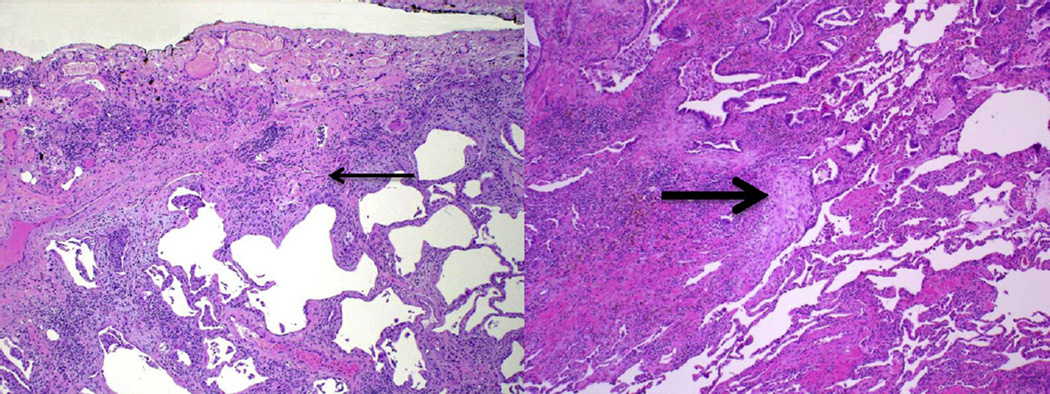
In those with an indication for SLB, pre-biopsy discussion with the thoracic surgeon should occur to ensure each lobe of a single lung is biopsied when possible, as considerable histologic variability between lobes can occur [65]. Furthermore, care should be taken to biopsy less diseased lung preferentially so as to enhance the pulmonary pathologist’s ability to survey areas undergoing active fibrosis. Patients with IPF generally demonstrate a histopathologic UIP pattern, characterized by areas of fibrosis with scarring and honeycomb change alternating with areas of less affected or normal parenchyma. This occurs in a predominant subpleural and paraseptal distribution with little inflammation and includes subepithelial foci of proliferating fibroblasts and myofibroblasts known as fibroblastic foci (Fig. 3) [66]. Like HRCT, varying levels of confidence in this pathologic diagnosis exist and a small minority of patients may demonstrate an unclassifiable fibrotic pattern.
Figure 3.
Histopathologic UIP pattern. Low-power photomicrographs showing findings characteristic of UIP, including subpleural interstitial fibrosis (thin arrow) with temporal heterogeneity (normal lung at the lower right of each slide) and fibroblastic foci (thick arrow). (Courtesy of Aliya Husain, M.D., Department of Pathology, The University of Chicago).
Multidisciplinary evaluation
Because non-IPF ILD tends to follow a more favorable clinical course than IPF, and may respond to corticosteroid and immunosuppressive therapy [3,67], both of which are strongly recommended against in IPF [4,68], establishing an accurate diagnosis is paramount. The 2011 ATS/ERS/JRS/ ALAT consensus statement on the diagnosis of IPF strongly recommends a multidisciplinary evaluation (MDE) in establishing the diagnosis [4]. The importance of such evaluation is highlighted by observations that isolated radiographic or histologic UIP can represent processes other than IPF and that IPF does not always demonstrate radiographic or histologic UIP [55,56,69]. MDE should include a clinician, radiologist and pathologist with considerable IPF and ILD experience. Discussion among these specialists leads to improved diagnostic agreement [70], and has become a cornerstone of evaluation at ILD referral centers.
Whether ILD is best diagnosed in a community or academic setting remains controversial. Flaherty and colleagues conducted an investigation of clinicians, radiologists and pathologists from community and academic settings to determine the level of diagnostic agreement in evaluating ILD [71]. Using a stepwise approach that provided an increasing amount of clinical, radiographic and histopathologic information, the authors demonstrated that diagnostic agreement, as determined by kappa score (<0 indicating poor agreement, 0–0.2 slight agreement, 0.2–0.4 fair agreement, 0.4–0.6 moderate agreement, 0.6–0.8 substantial agreement and >0.8 almost perfect agreement), increased in both groups with each step (Table 3). Though greater interobserver agreement was demonstrated among academic physicians compared with those from the community, considerable disagreement remained within both groups upon final diagnosis. The authors noted that the greater agreement among academic than community physicians was likely influenced by the fact that these individuals had collaborated on previous projects, including consensus statements, and that the community clinician with the most ILD experience tended to agree more often with the academicians. This study highlights the importance of MDE and experience in diagnosing ILD, but also underscores the need to improve the means by which we establish the diagnosis.
Table 3.
