Abstract
Elderly burn care represents a vast challenge. The elderly are one of the most susceptible populations to burn injuries, but also one of the fastest growing demographics, indicating a substantial increase in patient numbers in the near future. Despite the need and importance of elderly burn care, survival of elderly burn patients is poor. Additionally, little is known about the responses of elderly patients after burn. One central question that has not been answered is what age defines an elderly patient. The current study was conducted to determine whether there is a cut-off age for elderly burn patients that is correlated with an increased risk for mortality and to determine the burn size in modern burn care that is associated with increased mortality. To answer these questions, we applied appropriate statistical analyses to the Ross Tilley Burn Centre and the Inflammatory and Host Response to Injury databases. We could not find a clear cut-off age that differentiates or predicts between survival and death. Risk of death increased linearly with increasing age. Additionally, we found that the LD50 decreases from 45% total body surface area (TBSA) to 25% TBSA from the age of 55 years to the age of 70 years, indicating that even small burns lead to poor outcome in the elderly. We therefore concluded that age is not an ideal to predictor of burn outcome, but we strongly suggest that burn care providers be aware that if an elderly patient sustains even a 25% TBSA burn, the risk of mortality is 50% despite the implementation of modern protocolized burn care.
Keywords: burn, elderly, mortality, age
Introduction
A severe burn is an injury that affects every organ system, leading to significant morbidity and mortality [1, 2]. It has been shown that outcomes after burn are linked to age [3]. The best outcomes can be found in children, followed by adults and lastly, the elderly [3, 4]. While significant advances in outcomes have been made in children and adults [5], there have been minimal improvements in the outcomes for elderly burn patients with small burn which is recently reported in a historical cohort study [3]. The LD50 burn size in elderly has remained almost the same, at around 35% TBSA burn, over the last few decades. In general, the elderly have a thinning of the skin [6], decreased sensation, decreased metabolic resources and capacity [7], mental alterations, pre-existing medical conditions and other contributing factors [8– 10]. The failure of their immune system to fight off post-burn infections along with altered inflammatory and immune responses [11] contribute to worsened post-burn outcomes.
There is an ongoing effort to determine why the elderly have such poor outcomes on a cellular and mechanistic level; however, there are several essential questions that have not been addressed or well-defined. First, when is an elderly patient considered elderly? The definition of when a human becomes an elderly is not entirely clear. The World Health Organization (WHO) and the National Institutes of Health (NIH) have defined ‘elderly’ as 65 years or older (www.who.int/healthinfo/survey/ageingdefnolder/en/), but there is ongoing discussion about the age that defines an individual as elderly, varying from 55 to 75 years [12]. It is currently not clear what age can be defined as elderly in burn patients; therefore, the first aim of this study was to determine whether there is a cut off age for elderly burn patients. Second, we aimed to determine the minimum burn size that is associated with increased mortality. We tried to define elderly cut-off age by using the increase in mortality as an indicator for it. To do this, we looked at two databases: the Ross Tilley Burn Centre database and the Inflammation and Host Response to Injury (Glue Grant; https://www.gluegrant.org) database and compared their cut-off age and cut-off burn size. We hypothesized that elderly burn patients would have a cut-off age that is clearly associated with burn size and mortality. We further hypothesized that a particular burn size would be associated with increased morbidity and mortality after burn.
Methods
Patients
In this study, two existing databases were used to examine the cut-off age for elderly after burn and the cut-off burn size associated with increased morbidity and mortality. The first database was that of a single ABA-verified burn center, Ross Tilley Burn Centre (RTBC) at Sunnybrook Health Sciences Centre (SHSC). Research Ethics Board (REB) at Sunnybrook approved this study (# 003-2011). Patients from January 2006 to October 2014 with TBSA ≥20 and removing those cases that were futile (died within 2 days of admission).
The second database was that of the Inflammation and the Host Response to Injury Glue Grant (https://www.gluegrant.org). The study was approved by Institutional Review Boards (IRB) of the participating institutions (University of Texas Medical Branch, Galveston, TX; Loyola University Medical College, Chicago, IL; University of Texas Southwestern, Dallas, TX; University of Washington Seattle, Seattle, WA; Massachusetts General Hospital, Boston, MA). Over an 8-year period, 573 patients meeting all inclusion criteria were prospectively enrolled. Inclusion criteria were as follows: age of 0–99 years, admission to a participating hospital no later than 96 hours post-burn and ≥20% TBSA burns with the need for at least one surgical intervention. All hospitals followed standard operating procedures set forth by the burn patient-oriented research core [13, 14]. Each subject or a family member provided written informed consent before study participation. One of the main inclusion criteria was patient survival, but there were 5 patients that died within 3 days out of total of 86 cases that we did not remove.
