Abstract
Perinatal hypoxic-ischemic encephalopathy (HIE) is associated with high neonatal mortality and severe long-term neurologic morbidity. Yet the mechanisms of brain injury in infants with HIE remain largely elusive. The present study determined a novel mechanism of microRNA-210 (miR-210) in silencing endogenous neuroprotection and increasing hypoxicischemic brain injury in neonatal rats. The study further revealed a potential therapeutic effect of miR-210 inhibition using complementary locked nucleic acid oligonucleotides (miR-210-LNA) in 10-day-old neonatal rats in the Rice-Vannucci model. The underlying mechanisms were investigated with intracerebroventricular injection (i.c.v) of miR-210 mimic, miR-210-LNA, glucocorticoid receptor (GR) agonist and antagonist. Luciferase reporter gene assay was conducted for identification of miR-210 targeting GR 3’untranslated region. The results showed that the HI treatment significantly increased miR-210 levels in the brain, and miR-210 mimic significantly decreased GR protein abundance and exacerbated HI brain injury in the pups. MiR-210-LNA administration via i.c.v. 4 hours after the HI insult significantly decreased brain miR-210 levels, increased GR protein abundance, reduced HI-induced neuronal death and brain infarct size, and improved long-term neurological function recovery. Of importance, the intranasal delivery of miR-210-LNA 4 hours after the HI insult produced similar effects in decreasing HI-induced neonatal brain injury and improving neurological function later in life. Altogether, the present study provides evidence of a novel mechanism of miR-210 in a neonatal HI brain injury model, and suggests a potential therapeutic approach of miR-210 inhibition in the treatment of neonatal HIE.
Keywords: Neonatal hypoxic-ischemic brain injury, microRNA-210, glucocorticoid receptor, complementary locked nucleic acid (LNA) oligonucleotides, intranasal delivery, neuroprotection
1. Introduction
Perinatal hypoxic-ischemic encephalopathy (HIE) is associated with high neonatal mortality and severe long-term neurologic morbidity (Fernandez-Lopez et al., 2014; Li et al., 2012; Ma and Zhang, 2015; Verklan, 2009; Yager and Ashwal, 2009). The molecular mechanisms and the pathway of brain injury in infants with HIE remain largely elusive. Although therapeutic hypothermia is the current standard of care for newborns with moderate to severe HIE, nearly half of affected infants treated with hypothermia still die or suffer significant neurologic disability (Azzopardi et al., 2009; Higgins et al., 2011; Jacobs et al., 2011; Shankaran et al., 2005). Thus, there is an urgent need to investigate further the underlying mechanisms and to develop additional treatment strategies.
Recently, growing evidence has revealed microRNA (miR) signatures in various neurological disorders including ischemic stroke (Moon et al., 2013) and epilepsy (Jimenez-Mateos et al., 2012). MiRs are non-coding RNAs with about 21-22 nucleotides in length, which bind to 3’-untranslated region (3’UTR) of target mRNAs, leading to target transcript degradation and/or translational suppression (Kosik and Krichevsky, 2005; Ma and Zhang, 2015). Many of miRs are enriched in the nervous system and play key roles in the brain developmental plasticity (Kosik, 2006). Among them, miR-210 is The Master Hypoxamir of a specific group of miRs termed “Hypoxamirs” that are regulated by hypoxia (Chan et al., 2012). MiR-210 has been implicated playing an important role in multiple cellular processes, including angiogenesis (Fasanaro et al., 2008), mitochondrial metabolism (Chan et al., 2009) and apoptotic cell death (Chio et al., 2013). Yet, the functional significance of miR-210 in the pathophysiology of HI-induced brain injury in the developing brain remains elusive.
Herein, we present a novel finding that miR-210 targets the 3’UTR of glucocorticoid receptor (GR) transcript and down-regulates GR protein abundance in the neonatal brain in response to the HI insult, resulting in the increased brain susceptibility to HI injury in neonatal rats. Of critical importance, we demonstrate that the inhibition of miR-210 with itscomplementary locked nucleic acid (miR-210-LNA) administered through intracerebroventricular injection (i.c.v.) or intranasal delivery 4 hours after the HI insult significantly decreases brain miR-210 levels, reduces brain infarct size and improves long-term neurological function recovery, suggesting a novel therapeutic approach of miR-210 inhibition in the treatment of HIE in a neonatal rat model.
2. Material and methods
2.1. Hypoxic-ischemic (HI) treatment of rat pups
A modified Rice-Vannucci model was generated as described previously (Rice et al., 1981). Briefly, P10 rat pups (Charles River Laboratories, Portage, MI) were anesthetized with inhalation of 2-3% isoflurane. Right common carotid artery (CCA) was exposed, double ligated with a 5.0 silk surgical suture and then cut between two ligation sites. After surgery, pups were recuperated for 1 hour, and then placed in a hypoxic incubator containing humidified 8% oxygen balanced with 92% nitrogen for indicated time at 37°C. Pups were returned to their dams for recovery after the hypoxic treatment. For the sham treatment, CCA was exposed but without ligation and the hypoxic treatment. All procedures and protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Intracerebroventricular injection (i.c.v)
MiR-210 mimic, miR-210 scramble control (Qiagen), miR-210-LNA and LNA scramble control (Exiqon) were prepared according to manufacturer's instructions. A GR agonist, dexamethasone (DEX) (Sigma-Aldrich) was prepared in saline. A GR antagonist, RU486 (Tocris) was prepared in 10% ethanol. Drugs with the total volume of 2 μl were administered into the ipsilateral hemisphere of rat pups via i.c.v. (2 mm posterior, 1.5 mm lateral, 3 mm below the skull surface), as described previously (Gonzalez-Rodriguez et al., 2014b). In experimental protocol #1, pups were divided into two groups: 1) miR-210 mimic (20 pmol), and 2) miR-210 scramble control (20 pmol). The HI treatment was performed 48 hours after the injection. In experimental protocol #2, pups were divided into 6 groups: 1) saline control, 2) vehicle control (10% ethanol), 3) DEX (10 ng), 4) DEX (10 ng)+RU486 (500 ng), 5) DEX (10 ng)+RU486 (1000 ng), and 6) RU486 (500 ng). Drugs were injected into the ipsilateral hemisphere 24 hours before the HI treatment. In experimental protocol #3, pups were divided into 4 groups: 1) miR-210-LNA (50 pmol), 2) LNA scramble (50 pmol), 3) LNA (50 pmol)+RU486 (500 ng), and 4) LNA (50 pmol)+RU486 (1000 ng). Drugs were injected into the ipsilateral hemisphere 24 hours before the HI treatment. In experimental protocol #4, pups were divided into 2 groups: 1) miR-210-LNA (50 pmol), and 2) LNA scramble control (50 pmol). Drugs were injected into the ipsilateral hemisphere 4 hours after the HI treatment.