Interobserver agreement κ score stratified by center type and step of the evaluation process.
| Step | Clinicians |
Radiologists |
Pathologists |
|||
|---|---|---|---|---|---|---|
| Academic | Community | Academic | Community | Academic | Community | |
| 1 | 0.28 (0.03) | 0.20 (0.07) | 0.59 (0.11) | 0.38 (0.12) | 0.57 (0.06) | 0.14 (0.17) |
| 2 | 0.32 (0.03) | 0.23 (0.06) | 0.41 (0.08) | 0.34 (0.11) | NA | NA |
| 3 | 0.37 (0.02) | 0.27 (0.06) | 0.45 (0.08) | 0.40 (0.11) | 0.53 (0.06) | NA |
| 4 | 0.62 (0.03) | 0.47 (0.08) | 0.55 (0.08) | 0.31 (0.11) | 0.53 (0.05) | 0.14 (0.15) |
| 5 | 0.71 (0.03) | 0.44 (0.07) | 0.55 (0.08) | 0.32 (0.11) | 0.57 (0.05) | 0.41 (0.13) |
Definition of abbreviation: NA = not available.
Values are κ scores (SE).
Reprinted from Ref. [69] with permission of the American Thoracic Society. Copyright © 2014 American Thoracic Society.
Early referral
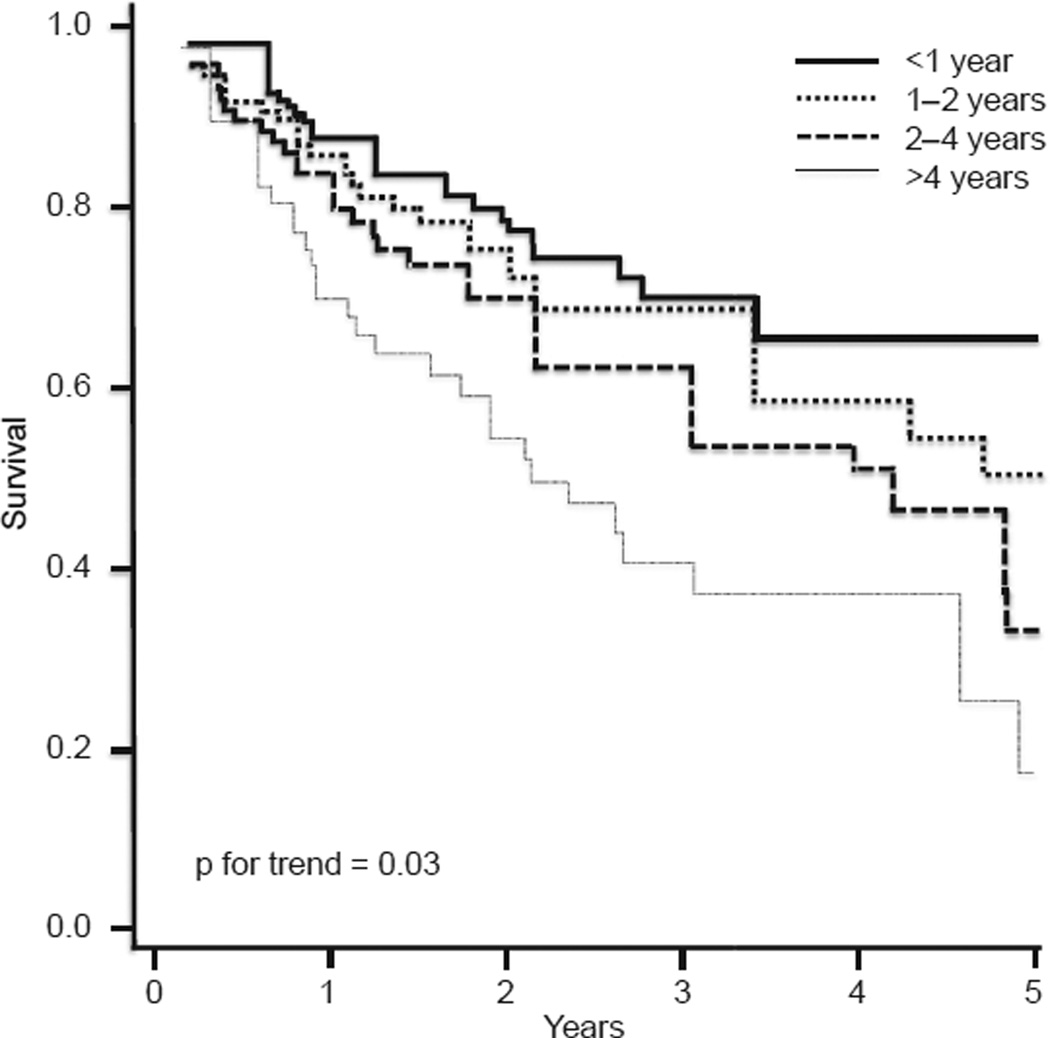
We recommend early referral to an ILD specialty center when MDE cannot be performed locally, or when diagnostic doubt exists following community MDE. Referral has the added advantage of providing patients access to specialized care, including clinical trial enrollment and lung transplant evaluation. Whether referral of patients to an ILD center improves outcomes is unknown, however, and remains an area ripe for further investigation. In 2011, Lamas and colleagues [72] conducted an investigation of 129 patients with IPF referred to a single ILD center to explore the association between time to referral and mortality. The authors showed that the median time from patient-reported onset of dyspnea to ILD center evaluation was 2.2 years and that a longer delay was associated with increased risk of death independent of disease severity (Fig. 4). Determining the cause of this observed difference was not possible by nature of the study design and warrants further investigation. Notably, referral delay did not affect the likelihood of receiving a lung transplant. The authors acknowledged that lead-time bias was a potential limitation to this study, but noted that age and disease severity were similar across quartiles and that adjusting for these potential confounders did not significantly alter their results.