Patients were treated according to treatment protocols specific to each study. Treatment protocols for both the RTBC and the Glue grant have been published previously [15–17].
Outcomes
The primary objectives of the study were 1) to determine the age that best defines elderly in burns; 2) to identify the LD50 burn size associated with increased mortality in the elderly after burn.
Statistics
Continuous variables were summarized using means and standard deviations or medians and interquartile ranges and compared using either a Student’s t test or Wilcoxon ranksum test, as appropriate. Discrete variables were presented using frequencies and percentages and tested using χ2 or Fisher’s exact test. As the cohorts were different with respect to age and % TBSA, we determined an age cut-off point separately for each cohort. Both cohorts were analyzed via Youden’s index and c-statistics, using mortality as an outcome. Youden’s index is maximizing J= (sensitivity+specificity−1) and therefore gives an equal weight to the false positives and false negatives. The c-statistic (equivalent to area under the Receiver Operating Characteristics curve) measures the predictive accuracy of a logistic regression. Age was dichotomized for each year from 25 to 75 and a logistic regression with outcome mortality adjusted for gender, % TBSA and inhalation injury was used to calculate the c-statistic. Confidence intervals were calculated for each c-statistic using the adjusted bootstrap percentile method.
Results
A total of 1457 patients were enrolled from the RTBC database and 573 patients from the Glue Grant database. In order to compare the two patient populations, we focused on burns over 20% TBSA and excluded pediatric burn patients, resulting in 235 patients from RTBC and 347 from the Glue Grant. Patient characteristics are presented in Table 1. Patients in the Glue Grant cohort were younger (40 years old vs. 48.0 years) and had a higher TBSA (42.1% vs.34.4%) compared to the RTBC patients. Both cohorts had a similar incidence of inhalation injuries and gender distribution.