2.3. Intranasal delivery
The intranasal delivery of miR-210-LNA was conducted 4 hours after the HI treatment. Pups were placed on their backs under light anesthesia with isoflurane (4% for induction and 2% for maintenance). After pups were sedated, miR-210-LNA (100 pmol; 200 pmol) or same dose of LNA scramble control in 5 μl saline were delivered into each naris using a fine tip. The pups were then maintained sedated with isoflurane for 2 minutes on their backs. All pups woke up within 1-2 min upon withdrawal of isoflurane and were returned to their dams.
2.4. Brain infarct size measurement
Brain infarct size was determined 48 hours after the HI treatment with 2, 3, 5-triphenyltetrazolium chloride monohydrate (TTC; Sigma-Aldrich) staining, as described previously (Gonzalez-Rodriguez et al., 2014b). Briefly, serial coronal slices of the pup brain (2-mm thickness) were cut and immersed into a 2% TTC solution for 5 minutes at 37°C and then fixed by 10% formaldehyde overnight. The caudal and the rostral surfaces of each slice were photographed, and the percentage of infarct area in the ipsilateral hemisphere for each slice was traced and analyzed by Image J software (NIH).
2.5. Assessment of cerebral cortical expansion
The degree of cortical cavitation was quantified by cortical width index as described previously (Zhao et al., 2006). Briefly, whole-brain images were captured using a digital camera. The distance from the midpoint of the forebrain to the edge of the cavitation and contralateral side was measured, and the ratio of ipsilateral width to contralateral width was defined as the cortical width index.
2.6. Real-time qRT-PCR for GR mRNA quantification
Total RNA was extracted using the TRIzol reagent (Invitrogen) and subjected to reverse transcription with Superscript III First-Strand Synthesis System (Invitrogen), following the manufacturer's instructions. The GR mRNA abundance was determined with real-time PCR using iQ SYBR Green Supermix (Bio-Rad) (Gonzalez-Rodriguez et al., 2014b). Primers included: GR, Forward: AGGTCTGAAGAGCCAAGAGTTA; Reverse: TGGAAGCAGTAGGTAAGGAGAT. Actin: Forward: TCAGGTCATCACTATCGGCAAT; Reverse: ACTGTGTTGGCATAGAGGTCTT. Real-time PCR was performed in a final volume of 25 μl and each PCR reaction mixture consisted of specific primers and iQ SYBR Green Supermix. Serial dilutions of the positive control were done on each plate to create a standard curve for the quantification. PCR was done in triplicate and threshold cycle numbers were averaged for each sample.
2.7. Real-time qRT-PCR for miR-210 quantification
MiR-210 levels were determined by miScript II RT kit (Qiagen) and miScript SYBR Green PCR kit with miScript Primer Assay kit (Qiagen) according to manufacturer's instructions. Primers included miScript Universal Primer, miR-210 miScript Primer Assay (Rn_miR-210_1; Cat#MS00000644; Qiagen) and SNORD61 miScript Primer Assay (Hs_SNORD61_11; Cat#MS00033705; Qiagen). Briefly, 1 μg of template RNA was mixed with reverse-transcription master mix in a final volume of 20 μl and incubated for 60 minutes at 37°C, and the reaction was stopped at 95°C. Two nanograms of template cDNA were used for miR-210 quantification in a final volume of 25 μl system containing specific primers and QuantiTect SYBR Green PCR master mix following manufacturer's instructions. Primers included miScript Universal Primer, miR-210 miScript Primer Assay and SNORD61 miScript Primer Assay (Qiagen). Serial dilutions of the positive control were done on each plate to create a standard curve for the quantification. PCR was done in triplicate and threshold cycle numbers were averaged for each sample.
2.8. GR 3′UTR cloning and reporter gene assay
As described previously (Dasgupta et al., 2012; Zhang et al., 2012), a 223-bp segment of 3’UTR of rat GR mRNA harboring the potential target region of mature miR-210 was PCR amplified from rat brain cDNA using the forward (5’-gagacccCTCGAGggctagacacccattttcaca) and the reverse (5’-gagacccTCTAGAgggctactactgcttctgttttg) primers, designed based on rat GR mRNA sequence (GENBANK accession #: M14053.1). The primers contained artificial XhoI (CTCGAG) and XbaI (TCTAGA) sites in forward and reverse primers respectively to facilitate cloning. Subsequently, XhoI-223bpGR-XbaI fragment was cloned between XhoI (5’) and XbaI (3’) sites in the pmirGLO luciferase vector (Promega) to generate pmirGLOXGRX reporter construct, which was used in reporter gene assay. For validation of the miR-210 target in rat GR 3’UTR, rat PC12 cells (ATCC) were transfected (500 ng/well) with empty pmirGLO (pmirGLO control vector), pmirGLOXGRX construct, pmirGLOXGRX plus 7 nM miR210-miScript miRNA mimic (miR-210) (Qiagen) or 7 nM scrambled miRNA mimic (S-miR210) (Qiagen) plus Attractene transfection reagent (Qiagen) following manufacturer's instructions. For inhibition experiments, in addition to pmirGLOXGRX plus miR-210 mimic, either 70 nM miR-210 inhibitor (Exiqon) or scrambled inhibitor (S-inhibitor) (Exiqon) was added during transfection complex formation as recommended by Qiagen. After 48 hours, the Firefly and Renilla reniformis luciferase activities in cell extracts were measured with the help of a luminometer using a dual-luciferase reporter assay system (Promega). The Firefly luciferase activity was normalized to Renilla reniformis luciferase activity and expressed as relative to control pmirGLO activity (% control), as described previously (Dasgupta et al., 2012).
2.9. Western blotting
Pups were euthanized at indicated time after the HI treatment. Brains were separated into ipsilateral and contralateral cerebrums and stored at −80°C immediately until analysis. Protein extraction was obtained by homogenization in RIPA lysis buffer (Santa Cruz Biotechnology) with further centrifugation at 14,000 g at 4°C for 30 minutes. Protein concentration was determined using a detergent compatible assay (Bio-Rad). Equal amounts of protein were loaded on an SDS-PAGE gel. After being electrophoresed and transferred to a nitrocellulose membrane, the membrane was blocked and incubated with the primary antibody of rabbit polyclonal anti-GR (Santa Cruz Biotechnology, 1:1000) overnight at 4°C. Nitrocellulose membranes were then incubated with secondary antibodies (Santa Cruz Biotechnology) for 1 hour at room temperature. Immunoblots were then probed with an ECL Plus chemiluminescence reagent kit (Amersham Biosciences) and visualized with the imagine system (Bio-Rad, Versa Doc, model 4000). The data were analyzed with the NIH Image J software. The values in the figures represent relative density of the bands normalized to β-actin.