Figure 4.
Survival from the time of evaluation at a tertiary care center adjusted for age and FVC across quartiles of delay. Entry time into the cohort began at study enrollment. Reprinted from Ref. [72] with permission of the American Thoracic Society. Copyright © 2014 American Thoracic Society.
When referral cannot be made due to inconvenience or other factors, local clinicians should screen for comorbidities and supplemental oxygen need, along with placing a referral to pulmonary rehabilitation (PR) for patients with IPF. In those who are referred, ILD centers provide additional benefits beyond routine care and therapeutics, including disease education and support group information. Several of these factors were cited among patients with IPF in a recent qualitative survey as reason for greater satisfaction when receiving care at an ILD center compared to a community setting [15]. A review of the core elements of IPF management at ILD referral centers is outlined below.
Comorbidity management
Patients with IPF are at increased risk for a host of comorbidities, including pulmonary malignancy [73], coronary artery disease [74], obstructive sleep apnea (OSA) [75], emphysema [76], pulmonary hypertension [77], diabetes mellitus [22], depression [78], hiatal hernia [26], and GERD [24]. These conditions have the potential to impact survival and quality of life, and it has therefore become common practice to manage comorbidities aggressively at many ILD centers. Though the effectiveness of this approach in modulating disease course is unknown, Lee and colleagues showed that the use of GERD therapy independently predicted longer survival in patients with IPF, while Mermigkis and colleagues demonstrated that patients treated for concurrent OSA reported improved quality of life. [79,80] An exception to this practice is in patients with IPF and pulmonary hypertension, as large clinical trials using pulmonary hypertension therapy have failed to show efficacy in patients with IPF [81,82]. Finally, it is important to prevent further morbidity by ensuring patients have received age-appropriate vaccinations, including those for influenza and pneumococcus.
Oxygen therapy
Hypoxemia at rest, with sleep and/or with exertion is common in patients with IPF. Though treatment of resting hypoxemia with supplemental oxygen has not been studied in a controlled fashion, the practice received a strong recommendation in the 2011 ATS/ERS/JRS/ALAT treatment guidelines [4]. This was partly due to robust evidence in patients with chronic obstructive lung disease, where a clear survival benefit was demonstrated with the use of long-term oxygen [83]. The need for supplemental oxygen when traveling by air or to altitude should also be assessed in this population.
Pulmonary rehabilitation
A mainstay of non-pharmacologic therapy for patients with IPF is PR. A systematic review performed by Holland and Hill showed that exercise training in patients with ILD, including IPF, was associated with an improvement in 6-min walk distance and increased quality of life scores [84]. Many PR programs also include educational components that teach breathing techniques and dyspnea coping strategies. As the benefits of PR appear to be most pronounced early in the disease course, this therapy should be offered to all patients at the time of diagnosis [85]. In patients who travel a considerable distance for evaluation, identifying a PR program close to home can add convenience and potentially improve adherence.
Pharmacotherapy
Unlike many other forms of ILD and IIP, IPF does not respond to, and should not be treated with, corticosteroid therapy [4,34,86]. A similar conclusion has now been reached with combination prednisone, azathioprine and N-acetylcysteine (NAC) after early termination of this arm in the PANTHER-IPF trial due to increased mortality and hospitalization risk compared to placebo [68]. The NAC and placebo arms of this trial are ongoing to investigate whether NAC leads to a smaller decline in lung function in patients with IPF, as suggested by previous studies [87,88].