Table 1.
Patient characteristics and outcomes.
| Variable (missing in each group) | Sunnybrook (N=235) |
Glue Grant (N=347) |
p-value |
|---|---|---|---|
| Age | 48 (17) | 40 (17) | <0.0001 |
| Male | 181 (77.0%) | 262 (75.5%) | 0..6737 |
| TBSA (%) | 34.4 (13.4) | 42.1 (19.1) | <0.0001 |
| Inhalation Injury (n, %) | 94 (40.0%) | 140 (40.5%) | 0.9112 |
| Outcomes | |||
| LOS (days) | 29 (19–59) | 33 (20.5–61) | 0.3043 |
| LOS/TBSA (days/%) | 1.03 (0.68–1.57) | 0.96 (0.60–1.55) | 0.1186 |
| Pneumonia (n, %) | 119 (50.6%) | 160 (46.1%) | 0.2833 |
| Burn Wound Infection (n, %) | 111 (47.2%) | 188 (54.2%) | 0.100 |
| Sepsis (n, %) | 82 (34.9%) | 37 (10.7%) | <0.0001 |
| Death (n, %) | 34 (14.5%) | 48 (19.6%) | 0.1154 |
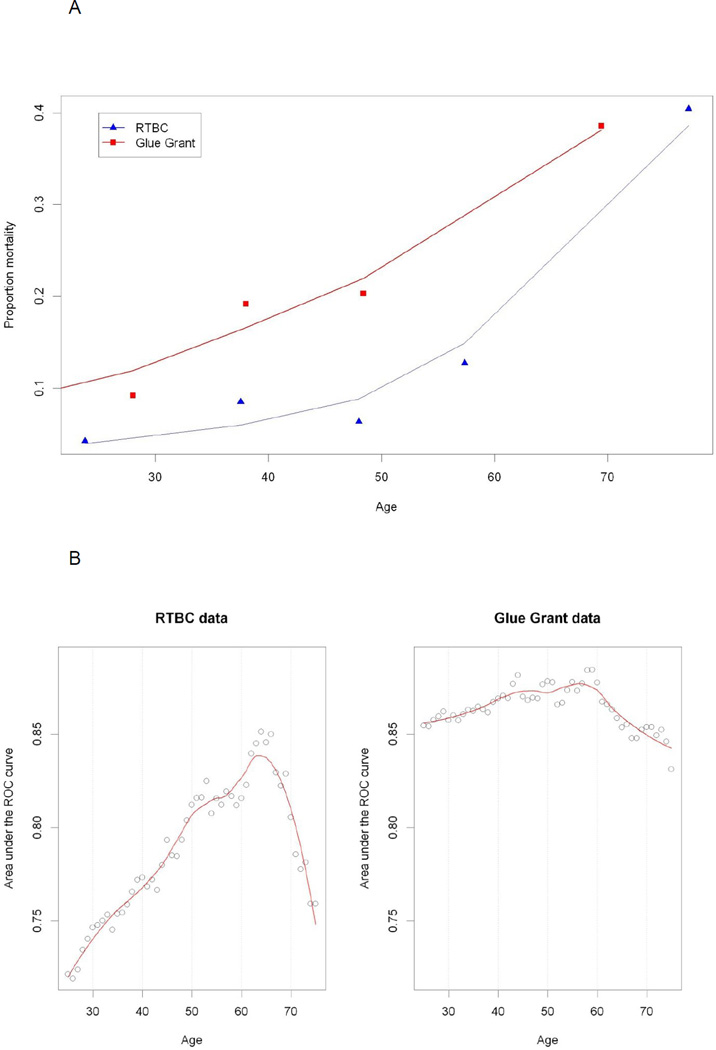
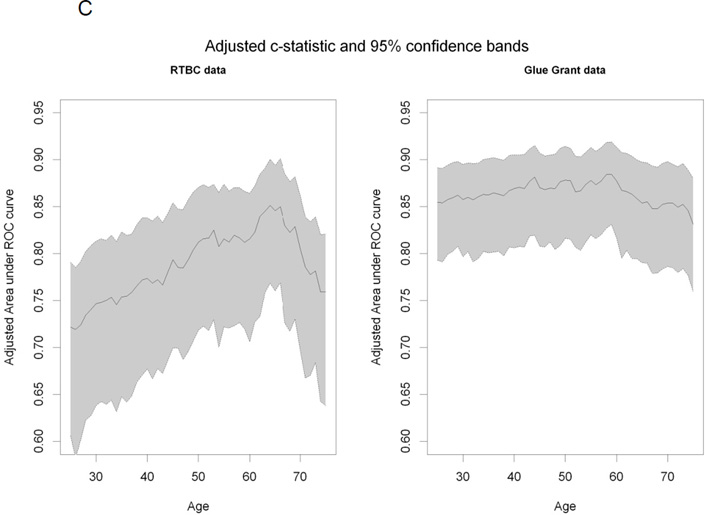
We found that mortality increased linearly with age in both groups (Figure 1A) and that no apparent threshold was present. We carried out a separate analysis for the two cohorts as they differed with respect to their clinical characteristics. Based on the Youden’s index analysis, the cut-off point was 54 years old for the RTBC cohort and 50 years old for the Glue Grant cohort. The analysis based on the c-statistics (adjusted for % TBSA, inhalation injury and gender) indicated some variation in the RTBC cohort from 0.72 to 0.85, with the maximum achieved around the age of 65 (Figure 1B, C). The c-statistics for Glue Grant seemed to be independent of the cut-off point for age, varying only slightly around 0.85 (Figure 1B, C).
Figure 1. Mortality plotted against age.
(A) Mortality versus age binned in 5 equal groups. (B): c-statistic adjusted for % TBSA, inhalation injury and gender for age dichotomized at every year. (C): c-statistic with 95% confidence using adjusted bootstrap confidence intervals.