2.10. Immunohistochemistry staining
Pups were transcardially perfused with 4% paraformaldehyde (PFA) in 0.1 M PBS (pH 7.4). Brains were post-fixed in 4% PFA overnight at 4°C, and then dehydrated with 30% sucrose in PBS. Samples were dissected and embedded into optimal cutting temperature compound (Tissue-Tek) on dry ice. The frozen coronal slices (10 μm thickness) were then sectioned using CM3050S cryostat (Leica Microsystems). Immunostaining with peroxidase-labelled streptavidin and DAB chromagen was carried out using a Vectastain Elite ABC system (Vector Laboratories) according to manufacturer's instructions. Brain slices were incubated with rabbit polyclonal anti-glucocorticoid receptor primary antibody (Novus, 1:50) overnight at 4 °C. Following incubation in biotinylated goat anti-rabbit secondary antibody (1:500) for 1 hour at room temperature, the slices were stained by DAB peroxidase substrate (Vector Laboratories) and counterstained using Hematoxylin QS (Vector Laboratories) to provide cytological detail. Images were captured using Zeiss bright field microscopy (Zeiss). The number of GR-positive cells was determined using the Image J software with color deconvolution plug-in and Cell Counter analysis tools (NIH), and was presented as a percentage of total cell number.
2.11. Immunofluorescence staining and confocal microscopy
After air dried and post-fixed in 4% PFA for 10 minutes at room temperature, the brain slices (10 μm) were blocked in 5% donkey serum (Jackson ImmunoResearch) containing 0.3% Triton X-100 (Sigma-Aldrich) for 1 hour at room temperature and then incubated with mouse monoclonal [BuGR2] anti-glucocorticoid receptor primary antibody (Abcam, 1:100) overnight at 4 °C. The brain slices were washed for 3 times and incubated with Alexa Fluor 647-conjugated donkey anti-mouse secondary antibody (Invitrogen, 1:200) for 1 h at room temperature. The nucleus was stained by Hoechst (Invitrogen). Brain slices were mounted and coverslipped using fluorescent mounting media (Dako). All slices were scanned with a Zeiss LSM 710 confocal microscopy (Zeiss). The number of GR-positive cells was determined using the Image J software with color deconvolution plug-in and Cell Counter analysis tools (NIH), and was presented as a percentage of total cell number. Low magnification images were gained using Image J stitching plug-in (Preibisch et al., 2009).
2.12. Terminal deoxynucleotidyltransferased UTP nick end labeling (TUNEL) assay
Cell death was detected using In Situ Cell Death Detection Kit, Fluorescein (Roche) following neuron staining. Brain slices were incubated with mouse anti-NeuN primary antibody (Millipore, 1:100) overnight at 4°C and then stained with donkey anti-mouse secondary antibody conjugated to Alexa Fluor 647 (Invitrogen) for 1 hour at room temperature. The TUNEL staining was completed according to manufacturer's instruction. Images were captured with a Zeiss LSM 710 confocal microscopy (Zeiss). For quantification of TUNEL-positive neuron cells, in each animal 4 randomly selected fields from the cortex or the hippocampus in the penumbra were analyzed in 3 nonadjacent sections (100 μm apart). Images were captured at 20 × magnification. NIH Image J software with nucleus counter particle analysis plug-in was used for cell counting. The TUNEL-positive neurons over the total number of neurons in selected field were calculated and present as a percentage.
2.13. Neurobehavioral assays
The following neurobehavioral tests were performed in rats 6 weeks after the HI treatment, as previously described (Hartman et al., 2012; Kamper et al., 2013): (i) Rotarod test for locomotor function evaluation: briefly, the rotarod (Columbus Instruments, Columbus, OH) consists of a horizontal cylinder (7 cm diameter) divided into four lanes. Three consecutive block trials were administered, in which the rotarod rotated at a constant speed of 5 RPM for 2 trials, followed by 2 trials of acceleration by 3 RPM every 5 seconds, and finally 2 trials of acceleration by 5 RPM every 3 seconds. Latency to fall was recorded as the time of walking on the cylinder. (ii) The water maze test consisted of a three day procedure including cued learning (day 1) and spatial learning (days 2 and 3), as well as probe trials for spatial memory evaluation (Kamper et al., 2013). Briefly, the animals were put into a metal pool filled with water. There was an escape platform, the surface of which was 1.5 cm above the water's surface (cued task), submerged 1.5 cm below the water's surface (spatial task) or removed (probe task). Each animal was allowed to find and climb onto the escape platform. Each animal was administered 10 trials (60 s max) per day in 5 blocks of 2 consecutive trials. Cumulative distance from the platform (measured 5× / s) and escape latency to find the platform in each test were recorded.
2.14. Statistics
Data were expressed as mean ± SEM. Experimental number (n) represents neonates from different dams. Comparisons between two groups were analyzed using Student's t test (unpaired, 2-tailed). Comparisons between multiple groups were analyzed using ANOVA or repeated-measures ANOVA analyses. Pearson correlation analysis was performed for regressions analysis of rotarod tests versus cortical width index. P value less than 0.05 was considered significant.
3. Results
3.1. MiR-210 increased neonatal HI brain injury
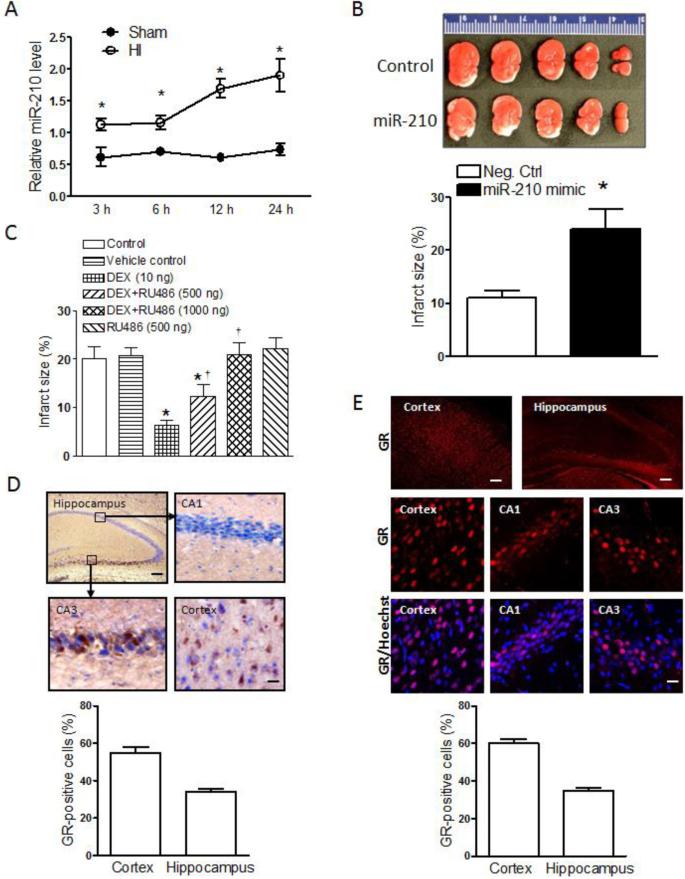
Brain HI insult was introduced in P10 rat pups with the ligation of right common carotid artery followed by 2.5 hours of hypoxic (8% O2) treatment. The HI treatment significantly increased miR-210 levels in the brain in a time-dependent manner (Fig. 1A). To determine the effect of miR-210 on HI brain injury in the pups, the miR-210 mimic or miR-210 negative control were delivered via i.c.v. into the ipsilateral hemisphere 48 hours before the mild HI treatment (1.5 hours of hypoxia, 8% O2). As shown in Figure 1B, the miR-210 mimic significantly exacerbated HI-induced brain injury by increasing approximate 2.5-fold in infarct size 48 hours after the HI treatment, as compared to the negative control.