The use of pirfenidone, an oral antifibrotic agent, in the treatment of IPF has been evaluated in two Japanese and two combined North American and European phase III trials [89–91]. These trials produced discordant results, but a meta-analysis showed that pirfenidone might slow disease progression and rate of FVC decline. [92] These results have supported the approval of pirfenidone in several countries and regions, including the European Union, Canada, India and Japan. The US Food and Drug Administration has yet to approve pirfenidone, pending the results of an ongoing large phase III U.S. multicenter clinical trial (ASCEND trial, NCT0136629). Dermatologic (photosensitivity and rash), neurologic (dizziness and fatigue) and gastro-intestinal (nausea, dyspepsia, diarrhea and anorexia) side effects have been reported with pirfenidone and should be routinely addressed [90,93].
While antifibrotic agents hold promise in treating IPF, the recently published ACE-IPF study has likely put to rest the question of warfarin therapy for IPF. This trial stemmed from a small open-label study showing mortality benefit in patients treated with anticoagulation in addition to corti-costeroids [94], but was terminated early due to a low probability of benefit and increased mortality in the warfarin arm compared to placebo [95]. Some authors argue that biologic plausibility still supports targeting the coagulation cascade, and that heparins, including inhaled heparin, may be a better choice of anticoagulant and warrant further investigation [96,97].
Numerous other phase II and III clinical trials are ongoing or recently completed and may provide additional treatment options in the near future. Information regarding clinical trial enrollment is provided to patients who qualify upon evaluation at ILD referral centers. While the decision to enroll in an IPF clinical trial must be weighed heavily by patient and physician, clinical trial availability has been shown to improve patient satisfaction [15], and should be available to as many patients as possible. Diagnostic and referral delays can result in clinical trial ineligibility due to advanced disease, underscoring the importance of active community physician involvement in this process.
Lung transplantation
Lung transplantation remains the sole therapy proven to prolong survival in IPF [98]. The first lung transplant for IPF was performed in 1983 [99], and IPF is now the leading cause of lung transplantation in the US, accounting for 38% of adult lung transplants in 2012 [100]. Following the introduction of the Lung Allocation Score (LAS), which prioritizes transplant candidates based on their probability of survival, there has been a drastic decrease in wait list time for patients with IPF, from a median of 997 days in 2002 to 2004 to 73 days in May 2005 to December 2007 [101]. Both single (SLT) and double lung transplants (DLT) are performed for IPF and while SLT is performed more commonly, the number of DLT procedures continues to rise. [102] This area remains controversial as studies show discordant results in long-term survival advantage between the procedures [103–107]. International societies including the American Thoracic Society and International Society for Heart and Lung Transplantation (ISHLT) strongly recommend early consideration of lung transplantation for patients with IPF [4,108].
Conclusion
Our understanding of IPF has grown immensely over the last two decades, yet the diagnostic process remains complex and misdiagnosis carries grave implications. Attention to common signs and symptoms, along with proper utilization of diagnostic tests can help diagnose IPF early in the disease course. A multidisciplinary approach in both academic and community settings is equally important in establishing the diagnosis. Referral to an ILD center should be considered in all patients with IPF to assist with diagnosis and/or provide access to the specialized care outlined above. Patients deserve the opportunity to participate in clinical trials and to be considered for lung transplant before advanced disease precludes eligibility, and underscores the importance of active clinician involvement in this process.
Acknowledgments
We thank Dr. Aliya Husain who contributed pathology images for this review. Medical writing assistance, supported financially by Boehringer Ingelheim Pharmaceuticals, Inc, was provided by Clare Ryles and Wendy Morris of Fleishman-Hillard Group, Ltd during the preparation of this manuscript. The authors drafted the article, were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version of the review, which solely reflects the authors’ interpretation and conclusions.
Footnotes
Conflict of interest statement
Dr. Noth has received honoraria for advisory boards with Boehringer Ingelheim, InterMune, Anthera within the last 12 months related to IPF. He has also received speaking honoraria from GSK and receives consulting fees for Immuneworks. He also has study contracts with the NIH, Stromedix, Sanofi, and Boehringer Ingelheim for the conduct of clinical trials in IPF. Boehringer Ingelheim Pharmaceuticals, Inc. paid the processing charge for this article and was allowed to review the manuscript for factual accuracy with no editorial input.