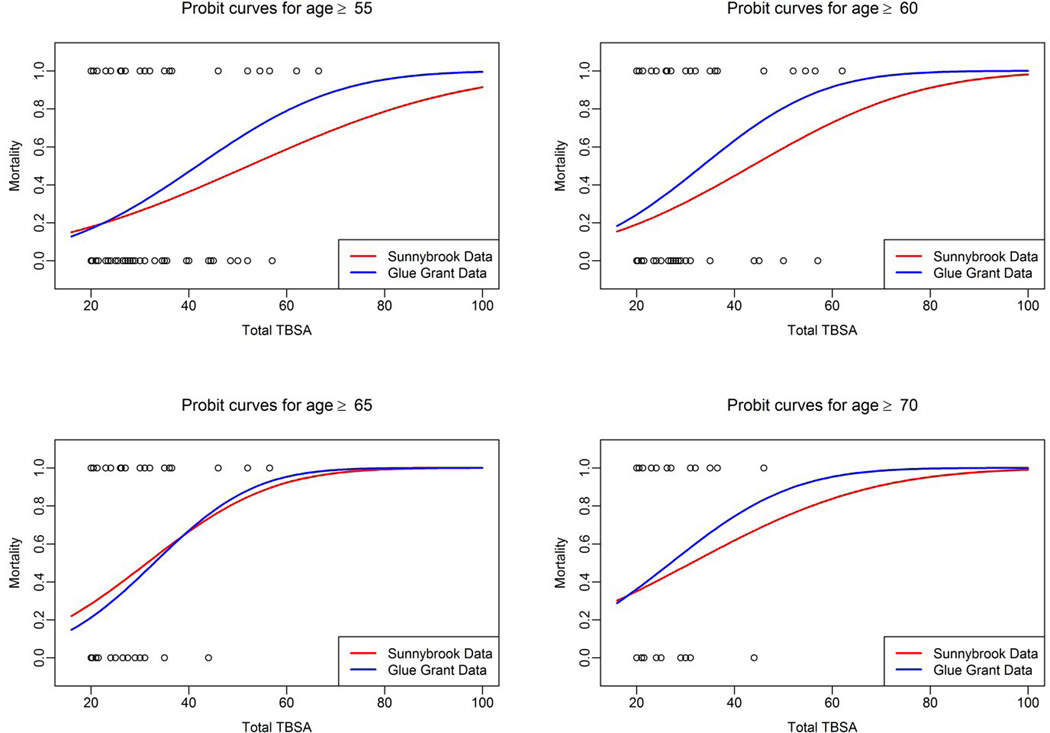
The second aim of this study was to determine the LD50 burn size associated with the cut-off age determined in our first aim. As we did not find a cut-off age, we conducted this analysis for four age groups: 55, 60, 65 and 70 years of age. We found similar LD50 burn sizes for the RTBC population as well as Glue Grant population (Table 2). The LD50 for 55 years of age was 45% TBSA, 36% TBSA for >60 years, 30% TBSA for >65 years and for 28% TBSA for >70 years of age. Burn size required for LD50 decreased with age (Table 2). The probit curves for age vs LD50 burn size are depicted for these 4 age groups in Figure 2.
Table 2.
LD50 burn size for various age groups
| LD50 | ||||
|---|---|---|---|---|
| RTBC | Glue Grant | |||
| Age | Logit Model | Probit Model | Logit Model | Probit Model |
| Age>=55 | 52.13 | 52.25 | 41.67 | 41.71 |
| Age>=60 | 43.62 | 43.59 | 33.36 | 33.43 |
| Age>=65 | 31.5 | 31.46 | 32.66 | 32.91 |
| Age>=70 | 31.19 | 31.23 | 27.04 | 26.99 |
Figure 2.
The probit curves for age vs LD50 burn size are depicted for different age groups.
Discussion
Over the last few decades, changes in the care of burned children and adults have significantly improved outcomes; however, these improvements have not been reflected in the elderly. The LD50 burn size has remained steady in this population at 35% TBSA burn over the last 2–3 decades[18] [4]. This lack of progress is of great concern in light of the substantially growing elderly population. In this study, we aimed to determine the LD50 as well as an age cutoff to predict the age at which elderly patients are at greater risk for mortality due to burn injury. To our surprise, we found that there is no cut-off age associated with increased morbidity and mortality after burn. Our vigorous statistical analysis and modeling indicated that the elderly have a linear increase in risk of dying: the older the patient, the higher the risk. In two datasets with over 500 patients, we could not find an inflection point indicating a cut-off value. Mortality increased linearly with age. One might speculate that the slope of the mortality curve steepens somewhat around 55 years of age, indicating a possible age limit for a higher risk for mortality, but there is only weak statistical support for this age.