Figure 1. Role of miR-210 and GR in neonatal HI brain injury.
(A) MiR-210 levels in the ipsilateral hemisphere in HI and Sham rat pups. n=4. * P<0.05, HI vs. Sham. (B) Pups received the miR-210 mimic or negative control (Neg. Ctrl) via i.c.v. 48 hours prior to the HI treatment (1.5 hours of hypoxia, 8% O2), and brain infarct size was determined 48 hours after HI. n=8. * P<0.05, miR-210 mimic vs. Neg. Ctrl. (C) Pups received the vehicle control, DEX, RU486, or DEX+RU486 via i.c.v. 24 hours prior to the HI treatment, and brain infarct size was determined 48 hours after HI treatment. n=6-7. * P<0.05, DEX or DEX+RU486 vs. control; † P<0.05 DEX+RU486 vs. DEX alone. (D) Representive immunohistochemical images and quantification of GR-positive cells (nine images per region, 3 sections/pup; n=4) in the cortex and hippocampus. Up-left, Scale bar = 100 μm; Others, Scale bar = 20 μm. (E) Representive immunofluorescence images and quantification of GR-positive cells (nine images per region, 3 sections/pup; n=4) in the cortex and hippocampus of rat pups. Up, Scale bar = 100 μm; Others, Scale bar = 20 μm.
3.2. GR mediated neuroprotection in neonatal HI brain injury
Our previous study demonstrated that DEX produced a neuroprotective effect in HI-induced brain injury in the pups (Gonzalez-Rodriguez et al., 2014a; Gonzalez-Rodriguez et al., 2014b). To determine whether this effect was mediated by GR, the DEX-mediated neuroprotection was investigated in the presence of a GR antagonist RU486. As shown in Fig. 1C, RU486 alone had no significant effect on HI-induced brain injury, but it dose-dependently inhibited the DEX-mediated neuroprotection, demonstrating a GR-mediated neuroprotective effect in HI-induced brain injury in the pups. The anatomical distribution of GR is rarely visualized in the neonatal brain. Using both immunohistochemistry (Fig. 1D) and immunofluorescence (Fig. 1E) staining, we found that the GR was highly expressed in the cortex and the CA3 region of the hippocampus close to dentate gyrus, with somewhat lowered expression in the CA1 region.
3.3. MiR-210 down-regulated GR expression in the neonatal brain
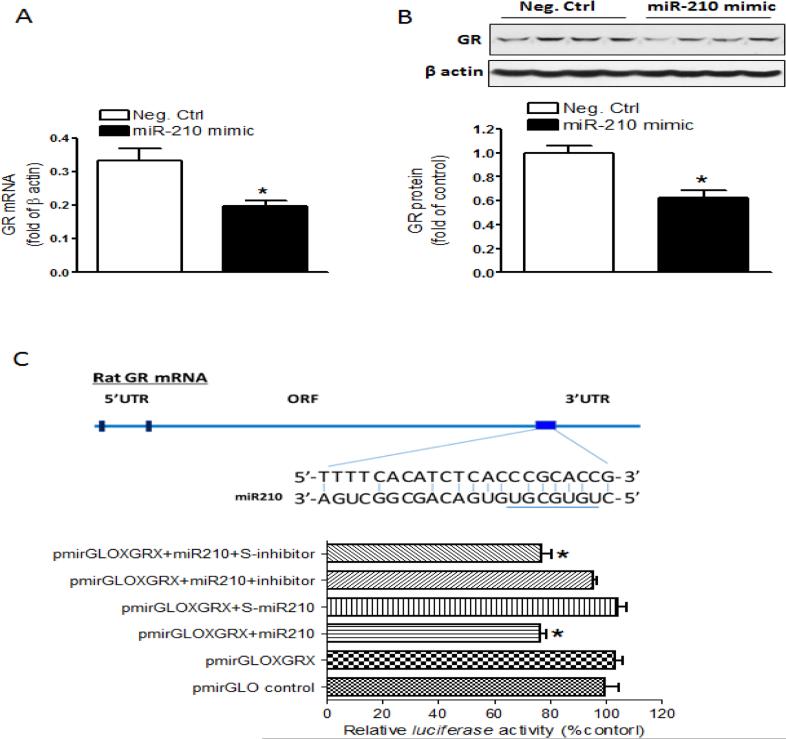
We then determined whether miR-210 down-regulated the GR expression in neonatal brains by i.c.v. administration of the miR-210 mimic or negative control. The results showed that the miR-210 mimic significantly decreased GR mRNA (Fig. 2A) and protein (Fig. 2B) abundance in the brain 48 hours after the treatment. MiR-210 has putative binding target sequences at the 3’UTR of GR transcript (Fig. 2C). We thus performed a luciferase assay to examine whether GR transcript was a direct target for miR-210. Using rat pheochromocytoma adherent variant PC12 cells transfected with pmirGLO-GR223 and treated with the miR-210 mimic, miR-210-LNA or the negative controls, we found that the miR-210 mimic, but not its negative control significantly decreased luciferase activity in the cells co-transfected with pmirGLO-GR223, which was blocked by miR-210-LNA (Fig. 2C).
Figure 2. MiR-210 down-regulated GR in the neonatal brain.
(A) GR mRNA and (B) protein abundance determined in the ipsilateral hemisphere 48 hours after miR-210 mimic or the negative control (Neg. Ctrl) via i.c.v. n=4. * P<0.05, miR-210 mimic vs. Neg. Ctrl. (C) Luciferase reporter gene assay of miR-210 targeting GR 3’UTR. The diagram shows GR mRNA 3’UTR with the binding sites of miR-210. The pmirGLO plasmid inserted with GR 3’UTR sequence containing putative miR-210 binding sites (pmirGLOXGRX) was transfected into PC12 cells, and was treated with miR-210 mimic, miR-210 scramble (S-miR-210), miR-210+miR-210 inhibitor, or miR-210+miR-210 inhibitor scramble (S-inhibitor). Firefly and Renilla reniformis luciferase activities were measured in a luminometer using a dual-luciferase reporter assay system. n=6. * P<0.05, treatmnet vs. pmirGLOXGRX alone.