References
- 1.King TE., Jr Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med. 2005;172:268–279. doi: 10.1164/rccm.200503-483OE. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- 3.Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. doi: 10.1164/ajrccm.157.1.9704130. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vries J, Kessels BL, Drent M. Quality of life of idiopathic pulmonary fibrosis patients. Eur Respir J. 2001;17:954–961. doi: 10.1183/09031936.01.17509540. [DOI] [PubMed] [Google Scholar]
- 6.Tomioka H, Imanaka K, Hashimoto K, Iwasaki H. Health-related quality of life in patients with idiopathic pulmonary fibrosis-cross-sectional and longitudinal study. Intern Med. 2007;46:1533–1542. doi: 10.2169/internalmedicine.46.6218. [DOI] [PubMed] [Google Scholar]
- 7.Nathan SD, Shlobin OA, Weir N, et al. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest. 2011;140:221–229. doi: 10.1378/chest.10-2572. [DOI] [PubMed] [Google Scholar]
- 8.Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012;21:355–361. doi: 10.1183/09059180.00002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez Perez ER, Daniels CE, Schroeder DR, et al. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest. 2010;137:129–137. doi: 10.1378/chest.09-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 11.Gribbin J, Hubbard RB, Le Jeune I, Smith CJ, West J, Tata LJ. Incidence and mortality of idiopathic pulmonary fibrosis and sarcoidosis in the UK. Thorax. 2006;61:980–985. doi: 10.1136/thx.2006.062836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navaratnam V, Fleming KM, West J, et al. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax. 2011;66:462–467. doi: 10.1136/thx.2010.148031. [DOI] [PubMed] [Google Scholar]
- 13.Mapel DW, Hunt WC, Utton R, Baumgartner KB, Samet JM, Coultas DB. Idiopathic pulmonary fibrosis: survival in population based and hospital based cohorts. Thorax. 1998;53:469–476. doi: 10.1136/thx.53.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghu G. Idiopathic pulmonary fibrosis: guidelines for diagnosis and clinical management have advanced from consensus-based in 2000 to evidence-based in 2011. Eur Respir J. 2011;37:743–746. doi: 10.1183/09031936.00017711. [DOI] [PubMed] [Google Scholar]
- 15.Schoenheit G, Becattelli I, Cohen AH. Living with idiopathic pulmonary fibrosis: an in-depth qualitative survey of European patients. Chron Respir Dis. 2011;8:225–231. doi: 10.1177/1479972311416382. [DOI] [PubMed] [Google Scholar]
- 16.Collard HR, Tino G, Noble PW, et al. Patient experiences with pulmonary fibrosis. Respir Med. 2007;101:1350–1354. doi: 10.1016/j.rmed.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 17.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 18.Crystal RG, Fulmer JD, Roberts WC, Moss ML, Line BR, Reynolds HY. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976;85:769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- 19.Fioret D, Perez RL, Roman J. Management of idiopathic pulmonary fibrosis. Am J Med Sci. 2011;341:450–453. doi: 10.1097/MAJ.0b013e31821fbdbc. [DOI] [PubMed] [Google Scholar]
- 20.Key AL, Holt K, Hamilton A, Smith JA, Earis JE. Objective cough frequency in idiopathic pulmonary fibrosis. Cough. 2010;6:4. doi: 10.1186/1745-9974-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 22.Enomoto T, Usuki J, Azuma A, Nakagawa T, Kudoh S. Diabetes mellitus may increase risk for idiopathic pulmonary fibrosis. Chest. 2003;123:2007–2011. doi: 10.1378/chest.123.6.2007. [DOI] [PubMed] [Google Scholar]
- 23.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc. 2006;3:293–298. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- 24.Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 25.Tobin RW, Pope CE, 2nd, Pellegrini CA, Emond MJ, Sillery J, Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158:1804–1808. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- 26.Noth I, Zangan SM, Soares RV, et al. Prevalence of hiatal hernia by blinded multidetector CT in patients with idio-pathic pulmonary fibrosis. Eur Respir J. 2012;39:344–351. doi: 10.1183/09031936.00099910. [DOI] [PubMed] [Google Scholar]
- 27.Awadalla NJ, Hegazy A, Elmetwally RA, Wahby I. Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Egypt: a multicenter case-control study. Int J Occup Environ Med. 2012;3:107–116. [PubMed] [Google Scholar]
- 28.Iwai K, Mori T, Yamada N, Yamaguchi M, Hosoda Y. Idiopathic pulmonary fibrosis. Epidemiologic approaches to occupational exposure. Am J Respir Crit Care Med. 1994;150:670–675. doi: 10.1164/ajrccm.150.3.8087336. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard R, Lewis S, Richards K, Johnston I, Britton J. Occupational exposure to metal or wood dust and aetiology of cryptogenic fibrosing alveolitis. Lancet. 1996;347:284–289. doi: 10.1016/s0140-6736(96)90465-1. [DOI] [PubMed] [Google Scholar]
- 30.Miyake Y, Sasaki S, Yokoyama T, et al. Occupational and environmental factors and idiopathic pulmonary fibrosis in Japan. Ann Occup Hyg. 2005;49:259–265. doi: 10.1093/annhyg/meh090. [DOI] [PubMed] [Google Scholar]
- 31.Hodgson U, Laitinen T, Tukiainen P. Nationwide prevalence of sporadic and familial idiopathic pulmonary fibrosis: evidence of founder effect among multiplex families in Finland. Thorax. 2002;57:338–342. doi: 10.1136/thorax.57.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Sancho C, Buendia-Roldan I, Fernandez-Plata MR, et al. Familial pulmonary fibrosis is the strongest risk factor for idiopathic pulmonary fibrosis. Respir Med. 2011;105:1902–1907. doi: 10.1016/j.rmed.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 33.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 34.Douglas WW, Ryu JH, Schroeder DR. Idiopathic pulmonary fibrosis: impact of oxygen and colchicine, prednisone, or no therapy on survival. Am J Respir Crit Care Med. 2000;161:1172–1178. doi: 10.1164/ajrccm.161.4.9907002. [DOI] [PubMed] [Google Scholar]