The lack of a cut-off age may be attributed several variables; however, there are two likely explanations for this phenomenon. First, it could be that despite 500 patients, our sample size was too small to detect a cut-off due to low power. Second, age may not be the best criterion to determine a cut-off for increased mortality post-burn. For example, Romanowski et al [19] recently showed that the frailty index is both a valid and good predictor of outcomes after burns. Using the frailty index makes sense, as patients of the same biological age may have vastly different physiological and metabolic characteristics. For example, a 65-year-old marathon runner would not have the same post-burn mortality risk as an obese, hypertensive, diabetic patient of the same age. It would therefore be desirable to determine if the frailty index correlates with post-burn mortality. Unfortunately, neither the RTBC nor the Glue Grant databases captured frailty index, but the relationship of frailty to post-burn mortality risk would be of great interest in future studies. While the comparison of these two databases revealed several similarities, it is important to mention that these two databases are quite different. RTBC study includes all admissions to one burn center, while the Glue grant possibly suffers from some extent of selection bias across multiple sites.
In the current study, we were able to determine that patients older than 65 years have an LD50 of 30–40% TBSA, which is significantly lower than the LD50 for children and adults under the age of 65. The LD50 decreases from almost 50% TBSA for a 50-year-old burn patient to 25% for a 70-year-old burn patient. Based on the LD50 results, it appears as though there is a change around 60 years of age. A burn over 35% TBSA in a patient of 65 years or older is associated with more than a 50% chance of non-survival. This is of great importance as the mean age of the population shifts upwards. We as well as others predict that burn injuries in this population will increase as well; therefore, novel therapeutic approaches must be developed to improve burn survival in the elderly.
How can the results of this study translate to the clinic and give burn care providers insights into treating elderly burn patients? First, age is not an ideal predictor of outcome. It would be better to rely on the use of scores such as the frailty score or other adjusted scores that do not reflect the biological age of a patient, but rather the aging progression and functionality of a patient. Secondly, burn care providers need to be aware that if an elderly patient has a burn of over 30% TBSA, their risk of mortality is 50%. We therefore hypothesize that an individualized approach should be developed over the next few years in order to improve elderly care in burn patients. Lastly, it is difficult to set a threshold age in order to classify adults as ‘elderly’ when it comes to burns. Based on our data, there is no clear inflection point and therefore we cannot define a clear cut-off point. Instead, we report that the risk of mortality simply increases by age, a variable that negatively correlate with outcome. This variability decreases with increasing age such that even small burns may lead to poorer outcomes along the aging axis. Clinical trials in which elderly patients are investigated should consider various aspects (e.g., admission numbers vs. age) to find a compromise to achieve sufficient power but at the same time accurately represents the elderly population. It appears that 60-year-old patients have a similar LD50 to 65-year-old and 70-year-old patients; therefore, 60 years may represent a better age to enrol burn patients into elderly studies.
Highlights.
In elderly, risk of death increased linearly with increasing age.
LD50 decreases from 45% TBSA to 25% TBSA from the age of 55 years to the age of 70 years.
Despite modern burn care protocols outcome remains poor in elderly patients.
Acknowledgments
The authors would like to acknowledge the participants of the Inflammation and Host Response to injury project:
Celeste C. Finnerty, PhD; Henry V. Baker, PhD; M. Cecilia Lopez, MSc; Richard L. Gamelli, MD; Nicole S. Gibran, MD; Matthew B. Klein, MD; Brett Arnoldo, MD; Ronald G. Tompkins, MD; David N. Herndon, MD. Ulysses G.J. Balis, M.D.; Paul E. Bankey, M.D., Ph.D.; Timothy R. Billiar, M.D.; Bernard H. Brownstein, Ph.D.; Steven E. Calvano, Ph.D.; David G. Camp II, Ph.D.; Irshad H. Chaudry, Ph.D.; J. Perren Cobb, M.D.; Joseph Cuschieri, M.D.; Ronald W. Davis, Ph.D.; Asit K. De, Ph.D.; Brian G. Harbrecht, M.D.; Laura Hennessy, R.N.; Jeffrey L. Johnson, M.D.; James A. Lederer, Ph.D.; Stephen F. Lowry, M.D.; Ronald V. Maier, M.D.; John A. Mannick, M.D.; Philip H. Mason, Ph.D.; Grace P. McDonald-Smith, M.Ed.; Carol L. Miller-Graziano, Ph.D.; Michael N. Mindrinos, Ph.D.; Joseph P. Minei, M.D.; Lyle L. Moldawer, Ph.D.; Ernest E. Moore, M.D.; Avery B. Nathens, M.D., Ph.D., M.P.H.; Grant E. O'Keefe, M.D., M.P.H.; Laurence G. Rahme, Ph.D.; Daniel G. Remick, M.D.; David A. Schoenfeld, Ph.D.; Michael B. Shapiro, M.D.; Geoffrey M. Silver, M.D.; Richard D. Smith, Ph.D.; Jason Sperry, M.D., Ph.D.; John D. Storey, Ph.D.; Robert Tibshirani, Ph.D.; Mehmet Toner, Ph.D.; H. Shaw Warren, M.D.; Michael A. West, M.D., PhD.; Bram Wispelwey, M.S.; and Wenzhong Xiao, Ph.D.