3.4. MiR-210-LNA treatment produced neuroprotection in neonatal HI brain injury
Next, we examined whether inhibition of miR-210 provided a neuroprotective effect against HI-induced brain injury in pups. MiR-210-LNA or its negative control was administered via i.c.v. 24 hours prior to the HI treatment. As shown in Fig. 3A, miR-210-LNA significantly decreased HI-induced brain injury by reducing the infarct size, as compared to the negative control. Moreover, this neuroprotective effect of miR-210-LNA was dose-dependently reversed by RU486 (Fig. 3A), indicating a GR-dependent effect. To test the therapeutic potential of miR-210 inhibition in neonatal HI brain injury, miR-210-LNA or the negative control was administered into the brain via i.c.v. 4 hours after the HI insult. The results showed that miR-210-LNA significantly down-regulated brain miR-210 levels at 24 hours after the HI treatment, as compared to control groups (Fig. 3B), which was similar to effects reported previously for miR-LNA in the brain (Jimenez-Mateos et al., 2012). Of importance, MiR-210-LNA significantly decreased brain infarct size at 48 hours after the HI treatment, as compared to negative control (Fig. 3C). We further examined the effect of miR-210-LNA post-treatment on HI-induced neuronal death in the brain and found that miR-210-LNA significantly reduced TUNEL-positive neurons (NeuN) in the cortex from 48% to 26%, as well as and the hippocampus from 56% to 30%, in the penumbra area (Fig. 3D,E,F).
Figure 3. MiR-210-LNA decreased neuronal cell death and infarct size in neonatal HI brain injury.
Rat pups received via i.c.v. the negative control (Neg. Ctrl), miR-210-LNA (LNA), or LNA+RU486 24 hours prior to the HI treatment. (A) Brain infarct size was determined 48 hours after HI. n=6-8. * P<0.05, LNA or LNA+RU486 vs. Neg.Ctrl; † P<0.05, LNA+RU486 vs. LNA alone. For panels B to F, pups received via i.c.v. the negative control or LNA 4 hours after the HI treatment. (B) MiR-210 levels in the ipsilateral hemisphere 24 hours after HI. n=5. (C) Brain infarct size 48 hours after HI. n=7-8. (D) Representive immunofluorescence images of TUNEL-positive neurons in the cortex. Scale bar = 20 μm. (E) Representive immunofluorescence images of TUNEL-positive neurons in the hippocampus. Scale bar = 20 μm. (F) Quantification of TUNEL-positive neurons (nine images per region, 3 sections/pup). n=4. * P<0.05, LNA vs. Neg. Ctrl.
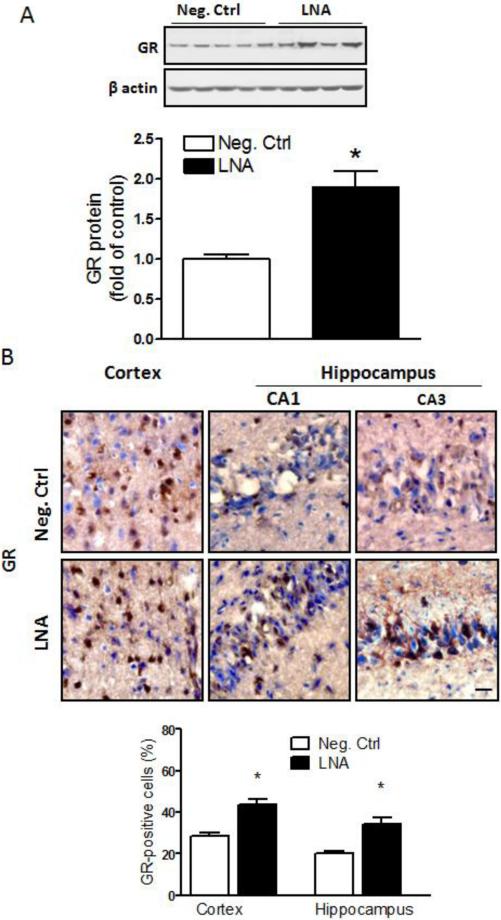
3.5. MiR-210-LNA treatment increased GR expression in neonatal brains
The western blotting results demonstrated that the miR-210-LNA post-HI treatment significantly increased GR protein abundance in the ipsilateral hemisphere 48 hours after the HI insult (Fig. 4A). Immunohistochemical staining also showed enhanced GR-positive signals in the cortex and hippocampal CA1 and CA3 regions in the ipsilateral hemisphere in miR-210-LNA-treated groups (Fig. 4B).
Figure 4. MiR-210-LNA increased GR protein abundance in neonatal brains.
Rat pups received via i.c.v. the negative control (Neg. Ctrl) or miR-210-LNA (LNA) 4 hours after the HI treatment. (A) GR protein abundance in the ipsilateral hemisphere 48 hours after HI. n=4. * P<0.05, LNA vs. Neg. Ctrl. (B) Representive immunohistochemical images and quantification of GR-positive cells in the cortex and hippocampus (nine images per region, 3 sections/pup; n=4) showed LNA-induced increase in GR expression in the cortex and hippocampal CA1 and CA3 regions 48 hours after the HI treatment. * P<0.05, LNA vs. Neg. Ctrl. Scale bar = 20 μm.
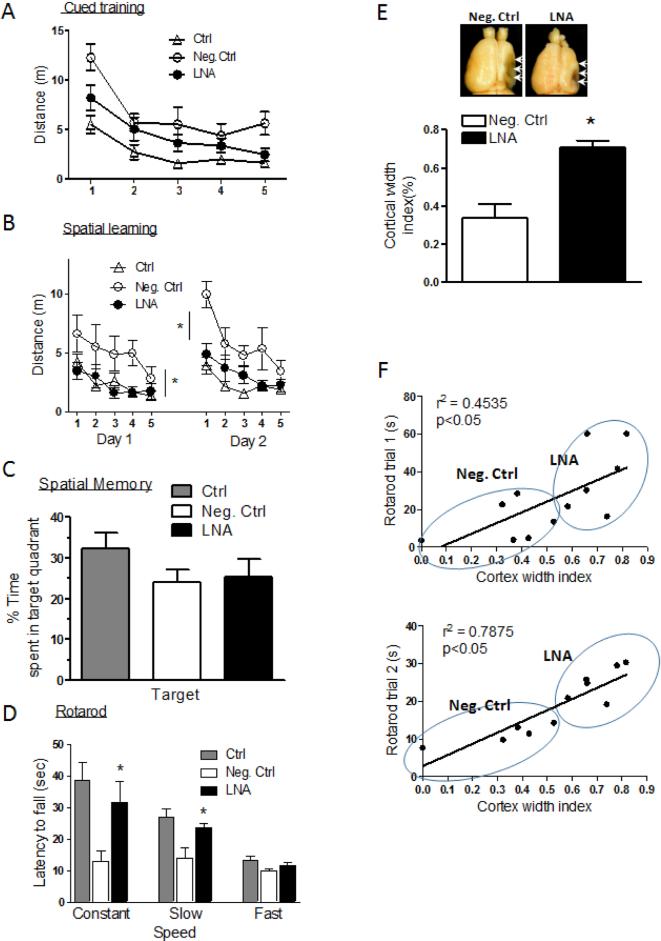
3.6. MiR-210-LNA treatment improved long-term neurological function recovery
The neurological function was evaluated six weeks after the HI brain injury in pups received the post-HI treatments with miR-210-LNA or the negative control. In the water maze test (Fig. 5A,B,C), miR-210-LNA did not significantly reduce swimming distance to find the platform in cued learning (Fig. 5A), but significantly improved spatial learning with a shorter swimming distance to find the platform (Fig. 5B). In the probe test for spatial memory, animals treated with miR-210-LNA did not show preference for the targeted quadrant (Fig. 5C). As shown in Fig. 5D, HI also resulted in motor performance deficits on the rotarod test. Animals received the negative control post-HI treatment fell off as quickly during the constant rotation and slow acceleration trials as during the more difficult fast acceleration trials. MiR-210-LNA post-HI treatment significantly improved motor functions during the constant and slow acceleration trials, but not during the fast acceleration trials (Fig. 5D). Consistent with the functional improvement, miR-210-LNA significantly reduced the brain atrophy with the increased cortex width index (Fig. 5E). A regression analysis between the rotarod performance and brain atrophy showed that motor performance was significantly correlated with the cortical width index (Fig. 5F).