- 35.Cottin V, Cordier J-F. Subclinical interstitial lung disease: no place for crackles? Am J Respir Crit Care Med. 2012;186:289. doi: 10.1164/ajrccm.186.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cottin V, Cordier JF. Velcro crackles: the key for early diagnosis of idiopathic pulmonary fibrosis? Eur Respir J. 2012;40:519–521. doi: 10.1183/09031936.00001612. [DOI] [PubMed] [Google Scholar]
- 37.Johnston ID, Prescott RJ, Chalmers JC, Rudd RM. British Thoracic Society study of cryptogenic fibrosing alveolitis: current presentation and initial management. Fibrosing Alveolitis Subcommittee of the Research Committee of the British Thoracic Society. Thorax. 1997;52:38–44. doi: 10.1136/thx.52.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JS, Kim EJ, Lynch KL, et al. Prevalence and clinical significance of circulating autoantibodies in idiopathic pulmonary fibrosis. Respir Med. 2013;107:249–255. doi: 10.1016/j.rmed.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama A, Kondo K, Nakajima M, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology. 2006;11:164–168. doi: 10.1111/j.1440-1843.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 41.Herazo-Maya JD, Noth I, Duncan SR, et al. Peripheral blood mononuclear cell gene expression profiles predict poor outcome in idiopathic pulmonary fibrosis. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005964. 205ra136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King TE, Jr, Schwarz MI, Brown K, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt SL, Tayob N, Han MK, et al. Predicting pulmonary fibrosis disease course from past trends in pulmonary function. Chest March. 2014;145(3):579–585. doi: 10.1378/chest.13-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akagi T, Matsumoto T, Harada T, et al. Coexistent emphysema delays the decrease of vital capacity in idiopathic pulmonary fibrosis. Respir Med. 2009;103:1209–1215. doi: 10.1016/j.rmed.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Aduen JF, Zisman DA, Mobin SI, et al. Retrospective study of pulmonary function tests in patients presenting with isolated reduction in single-breath diffusion capacity: implications for the diagnosis of combined obstructive and restrictive lung disease. Mayo Clin Proc. 2007;82:48–54. doi: 10.4065/82.1.48. [DOI] [PubMed] [Google Scholar]
- 46.Latsi PI, du Bois RM, Nicholson AG, et al. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–537. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 47.Richeldi L, Ryerson CJ, Lee JS, et al. Relative versus absolute change in forced vital capacity in idiopathic pulmonary fibrosis. Thorax. 2012;67:407–411. doi: 10.1136/thoraxjnl-2011-201184. [DOI] [PubMed] [Google Scholar]
- 48.Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:830–836. doi: 10.1183/09031936.00155108. [DOI] [PubMed] [Google Scholar]
- 49.Collard HR, King TE, Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 50.du Bois RM, Weycker D, Albera C, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183:1231–1237. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 51.Guerry-Force ML, Muller NL, Wright JL, et al. A comparison of bronchiolitis obliterans with organizing pneumonia, usual interstitial pneumonia, and small airways disease. Am Rev Respir Dis. 1987;135:705–712. doi: 10.1164/arrd.1987.135.3.705. [DOI] [PubMed] [Google Scholar]
- 52.Muller NL, Guerry-Force ML, Staples CA, et al. Differential diagnosis of bronchiolitis obliterans with organizing pneumonia and usual interstitial pneumonia: clinical, functional, and radiologic findings. Radiology. 1987;162:151–156. doi: 10.1148/radiology.162.1.3786754. [DOI] [PubMed] [Google Scholar]
- 53.Mathieson JR, Mayo JR, Staples CA, Muller NL. Chronic diffuse infiltrative lung disease: comparison of diagnostic accuracy of CT and chest radiography. Radiology. 1989;171:111–116. doi: 10.1148/radiology.171.1.2928513. [DOI] [PubMed] [Google Scholar]
- 54.Epler GR, McLoud TC, Gaensler EA, Mikus JP, Carrington CB. Normal chest roentgenograms in chronic diffuse infiltrative lung disease. N Engl J Med. 1978;298:934–939. doi: 10.1056/NEJM197804272981703. [DOI] [PubMed] [Google Scholar]
- 55.Sumikawa H, Johkoh T, Colby TV, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med. 2008;177:433–439. doi: 10.1164/rccm.200611-1696OC. [DOI] [PubMed] [Google Scholar]
- 56.Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax. 2003;58:143–148. doi: 10.1136/thorax.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raghu G, Mageto YN, Lockhart D, Schmidt RA, Wood DE, Godwin JD. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest. 1999;116:1168–1174. doi: 10.1378/chest.116.5.1168. [DOI] [PubMed] [Google Scholar]
- 58.Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 59.Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164:193–196. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]
- 60.ACR practice guideline for the performance of high-resolution computed tomography (HRCT) of the lungs in adults. 2010 at, http://www.acr.org/~/media/ACR/Documents/PGTS/guidelines/HRCT_Lungs.pdf.