Source of Funding: This study was supported by - Canadian Institutes of Health Research # 123336. CFI Leader’s Opportunity Fund: Project # 25407 NIH RO1 GM087285-01
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
There is no conflict of interest.
References
- 1.Jeschke MG, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248(3):387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6(7):e21245. doi: 10.1371/journal.pone.0021245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wearn C, et al. Outcomes of burns in the elderly: revised estimates from the Birmingham Burn Centre. Burns. 2015;41(6):1161–1168. doi: 10.1016/j.burns.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Pereira CT, et al. Age-dependent differences in survival after severe burns: a unicentric review of 1,674 patients and 179 autopsies over 15 years. J Am Coll Surg. 2006;202(3):536–548. doi: 10.1016/j.jamcollsurg.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Kraft R, et al. Burn size and survival probability in paediatric patients in modern burn care: a prospective observational cohort study. Lancet. 2012;379(9820):1013–1021. doi: 10.1016/S0140-6736(11)61345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albornoz CR, et al. Burns are more aggressive in the elderly: proportion of deep burn area/total burn area might have a role in mortality. Burns. 2011;37(6):1058–1061. doi: 10.1016/j.burns.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Grimble RF. Inflammatory response in the elderly. Curr Opin Clin Nutr Metab Care. 2003;6(1):21–29. doi: 10.1097/00075197-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein AD, et al. Wound healing and aging. Dermatol Clin. 1993;11(4):749–757. [PubMed] [Google Scholar]
- 9.Lundgren RS, et al. Influence of comorbidities and age on outcome following burn injury in older adults. J Burn Care Res. 2009;30(2):307–314. doi: 10.1097/BCR.0b013e318198a416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham TN, et al. Epidemiology and outcomes of older adults with burn injury: an analysis of the National Burn Repository. J Burn Care Res. 2009;30(1):30–36. doi: 10.1097/BCR.0b013e3181921efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rani M, Schwacha MG. Aging and the pathogenic response to burn. Aging Dis. 2012;3(2):171–180. [PMC free article] [PubMed] [Google Scholar]
- 12.Orimo H. Reviewing the definition of elderly. Nihon Ronen Igakkai Zasshi. 2006;43(1):27–34. doi: 10.3143/geriatrics.43.27. [DOI] [PubMed] [Google Scholar]
- 13.Finnerty CC, et al. Temporal cytokine profiles in severely burned patients: a comparison of adults and children. Mol Med. 2008;14(9–10):553–560. doi: 10.2119/2007-00132.Finnerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein MB, et al. Inflammation and the host response to injury: an overview of the multicenter study of the genomic and proteomic response to burn injury. J Burn Care Res. 2006;27(4):448–451. doi: 10.1097/01.BCR.0000227477.33877.E6. [DOI] [PubMed] [Google Scholar]
- 15.Jeschke MG, et al. Morbidity and survival probability in burn patients in modern burn care. Crit Care Med. 2015;43(4):808–815. doi: 10.1097/CCM.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanojcic M, et al. Leukocyte infiltration and activation of the NLRP3 inflammasome in white adipose tissue following thermal injury. Crit Care Med. 2014;42(6):1357–1364. doi: 10.1097/CCM.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein MB, et al. Benchmarking outcomes in the critically injured burn patient. Ann Surg. 2014;259(5):833–841. doi: 10.1097/SLA.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oreffo RO, Triffitt JT. Future potentials for using osteogenic stem cells and biomaterials in orthopedics. Bone. 1999;25(2 Suppl):5S–9S. doi: 10.1016/s8756-3282(99)00124-6. [DOI] [PubMed] [Google Scholar]
- 19.Romanowski KS, et al. Frailty score on admission predicts outcomes in elderly burn injury. J Burn Care Res. 2015;36(1):1–6. doi: 10.1097/BCR.0000000000000190. [DOI] [PubMed] [Google Scholar]