Figure 5. MiR-210-LNA improved long-term neurological function recovery after neonatal HI brain injury.
Rat pups received via i.c.v. the negative control (Neg. Ctrl) or miR-210-LNA (LNA) 4 hours after the HI treatment, and neurological function was evaluated six weeks after HI. (A-C) Water maze tests of cued training (A), spatial leaning (B) and spatial memory (C). (D) Rotarod tests. Ctrl, sham control without HI. (E) Brain cortex width index analysis. n=9. * P<0.05, LNA vs. Neg. Ctrl. (F) Regression analysis of the correlation between the rotarod performances and cortex width index.
3.7. Intranasal delivery of miR-210-LNA produced a neuroprotective effect
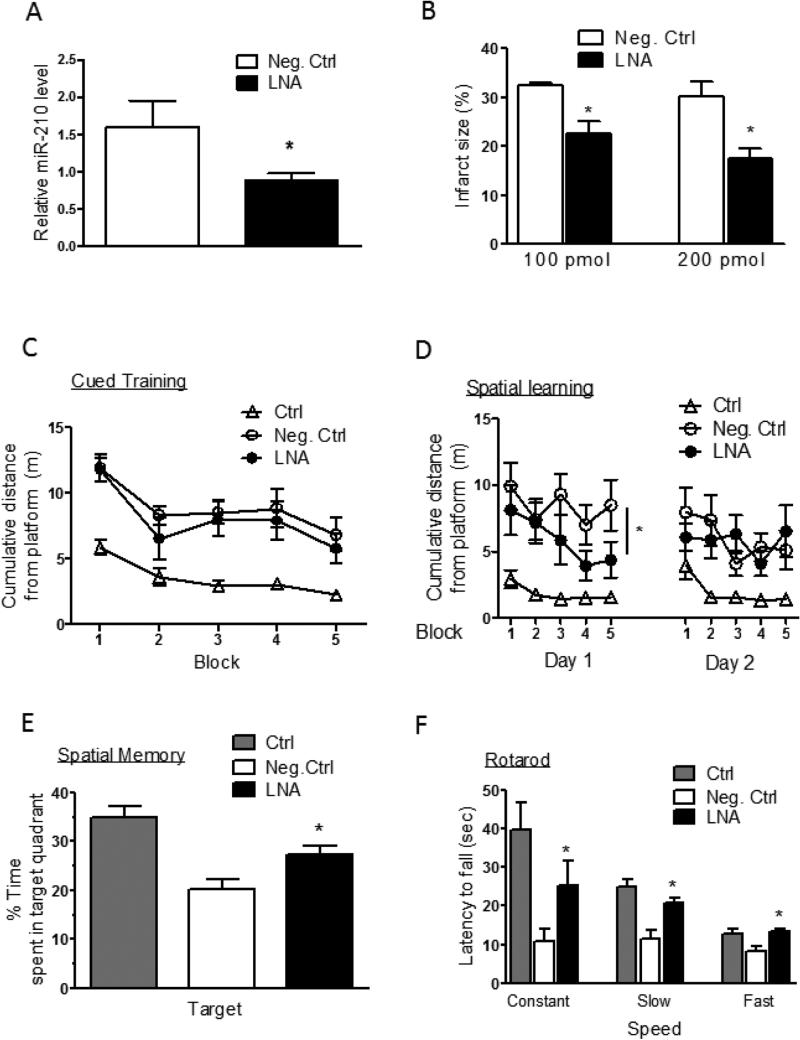
To further determine the clinical therapeutic relevance of miR-210 inhibition in neonatal HI brain injury, we administered miR-210-LNA into each naris of rat pups 4 hours after the HI insult. The results demonstrated that intranasal administration of miR-210-LNA significantly down-regulated miR-210 levels in the brain, as compared to the negative control (Fig. 6A). Of critical importance, intranasal administration of miR-210-LNA after the HI treatment dose-dependently decreased brain infarct size, as compared to the negative control (Fig. 6B). We further determined the effect of intranasal delivery of miR-210-LNA on neurobehavioral function recovery six weeks after the HI treatment. In the water maze test, miR-210-LNA did not affect the performance in cued learning (Fig. 6C), but significantly improved spatial learning with a shorter swimming distance (Fig. 6D). In the probe test for spatial memory, animals treated with miR-210-LNA showed a strong inclination for the targeted quadrant, but animals treated with the negative control did not (Fig. 6E). In the rotarod test, treatment with miR-210-LNA significantly improved motor functions during the constant, slow acceleration trials and fast acceleration trials with the increased latency of fall off, as compared to negative control animals (Fig. 6F).
Figure 6. Intranasal administration of miR-210-LNA produced a neuroprotective effect.
Rat pups received via intranasal administration of the negative control (Neg. Ctrl) or miR-210-LNA (LNA) 4 hours after the HI treatment. Ctrl, sham control without HI. (A) MiR-210 levels in the ipsilateral hemisphere 24 hours after HI. n=5. (B) Brain infarct size 48 hours after HI. n=7-8. For panels C to F, neurological function recovery determined six weeks after the HI. (C-E) Water maze tests of cued training (C), spatial leaning (D) and spatial memory (E). (F) Rotarod tests. n=9-10. * P<0.05, LNA vs. Neg. Ctrl.
4. Discussion
The present study demonstrated that increased miR-210 exacerbated HI-induced brain injury in rat pups. We further identified that the inhibition of GR transcript was a novel target of miR-210 and GR down-regulation mediated the effect of miR-210 in HI-induced brain injury. Of importance, silencing miR-210 with miR-210-LNA via intracerebroventricular or intranasal delivery provided a neuroprotective effect on neonatal HI insult, implicating a novel target of potential therapeutic intervention in the treatment of neonatal HIE.
It has been reported that miR-210 is upregulated at 24 hours upon brain transient focal ischemia in adult rats (Jeyaseelan et al., 2008). However, the biologic function of miR-210 in cerebral ischemia remains unclear. It was reported in vitro that miR-210 mediated hypoxia-induced apoptotic death in neural cell cultures, and silencing miR-210 reduced apoptotic cell death (Chio et al., 2013). Other in vitro studies showed that overexpression of miR-210 reduced apoptosis in cultured progenitor cells exposed to oxygen and glucose deprivation (Kim et al., 2009). These results suggest that the effects of miR-210 are cell type and tissue specific, and may also vary with the different insults in vivo. The present study demonstrated the in vivo effect of miR-210 in regulating HI-induced brain injury in rat pups. We found that the HI insult increased miR-210 in the brain, and miR-210 mimic increased the vulnerability of the neonatal brain to HI-induced brain injury, demonstrating a detrimental effect of elevated endogenous miR-210 in HI-induced brain injury in neonates.