- 61.Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 62.Park JH, Kim DK, Kim DS, et al. Mortality and risk factors for surgical lung biopsy in patients with idiopathic interstitial pneumonia. Eur J Cardiothorac Surg. 2007;31:1115–1119. doi: 10.1016/j.ejcts.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 63.Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J. 2001;17:175–179. doi: 10.1183/09031936.01.17201750. [DOI] [PubMed] [Google Scholar]
- 64.Luo Q, Han Q, Chen X, Xie J, Wu L, Chen R. The diagnosis efficacy and safety of video-assisted thoracoscopy surgery (VATS) in undefined interstitial lung diseases: a retrospective study. J Thorac Dis. 2013;5:283–288. doi: 10.3978/j.issn.2072-1439.2013.04.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164:1722–1727. doi: 10.1164/ajrccm.164.9.2103074. [DOI] [PubMed] [Google Scholar]
- 66.Katzenstein AL, Mukhopadhyay S, Myers JL. Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases. Hum Pathol. 2008;39:1275–1294. doi: 10.1016/j.humpath.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 67.Flaherty KR, Toews GB, Travis WD, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J. 2002;19:275–283. doi: 10.1183/09031936.02.00182002. [DOI] [PubMed] [Google Scholar]
- 68.Raghu G, Anstrom KJ, King TE, Jr, Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:322–329. doi: 10.1513/pats.200602-019TK. [DOI] [PubMed] [Google Scholar]
- 70.Flaherty KR, King TE, Jr, Raghu G, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170:904–910. doi: 10.1164/rccm.200402-147OC. [DOI] [PubMed] [Google Scholar]
- 71.Flaherty KR, Andrei AC, King TE, Jr, et al. Idiopathic interstitial pneumonia: do community and academic physicians agree on diagnosis? Am J Respir Crit Care Med. 2007;175:1054–1060. doi: 10.1164/rccm.200606-833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lamas DJ, Kawut SM, Bagiella E, Philip N, Arcasoy SM, Lederer DJ. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. Am J Respir Crit Care Med. 2011;184:842–847. doi: 10.1164/rccm.201104-0668OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma Y, Seneviratne CK, Koss M. Idiopathic pulmonary fibrosis and malignancy. Curr Opin Pulm Med. 2001;7:278–282. doi: 10.1097/00063198-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 74.Nathan SD, Basavaraj A, Reichner C, et al. Prevalence and impact of coronary artery disease in idiopathic pulmonary fibrosis. Respir Med. 2010;104:1035–1041. doi: 10.1016/j.rmed.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Lancaster LH, Mason WR, Parnell JA, et al. Obstructive sleep apnea is common in idiopathic pulmonary fibrosis. Chest. 2009;136:772–778. doi: 10.1378/chest.08-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cottin V, Nunes H, Brillet PY, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26:586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 77.Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30:715–721. doi: 10.1183/09031936.00107206. [DOI] [PubMed] [Google Scholar]
- 78.Akhtar AA, Ali MA, Smith RP. Depression in patients with idiopathic pulmonary fibrosis. Chron Respir Dis. 2013;10:127–133. doi: 10.1177/1479972313493098. [DOI] [PubMed] [Google Scholar]
- 79.Lee JS, Ryu JH, Elicker BM, et al. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1390–1394. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mermigkis C, Bouloukaki I, Antoniou KM, et al. CPAP therapy in patients with idiopathic pulmonary fibrosis and obstructive sleep apnea: does it offer a better quality of life and sleep? Sleep Breath. 2013;17:1137–1143. doi: 10.1007/s11325-013-0813-8. [DOI] [PubMed] [Google Scholar]
- 81.Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, Hunninghake GW. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.King TE, Jr, Brown KK, Raghu G, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 83.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 84.Holland A, Hill C. Physical training for interstitial lung disease. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD006322.pub2. CD006322. [DOI] [PubMed] [Google Scholar]
- 85.Holland AE, Hill CJ, Glaspole I, Goh N, McDonald CF. Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir Med. 2012;106:429–435. doi: 10.1016/j.rmed.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 86.Nagai S, Kitaichi M, Hamada K, et al. Hospital-based historical cohort study of 234 histologically proven Japanese patients with IPF. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:209–214. [PubMed] [Google Scholar]