The miR-210-mediated brain injury is possibly due to an increase in cell death. In agreement, previous studies showed that miR-210 overexpression resulted in the expression of pro-apoptotic genes and thereafter apoptotic cell death in endothelial cells (Chan et al., 2009). Indeed, the present study revealed that the inhibition of miR-210 reduced neuronal cell death in both the cortex and hippocampus. While the mechanisms of miR-210 in regulating neuronal cell death remain elusive, the present study identified the inhibition of GR transcript and GR down-regulation in the brain as a novel target of miR-210. MiRs silence gene expression by binding to the 3’UTR of the transcript via their seed sequences at 5’ ends (nucleotides 2-8), resulting in transcript degradation or translational inhibition of the target genes. Rat GR mRNA 3’UTR contains the binding sequences for miR-210. We have shown that intracerebroventricular injection of the miR-210 mimic significantly reduced both GR mRNA and protein abundance in the brain. These in vivo findings are supported by the results of luciferase reporter gene assay, showing that miR-210 significantly decreased luciferase activity in PC12 cells co-transfected with pmirGLO-GR223, which was blocked by miR-210-LNA. Thus, miR-210 suppressed luciferase expression by binding to the downstream GR transcript 3’UTR, providing the evidence that the GR transcript is indeed a direct target of miR-210.
The finding that inhibition of miR-210 with miR-210-LNA 4 hours after the HI insult significantly increased GR protein abundance in the brain further support the notion that GR is a downstream target of miR-210 in the neonatal brain. The functional significance of increased GR in miR-210-LNA-induced neuroprotection after the HI insult is suggested by the finding of co-localization of the GR and TUNEL-positive cell distribution in the penumbra region of brain infarct site. Cell death in the penumbra region is an important marker for the evaluation of therapeutic consequence. Thus, the increased GR immunoreactivity in the CA1 and CA3 regions of the hippocampus and in the cortex after the LNA-miR-210 treatment corresponded to a significant reduction of TUNEL-positive staining in these regions, suggesting that the LNA-miR-210 treatment exerts neuroprotection through the upregulation of GR. Indeed, the finding that inhibiting the GR by RU486 countered the neuroprotective effect produced by LNA-miR-210 in HI-induced brain injury, demonstrates a key role of GR in miR-210-mediated regulation of HI-induced brain injury in neonates. Our recent studies demonstrated that fetal hypoxia-mediated down-regulation of GR in the developing brain resulted in the increased brain susceptibility to HI injury in neonatal rats (Gonzalez-Rodriguez et al., 2014b), and the interaction of glucocorticoids-GR signaling and L-PGDS-PGD2-DP1-pERK-mediated pathway contributed to the neuroprotective effects (Gonzalez-Rodriguez et al., 2014a).
Of importance, the miR-210-LNA treatment after neonatal brain HI insult significantly improved long-term neurological function recovery. Neurological function is one of the major indicators to reflect the degree of brain injury, thereby to evaluate the therapeutic effect in the treatment of neonatal HI brain injury. In the present study, the water maze and rotarod tests were performed to evaluate the long-term effect of miR-210-LNA through intracerebroventricular or intranasal delivery on the HI-induced brain injury. The result of water maze test showed that the miR-210-LNA treatment significantly improved spatial learning ability. However, spatial memory in the probe trial was significantly improved in the intranasal but not in intracerebroventricular delivery. This is possibly due to the lower dosage of miR-210-LNA delivered through intracerebroventricular. Extending water maze training by another day or two may also be needed to further evaluate long-term memory alterations in this model. The water maze test used in the present study was a standard method that primarily detected the changes of spatial learning and memory functions (D'Hooge and De Deyn, 2001; Prusky et al., 2000). The cued training in this protocol was usually used to exclude animals with non-cognitive impairments, such as visual function damage, which might negatively affect the navigation in the pool during the spatial learning and memory test (Brandeis et al., 1989; Chen et al., 1995; Prusky et al., 2000). Therefore, this protocol may not accurately reflect the function changes of striatum-dependent cued learning. A modified protocol of two-cue water maze task with extended training time and more visible cues (Lee et al., 2008) would be needed to measure the cued learning after miR-210-LNA treatment in the neonatal HI model.
The HI insult also resulted in impaired locomotor function as shown by the rotarod test. The miR-210-LNA intracerebroventricular delivery significantly improved the performance during the constant speed and slow acceleration trials and intranasal delivery also improved the performance during high acceleration trial, suggesting that treatment promoted motor function recovery after neonatal HI brain injury. Moreover, the positive correlation between the improvement of motor function and the reduction of brain tissue loss indicated that the long-term neuroprotective effect of miR-210-LNA is through the decrease of brain tissue loss.
Although intracerebroventricular injection of miR-210 antagomir LNA demonstrated exciting and novel insights about the molecular mechanisms underlying perinatal HI brain injury, this invasive procedure is unsuited for broad clinical application. The intranasal delivery is a powerful, non-invasive method to directly deliver chemicals and peptides to the brain, which is not obstructed by the blood-brain barrier, avoids fast systemic clearance, and limits potential secondary effects. It has been used to deliver chemicals, peptides, oligonucleotides, proteins and stem cells into the brain (Cai et al., 2011; Cooper et al., 2013; Kim et al., 2012; Perez et al., 2012; Rat et al., 2011; van Velthoven et al., 2013; Yang et al., 2013a; Yang et al., 2013b). A recent study demonstrated that silencing miR-134 with intranasal administration of miR-134 antagomir LNA in each nostril produced neuroprotective and prolonged seizure-suppressive effects in mice (Jimenez-Mateos et al., 2012). Our results showed that miR-210-LNA delivery through intranasal route dose-dependently reduced infarct size and improved long-term neurological functions by the down-regulation of brain miR-210 levels in neonatal HI brain injury, which may facilitate translation to the clinic for the treatment of neonatal brain injury.
5. Conclusion
In conclusion, the present study provides novel evidence of a causative mechanism of increased miR-210 in neonatal HI brain injury and identifies a novel target of GR down-regulation in miR-210-mediated neural injury. Of critical importance, the finding that the inhibition of miR-210 with miR-210-LNA through both intracerebroventricular injection and intranasal delivery 4 hours after the HI insult significantly reduced the brain infarct size and improved the neurological function recovery following the HI insult provides a proof of concept for a novel target of potential therapeutic intervention in the treatment of neonatal HIE. This is in agreement with recent findings that miRs are potential targets for neurological disease therapy (Hollander et al., 2010; Jimenez-Mateos et al., 2012), and that the LNA-mediated effect in the brain tissue can last at least four weeks (Jimenez-Mateos et al., 2012). While the caution should be observed in extrapolating the findings of animal studies directly to the humans, the present study suggests new insights for the development of therapeutic strategies that may be beneficial for the treatment of newborns with HIE given the current lack of effective therapeutic interventions.
Highlights.
hypoxic-ischemic insult upregulated miR-210 levels in neonatal rat brains.
miR-210 exacerbated hypoxic-ischemic brain injury in neonatal rats.
miR-210 downregulated the glucocorticoid receptor in the brain.
miR-210 inhibition provided neuroprotection by upregulation of the glucocorticoid receptor.
ACKNOWLEDGEMENTS
A portion of this research used the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core, a facility supported in part by the National Science Foundation through the Major Research Instrumentation program of the Division of Biological Infrastructure Grant No. 0923559 and the Loma Linda University School of Medicine.
Sources of Funding:
This work was supported by National Institutes of Health grants HL82779 (L.Z.), HL83966 (L.Z.), HL118861 (L.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
References
- Azzopardi DV, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- Brandeis R, et al. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Cai Z, et al. Intranasal administration of insulin-like growth factor-1 protects against lipopolysaccharide-induced injury in the developing rat brain. Neuroscience. 2011;194:195–207. doi: 10.1016/j.neuroscience.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SY, et al. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–84. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YC, et al. miR-210: the master hypoxamir. Microcirculation. 2012;19:215–23. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KS, et al. Synaptic loss in cognitively impaired aged rats is ameliorated by chronic human nerve growth factor infusion. Neuroscience. 1995;68:19–27. doi: 10.1016/0306-4522(95)00099-5. [DOI] [PubMed] [Google Scholar]
- Chio CC, et al. MicroRNA-210 targets antiapoptotic Bcl-2 expression and mediates hypoxia-induced apoptosis of neuroblastoma cells. Arch Toxicol. 2013;87:459–68. doi: 10.1007/s00204-012-0965-5. [DOI] [PubMed] [Google Scholar]
- Cooper PR, et al. Efflux of monoclonal antibodies from rat brain by neonatal Fc receptor, FcRn. Brain Res. 2013;1534:13–21. doi: 10.1016/j.brainres.2013.08.035. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dasgupta C, et al. Chronic hypoxia during gestation causes epigenetic repression of the estrogen receptor-alpha gene in ovine uterine arteries via heightened promoter methylation. Hypertension. 2012;60:697–704. doi: 10.1161/HYPERTENSIONAHA.112.198242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanaro P, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–83. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lopez D, et al. Mechanisms of perinatal arterial ischemic stroke. J Cereb Blood Flow Metab. 2014;34:921–32. doi: 10.1038/jcbfm.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rodriguez PJ, et al. Dexamethasone protects neonatal hypoxic-ischemic brain injury via L-PGDS-dependent PGD2-DP1-pERK signaling pathway. PLoS One. 2014a;9:e114470. doi: 10.1371/journal.pone.0114470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rodriguez PJ, et al. Fetal hypoxia increases vulnerability of hypoxicischemic brain injury in neonatal rats: role of glucocorticoid receptors. Neurobiol Dis. 2014b;65:172–9. doi: 10.1016/j.nbd.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, et al. Motor and cognitive deficits in mice bred to have low or high blood pressure. Physiol Behav. 2012;105:1092–7. doi: 10.1016/j.physbeh.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Higgins RD, et al. Hypothermia and other treatment options for neonatal encephalopathy: an executive summary of the Eunice Kennedy Shriver NICHD workshop. J Pediatr. 2011;159:851–858. e1. doi: 10.1016/j.jpeds.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SE, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. 2011;165:692–700. doi: 10.1001/archpediatrics.2011.43. [DOI] [PubMed] [Google Scholar]
- Jeyaseelan K, et al. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–66. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- Jimenez-Mateos EM, et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med. 2012;18:1087–94. doi: 10.1038/nm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper JE, et al. Juvenile traumatic brain injury evolves into a chronic brain disorder: behavioral and histological changes over 6months. Exp Neurol. 2013;250:8–19. doi: 10.1016/j.expneurol.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, et al. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–8. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ID, et al. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol Ther. 2012;20:829–39. doi: 10.1038/mt.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–20. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- Kosik KS, Krichevsky AM. The Elegance of the MicroRNAs: A Neuronal Perspective. Neuron. 2005;47:779–82. doi: 10.1016/j.neuron.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Lee AS, et al. A double dissociation revealing bidirectional competition between striatum and hippocampus during learning. Proc Natl Acad Sci U S A. 2008;105:17163–8. doi: 10.1073/pnas.0807749105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: mechanisms and possible interventions. Prog Neurobiol. 2012;98:145–65. doi: 10.1016/j.pneurobio.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Zhang L. Epigenetic programming of hypoxic-ischemic encephalopathy in response to fetal hypoxia. Prog Neurobiol. 2015;124:28–48. doi: 10.1016/j.pneurobio.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JM, et al. Inhibition of microRNA-181 reduces forebrain ischemia-induced neuronal loss. J Cereb Blood Flow Metab. 2013;33:1976–82. doi: 10.1038/jcbfm.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez AP, et al. Increased brain radioactivity by intranasal P-labeled siRNA dendriplexes within in situ-forming mucoadhesive gels. Int J Nanomedicine. 2012;7:1373–85. doi: 10.2147/IJN.S28261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch S, et al. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics. 2009;25:1463–5. doi: 10.1093/bioinformatics/btp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, et al. Reduced visual acuity impairs place but not cued learning in the Morris water task. Behav Brain Res. 2000;116:135–40. doi: 10.1016/s0166-4328(00)00267-9. [DOI] [PubMed] [Google Scholar]
- Rat D, et al. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer's disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011;25:3208–18. doi: 10.1096/fj.10-180133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JE, 3rd, et al. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–41. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Shankaran S, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- van Velthoven CT, et al. Mesenchymal stem cell transplantation attenuates brain injury after neonatal stroke. Stroke. 2013;44:1426–32. doi: 10.1161/STROKEAHA.111.000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verklan MT. The chilling details: hypoxic-ischemic encephalopathy. J Perinat Neonatal Nurs. 2009;23:59–68. doi: 10.1097/01.JPN.0000346221.48202.7e. quiz 69-70. [DOI] [PubMed] [Google Scholar]
- Yager JY, Ashwal S. Animal models of perinatal hypoxic-ischemic brain damage. Pediatr Neurol. 2009;40:156–67. doi: 10.1016/j.pediatrneurol.2008.10.025. [DOI] [PubMed] [Google Scholar]
- Yang D, et al. Intranasal delivery of cell-penetrating anti-NF-kappaB peptides (Tat-NBD) alleviates infection-sensitized hypoxic-ischemic brain injury. Exp Neurol. 2013a;247:447–55. doi: 10.1016/j.expneurol.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, et al. Taming neonatal hypoxic-ischemic brain injury by intranasal delivery of plasminogen activator inhibitor-1. Stroke. 2013b;44:2623–7. doi: 10.1161/STROKEAHA.113.001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. J Cell Mol Med. 2012;16:249–59. doi: 10.1111/j.1582-4934.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao BQ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–5. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]