- 87.Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 88.Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med. 1997;156:1897–1901. doi: 10.1164/ajrccm.156.6.9706065. [DOI] [PubMed] [Google Scholar]
- 89.Taniguchi H, Ebina M, Kondoh Y, et al. Pirfenidone in idio-pathic pulmonary fibrosis. Eur Respir J. 2010;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 90.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 91.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 92.Spagnolo P, Del Giovane C, Luppi F, et al. Non-steroid agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD003134.pub2. CD003134. [DOI] [PubMed] [Google Scholar]
- 93.Jiang C, Huang H, Liu J, Wang Y, Lu Z, Xu Z. Adverse events of pirfenidone for the treatment of pulmonary fibrosis: a meta-analysis of randomized controlled trials. PLoS One. 2012;7:e47024. doi: 10.1371/journal.pone.0047024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kubo H, Nakayama K, Yanai M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128:1475–1482. doi: 10.1378/chest.128.3.1475. [DOI] [PubMed] [Google Scholar]
- 95.Noth I, Anstrom KJ, Calvert SB, et al. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:88–95. doi: 10.1164/rccm.201202-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bendstrup KE, Gram J, Jensen JI. Effect of inhaled heparin on lung function and coagulation in healthy volunteers. Eur Respir J. 2002;19:606–610. doi: 10.1183/09031936.02.00105202. [DOI] [PubMed] [Google Scholar]
- 97.Bendstrup E, Hilberg O. Is warfarin the right anticoagulant in idiopathic pulmonary fibrosis? Am J Respir Crit Care Med. 2012;186:693. doi: 10.1164/ajrccm.186.7.693a. author reply −4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thabut G, Mal H, Castier Y, et al. Survival benefit of lung transplantation for patients with idiopathic pulmonary fibrosis. J Thorac Cardiovasc Surg. 2003;126:469–475. doi: 10.1016/s0022-5223(03)00600-7. [DOI] [PubMed] [Google Scholar]
- 99.Unilateral lung transplantation for pulmonary fibrosis. Toronto Lung Transplant Group. N Engl J Med. 1986;314:1140–1145. doi: 10.1056/NEJM198605013141802. [DOI] [PubMed] [Google Scholar]
- 100.Organ Procurement and Transplantation Network (OPTN) 2012 at http://optn.transplant.hrsa.gov.
- 101.Nathan SD, Shlobin OA, Ahmad S, Burton NA, Barnett SD, Edwards E. Comparison of wait times and mortality for idiopathic pulmonary fibrosis patients listed for single or bilateral lung transplantation. J Heart Lung Transplant. 2010;29:1165–1171. doi: 10.1016/j.healun.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 102.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31:1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 103.Thabut G, Christie JD, Ravaud P, et al. Survival after bilateral versus single-lung transplantation for idiopathic pulmonary fibrosis. Ann Intern Med. 2009;151:767–774. doi: 10.7326/0003-4819-151-11-200912010-00004. [DOI] [PubMed] [Google Scholar]
- 104.Mason DP, Brizzio ME, Alster JM, et al. Lung transplantation for idiopathic pulmonary fibrosis. Ann Thorac Surg. 2007;84:1121–1128. doi: 10.1016/j.athoracsur.2007.04.096. [DOI] [PubMed] [Google Scholar]
- 105.De Oliveira NC, Osaki S, Maloney J, Cornwell RD, Meyer KC. Lung transplant for interstitial lung disease: outcomes for single versus bilateral lung transplantation. Interact Car-diovasc Thorac Surg. 2012;14:263–267. doi: 10.1093/icvts/ivr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.George TJ, Arnaoutakis GJ, Shah AS. Lung transplant in idiopathic pulmonary fibrosis. Arch Surg. 2011;146:1204–1209. doi: 10.1001/archsurg.2011.239. [DOI] [PubMed] [Google Scholar]
- 107.Keating D, Levvey B, Kotsimbos T, et al. Lung transplantation in pulmonary fibrosis: challenging early outcomes counterbalanced by surprisingly good outcomes beyond 15 years. Transplant Proc. 2009;41:289–291. doi: 10.1016/j.transproceed.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 108.Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update-a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25:745–755. doi: 10.1016/j.healun.2006.03.011. [DOI] [PubMed] [Google Scholar]